Sex-Dependent Effects of Chronic Restraint Stress on Mood-Related Behaviours and Neurochemistry in Mice
Abstract
:1. Introduction
2. Results
2.1. Behavioural Tests
2.1.1. Open Field Test
2.1.2. Elevated Plus-Maze Test
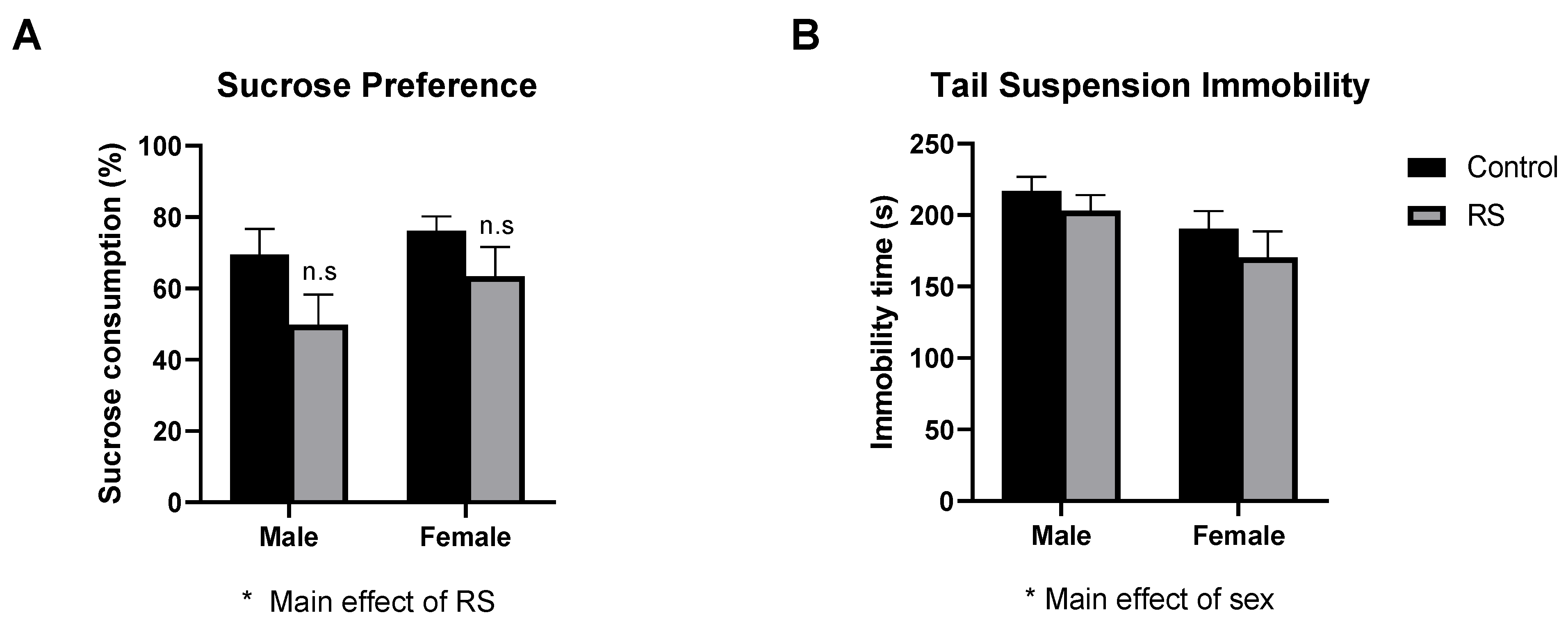
2.1.3. Sucrose Preference Test and Tail Suspension Test
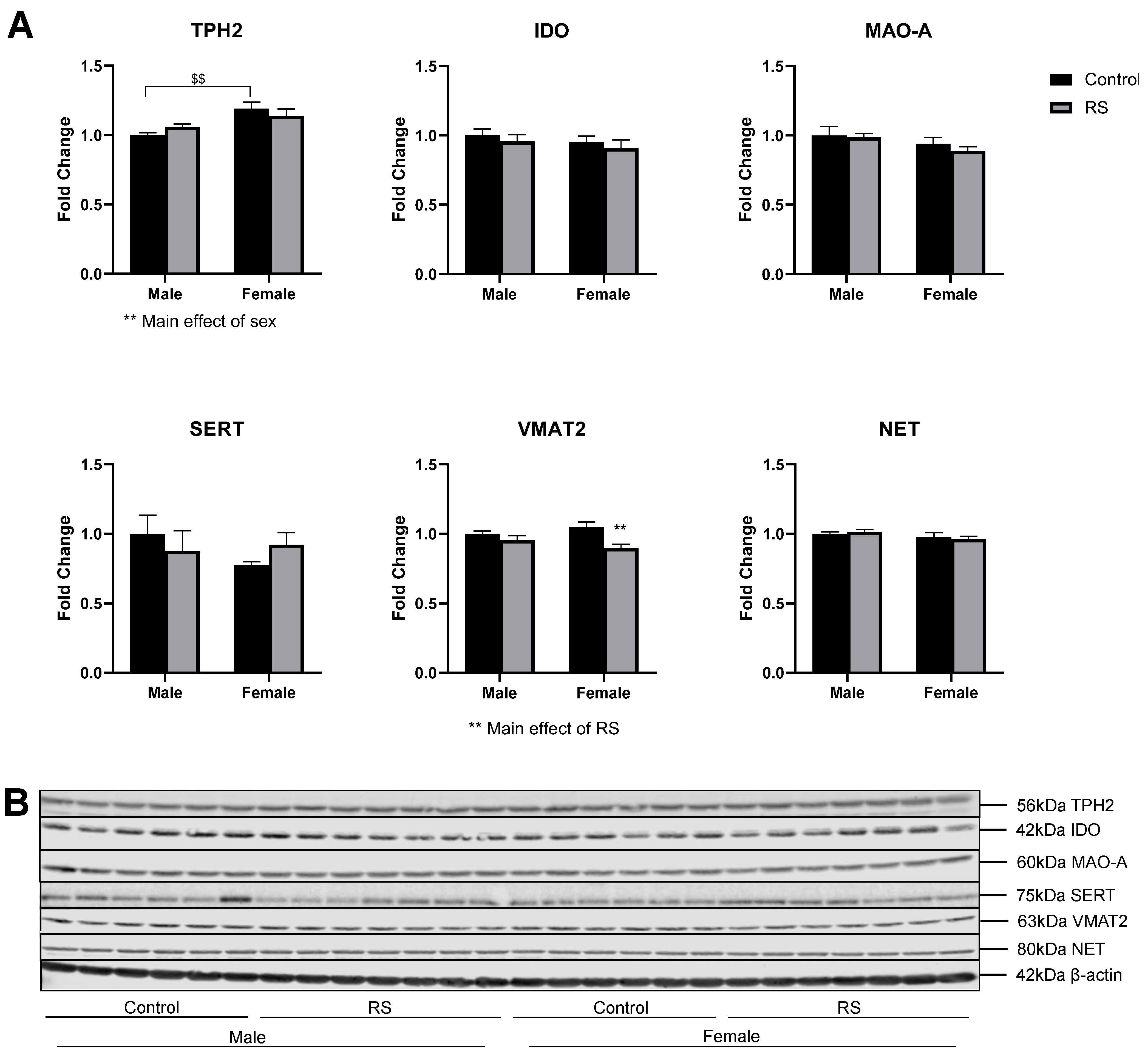
2.2. Serotonergic and Noradrenergic Regulation in the Prefrontal Cortex
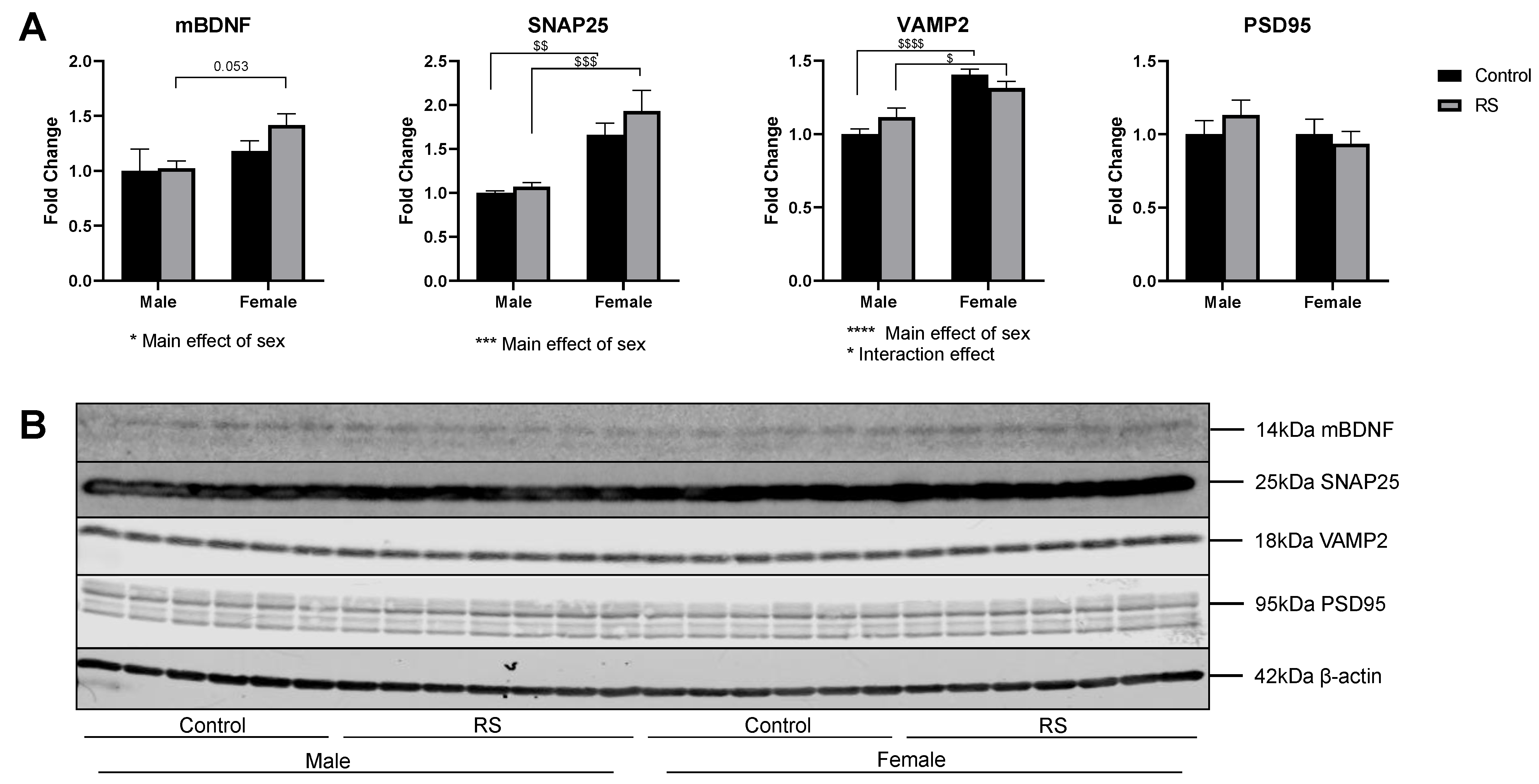
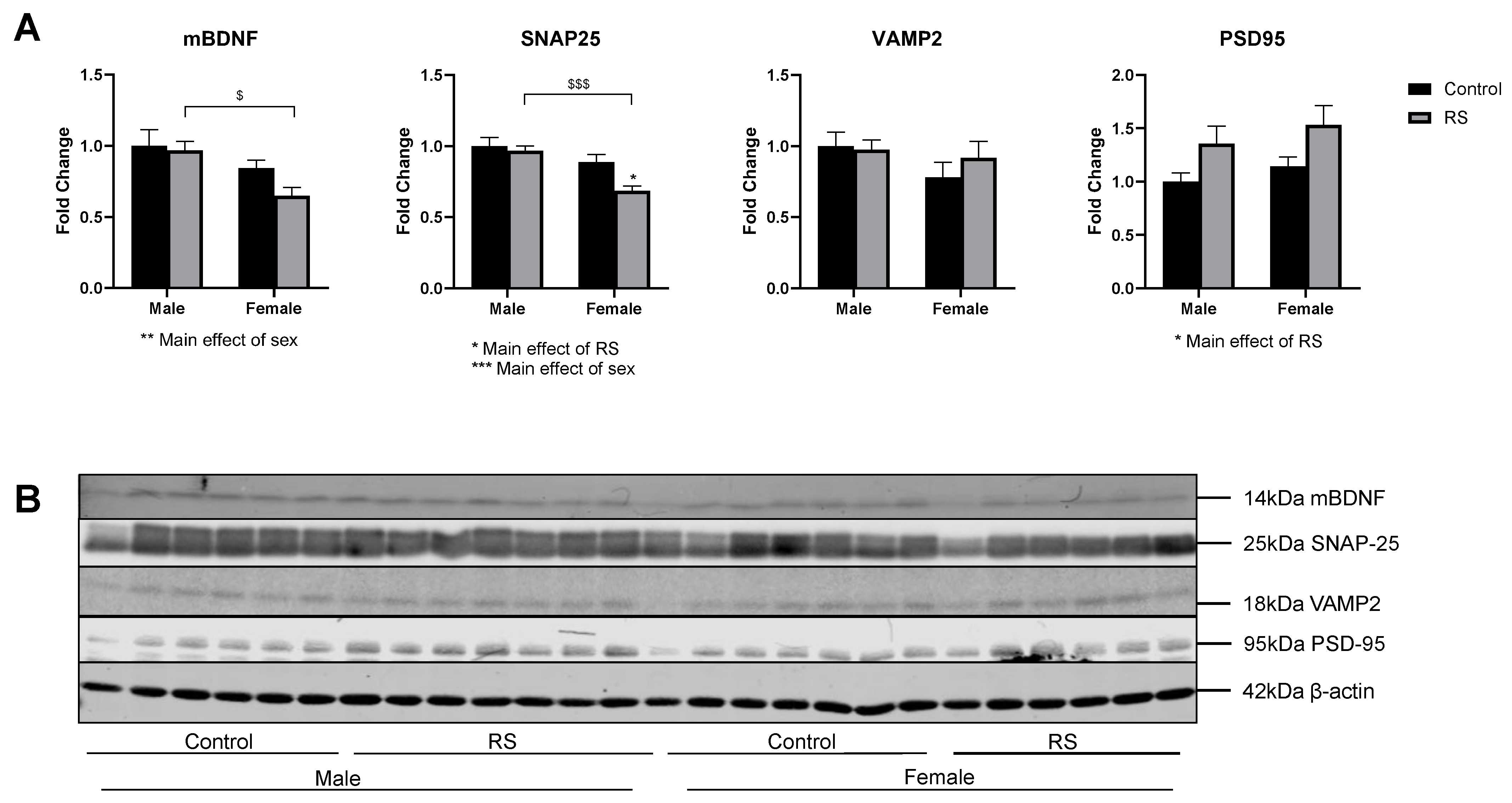
2.3. mBDNF and Synaptic Protein Expression in the Prefrontal Cortex
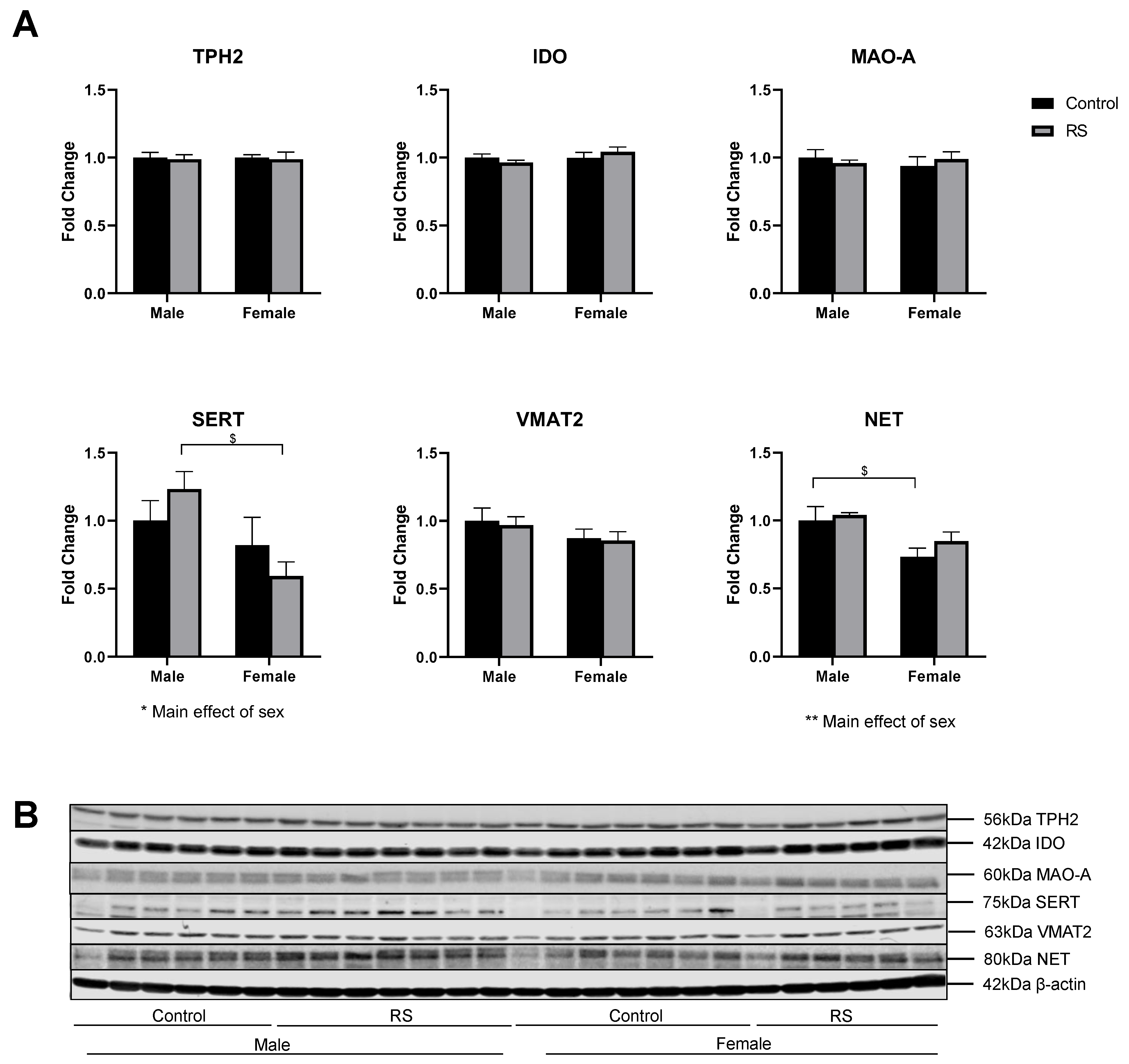
2.4. Serotonergic and Noradrenergic Regulation in the Hippocampus
2.5. mBDNF and Synaptic Protein Expression in the Hippocampus
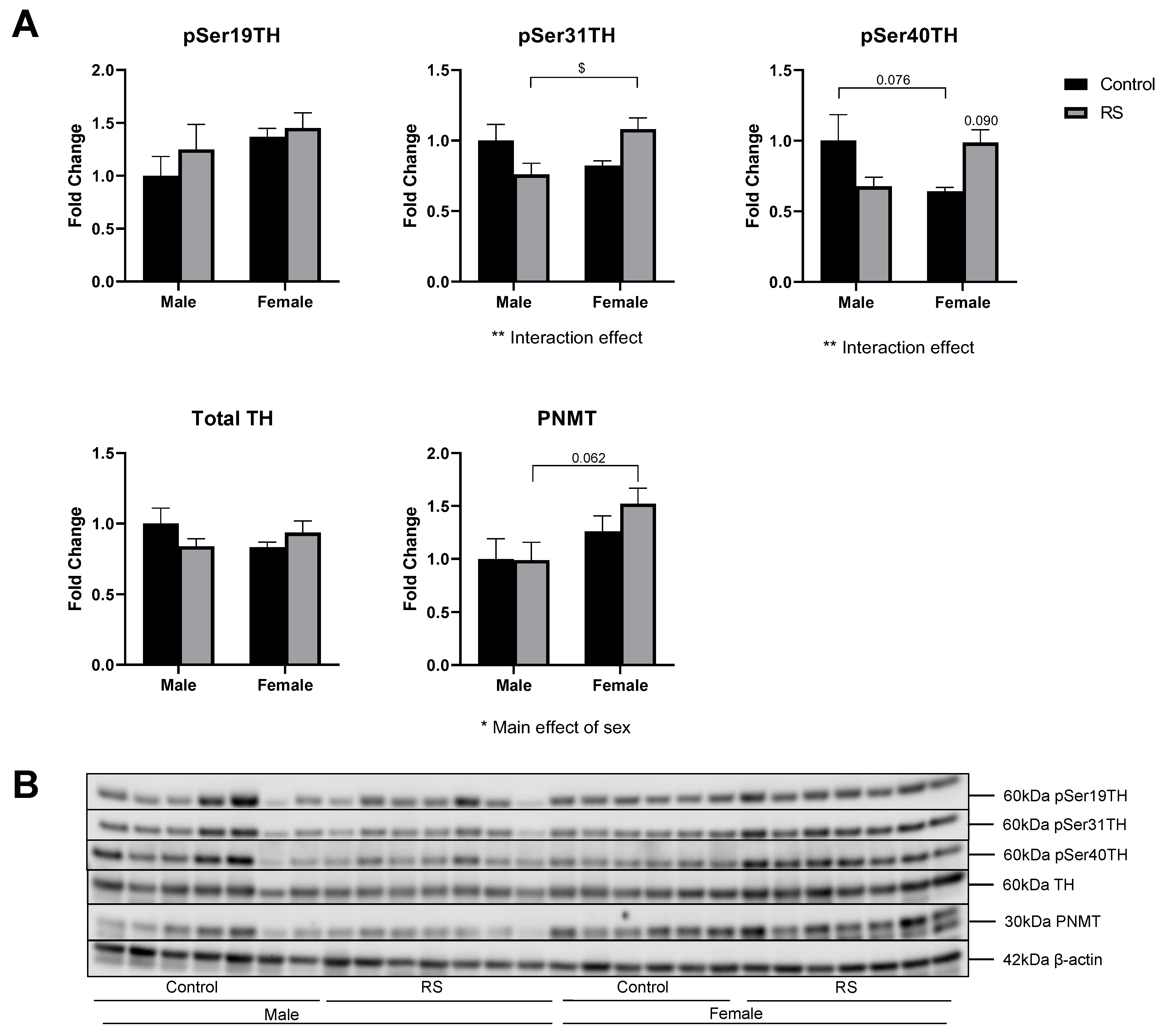
2.6. Regulation of Adrenal Catecholamine Synthesis
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Behavioural Tests
4.3.1. Open Field Test (OFT)
4.3.2. Elevated Plus-Maze (EPM) Test
4.3.3. Tail Suspension Test (TST)
4.3.4. Sucrose Preference Test (SPT)
4.4. Fresh Tissue Collection and Homogenisation
4.5. Western Blot
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Primary Antibodies | |||
|---|---|---|---|
| Specificity | Species | Manufacturer | Dilution |
| IDO | Mouse | Santa Cruz (Cat# sc-137012, RRID:AB_2123436) | 1:5000 in 1% milk/1% BSA/TBST |
| Anti-monoamine oxidase-A (MAO-A) | Monoclonal Rabbit | ABclonal (Cat# A4105, RRID:AB_2863189) | 1:2000 in 5% BSA/TBST |
| Anti-mature brain-derived neurotrophic factor (mBDNF) | Polyclonal Rabbit | Osenses (Cat# 2728, RRID: Not available) | 1:4000 in 5% BSA/TBST |
| Phenylethanolamine N-methyltransferase (PNMT) | Polyclonal Rabbit | Abcam (Cat# Ab69579, RRID:AB_2050200) | 1:3000 in 5% BSA/TBST |
| Anti-phospho-Serine 19-tyrosine hydroxylase (pSer19TH) | Rabbit | Homemade † | 1:6000 in TBST/Azide |
| Anti-phospho-Serine 31-tyrosine hydroxylase (pSer31TH) | Rabbit | Homemade † | 1:4000 in BSA/Azide |
| Anti-phospho-Serine 40-tyrosine hydroxylase (pSer40TH) | Sheep | Homemade † | 1:2000 in TBST/Azide |
| Anti-serotonin transporter (SERT) | Polyclonal Rabbit | ABclonal (Cat# A14171, RRID:AB_2761030) | 1:1000 in TBST |
| Anti-tyrosine hydroxylase (TH) | Monoclonal Mouse | Sigma (Cat# T1299, RRID:AB_477560) | 1:7000 in 5% BSA/TBST |
| Anti-tryptophan hydroxylase 1 (TPH1) | Polyclonal Rabbit | ABclonal (Cat# A1569, RRID:AB_2763100) | 1:2000 in 5% BSA/Azide |
| Anti-tryptophan hydroxylase 2 (TPH2) | Polyclonal Rabbit | Sigma (Cat# SAB2102520, RRID:AB_10606926) | 1:1000 in 5% BSA/Azide |
| Anti-vesicular monoamine transporter 2 (VMAT2) | Monoclonal Mouse | Santa Cruz (Cat# sc-374079, RRID:AB_10917928) | 1:1000 in 5% BSA/Azide |
| Noradrenaline transporter (NET) | Polyclonal Rabbit | Invitrogen (Cat# PA5-89680, RRID:AB_2805725) | 1:1000 in 5% BSA/Azide |
| Synaptosome-associated protein 25 (SNAP25) | Monoclonal Mouse | Santa Cruz (Cat# sc-20038, RRID:AB_628264) | 1:1000 in 5% BSA/Azide |
| Vesicle-associated membrane protein 2 (VAMP2) | Polyclonal Rabbit | Osenses Australia (Cat# OSS00035W, RRID: Not available) | 1:1500 in 5% BSA/Azide |
| Postsynaptic density protein 95 (PSD95) | Monoclonal Mouse | Sigma (Cat# P246, RRID:AB_260911) | 1:2000 in 5% BSA/Azide |
| β-actin | Monoclonal Rabbit (High Dilution) | ABclonal (Cat# AC026, RRID:AB_2768234) | 1:20,000 in TBST |
| β-actin | Monoclonal Mouse | Abcam (Cat# ab66338, RRID:AB_2289239) | 1:8000 in TBST |
| Secondary Antibodies | |||
| anti-rabbit IgG | Polyclonal Donkey | Li-Cor Biosciences (Cat# 926-32213, RRID:AB_621848) | 1:20,000 in TBST |
| anti-mouse IgG | Polyclonal Goat | Li-Cor Biosciences (Cat# 926-32210, RRID:AB_621842) | 1:20,000 in TBST |
| anti-goat IgG | Polyclonal Donkey | Li-Cor Biosciences (Cat# 926-32214, RRID:AB_621846) | 1:20,000 in TBST |
References
- Nemeroff, C.B. The state of our understanding of the pathophysiology and optimal treatment of depression: Glass half full or half empty? Am. J. Psychiatry 2020, 177, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Brigitta, B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin. Neurosci. 2002, 4, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S. Pathophysiology of depression and innovative treatments: Remodeling glutamatergic synaptic connections. Dialogues Clin. Neurosci. 2014, 16, 11–27. [Google Scholar] [CrossRef]
- Adwas, A.A.; Jbireal, J.; Azab, A.E. Anxiety: Insights into signs, symptoms, etiology, pathophysiology, and treatment. East Afr. Sch. J. Med. Sci. 2019, 2, 580–591. [Google Scholar]
- Menke, A. Is the hpa axis as target for depression outdated, or is there a new hope? Front. Psychiatry 2019, 10, 101. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022, 27, 9004. [Google Scholar] [CrossRef]
- Brown, S.J.; Huang, X.-F.; Newell, K.A. The kynurenine pathway in major depression: What we know and where to next. Neurosci. Biobehav. Rev. 2021, 127, 917–927. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Tryptophan metabolism in depression: A narrative review with a focus on serotonin and kynurenine pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef]
- Agorastos, A.; Chrousos, G.P. The neuroendocrinology of stress: The stress-related continuum of chronic disease development. Mol. Psychiatry 2022, 27, 502–513. [Google Scholar] [CrossRef]
- Gulyaeva, N.V. Glucocorticoid regulation of the glutamatergicsynapse: Mechanisms of stress-dependent neuroplasticity. J. Evol. Biochem. Physiol. 2021, 57, 564–576. [Google Scholar] [CrossRef]
- Saaltink, D.-J.; Vreugdenhil, E. Stress, glucocorticoid receptors, and adult neurogenesis: A balance between excitation and inhibition? Cell. Mol. Life Sci. 2014, 71, 2499–2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Chen, H.; Chen, Y.; Wei, W.; Sun, Y.; Zhang, L.; Cui, L.; Wang, Y. Dysfunction of the snare complex in neurological and psychiatric disorders. Pharmacol. Res. 2021, 165, 105469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, I. The impact of emotional arousal on amygdala activity, memory consolidation, and long-term potentiation in the hippocampus. J. Stud. Res. 2022, 11, 1–18. [Google Scholar] [CrossRef]
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatry 2017, 27, 101–111. [Google Scholar] [CrossRef]
- Amano, A.; Tsunoda, M.; Aigaki, T.; Maruyama, N.; Ishigami, A. Age-related changes of dopamine, noradrenaline and adrenaline in adrenal glands of mice. Geriatr. Gerontol. Int. 2013, 13, 490–496. [Google Scholar] [CrossRef]
- Dunkley, P.R.; Dickson, P.W. Tyrosine hydroxylase phosphorylation in vivo. J. Neurochem. 2019, 149, 706–728. [Google Scholar] [CrossRef] [Green Version]
- Kumer, S.C.; Vrana, K.E. Intricate regulation of tyrosine hydroxylase activity and gene expression. J. Neurochem. 1996, 67, 443–462. [Google Scholar] [CrossRef]
- Stefanovic, B.; Spasojevic, N.; Jovanovic, P.; Dronjak, S.J. Pharmacology. Melatonin treatment affects changes in adrenal gene expression of catecholamine biosynthesizing enzymes and norepinephrine transporter in the rat model of chronic-stress-induced depression. Can. J. Physiol. Pharmacol. 2019, 97, 685–690. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, P.; Stefanovic, B.; Spasojevic, N.; Puskas, N.; Dronjak, S.J.E.R. Effects of oxytocin on adreno-medullary catecholamine synthesis, uptake and storage in rats exposed to chronic isolation stress. Endocr. Res. 2016, 41, 124–131. [Google Scholar] [CrossRef]
- Gavrilovic, L.; Spasojevic, N.; Tanic, N.; Dronjak, S.J.N.L. Chronic isolation of adult rats decreases gene expression of catecholamine biosynthetic enzymes in adrenal medulla. Neuroendocrinol. Lett. 2008, 29, 1015. [Google Scholar]
- Manni, L.; Di Fausto, V.; Fiore, M.; Aloe, L. Repeated restraint and nerve growth factor administration in male and female mice: Effect on sympathetic and cardiovascular mediators of the stress response. Curr. Neurovascular Res. 2008, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, T.P.; Gunn, R.K.; Brown, R.E. What are we measuring when we test strain differences in anxiety in mice? Behav. Genet. 2013, 43, 34–50. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, M.; Wang, J.; Yao, J.; Yu, J.; Liu, W.; Wu, L.; Wang, J.; Gao, R. Involvement of the microbiota-gut-brain axis in chronic restraint stress: Disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 2021, 13, 1869501. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Yang, J.; Zhu, T.; Liu, P.; Yang, J. Bdnf-trkb signaling-mediated upregulation of narp is involved in the antidepressant-like effects of (2r,6r)-hydroxynorketamine in a chronic restraint stress mouse model. BMC Psychiatry 2022, 22, 182. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.; Ryan, C.; Curley, A.; Mulcaire, J.; Kelly, J.P. Sex differences in baseline and drug-induced behavioural responses in classical behavioural tests. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 37, 227–236. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, X.; Zhou, Y.; Zheng, Q.; Chen, Z.; Zhang, H.; Sun, Z.; Xu, G.; Hu, G. Hypothalamus-pituitary-adrenal axis imbalance and inflammation contribute to sex differences in separation- and restraint-induced depression. Horm. Behav. 2020, 122, 104741. [Google Scholar] [CrossRef]
- Bittar, T.P.; Pelaez, M.C.; Hernandez Silva, J.C.; Quessy, F.; Lavigne, A.-A.; Morency, D.; Blanchette, L.-J.; Arsenault, E.; Cherasse, Y.; Seigneur, J.; et al. Chronic stress induces sex-specific functional and morphological alterations in corticoaccumbal and corticotegmental pathways. Biol. Psychiatry 2021, 90, 194–205. [Google Scholar] [CrossRef]
- Olave, F.A.; Aguayo, F.I.; Román-Albasini, L.; Corrales, W.A.; Silva, J.P.; González, P.I.; Lagos, S.; García, M.A.; Alarcón-Mardones, M.; Rojas, P.S.; et al. Chronic restraint stress produces sex-specific behavioral and molecular outcomes in the dorsal and ventral rat hippocampus. Neurobiol. Stress 2022, 17, 100440. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, W.; Li, C.; Su, Z.; Guo, Z.; Li, Z.; Wang, C.; Xu, F.J.P. Sex differences in peripheral monoamine transmitter and related hormone levels in chronic stress mice with a depression-like phenotype. PeerJ 2022, 10, e14014. [Google Scholar] [CrossRef]
- Liu, L.-L.; Li, J.-M.; Su, W.-J.; Wang, B.; Jiang, C.-L. Sex differences in depressive-like behaviour may relate to imbalance of microglia activation in the hippocampus. Brain Behav. Immun. 2019, 81, 188–197. [Google Scholar] [CrossRef]
- Fonseca-Rodrigues, D.; Gonçalves, J.; Laranjeira, I.; Almeida, A.; Pinto-Ribeiro, F. Sucrose intake and preference by wistar han rats are not influenced by sex or food/water deprivation. Pharmacol. Biochem. Behav. 2022, 216, 173387. [Google Scholar] [CrossRef] [PubMed]
- Colelli, V.; Campus, P.; Conversi, D.; Orsini, C.; Cabib, S. Either the dorsal hippocampus or the dorsolateral striatum is selectively involved in consolidation of forced swim-induced immobility depending on genetic background. Neurobiol. Learn. Mem. 2014, 111, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, Z.-H.; Ge, M.-J.; Shen, J.-X.; Han, F.; Pan, C.; Chen, J.-J.; Zhu, X.-L.; Hou, W.-Y.; Hou, Y.-Q.; et al. Estradiol attenuates chronic restraint stress-induced dendrite and dendritic spine loss and cofilin1 activation in ovariectomized mice. Horm. Behav. 2021, 135, 105040. [Google Scholar] [CrossRef] [PubMed]
- Bangasser, D.A.; Cuarenta, A. Sex differences in anxiety and depression: Circuits and mechanisms. Nat. Rev. Neurosci. 2021, 22, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Pais, M.L.; Martins, J.; Castelo-Branco, M.; Gonçalves, J. Sex differences in tryptophan metabolism: A systematic review focused on neuropsychiatric disorders. Int. J. Mol. Sci. 2023, 24, 6010. [Google Scholar] [CrossRef]
- Xing, H.; Zhang, X.; Xing, N.; Qu, H.; Zhang, K. Uncovering pharmacological mechanisms of zhi-zi-hou-po decoction in chronic unpredictable mild stress induced rats through pharmacokinetics, monoamine neurotransmitter and neurogenesis. J. Ethnopharmacol. 2019, 243, 112079. [Google Scholar] [CrossRef]
- Couch, Y.; Anthony, D.C.; Dolgov, O.; Revischin, A.; Festoff, B.; Santos, A.I.; Steinbusch, H.W.; Strekalova, T. Microglial activation, increased tnf and sert expression in the prefrontal cortex define stress-altered behaviour in mice susceptible to anhedonia. Brain Behav. Immun. 2013, 29, 136–146. [Google Scholar] [CrossRef]
- Gavrilović, L.; Popović, N.M.; Stojiljković, V.; Pejić, S.; Todorović, A.; Pavlović, I.; Pajović, S.B.J.F.B. Changes of hippocampal noradrenergic capacity in stress condition. Folia Biol. 2020, 66, 81–84. [Google Scholar]
- Eiden, L.E.; Weihe, E. Vmat2: A dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann. New York Acad. Sci. 2011, 1216, 86–98. [Google Scholar] [CrossRef] [Green Version]
- Müller, H.K.; Wegener, G.; Popoli, M.; Elfving, B. Differential expression of synaptic proteins after chronic restraint stress in rat prefrontal cortex and hippocampus. Brain Res. 2011, 1385, 26–37. [Google Scholar] [CrossRef]
- Notaras, M.; van den Buuse, M. Neurobiology of bdnf in fear memory, sensitivity to stress, and stress-related disorders. Mol. Psychiatry 2020, 25, 2251–2274. [Google Scholar] [CrossRef]
- Chan, C.B.; Ye, K. Sex differences in brain-derived neurotrophic factor signaling and functions. J. Neurosci. Res. 2017, 95, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Yang, L.; Wei, W. Role of fto on camkii/creb signaling pathway of hippocampus in depressive-like behaviors induced by chronic restraint stress mice. Behav. Brain Res. 2021, 406, 113227. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.K.; Guan, L.; Stutz, B.; Dickson, P.W.; Dunkley, P.R.; Bobrovskaya, L. The effects of footshock and immobilization stress on tyrosine hydroxylase phosphorylation in the rat locus coeruleus and adrenal gland. Neuroscience 2011, 192, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, X.; Sun, B.; Sterling, C.; Tank, A.W. Evidence for regulation of tyrosine hydroxylase mrna translation by stress in rat adrenal medulla. Brain Res. 2007, 1158, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabban, E.L.; Kvetňanský, R. Stress-triggered activation of gene expression in catecholaminergic systems: Dynamics of transcriptional events. Trends Neurosci. 2001, 24, 91–98. [Google Scholar] [CrossRef]
- Stroth, N.; Eiden, L.E. Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience 2010, 165, 1025–1030. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.L.; Tai, T.C.; Wong-Faull, D.C.; Claycomb, R.; Meloni, E.G.; Myers, K.M.; Carlezon, W.A.; Kvetnansky, R. Epinephrine: A short- and long-term regulator of stress and development of illness. Cell. Mol. Neurobiol. 2012, 32, 737–748. [Google Scholar] [CrossRef]
- Sadler, A.M.; Bailey, S.J. Repeated daily restraint stress induces adaptive behavioural changes in both adult and juvenile mice. Physiol. Behav. 2016, 167, 313–323. [Google Scholar] [CrossRef]
- Brivio, P.; Gallo, M.T.; Gruca, P.; Lason, M.; Litwa, E.; Fumagalli, F.; Papp, M.; Calabrese, F. Resilience to chronic mild stress-induced anhedonia preserves the ability of the ventral hippocampus to respond to an acute challenge. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 1–10. [Google Scholar] [CrossRef]
- Taliaz, D.; Loya, A.; Gersner, R.; Haramati, S.; Chen, A.; Zangen, A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 4475–4483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Herselman, M.F.; Zhou, X.-F.; Bobrovskaya, L. Effects of corticosterone on bdnf expression and mood behaviours in mice. Physiol. Behav. 2022, 247, 113721. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.L.; Bobrovskaya, L.; Dunkley, P.R.; Dickson, P.W. Differential regulation of human tyrosine hydroxylase isoforms 1 and 2 in situ: Isoform 2 is not phosphorylated at ser35. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 1860–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herselman, M.F.; Lin, L.; Luo, S.; Yamanaka, A.; Zhou, X.-F.; Bobrovskaya, L. Sex-Dependent Effects of Chronic Restraint Stress on Mood-Related Behaviours and Neurochemistry in Mice. Int. J. Mol. Sci. 2023, 24, 10353. https://doi.org/10.3390/ijms241210353
Herselman MF, Lin L, Luo S, Yamanaka A, Zhou X-F, Bobrovskaya L. Sex-Dependent Effects of Chronic Restraint Stress on Mood-Related Behaviours and Neurochemistry in Mice. International Journal of Molecular Sciences. 2023; 24(12):10353. https://doi.org/10.3390/ijms241210353
Chicago/Turabian StyleHerselman, Mauritz Frederick, Liying Lin, Shayan Luo, Akihiro Yamanaka, Xin-Fu Zhou, and Larisa Bobrovskaya. 2023. "Sex-Dependent Effects of Chronic Restraint Stress on Mood-Related Behaviours and Neurochemistry in Mice" International Journal of Molecular Sciences 24, no. 12: 10353. https://doi.org/10.3390/ijms241210353
APA StyleHerselman, M. F., Lin, L., Luo, S., Yamanaka, A., Zhou, X.-F., & Bobrovskaya, L. (2023). Sex-Dependent Effects of Chronic Restraint Stress on Mood-Related Behaviours and Neurochemistry in Mice. International Journal of Molecular Sciences, 24(12), 10353. https://doi.org/10.3390/ijms241210353






