Taurine Protects against the Fatty Liver Hemorrhagic Syndrome in Laying Hens through the Regulation of Mitochondrial Homeostasis
Abstract
:1. Introduction
2. Results
2.1. Effects of Taurine on Liver Index, Pathological Anatomy Changes and Incidence of FLHS
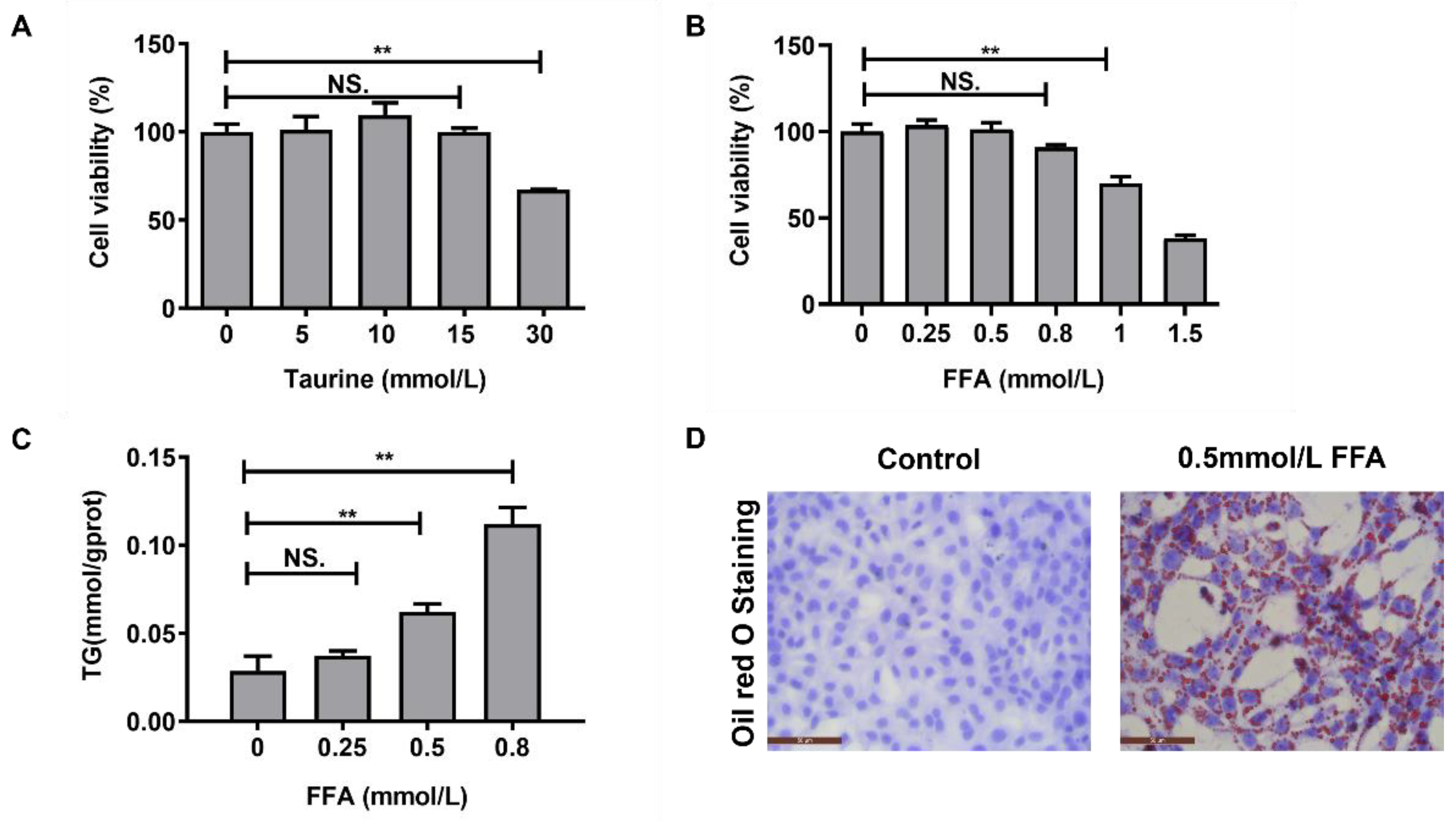
2.2. Screening the Concentrations of Taurine and FFA In Vitro
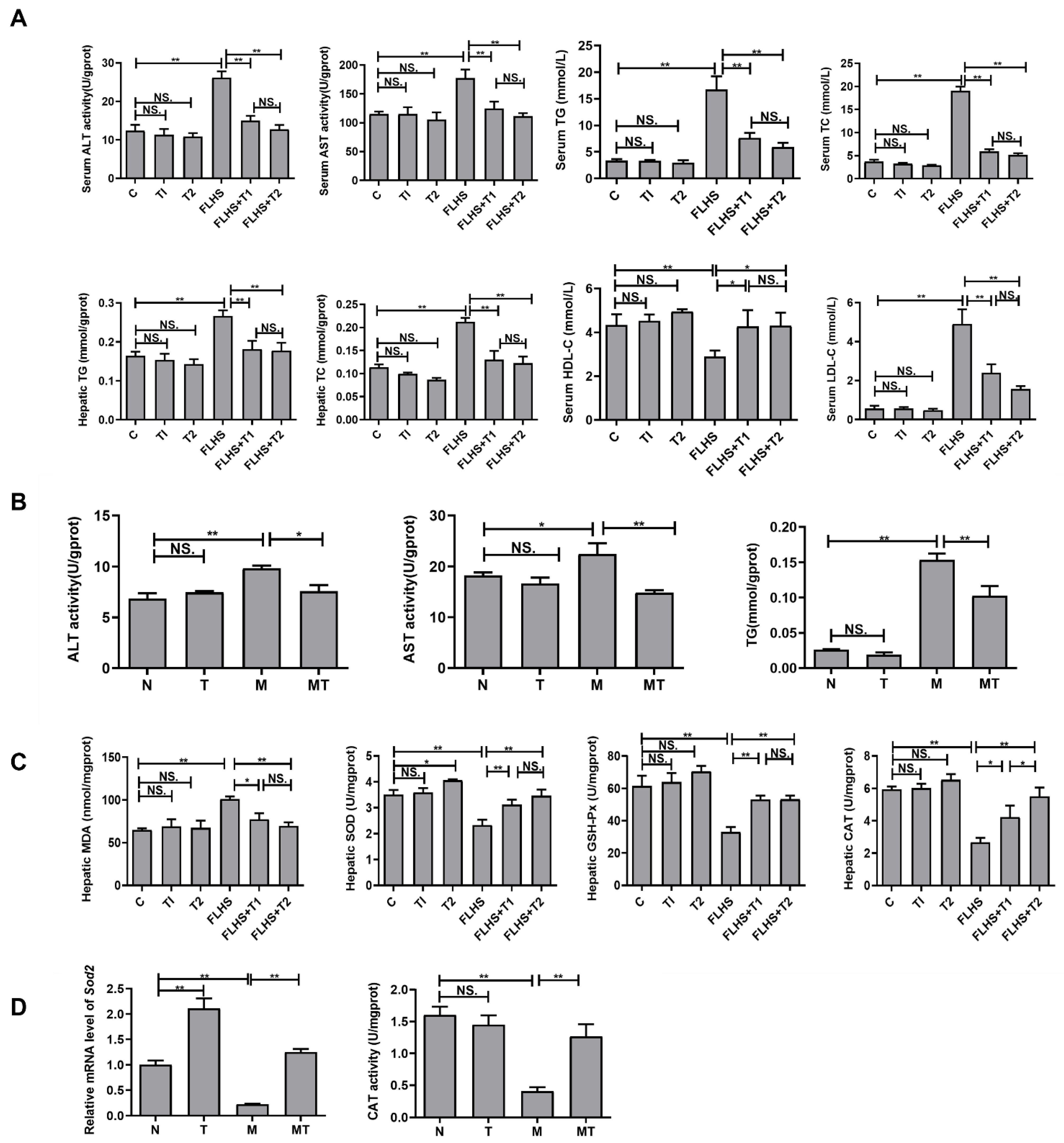
2.3. Effects of Taurine on Liver Function, Lipid Parameters, and Antioxidant Capacity Both In Vivo and In Vitro
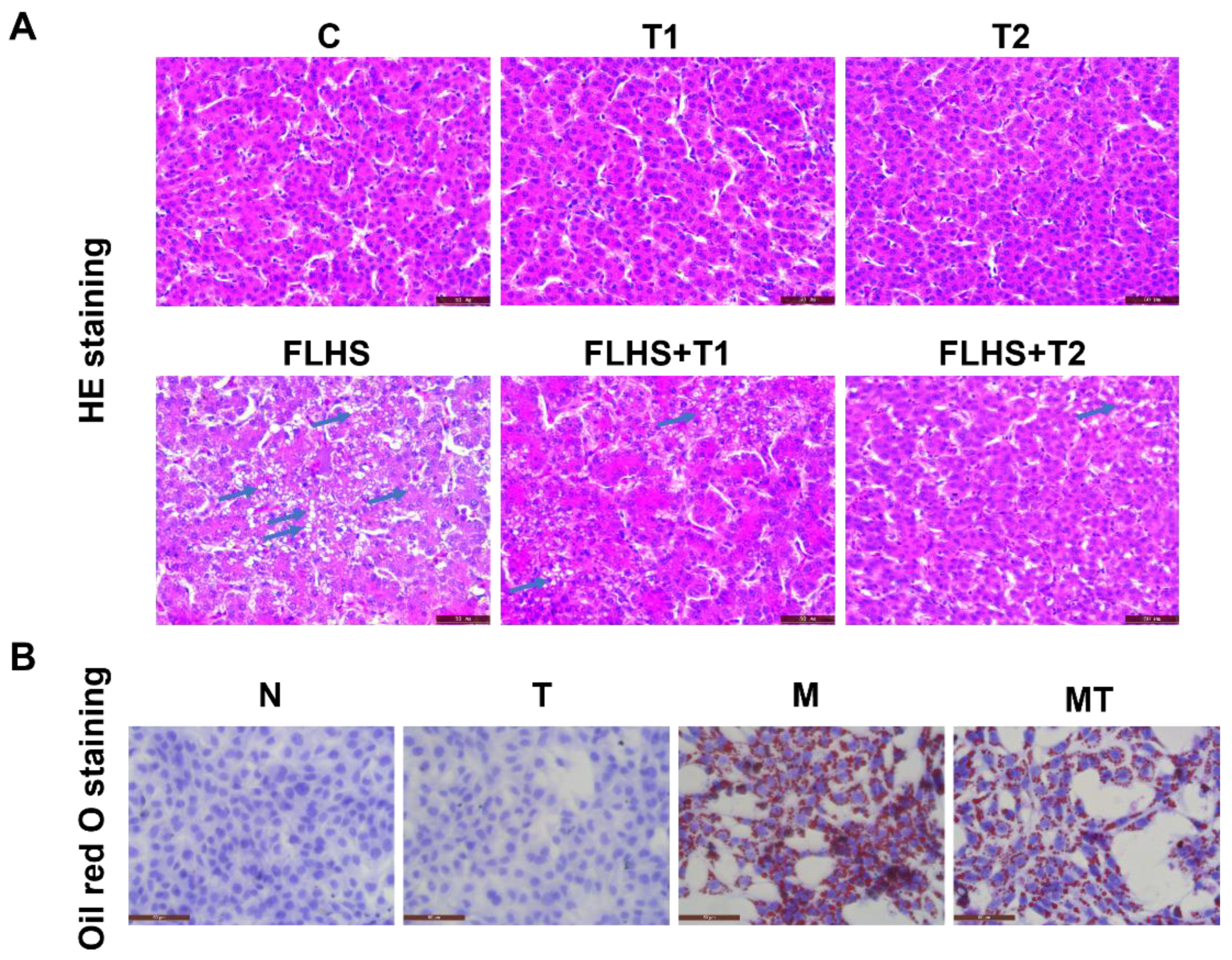
2.4. Effects of Taurine on Pathological Changes and Lipid Accumulation of Hepatocytes Both In Vivo and In Vitro
2.5. Effects of Taurine on Mitochondrial Function Both In Vivo and In Vitro
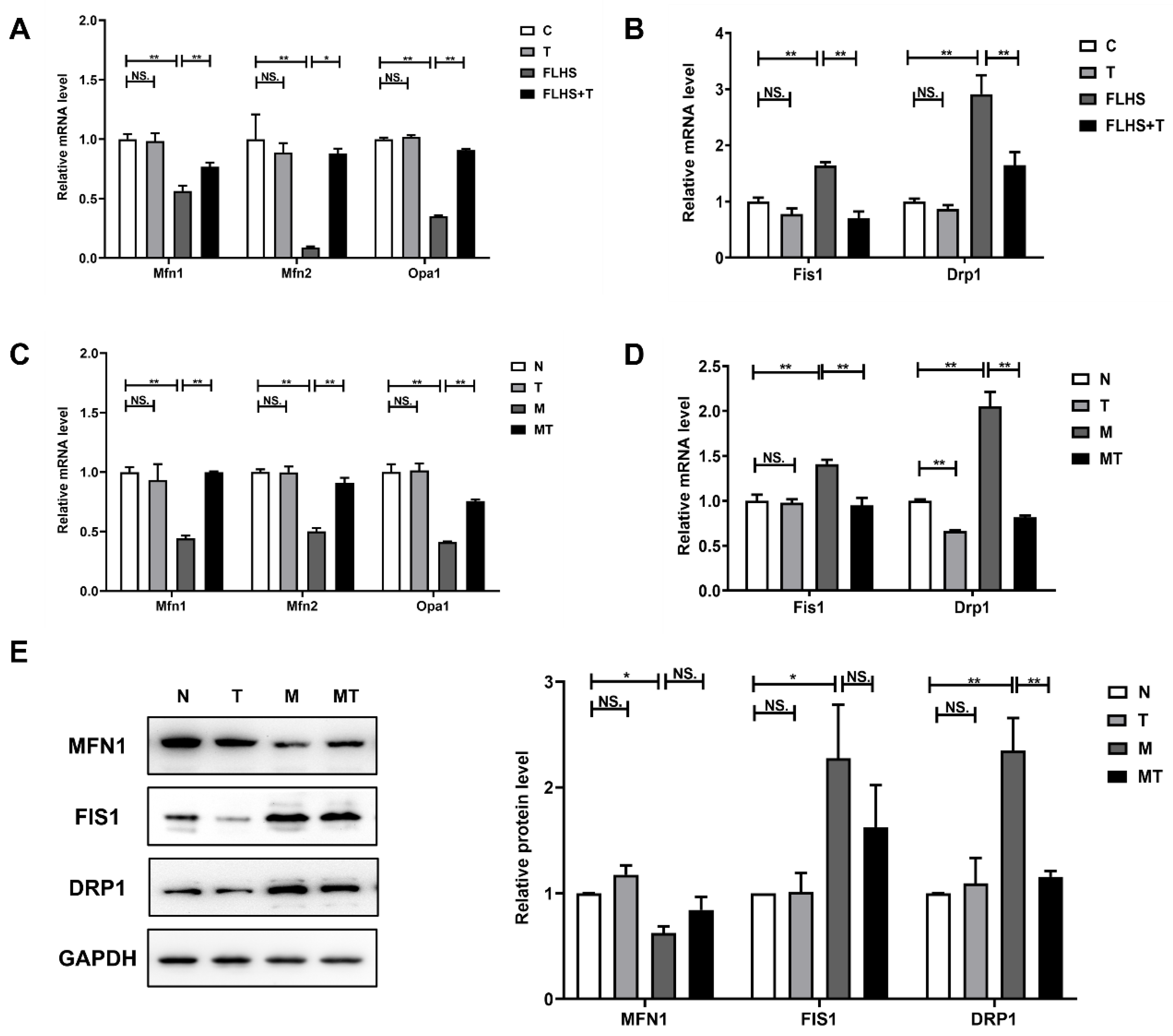
2.6. Effects of Taurine on Mitochondrial Homeostasis Both In Vivo and In Vitro
2.6.1. Effects of Taurine on Mitochondrial Dynamic Balance
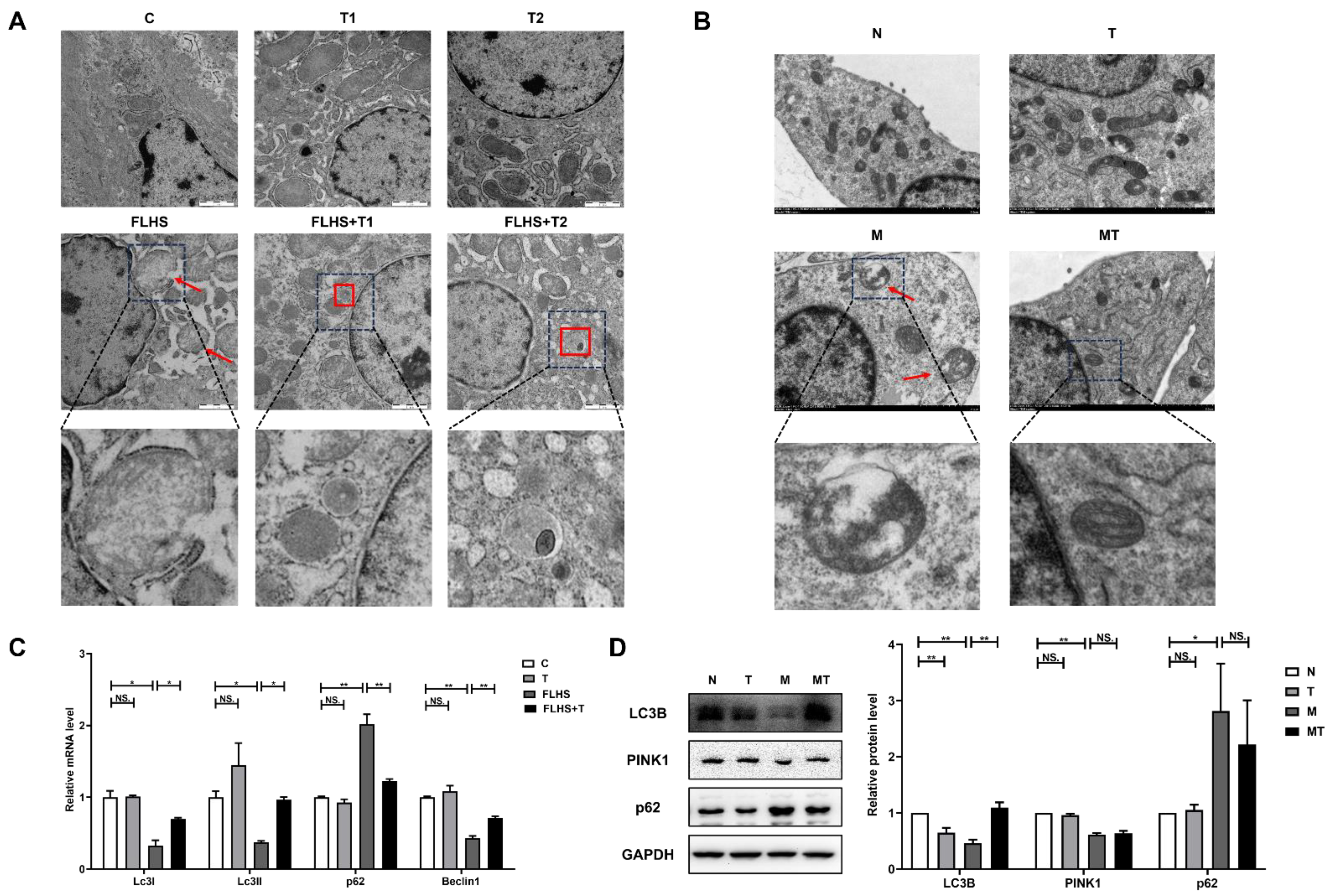
2.6.2. Effects of Taurine on Mitochondrial Autophagy
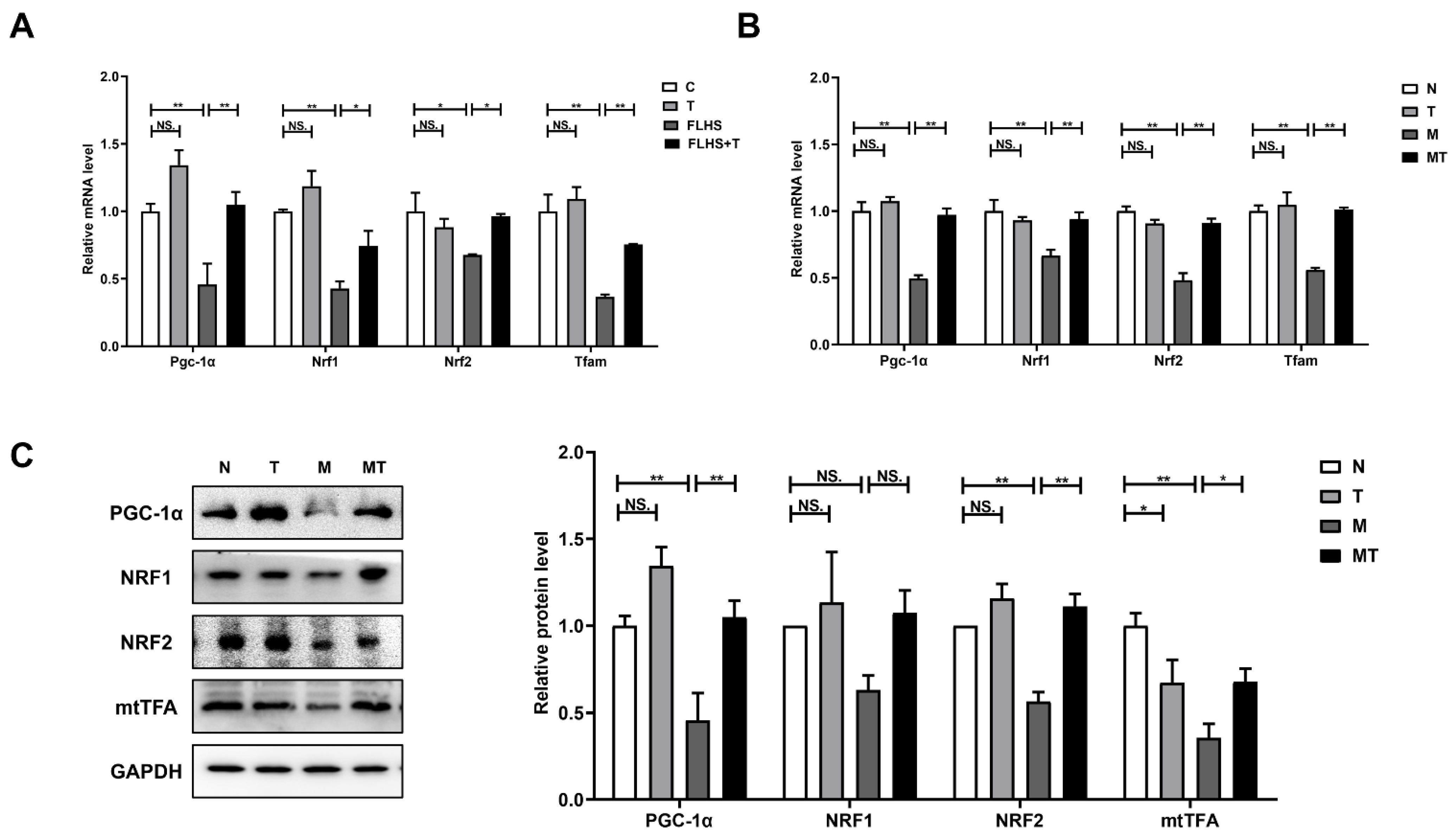
2.6.3. Effects of Taurine on Mitochondrial Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Hens, Diets, and Experimental Design
4.2. Cell Culture and Treatments
4.3. Cell Viability Assay
4.4. Biochemical Analysis
4.5. Hematoxylin and Eosin (H&E) Staining
4.6. Oil Red O Staining
4.7. Transmission Electron Microscopy (TEM)
4.8. Real-Time Quantitative PCR (RT-qPCR)
4.9. Western Blot
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eslam, M.; Sanyal, A.J.; George, J.; Int Consensus, P. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Brizi, M.; Bianchi, G.; Tomassetti, S.; Bugianesi, E.; Lenzi, M.; McCullough, A.J.; Natale, S.; Forlani, G.; Melchionda, N. Nonalcoholic fatty liver disease: A feature of the metabolic syndrome. Diabetes 2001, 50, 1844–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuppalanchi, R.; Chalasani, N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology 2009, 49, 306–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini, M.; Summers, P.; Faita, F.; Brunetto, M.R.; Callea, F.; De Nicola, A.; Di Lascio, N.; Farinati, F.; Gastaldelli, A.; Gridelli, B.; et al. Digital liver biopsy: Bio-imaging of fatty liver for translational and clinical research. World J. Hepatol. 2018, 10, 231–245. [Google Scholar] [CrossRef]
- Dai, H.; Chu, L.; Song, S.; Li, W.; Zhang, L.; Wu, Z.; Zeng, J.; Duan, Q. Prevalence of and risk factors for fatty liver disease in a professional population of Wuhan, China. Public Health 2009, 123, 545–548. [Google Scholar] [CrossRef]
- Wong, V.W.; Chu, W.C.; Wong, G.L.; Chan, R.S.; Chim, A.M.; Ong, A.; Yeung, D.K.; Yiu, K.K.; Chu, S.H.; Woo, J.; et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: A population study using proton-magnetic resonance spectroscopy and transient elastography. Gut 2012, 61, 409–415. [Google Scholar] [CrossRef]
- Zhai, H.L.; Wang, N.J.; Han, B.; Li, Q.; Chen, Y.; Zhu, C.F.; Chen, Y.C.; Xia, F.Z.; Cang, Z.; Zhu, C.X.; et al. Low vitamin D levels and non-alcoholic fatty liver disease, evidence for their independent association in men in East China: A cross-sectional study (Survey on Prevalence in East China for Metabolic Diseases and Risk Factors (SPECT-China)). Br. J. Nutr. 2016, 115, 1352–1359. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Lee, E.Y.; Li, J.; Jun, M.J.; Yoon, E.; Ahn, S.B.; Liu, C.L.; Yang, H.L.; Rui, F.J.; Zou, B.Y.; et al. NASH/Liver Fibrosis Prevalence and Incidence of Nonliver Comorbidities among People with NAFLD and Incidence of NAFLD by Metabolic Comorbidities: Lessons from South Korea. Dig. Dis. 2021, 39, 634–645. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Meng, J.C.; Ma, N.; Liu, H.L.; Liu, J.; Liu, J.X.; Wang, J.P.; He, X.; Zhao, X.H. Untargeted and targeted metabolomics profiling reveals the underlying pathogenesis and abnormal arachidonic acid metabolism in laying hens with fatty liver hemorrhagic syndrome. Poult. Sci. 2021, 100, 10. [Google Scholar] [CrossRef]
- Chen, W.; Shi, Y.; Li, G.Y.; Huang, C.; Zhuang, Y.; Shu, B.; Cao, X.H.; Li, Z.Q.; Hu, G.L.; Liu, P.; et al. Preparation of the peroxisome proliferator-activated receptor alpha polyclonal antibody: Its application in fatty liver hemorrhagic syndrome. Int. J. Biol. Macromol. 2021, 182, 179–186. [Google Scholar] [CrossRef]
- Guo, L.Y.; Kuang, J.; Zhuang, Y.; Jiang, J.L.; Shi, Y.; Huang, C.; Zhou, C.M.; Xu, P.Z.; Liu, P.; Wu, C.; et al. Serum Metabolomic Profiling to Reveal Potential Biomarkers for the Diagnosis of Fatty Liver Hemorrhagic Syndrome in Laying Hens. Front. Physiol. 2021, 12, 13. [Google Scholar] [CrossRef]
- Rector, R.S.; Thyfault, J.P.; Uptergrove, G.M.; Morris, E.M.; Naples, S.P.; Borengasser, S.J.; Mikus, C.R.; Laye, M.J.; Laughlin, M.H.; Booth, F.W.; et al. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J. Hepatol. 2010, 52, 727–736. [Google Scholar] [CrossRef] [Green Version]
- Scorrano, L. Keeping mitochondria in shape: A matter of life and death. Eur. J. Clin. Investig. 2013, 43, 886–893. [Google Scholar] [CrossRef]
- Benard, G.; Bellance, N.; James, D.; Parrone, P.; Fernandez, H.; Letellier, T.; Rossignol, R. Mitochondrial bioenergetics and structural network organization. J. Cell Sci. 2007, 120 Pt 5, 838–848. [Google Scholar] [CrossRef] [Green Version]
- Delettre, C.; Lenaers, G.; Griffoin, J.M.; Gigarel, N.; Lorenzo, C.; Belenguer, P.; Pelloquin, L.; Grosgeorge, J.; Turc-Carel, C.; Perret, E.; et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat. Genet. 2000, 26, 207–210. [Google Scholar] [CrossRef]
- Shirihai, O.S.; Song, M.; Dorn, G.W. 2nd, How mitochondrial dynamism orchestrates mitophagy. Circ. Res. 2015, 116, 1835–1849. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Czaja, M.J. Regulation of lipid droplets by autophagy. Trends Endocrinol. Metab. 2011, 22, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Tang, X.; Zhang, P.; Liu, G.; Guo, H. Cyanidin-3-O-β-glucoside protects primary mouse hepatocytes against high glucose-induced apoptosis by modulating mitochondrial dysfunction and the PI3K/Akt pathway. Biochem. Pharmacol. 2014, 90, 135–144. [Google Scholar] [CrossRef]
- Roysommuti, S.; Wyss, J.M. Perinatal taurine exposure affects adult arterial pressure control. Amino Acids 2014, 46, 57–72. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.Y.; Chou, C.H.; Chiu, C.H.; Yang, K.T.; Lin, Y.L.; Weng, W.L.; Chen, Y.C. Preventive effects of taurine on development of hepatic steatosis induced by a high-fat/cholesterol dietary habit. J. Agric. Food Chem. 2011, 59, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Fujita, M.; Nakamura, M.; Sakono, M.; Nishizono, S.; Sato, M.; Imaizumi, K.; Mori, M.; Fukuda, N. Taurine ameliorates cholesterol metabolism by stimulating bile acid production in high-cholesterol-fed rats. Clin. Exp. Pharmacol. Physiol. 2016, 43, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yoshikawa, N.; Ito, H.; Schaffer, S.W. Impact of taurine depletion on glucose control and insulin secretion in mice. J. Pharmacol. Sci. 2015, 129, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.C.; Sebastian, B.M.; Tang, H.; McMullen, M.M.; Axhemi, A.; Jacobsen, D.W.; Nagy, L.E. Taurine Supplementation Prevents Ethanol-Induced Decrease in Serum Adiponectin and Reduces Hepatic Steatosis in Rats. Hepatology 2009, 49, 1554–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Zhang, J.; Ma, H.; Dai, B.; Zheng, L.; Miao, J.; Zhang, Y. The influence of dietary taurine and reduced housing density on hepatic functions in laying hens. Poult. Sci. 2014, 93, 1724–1736. [Google Scholar] [CrossRef]
- Tang, R.Y.; Yang, Q.H.; Lin, S.M.; Feng, Y.; Yang, J.C.; Lv, Q.F.; Wu, G.F.; Hu, J.M. Preventive or Curative Administration of Taurine Regulates Lipid Metabolism in the Liver of Rats with Alcoholic Liver Disease. In Taurine 11; Hu, J., Piao, F., Schaffer, S.W., ElIdrissi, A., Wu, J.Y., Eds.; Springer International Publishing Ag: Cham, Switzerland, 2019; Volume 1155, pp. 119–131. [Google Scholar]
- Wu, G.F.; Tang, R.Y.; Yang, J.C.; Tao, Y.; Liu, Z.Y.; Feng, Y.; Lin, S.M.; Yang, Q.H.; Lv, Q.F.; Hu, J.M. Taurine Accelerates Alcohol and Fat Metabolism of Rats with Alcoholic Fatty Liver Disease. In Taurine 9; Marcinkiewicz, J., Schaffer, S.W., Eds.; Springer: Berlin, Germany, 2015; Volume 803, pp. 793–805. [Google Scholar]
- Wu, G.F.; Yang, J.C.; Lv, H.; Jing, W.Y.; Zhou, J.Q.; Feng, Y.; Lin, S.M.; Yang, Q.H.; Hu, J.M. Taurine prevents ethanol-induced apoptosis mediated by mitochondrial or death receptor pathways in liver cells. Amino Acids 2018, 50, 863–875. [Google Scholar] [CrossRef]
- Gentile, C.L.; Nivala, A.M.; Gonzales, J.C.; Pfaffenbach, K.T.; Wang, D.; Wei, Y.; Jiang, H.; Orlicky, D.J.; Petersen, D.R.; Pagliassotti, M.J.; et al. Experimental evidence for therapeutic potential of taurine in the treatment of nonalcoholic fatty liver disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1710–R1722. [Google Scholar] [CrossRef]
- Khalili Fard, J.; Hamzeiy, H.; Sattari, M.; Eghbal, M.A. Protective Roles of N-acetyl Cysteine and/or Taurine against Sumatriptan-Induced Hepatotoxicity. Adv. Pharm. Bull. 2016, 6, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Jamshidzadeh, A.; Heidari, R.; Abasvali, M.; Zarei, M.; Ommati, M.M.; Abdoli, N.; Khodaei, F.; Yeganeh, Y.; Jafari, F.; Zarei, A.; et al. Taurine treatment preserves brain and liver mitochondrial function in a rat model of fulminant hepatic failure and hyperammonemia. Biomed. Pharmacother. 2017, 86, 514–520. [Google Scholar] [CrossRef]
- Heidari, R.; Babaei, H.; Eghbal, M.A. Amodiaquine-induced toxicity in isolated rat hepatocytes and the cytoprotective effects of taurine and/or N-acetyl cysteine. Res. Pharm. Sci. 2014, 9, 97–105. [Google Scholar]
- Warskulat, U.; Borsch, E.; Reinehr, R.; Heller-Stilb, B.; Mönnighoff, I.; Buchczyk, D.; Donner, M.; Flögel, U.; Kappert, G.; Soboll, S.; et al. Chronic liver disease is triggered by taurine transporter knockout in the mouse. FASEB J. 2006, 20, 574–576. [Google Scholar] [CrossRef]
- Walzem, R.L.; Simon, C.; Morishita, T.; Lowenstine, L.; Hansen, R.J. Fatty liver hemorrhagic syndrome in hens overfed a purified diet. Selected enzyme activities and liver histology in relation to liver hemorrhage and reproductive performance. Poult. Sci. 1993, 72, 1479–1491. [Google Scholar] [CrossRef]
- Haghighi-Rad, F.; Polin, D. The relationship of plasma estradiol and progesterone levels to the fatty liver hemorrhagic syndrome in laying hens. Poult. Sci. 1981, 60, 2278–2283. [Google Scholar] [CrossRef]
- Stake, P.E.; Fredrickson, T.N.; Bourdeau, C.A. Induction of fatty liver-hemorrhagic syndrome in laying hens by exogenous beta-estradiol. Avian. Dis. 1981, 25, 410–422. [Google Scholar] [CrossRef]
- Rozenboim, I.; Mahato, J.; Cohen, N.A.; Tirosh, O. Low protein and high-energy diet: A possible natural cause of fatty liver hemorrhagic syndrome in caged White Leghorn laying hens. Poult. Sci. 2016, 95, 612–621. [Google Scholar] [CrossRef]
- Yang, F.; Ruan, J.; Wang, T.; Luo, J.; Cao, H.; Song, Y.; Huang, J.; Hu, G. Improving effect of dietary soybean phospholipids supplement on hepatic and serum indexes relevant to fatty liver hemorrhagic syndrome in laying hens. Anim. Sci. J. 2017, 88, 1860–1869. [Google Scholar] [CrossRef]
- Baylin, A.; Kabagambe, E.K.; Siles, X.; Campos, H. Adipose tissue biomarkers of fatty acid intake. Am. J. Clin. Nutr. 2002, 76, 750–757. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.R.; Liu, J.; Plumeri, D.; Cao, Y.B.; He, T.; Lin, L.; Li, Y.; Jiang, Y.Y.; Li, J.; Shang, J. Lipotoxicity in HepG2 cells triggered by free fatty acids. Am. J. Transl. Res. 2011, 3, 284–291. [Google Scholar]
- Malhi, H.; Bronk, S.F.; Werneburg, N.W.; Gores, G.J. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 2006, 281, 12093–12101. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.J.; Wang, M.Y.; Liu, L.; Peng, J.F.; Guo, C.X.; Chen, X.P.; Huang, L.; Tan, J.Q.; Yang, G.P. Transcriptional Repression of CYP3A4 by Increased miR-200a-3p and miR-150-5p Promotes Steatosis in vitro. Front. Genet. 2019, 10, 8. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.R.; Xu, J.H.; Wei, G.H.; Zhang, L.J.; Sun, L.E.; Wang, G.Y.; Li, F.; Jiang, F.X. HNF1 alpha Controls Liver Lipid Metabolism and Insulin Resistance via Negatively Regulating the SOCS-3-STAT3 Signaling Pathway. J. Diabetes Res. 2019, 2019, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.R.; Mao, Y.Q.; Fan, X.M. Inhibition of ghrelin o-acyltransferase attenuated lipotoxicity by inducing autophagy via AMPK-mTOR pathway. Drug Des. Dev. Ther. 2018, 12, 873–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, J.H.; Wang, H.; Ye, Y.; Chan, P.K.; Pan, S.Y.; Fong, W.F.; Yu, Z.L. Inhibitory effect of schisandrin B on free fatty acid-induced steatosis in L-02 cells. World J. Gastroenterol. 2011, 17, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Kalaz, E.B.; Çoban, J.; Aydın, A.F.; Doğan-Ekici, I.; Doğru-Abbasoğlu, S.; Öztezcan, S.; Uysal, M. Carnosine and taurine treatments decreased oxidative stress and tissue damage induced by D-galactose in rat liver. J. Physiol. Biochem. 2014, 70, 15–25. [Google Scholar] [CrossRef]
- Park, T.; Lee, K. Dietary taurine supplementation reduces plasma and liver cholesterol and triglyceride levels in rats fed a high-cholesterol or a cholesterol-free diet. Adv. Exp. Med. Biol. 1998, 442, 319–325. [Google Scholar]
- Choi, M.J.; Kim, J.H.; Chang, K.J. The effect of dietary taurine supplementation on plasma and liver lipid concentrations and free amino acid concentrations in rats fed a high-cholesterol diet. Adv. Exp. Med. Biol. 2006, 583, 235–242. [Google Scholar]
- Murakami, S.; Ono, A.; Kawasaki, A.; Takenaga, T.; Ito, T. Taurine attenuates the development of hepatic steatosis through the inhibition of oxidative stress in a model of nonalcoholic fatty liver disease in vivo and in vitro. Amino Acids 2018, 50, 1279–1288. [Google Scholar] [CrossRef]
- Wu, Q.; Tang, H.Q.; Wang, H.B. The anti-oxidation and mechanism of essential oil of paederia scandens in the NAFLD model of chicken. Animals 2019, 9, 850. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.Z.; Chen, L.; Wang, H.; Narisi, B.; Chen, B. Li-Gan-Shi-Liu-Ba-Wei-San improves non-alcoholic fatty liver disease through enhancing lipid oxidation and alleviating oxidation stress. J. Ethnopharmacol. 2015, 176, 499–507. [Google Scholar] [CrossRef]
- Doc, Z.; Kapusta, E.; Formicki, G.; Martiniakova, M.; Omelka, R. Effect of Taurine on Ethanol-Induced Oxidative Stress in Mouse Liver and Kidney. Chin. J. Physiol. 2019, 62, 148–156. [Google Scholar]
- Zhu, W.H.; Chen, S.W.; Chen, R.G.; Peng, Z.Q.; Wan, J.; Wu, B.Y. Taurine and tea polyphenols combination ameliorate nonalcoholic steatohepatitis in rats. BMC Complement. Altern. Med. 2017, 17, 12. [Google Scholar] [CrossRef] [Green Version]
- Rines, A.K.; Ardehali, H. Transition metals and mitochondrial metabolism in the heart. J. Mol. Cell. Cardiol. 2013, 55, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Sanyal, A.J.; Campbell-Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef]
- Wei, Y.; Rector, R.S.; Thyfault, J.P.; Ibdah, J.A. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J. Gastroenterol. 2008, 14, 193–199. [Google Scholar] [CrossRef]
- Pessayre, D.; Fromenty, B. NASH: A mitochondrial disease. J. Hepatol. 2005, 42, 928–940. [Google Scholar] [CrossRef]
- Du, J.; Zhang, X.; Han, J.; Man, K.; Zhang, Y.; Chu, E.S.; Nan, Y.; Yu, J. Pro-Inflammatory CXCR3 Impairs Mitochondrial Function in Experimental Non-Alcoholic Steatohepatitis. Theranostics 2017, 7, 4192–4203. [Google Scholar] [CrossRef]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free. Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef]
- Lim, C.Y.; Jun, D.W.; Jang, S.S.; Cho, W.K.; Chae, J.D.; Jun, J.H. Effects of carnitine on peripheral blood mitochondrial DNA copy number and liver function in non-alcoholic fatty liver disease. Korean J. Gastroenterol. 2010, 55, 384–389. [Google Scholar] [CrossRef] [Green Version]
- Serviddio, G.; Bellanti, F.; Tamborra, R.; Rollo, T.; Capitanio, N.; Romano, A.D.; Sastre, J.; Vendemiale, G.; Altomare, E. Uncoupling protein-2 (UCP2) induces mitochondrial proton leak and increases susceptibility of non-alcoholic steatohepatitis (NASH) liver to ischaemia-reperfusion injury. Gut 2008, 57, 957–965. [Google Scholar] [CrossRef]
- Sihali-Beloui, O.; El-Aoufi, S.; Maouche, B.; Marco, S. Psammomys obesus, a unique model of metabolic syndrome, inflammation and autophagy in the pathologic development of hepatic steatosis. C. R. Biol. 2016, 339, 475–486. [Google Scholar] [CrossRef]
- Rautou, P.E.; Mansouri, A.; Lebrec, D.; Durand, F.; Valla, D.; Moreau, R. Autophagy in liver diseases. J. Hepatol. 2010, 53, 1123–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amir, M.; Czaja, M.J. Autophagy in nonalcoholic steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legros, F.; Lombes, A.; Frachon, P.; Rojo, M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol. Biol. Cell 2002, 13, 4343–4354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olichon, A.; Baricault, L.; Gas, N.; Guillou, E.; Valette, A.; Belenguer, P.; Lenaers, G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J. Biol. Chem. 2003, 278, 7743–7746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fekkes, P.; Shepard, K.A.; Yaffe, M.P. Gag3p, an outer membrane protein required for fission of mitochondrial tubules. J. Cell Biol. 2000, 151, 333–340. [Google Scholar] [CrossRef]
- Tong, W.; Ju, L.; Qiu, M.; Xie, Q.; Chen, Y.; Shen, W.; Sun, W.; Wang, W.; Tian, J. Liraglutide ameliorates non-alcoholic fatty liver disease by enhancing mitochondrial architecture and promoting autophagy through the SIRT1/SIRT3-FOXO3a pathway. Hepatol. Res. 2016, 46, 933–943. [Google Scholar] [CrossRef]
- Gong, F.; Gao, L.; Ding, T. IDH2 protects against nonalcoholic steatohepatitis by alleviating dyslipidemia regulated by oxidative stress. Biochem. Biophys. Res. Commun. 2019, 514, 593–600. [Google Scholar] [CrossRef]
- Sasi, U.S.S.; Sindhu, G.; Raghu, K.G. Fructose-palmitate based high calorie induce steatosis in HepG2 cells via mitochondrial dysfunction: An in vitro approach. Toxicol. Vitr. 2020, 68, 13. [Google Scholar]
- Eid, N.; Ito, Y.; Otsuki, Y. Triggering of Parkin Mitochondrial Translocation in Mitophagy: Implications for Liver Diseases. Front. Pharmacol. 2016, 7, 100. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.Y.; Lin, H.K.; Xu, Y.Y.; Zhou, F.; Wang, J.; Liu, J.J.; Zhu, X.H.; Guo, X.P.; Tang, Y.H.; Yao, P. Frataxin-Mediated PINK1-Parkin-Dependent Mitophagy in Hepatic Steatosis: The Protective Effects of Quercetin. Mol. Nutr. Food Res. 2018, 62, 11. [Google Scholar] [CrossRef]
- Gonçalves, I.O.; Passos, E.; Diogo, C.V.; Rocha-Rodrigues, S.; Santos-Alves, E.; Oliveira, P.J.; Ascensão, A.; Magalhães, J. Exercise mitigates mitochondrial permeability transition pore and quality control mechanisms alterations in nonalcoholic steatohepatitis. Appl. Physiol. Nutr. Metab. 2016, 41, 298–306. [Google Scholar] [CrossRef]
- Martinez-Vicente, M.; Talloczy, Z.; Wong, E.; Tang, G.; Koga, H.; Kaushik, S.; de Vries, R.; Arias, E.; Harris, S.; Sulzer, D.; et al. Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease. Nat. Neurosci. 2010, 13, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Lee, M.S. Autophagy as a crosstalk mediator of metabolic organs in regulation of energy metabolism. Rev. Endocr. Metab. Disord. 2014, 15, 11–20. [Google Scholar] [CrossRef]
- Begriche, K.; Sutton, G.M.; Fang, J.; Butler, A.A. The role of melanocortin neuronal pathways in circadian biology: A new homeostatic output involving melanocortin-3 receptors? Obes. Rev. 2009, 10 (Suppl. 2), 14–24. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
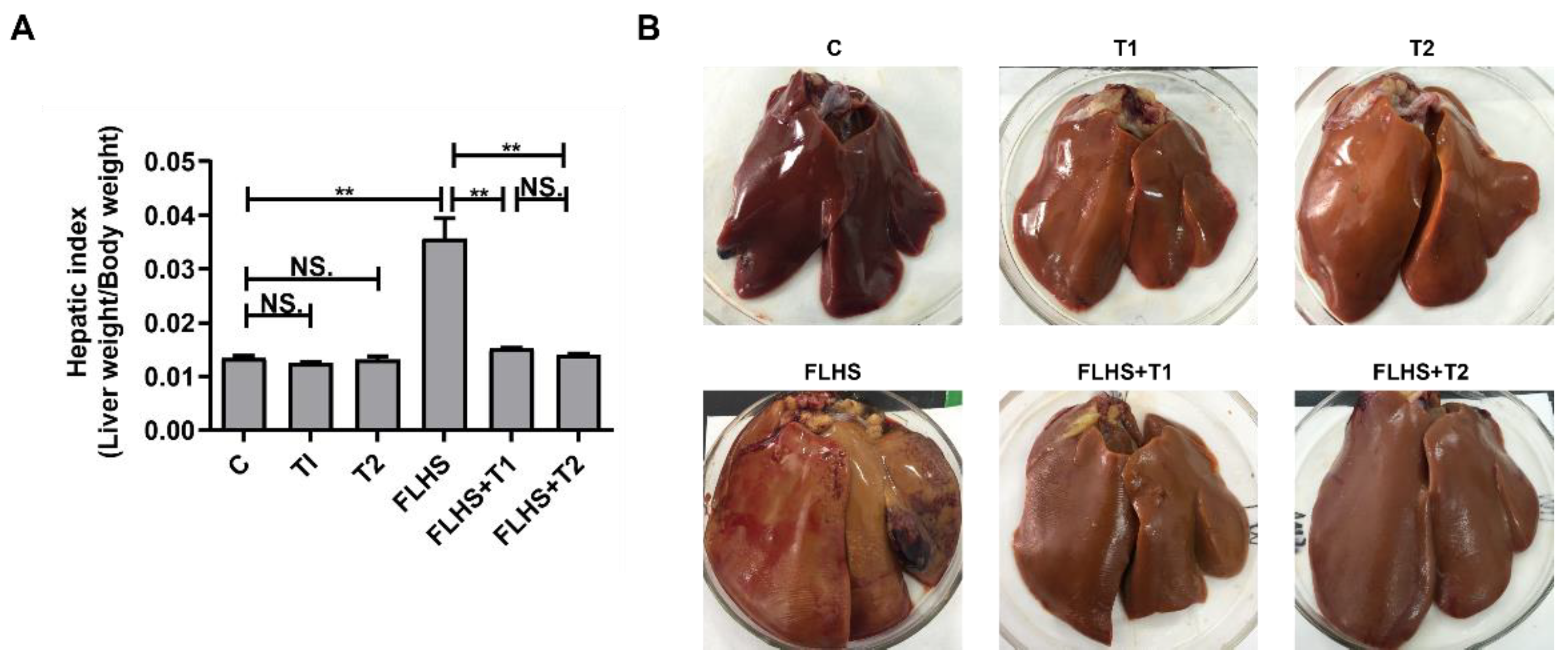

| Grouping | C | T1 | T2 | FLHS | FLHS + T1 | FLHS + T2 |
|---|---|---|---|---|---|---|
| Incidence of FLHS | 4% | 3% | 3% | 70% | 10% | 12% |
| Ingredient, % | Standard Diet | High-Fat and Low-Protein Diet |
|---|---|---|
| Corn | 61.5 | 61.5 |
| Soybean meal | 26.0 | 18.0 |
| Soybean oil | 0 | 8.0 |
| Dicalcium Phosphate | 1.2 | 1.2 |
| Limestone | 9.0 | 9.0 |
| Premix | 2.0 | 2.0 |
| Salt | 0.3 | 0.3 |
| Total | 100 | 100 |
| Calculated composition | ||
| Metabolizable energy, MJ/kg | 11.09 | 12.85 |
| Crude protein | 16.85 | 13.51 |
| Calcium | 3.68 | 3.65 |
| Available P | 0.38 | 0.37 |
| Lysine | 0.93 | 0.69 |
| Threonine (%) | 0.67 | 0.52 |
| Gene | Primer Sequence |
|---|---|
| Gallus-Cpt1 | Forward: 5′-GCCAAGTCGCTCGCTGATGAC-3′ Reverse: 5′-ACGCCTCGTAGGTCAGACAGAAC-3′ |
| Gallus-Mfn1 | Forward: 5′-TCCTGCTGCAACTCCAGAGA-3′ Reverse: 5′-ATCACTCCGCCAACAACGAT-3′ |
| Gallus-Mfn2 | Forward: 5′-CTGGCTCATGCCCTCCATCA-3′ Reverse: 5′-AATGCCAGGGCTGTCCATGA-3′ |
| Gallus-Opa1 | Forward: 5′-AGTCTGTGGAGCAGCAAGCA-3′ Reverse: 5′-TCCTGTCAAGCTCTCGCAAC-3′ |
| Gallus-Fis1 | Forward: 5′-TGTCTGTGGAGGACCTGCTGAAG-3′ Reverse: 5′-ACCAGGCACCAGGCGTACTC-3′ |
| Gallus-Drp1 | Forward: 5′- GAGCTCAACACTGCTATTTACG-3′ Reverse: 5′-TCAAAACAACCTTTCCGATCAC-3′ |
| Gallus-Lc3I | Forward: 5′-TTACACCCATATCAGATTCTTG-3′ Reverse: 5′-ATTCCAACCTGTCCCTCA-3′ |
| Gallus-Lc3II | Forward: 5′-CTTCTTCCTCCTGGTGAACG-3′ Reverse: 5′-GCACTCCGAAAGTCTCCTGA-3′ |
| Gallus-P62 | Forward: 5′-GACCCAGCCAAGACTACCAT-3′ Reverse: 5′-CAGAGGCATGTAGTTTCGGC-3′ |
| Gallus-Beclin1 | Forward: 5′-CGACTGGAGCAGGAAGAAG-3′ Reverse: 5′-TCTGAGCATAACGCATCTGG-3′ |
| Gallus-Pgc1α | Forward: 5′-ATGTGTCGCCTTCTTGCTCTTC-3′ Reverse: 5′-GGACCTTGATCTTGACCTGGAA-3′ |
| Gallus-Nrf1 | Forward: 5′-AGCATTGAGGACTATCGTGAAG-3′ Reverse: 5′-TGGTTGTGGTCTGTTGCTGTGT-3′ |
| Gallus-Nrf2 | Forward: 5′-GCCTTCCTCTGCTGCCATTAGT-3′ Reverse: 5′-TGCCTTCAGTGTGCTTCTGGTT-3′ |
| Gallus-Tfam | Forward: 5′-TTCCAGGAGGCTAAGGATGAG-3′ Reverse: 5′-CACTGCGACGGATGAGATCACT-3′ |
| Gallus-β-actin | Forward: 5′-GAGAAATTGTGCGTGACATCA-3′ Reverse: 5′-CCTGAACCTCTCATTGCCA-3′ |
| Homo-Sod2 | Forward: 5′-GAGATGTTACACGCCCAGATAGC-3′ Reverse: 5′-AATCCCCAGCAGTGGAATAAGG-3′ |
| Homo-Mfn1 | Forward: 5′-TGCTGGCTAAGAAGGCGATTACTG-3′ Reverse: 5′-TGTCTCCGAGATAGCACCTCACC-3′ |
| Homo-Mfn2 | Forward: 5′-GTGCTTCTCCCTCAACTATGAC-3′ Reverse: 5′-ATCCGAGAGAGAAATGGAACTC-3 |
| Homo-Opa1 | Forward: 5′-TCTGCACACTCAGTTGAAGTAT-3′ Reverse: 5′-GCCTTTGTCATCTTTCTGCAAT-3′ |
| Homo-Fis1 | Forward: 5′-TGTCTGTGGAGGACCTGCTGAAG-3′ Reverse: 5′-ACCAGGCACCAGGCGTACTC-3′ |
| Homo-Drp1 | Forward: 5′-GAGCTCAACACTGCTATTTACG-3′ Reverse: 5′-TCAAAACAACCTTTCCGATCAC-3′ |
| Homo-Pgc1α | Forward: 5′-CAGAGAGTATGAGAAGCGAGAG-3′ Reverse: 5′-AGCATCACAGGTATAACGGTAG-3 |
| Homo-Nrf1 | Forward: 5′-GCTACTTACACCGAGCATAGTA-3′ Reverse: 5′-CTCAAATACATGAGGCCGTTTC-3′ |
| Homo-Nrf2 | Forward: 5′-ACGGTATGCAACAGGACATTGAGC-3′ Reverse: 5′-TTGGCTTCTGGACTTGGAACCATG-3′ |
| Homo-Tfam | Forward: 5′-AGCTCAGAACCCAGATGCAA-3′ Reverse: 5′-CCTGCCACTCCGCCCTATAA-3′ |
| Homo-β-actin | Forward: 5′-GAGAGGGAAATCGTGCGTGACA-3′ Reverse: 5′-CGATAGTGACCTGACCGTCA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San, J.; Hu, J.; Pang, H.; Zuo, W.; Su, N.; Guo, Z.; Wu, G.; Yang, J. Taurine Protects against the Fatty Liver Hemorrhagic Syndrome in Laying Hens through the Regulation of Mitochondrial Homeostasis. Int. J. Mol. Sci. 2023, 24, 10360. https://doi.org/10.3390/ijms241210360
San J, Hu J, Pang H, Zuo W, Su N, Guo Z, Wu G, Yang J. Taurine Protects against the Fatty Liver Hemorrhagic Syndrome in Laying Hens through the Regulation of Mitochondrial Homeostasis. International Journal of Molecular Sciences. 2023; 24(12):10360. https://doi.org/10.3390/ijms241210360
Chicago/Turabian StyleSan, Jishuang, Jianmin Hu, Huiping Pang, Wenjun Zuo, Na Su, Zimeng Guo, Gaofeng Wu, and Jiancheng Yang. 2023. "Taurine Protects against the Fatty Liver Hemorrhagic Syndrome in Laying Hens through the Regulation of Mitochondrial Homeostasis" International Journal of Molecular Sciences 24, no. 12: 10360. https://doi.org/10.3390/ijms241210360






