The Impact of Gold Nanoparticles on Somatic Embryogenesis Using the Example of Arabidopsis thaliana
Abstract
:1. Introduction
2. Results
2.1. Developmental Changes in Explants during Culture
2.2. Histological Changes in Explants
2.3. Spatio-Temporal Changes in the Wall Components
2.4. Distribution of Pectic Epitopes during Culture
2.5. Distribution of AGP Epitopes during Culture
2.6. Distribution of Extensin Epitopes during Culture
3. Discussion
3.1. Nanoparticles and Somatic Embryogenesis
3.2. Cell Wall Composition during SE in Control
3.3. Differences in Wall Composition between Control and NP-Treated Explants
4. Materials and Methods
4.1. Nanoparticle Characterization
4.2. Plant Material and Growth Conditions
4.3. Sample Preparation
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ndlovu, N.; Mayaya, T.; Muitire, C.; Munyengwa, N. Nanotechnology applications in crop production and food systems. Int. J. Plant Breed. 2020, 7, 624–634. [Google Scholar]
- Yasmeen, R. Biological and clinical perspectives of nanobiotechnology. Res. J. Adv. Sci. 2021, 2, 1–8. [Google Scholar]
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Bajguz, A. Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 2020, 9, 825. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, M.; Bhandari, B. Nanotechnology—A shelf life extension strategy for fruits and vegetables. Crit. Rev. Food Sci. 2020, 60, 1706–1721. [Google Scholar] [CrossRef] [PubMed]
- Tarrahi, R.; Mahjouri, S.; Khataee, A. A review on in vivo and in vitro nanotoxicological studies in plants: A headlight for future targets. Ecotoxi. Environ. Safe 2021, 208, 111697. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Firoz, M.; Al-Khaishany, M.Y. Role of nanoparticles in plants. In Nanotechnology and Plant Sciences: Nanoparticles and Their Impact on Plants; Springer: Berlin/Heidelberg, Germany, 2015; pp. 19–35. [Google Scholar]
- Martinez-Ballesta, M.C.; Chelbi, N.; Lopez-Zaplana, A.; Carvajal, M. Discerning the mechanism of the multiwalled carbon nanotubes effect on root cell water and nutrient transport. Plant Physiol. Bioch. 2020, 146, 23–30. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe nanoparticles: Are we there yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Kumari, P.; Seth, R.; Meena, A. Consequences of nanomaterials on human health and ecosystem. In Nanotoxicology; CRC Press: Boca Raton, FL, USA, 2021; pp. 157–200. [Google Scholar]
- Mohana, N.C.; Mithun, P.R.; Rao, H.Y.; Mahendra, C.; Satish, S. Nanoparticle applications in sustainable agriculture, poultry, and food: Trends and perspective. In Nanotoxicity; Elsevier: Amsterdam, The Netherlands, 2020; pp. 341–353. [Google Scholar]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Milewska-Hendel, A.; Gepfert, W.; Zubko, M.; Kurczyńska, E. Morphological, Histological and Ultrastructural Changes in Hordeum vulgare (L.) Roots That Have Been Exposed to Negatively Charged Gold Nanoparticles. Appl. Sci. 2022, 12, 3265. [Google Scholar] [CrossRef]
- Parzymies, M. Nano-silver particles reduce contaminations in tissue culture but decrease regeneration rate and slows down growth and development of Aldrovanda vesiculosa explants. Appl. Sci. 2021, 11, 3653. [Google Scholar] [CrossRef]
- Zanelli, D.; Candotto Carniel, F.; Tretiach, M. The Interaction of Graphene Oxide with the Pollen− Stigma System: In Vivo Effects on the Sexual Reproduction of Cucurbita pepo L. Appl. Sci. 2021, 11, 6150. [Google Scholar] [CrossRef]
- Kurczyńska, E.; Godel-Jędrychowska, K.; Sala, K.; Milewska-Hendel, A. Nanoparticles—Plant interaction: What we know, where we are? Appl. Sci. 2021, 11, 5473. [Google Scholar] [CrossRef]
- Kralova, K.; Jampilek, J. Responses of medicinal and aromatic plants to engineered nanoparticles. App. Sci. 2021, 11, 1813. [Google Scholar] [CrossRef]
- Sivanesan, I.; Nayeem, S.; Venkidasamy, B.; Kuppuraj, S.P.; Rn, C.; Samynathan, R. Genetic and epigenetic modes of the regulation of somatic embryogenesis: A review. Biol. Fut. 2022, 73, 259–277. [Google Scholar] [CrossRef]
- Fehér, A. Callus, dedifferentiation, totipotency, somatic embryogenesis: What these terms mean in the era of molecular plant biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef] [Green Version]
- Boutilier, K.; Offringa, R.; Sharma, V.K.; Kieft, H.; Ouellet, T.; Zhang, L.; van Lookeren Campagne, M.M. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 2002, 14, 1737–1749. [Google Scholar] [CrossRef] [Green Version]
- Kokina, I.; Gerbreders, V.; Sledevskis, E.; Bulanovs, A. Penetration of nanoparticles in flax (Linum usitatissimum L.) calli and regenerants. J. Biotechnol. 2013, 165, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Saranya, S.; Aswani, R.; Remakanthan, A.; Radhakrishnan, E.K. Nanotechnology in agriculture. In Nanotechnology for Agriculture: Advances for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–17. [Google Scholar]
- Kokina, I.; Jahundoviča, I.; Mickeviča, I.; Jermaļonoka, M.; Strautiņš, J.; Popovs, S.; Gerbreders, V. Target transportation of auxin on mesoporous Au/SiO2 nanoparticles as a method for somaclonal variation increasing in flax (L. usitatissimum L.). J. Nanomater. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.S.; Fahmy, A.H.; Ahmed, S.S. Copper nanoparticles elevate regeneration capacity of (Ocimum basilicum L.) plant via somatic embryogenesis. Plant Cell Tiss. Organ 2019, 136, 41–50. [Google Scholar] [CrossRef]
- Nalci, O.B.; Nadaroglu, H.; Pour, A.H.; Gungor, A.A.; Haliloglu, K. Effects of ZnO, CuO and γ-Fe 3 O 4 nanoparticles on mature embryo culture of wheat (Triticum aestivum L.). Plant Cell Tiss. Organ 2019, 136, 269–277. [Google Scholar] [CrossRef]
- Giorgetti, L.; Castiglione, M.R.; Bernabini, M.; Geri, C. Nanoparticles effects on growth and differentiation in cell culture of carrot (Daucus carota L.). Agrochimica 2011, 55, 45–53. [Google Scholar]
- Elsayh, S.A.; Arafa, R.N.; Ali, G.A.; Abdelaal, W.B.; Sidky, R.A.; Ragab, T.I. Impact of silver nanoparticles on multiplication, rooting of shoots and biochemical analyses of date palm Hayani cv. by in vitro. J. Agric. Biotech. 2022, 43, 102400. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X. Regulation of somatic embryogenesis in higher plants. Crit. Rev. Plant Sci. 2010, 29, 36–57. [Google Scholar] [CrossRef]
- Rui, Y.; Dinneny, J.R. A wall with integrity: Surveillance and maintenance of the plant cell wall under stress. New Phytol. 2020, 225, 1428–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sala, K.; Malarz, K.; Barlow, P.W.; Kurczyńska, E.U. Distribution of some pectic and arabinogalactan protein epitopes during Solanum lycopersicum (L.) adventitious root development. Plant Biol. 2017, 17, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sala, K.; Karcz, J.; Rypień, A.; Kurczyńska, E.U. Unmethyl-esterified homogalacturonan and extensins seal Arabidopsis graft union. Plant Biol. 2019, 19, 151. [Google Scholar] [CrossRef]
- Popielarska-Konieczna, M.; Sala, K.; Abdullah, M.; Tuleja, M.; Kurczyńska, E. Extracellular matrix and wall composition are diverse in the organogenic and non-organogenic calli of Actinidia arguta. Plant Cell Rep. 2020, 39, 779–798. [Google Scholar] [CrossRef] [Green Version]
- Milewska-Hendel, A.; Sala, K.; Gepfert, W.; Kurczyńska, E. Gold nanoparticles-induced modifications in cell wall composition in barley roots. Cells 2021, 10, 1965. [Google Scholar] [CrossRef]
- Potocka, I.; Godel, K.; Dobrowolska, I.; Kurczyńska, E.U. Spatio-temporal localization of selected pectic and arabinogalactan protein epitopes and the ultrastructural characteristics of explant cells that accompany the changes in the cell fate during somatic embryogenesis in Arabidopsis thaliana. Plant Physiol. Biochem. 2018, 127, 573–589. [Google Scholar] [CrossRef]
- Kuczak, M.; Kurczyńska, E. Cell wall composition as a marker of the reprogramming of the cell fate on the example of a Daucus carota (L.) hypocotyl in which somatic embryogenesis was induced. Inter. J. Mol. Sci. 2020, 21, 8126. [Google Scholar] [CrossRef]
- Betekhtin, A.; Rojek, M.; Milewska-Hendel, A.; Gawecki, R.; Karcz, J.; Kurczynska, E.; Hasterok, R. Spatial distribution of selected chemical cell wall components in the embryogenic callus of Brachypodium distachyon. PLoS ONE 2016, 11, e0167426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurczynska, E.U.; Potocka, I.; Dobrowolska, I.; Kulinska-Lukaszek, K.; Sala, K.; Wrobel, J. Cellular markers for somatic embryogenesis. In Embryogenesis; IntechOpen: London, UK, 2012. [Google Scholar]
- Li, H.; Ye, X.; Guo, X.; Geng, Z.; Wang, G. Effects of surface ligands on the uptake and transport of gold nanoparticles in rice and tomato. J. Hazard. Mater. 2016, 314, 188–196. [Google Scholar] [CrossRef]
- Sun, H.; Lei, C.; Xu, J.; Li, R. Foliar uptake and leaf-to-root translocation of nanoplastics with different coating charge in maize plants. J. Hazard. Mater. 2021, 416, 125854. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Wang, H.; Yan, B.; Zheng, H.; Jiang, Y.; Miranda, O.R.; Vachet, R.W. Effect of surface charge on the uptake and distribution of gold nanoparticles in four plant species. Environ. Sci. Technol. 2012, 46, 12391–12398. [Google Scholar] [CrossRef]
- Liu, M.; Feng, S.; Ma, Y.; Xie, C.; He, X.; Ding, Y.; Zhang, Z. Influence of surface charge on the phytotoxicity, transformation, and translocation of CeO2 nanoparticles in cucumber plants. ACS Appl. Mater. Interfaces 2019, 11, 16905–16913. [Google Scholar] [CrossRef] [PubMed]
- Spielman-Sun, E.; Lombi, E.; Donner, E.; Howard, D.; Unrine, J.M.; Lowry, G.V. Impact of surface charge on cerium oxide nanoparticle uptake and translocation by wheat (Triticum aestivum). Sci. Technol. Stud. 2017, 51, 7361–7368. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Dang, F.; Huang, Y.; Chen, N.; Zhou, D. Uptake, translocation, and transformation of silver nanoparticles in plants. Environ. Sci. Nano. 2022, 9, 12–39. [Google Scholar] [CrossRef]
- Spielman-Sun, E.; Avellan, A.; Bland, G.D.; Tappero, R.V.; Acerbo, A.S.; Unrine, J.M.; Lowry, G.V. Nanoparticle surface charge influences translocation and leaf distribution in vascular plants with contrasting anatomy. Environ. Sci. Nano. 2019, 6, 2508–2519. [Google Scholar] [CrossRef]
- Godel-Jedrychowska, K.; Kulinska-Lukaszek, K.; Horstman, A.; Soriano, M.; Li, M.; Malota, K.; Kurczynska, E.U. Symplasmic isolation marks cell fate changes during somatic embryogenesis. J. Exp. Bot. 2020, 71, 2612–2628. [Google Scholar] [CrossRef] [Green Version]
- Aghdaei, M.; Sarmast, M.K.; Salehi, H. Effects of silver nanoparticles on Tecomella undulata (Roxb.) Seem. Micropropagation. Adv. Hortic. Sci. 2012, 26, 21–24. [Google Scholar]
- Sarmast, M.K.; Niazi, A.; Salehi, H.; Abolimoghadam, A. Silver nanoparticles affect ACS expression in Tecomella undulata in Vitro culture. Plant Cell Tiss. Organ 2015, 121, 227–236. [Google Scholar] [CrossRef]
- Helaly, M.N.; El-Metwally, M.A.; El-Hoseiny, H.; Omar, S.A.; El-Sheery, N.I. Effect of nanoparticles on biological contamination of in Vitro cultures and organogenic regeneration of banana. Aust. J. Crop. Sci. 2014, 8, 612–624. [Google Scholar]
- Mahendran, D.; Kavi Kishor, P.B.; Geetha, N.; Venkatachalam, P. Phycomolecule-coated silver nanoparticles and seaweed extracts induced high-frequency somatic embryogenesis and plant regeneration from Gloriosa superba L. J. Appl. Phycol. 2018, 30, 1425–1436. [Google Scholar] [CrossRef]
- Devasia, J.; Muniswamy, B.; Mishra, M.K. Investigation of ZnO Nanoparticles on In Vitro Cultures of Coffee (Coffea Arabica L.). Int. J. Nanosci. Nanotechnol. 2020, 16, 271–277. [Google Scholar]
- Nhut, D.T.; Duc, H.H.; Hoang, N.H.; Ngan, H.T.M.; Diem, L.T.; Tung, H.T.; Huong, T.T. Efficient transgenic plantlet regeneration from hairy roots via somatic embryogenesis and hardening plantlets of Panax vietnamensis by iron nanoparticles-supplied culture. Plant Cell Tiss. Organ 2022, 151, 335–345. [Google Scholar] [CrossRef]
- Manh Cuong, D.; Cong Du, P.; Tung, H.T.; Ngan, H.T.M.; Luan, V.Q.; Phong, T.H.; Khai, H.D.; Phuong, T.T.B.; Nhut, D.T. Silver nanoparticles as an effective stimulant in micropropagation of Panax vietnamensis—A valuable medicinal plant. Plant Cell Tiss. Organ Cult. 2021, 146, 577–588. [Google Scholar] [CrossRef]
- Taha, R.A.; Hassan, M.M.; Ibrahim, E.A.; Abou Baker, N.H.; Shaaban, E.A. Carbon nanotubes impact on date palm in Vitro cultures. Plant Cell Tiss. Organ Cult. 2016, 127, 525–534. [Google Scholar] [CrossRef]
- Van Hengel, A.J.; Tadesse, Z.; Immerzeel, P.; Schols, H.; Van Kammen, A.B.; de Vries, S.C. N-acetylglucosamine and glucosamine-containing arabinogalactan proteins control somatic embryogenesis. Plant Physiol. 2001, 125, 1880–1890. [Google Scholar] [CrossRef] [Green Version]
- Baluška, F.; Hlavacka, A.; Sˇamaj, J.; Palme, K.; Robinson, D.G.; Matoh, T.; Volkmann, D. F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments. Plant Physiol. 2002, 130, 422–431. [Google Scholar] [CrossRef] [Green Version]
- Baluška, F.; Liners, F.; Hlavačka, A.; Schlicht, M.; Van Cutsem, P.; McCurdy, D.W.; Menzel, D. Cell wall pectins and xyloglucans are internalized into dividing root cells and accumulate within cell plates during cytokinesis. Protoplasma 2005, 225, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Van Hengel, A.J.; Van Kammen, A.B.; De Vries, S.C. A relationship between seed development, arabinogalactan-proteins (AGPs) and the AGP mediated promotion of somatic embryogenesis. Physiol. Plant 2002, 114, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Šamaj, J.; Bobák, M.; Blehová, A.; Pret’ová, A. Importance of cytoskeleton and cell wall in somatic embryogenesis. In Somatic Embryogenesis; Springer: Berlin/Heidelberg, Germany, 2006; pp. 35–50. [Google Scholar]
- Seifert, G.J.; Roberts, K. The biology of arabinogalactan proteins. Annu. Rev. Plant Biol. 2007, 58, 137–161. [Google Scholar] [CrossRef]
- Geshi, N.; Johansen, J.N.; Dilokpimol, A.; Rolland, A.; Belcram, K.; Verger, S.; Mouille, G. A galactosyltransferase acting on arabinogalactan protein glycans is essential for embryo development in Arabidopsis. Plant J. 2013, 76, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Smertenko, A.; Bozhkov, P.V. Somatic embryogenesis: Life and death processes during apical—Basal patterning. J. Exp. Bot. 2014, 65, 1343–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testillano, P.S. Microspore embryogenesis: Targeting the determinant factors of stress-induced cell reprogramming for crop improvement. J. Exp. Bot. 2019, 70, 2965–2978. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Building an extensible cell wall. Plant Physiol. 2022, 189, 1246–1277. [Google Scholar] [CrossRef]
- Pilarska, M.; Czaplicki, A.Z.; Konieczny, R. Patterns of pectin epitope expression during shoot and root regeneration in androgenic cultures of two wheat cultivars. Acta. Biol. Crac. Ser. Bot. 2007, 49, 69–72. [Google Scholar]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef] [Green Version]
- Portillo, L.; Olmedilla, A.; Santacruz-Ruvalcaba, F. Cellular and molecular changes associated with somatic embryogenesis induction in Agave tequilana. Protoplasma 2012, 249, 1101–1107. [Google Scholar] [CrossRef]
- Corral-Martínez, P.; Driouich, A.; Seguí-Simarro, J.M. Dynamic Changes in Arabinogalactan-Protein, Pectin, Xyloglucan and Xylan Composition of the Cell Wall During Microspore Embryogenesis in Brassica napus. Front. Plant Sci. 2019, 10, 332. [Google Scholar] [CrossRef] [Green Version]
- Filipović, B.K.; Trifunović-Momčilov, M.M.; Simonović, A.D.; Jevremović, S.B.; Milošević, S.M.; Subotić, A.R. Immunolocalization of some arabinogalactan protein epitopes during indirect somatic embryogenesis and shoot organogenesis in leaf culture of centaury (Centaurium erythraea Rafn). In Vitro Cell Dev. Biol. 2021, 57, 470–480. [Google Scholar] [CrossRef]
- Xu, C.; Takáč, T.; Burbach, C.; Menzel, D.; Šamaj, J. Developmental localization and the role of hydroxyproline rich glycoproteins during somatic embryogenesis of banana (Musa spp. AAA). Plant Biol. 2011, 11, 38. [Google Scholar]
- Varhanikova, M.; Uvackova, L.; Pretova, A.; Obert, B. Arabinogalactan proteins in embryogenic and non-embryogenic maize calli. J. Microbiol. Biotechnol. Food Sci. 2013, 2, 2262–2271. [Google Scholar]
- Pilarska, M.; Knox, J.P.; Konieczny, R. Arabinogalactan-protein and pectin epitopes in relation to an extracellular matrix surface network and somatic embryogenesis and callogenesis in Trifolium nigrescens Viv. Plant Cell Tiss. Organ 2013, 115, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Zagorchev, L.; Stoineva, R.; Odjakova, M. Changes in arabinogalactan proteins during somatic embryogenesis in suspension in vitro cultures of Dactylis glomerata L. Bulg. J. Agric. Sci. 2013, 19, 35–38. [Google Scholar]
- Zagorchev, L.; Odjakova, M. Hydroxyproline rich proteins in salt adapted embryogenic suspension cultures of Dactylis glomerata L. Biotechnol. Biotechnol. Equip. 2011, 25, 2321–2328. [Google Scholar] [CrossRef]
- Lee, Y.I.; Hsu, S.T.; Yeung, E.C. Orchid protocorm-like bodies are somatic embryos. Am. J. Bot. 2013, 100, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.D.; MTrifunović-Momčilov, M.; Filipović, B.K.; Marković, M.P.; Bogdanović, M.D.; Subotić, A.R. Somatic embryogenesis in Centaurium erythraea Rafn—Current status and perspectives: A review. Plants 2020, 10, 70. [Google Scholar]
- Kim, J.H.; Lee, Y.; Kim, E.J.; Gu, S.; Sohn, E.J.; Seo, Y.S.; Chang, Y.S. Exposure of iron nanoparticles to Arabidopsis thaliana enhances root elongation by triggering cell wall loosening. Environ. Sci. Technol. 2014, 48, 3477–3485. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Zhao, J.; He, R.; Tang, Y. CuO nanoparticle exposure impairs the root tip cell walls of Arabidopsis thaliana seedlings. Wat. Air Soil Poll 2020, 231, 324. [Google Scholar] [CrossRef]
- Castilleux, R.; Plancot, B.; Ropitaux, M.; Carreras, A.; Leprince, J.; Boulogne, I.; Vicré, M. Cell wall extensins in root—Microbe interactions and root secretions. J. Exp. Bot. 2018, 69, 4235–4247. [Google Scholar] [CrossRef] [Green Version]
- Lamport, D.T.; Kieliszewski, M.J.; Chen, Y.; Cannon, M.C. Role of the extensin superfamily in primary cell wall architecture. Plant Physiol. 2011, 56, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Matras, E.; Gorczyca, A.; Pociecha, E.; Przemieniecki, S.W.; Oćwieja, M. Phytotoxicity of silver nanoparticles with different surface properties on monocots and dicots model plants. J. Soil Sci. Plant Nutr. 2022, 22, 1647–1664. [Google Scholar] [CrossRef]
- Siegel, J.; Záruba, K.; Švorčík, V.; Kroumanová, K.; Burketová, L.; Martinec, J. Round-shape gold nanoparticles: Effect of particle size and concentration on Arabidopsis thaliana root growth. Nanoscale Res. Lett. 2018, 13, 95. [Google Scholar] [CrossRef] [Green Version]
- Husted, S.; Minutello, F.; Pinna, A.; Le Tougaard, S.; Møs, P.; Kopittke, P.M. What is missing to advance foliar fertilization using nanotechnology? Trends Plant Sci. 2023, 28, 90–105. [Google Scholar] [CrossRef]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Wiesner, M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants—Critical review. Nanotoxicology 2016, 10, 257–278. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Jones, L.; Seymour, G.B.; Knox, J.P. Localization of Pectic Galactan in Tomato Cell Walls Using a Monoclonal Antibody Specific to (1 [->] 4)-β-D-Galactan. Plant Physiol. 1997, 113, 1405–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willats, W.G.; Marcus, S.E.; Knox, J.P. Generation of a monoclonal antibody specific to (1→5)-α-l-arabinan. Carbohydr. Res. 1998, 308, 149–152. [Google Scholar] [CrossRef]
- Knox, J.P.; Linstead, P.J.; Cooper, J.P.C.; Roberts, K. Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J. 1991, 1, 317–326. [Google Scholar] [CrossRef]
- Smallwood, M.; Yates, E.A.; Willats, W.G.; Martin, H.; Knox, J.P. Immunochemical comparison of membrane-associated and secreted arabinogalactan-proteins in rice and carrot. Planta 1996, 198, 452–459. [Google Scholar] [CrossRef]
- Pennell, R.I.; Knox, J.P.; Scofield, G.N.; Selvendran, R.R.; Roberts, K. A family of abundant plasma membrane-associated glycoproteins related to the arabinogalactan proteins is unique to flowering plants. J. Cell Biol. 1989, 108, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, M.; Beven, A.; Donovan, N.; Neill, S.J.; Peart, J.; Roberts, K.; Knox, J.P. Localization of cell wall proteins in relation to the developmental anatomy of the carrot root apex. Plant J. 1994, 5, 237–246. [Google Scholar] [CrossRef]
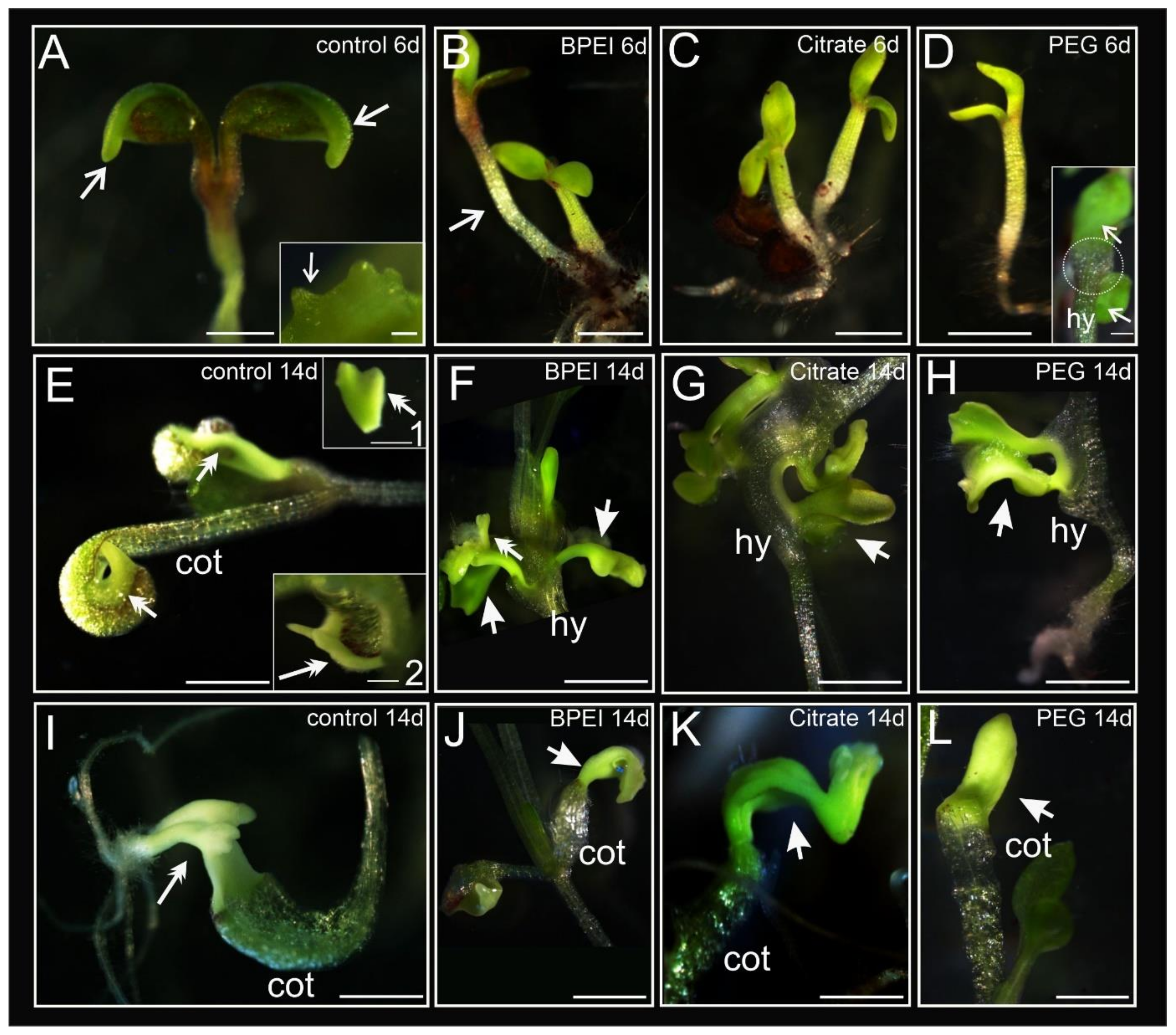


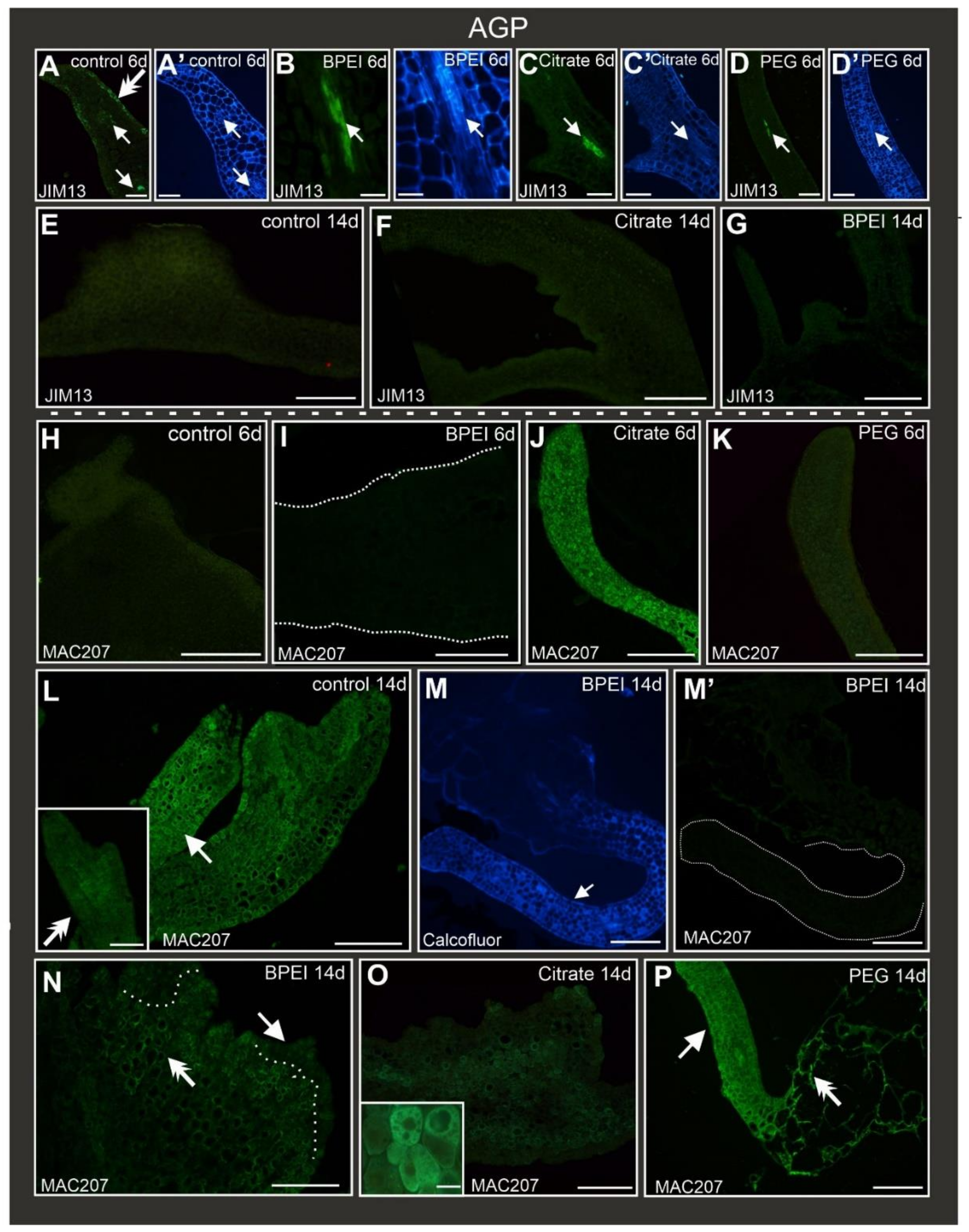


| N | Presence of Cotyledons Margin | Somatic Embryos | Bulge on Cotyledons | Bulge on Hypocotyl | Without Changes in Cell Fate | |
|---|---|---|---|---|---|---|
| 35S:BBM | 146 | 90.4 a | 84.2 a | 2.1 a | 0 a | 11.0 a |
| 35S:BBM + BPEI Au NPs | 146 | 19.2 b | 2.1 b | 37.7 b | 23.3 b | 43.2 b |
| 35S:BBM + Citrate Au NPs | 144 | 21.5 b | 0 b | 25.0 b | 20.8 b | 50.0 b |
| 35S:BBM + PEG Au NPs | 144 | 9.0 c | 0 b | 34.0 b | 16.7 b | 46.5 b |
| LM5 | LM6 | JIM13 | MAC207 | LM2 | JIM16 | JIM11 | LM5 | LM6 | JIM13 | MAC207 | LM2 | JIM16 | JIM11 | LM5 | LM6 | JIM13 | MAC207 | LM2 | JIM16 | JIM11 | LM5 | LM6 | JIM13 | MAC702 | LM2 | JIM16 | JIM11 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EXPLANT CELLS | MARGIN CELLS | PROTRUSION | SOMATIC EMBRYOS | ||||||||||||||||||||||||||
| CONTROL | EARLY | + | + | − | − | + | + | − | + | + | + | − | + | − | − | + | + | − | − | + | − | − | Not applicable | ||||||
| LATE | + | + | − | − | + | + | + | + | + | − | − | + | + | − | + | + | − | − | + | + | − | + | − | − | + | + | + | − | |
| EXPLANT CELLS | SURFACE CELLS | BULGES | OGRAN-LIKE STRUCTURE | ||||||||||||||||||||||||||
| BPEI | EARLY | + | + | − | − | + | + | − | Not applicable | Not applicable | Not applicable | ||||||||||||||||||
| LATE | − | + | − | − | + | + | + | + | − | − | − | + | + | − | + | − | − | + | + | + | − | − | + | − | − | − | + | − | |
| CITRATE | EARLY | + | + | − | + | + | + | - | Not applicable | Not applicable | Not applicable | ||||||||||||||||||
| LATE | − | + | − | + | + | − | + | − | + | − | − | − | − | − | − | + | − | − | + | − | − | − | + | − | − | − | − | − | |
| PEG | EARLY | − | + | − | − | + | + | − | Not applicable | Not applicable | Not applicable | ||||||||||||||||||
| LATE | − | − | − | + | + | − | − | + | − | − | − | − | − | − | + | − | − | − | − | + | − | + | + | − | + | + | + | − | |
| Antibody | Recognized Epitope | References |
|---|---|---|
| Pectins | ||
| LM5 | Linear tetrasaccharide in (1→4)-β-D-galactans (RG I side chain) | [84] |
| LM6 | Linear pentasaccharide in (1→5)-α-L-arabinans (RG I side chain) | [85] |
| AGP | ||
| JIM13 | Arabinogalactan/arabinogalactan protein, carbohydrate epitope (β)GlcA1→3(α)GalA1→2Rha | [86] |
| LM2 | Arabinogalactan/arabinogalactan protein, carbohydrate epitope containing β→linked GlcA | [87] |
| MAC207 | Arabinogalactan protein, (β)GlcA1→3(α)GalA1→2Rha | [88] |
| JIM16 | AGP glycan | [86] |
| Extensins | ||
| JIM11 | Extensin/HRGP glycoprotein | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godel-Jędrychowska, K.; Milewska-Hendel, A.; Sala, K.; Barański, R.; Kurczyńska, E. The Impact of Gold Nanoparticles on Somatic Embryogenesis Using the Example of Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 10356. https://doi.org/10.3390/ijms241210356
Godel-Jędrychowska K, Milewska-Hendel A, Sala K, Barański R, Kurczyńska E. The Impact of Gold Nanoparticles on Somatic Embryogenesis Using the Example of Arabidopsis thaliana. International Journal of Molecular Sciences. 2023; 24(12):10356. https://doi.org/10.3390/ijms241210356
Chicago/Turabian StyleGodel-Jędrychowska, Kamila, Anna Milewska-Hendel, Katarzyna Sala, Rafał Barański, and Ewa Kurczyńska. 2023. "The Impact of Gold Nanoparticles on Somatic Embryogenesis Using the Example of Arabidopsis thaliana" International Journal of Molecular Sciences 24, no. 12: 10356. https://doi.org/10.3390/ijms241210356
APA StyleGodel-Jędrychowska, K., Milewska-Hendel, A., Sala, K., Barański, R., & Kurczyńska, E. (2023). The Impact of Gold Nanoparticles on Somatic Embryogenesis Using the Example of Arabidopsis thaliana. International Journal of Molecular Sciences, 24(12), 10356. https://doi.org/10.3390/ijms241210356










