Comparative Analysis of Water-Soluble Polysaccharides from Dendrobium Second Love ‘Tokimeki’ and Dendrobium nobile in Structure, Antioxidant, and Anti-Tumor Activity In Vitro
Abstract
:1. Introduction
2. Results and Discussion
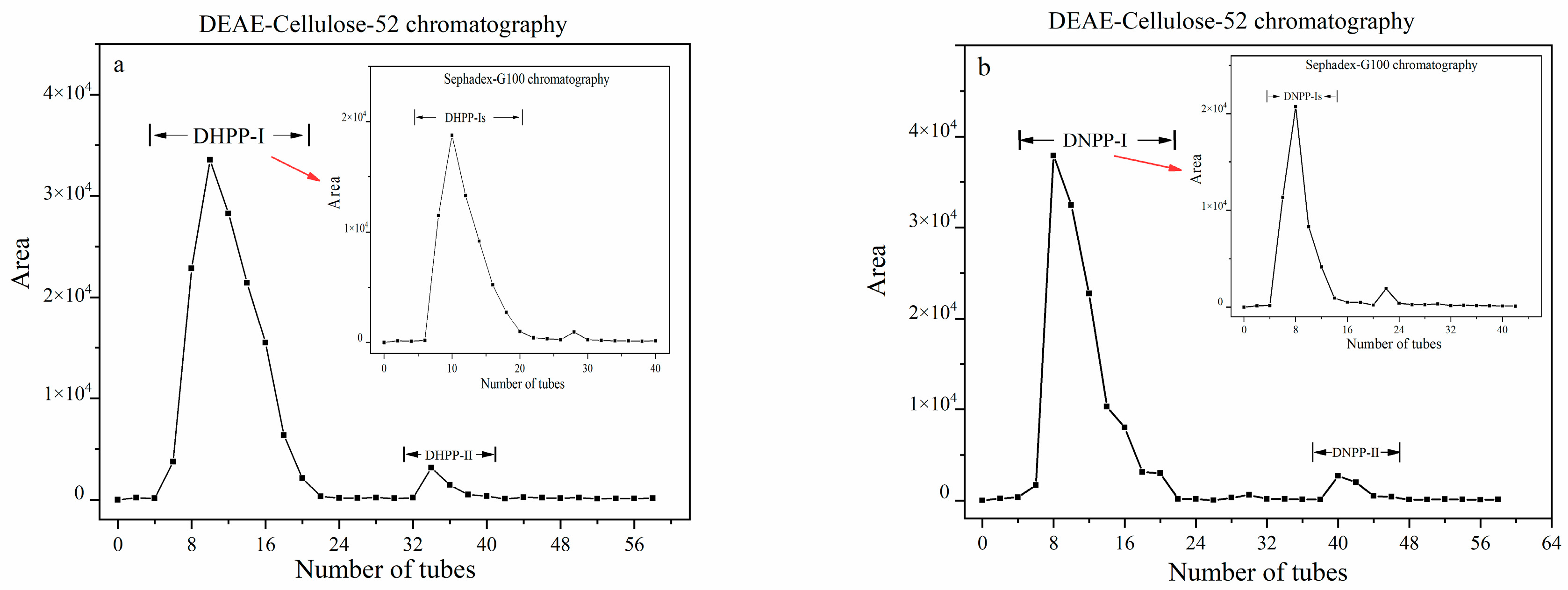
2.1. Isolation and Purification of Polysaccharides
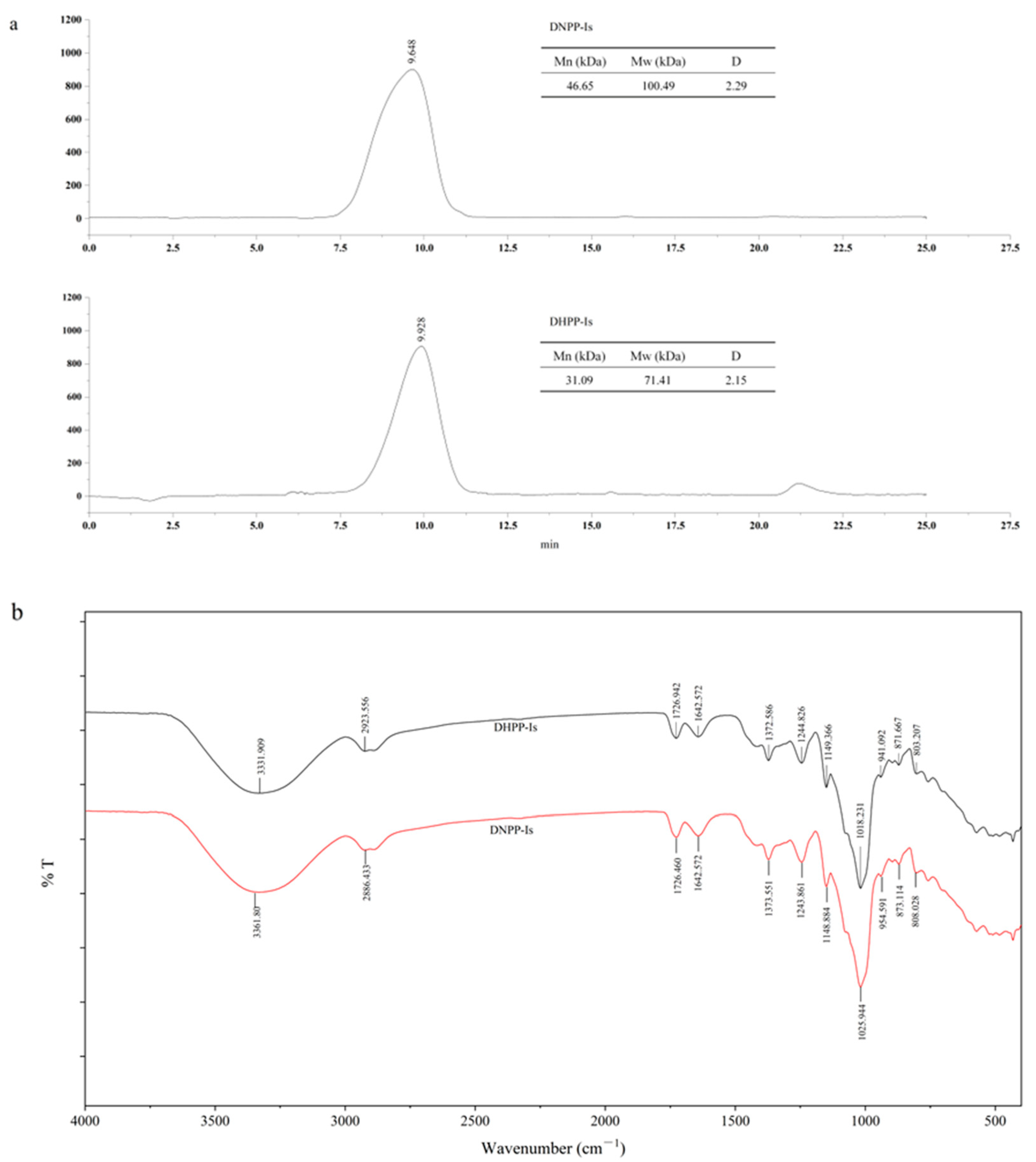
2.2. Molecular Weight (Mw) Determination and Infrared Spectroscopy
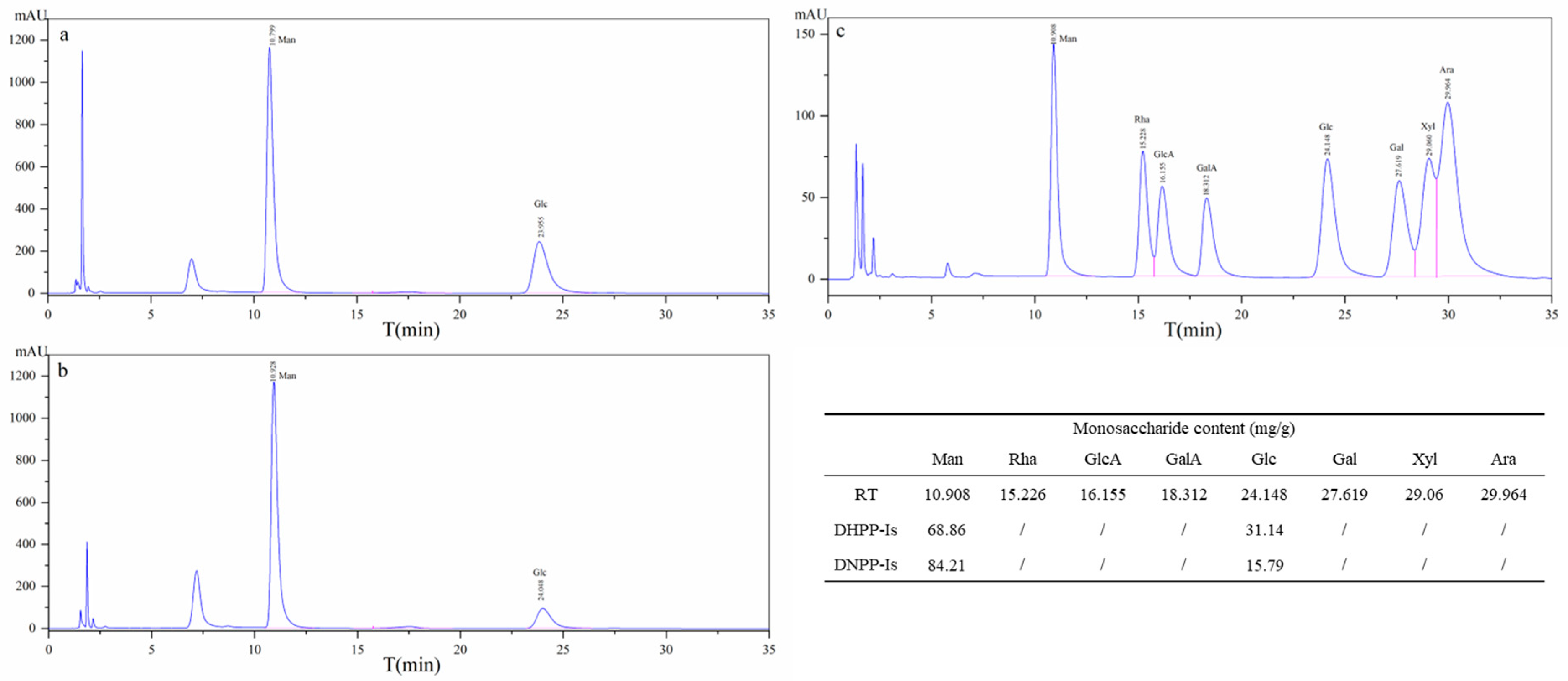
2.3. Monosaccharide and Methylation Analysis
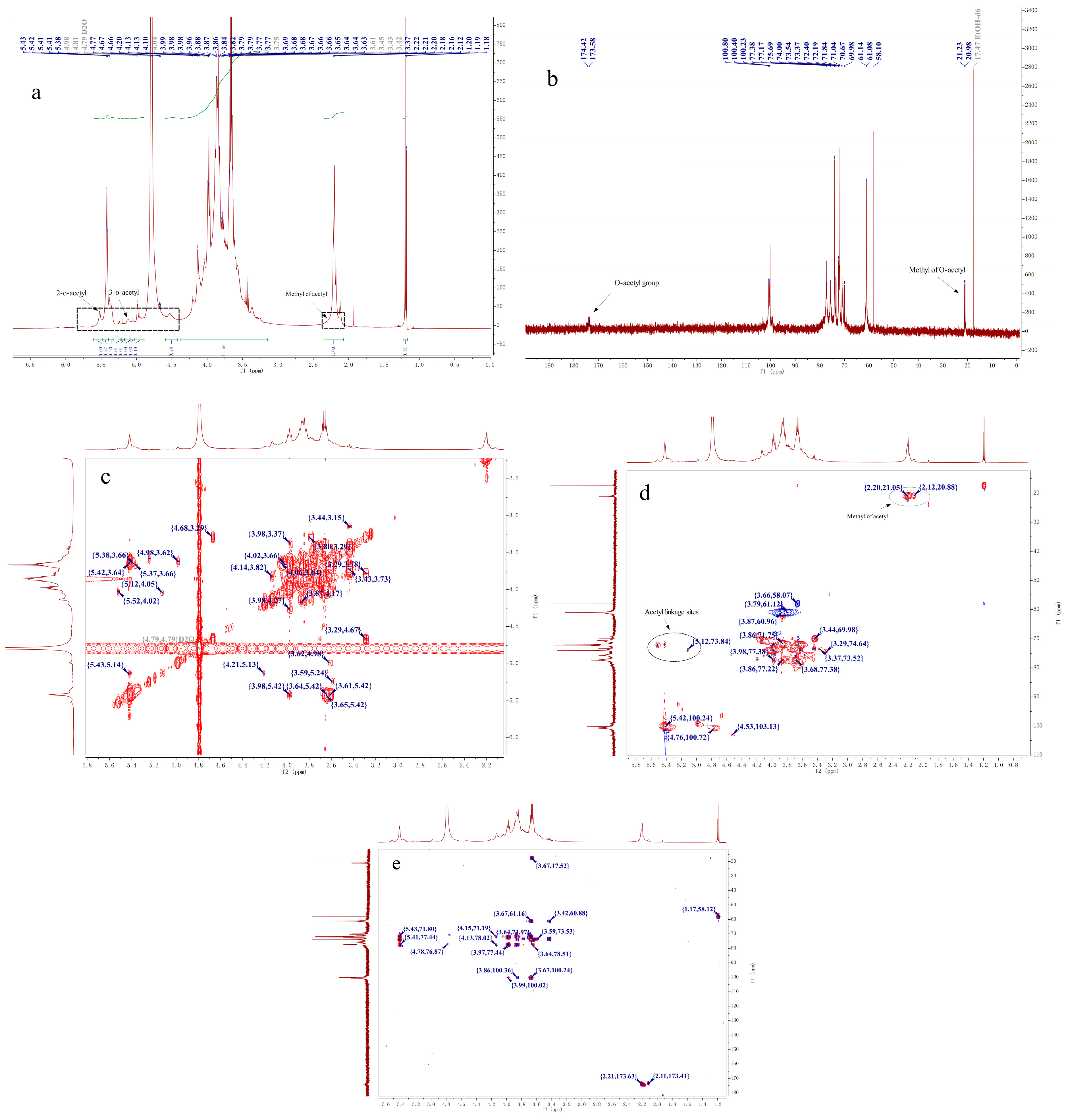
2.4. NMR Analysis
2.5. Antioxidant Activity
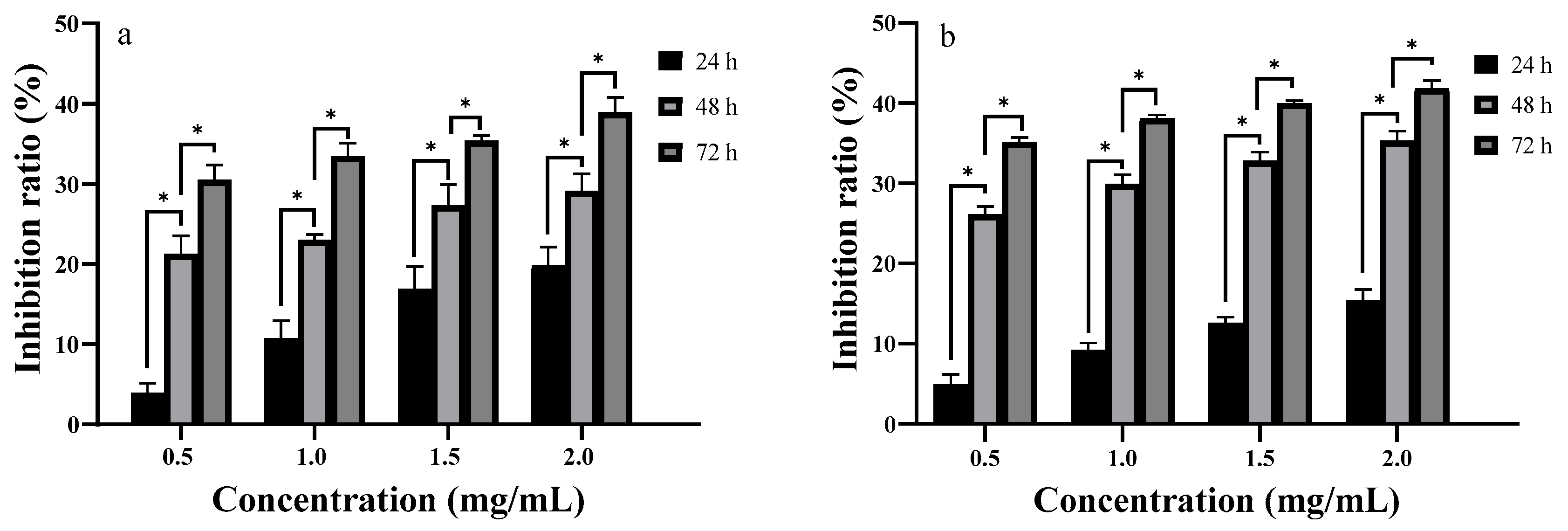
2.6. In Vitro Anti-Proliferative Activity
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Composition and Structure Analysis of Polysaccharide
4.2.1. Extraction and Purification of Polysaccharide
4.2.2. Determination of Purity, Uronic Acid, and Molecular Weight
4.2.3. Determination of Monosaccharide Composition
4.2.4. Methylation Analysis
4.2.5. IR and NMR Spectroscopy
4.3. Bioactivities of Polysaccharide
4.3.1. Antioxidant Activity Test
4.3.2. Proliferative Inhibition of SPC-A-1 Cells In Vitro
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varki, A. Biological roles of glycans. Glycobiology 2016, 27, 3–49. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.-M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Ernst, B.; Magnani, J.L. From carbohydrate leads to glycomimetic drugs. Nat. Rev. Drug. Discov. 2009, 8, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Galan, M.C.; Benito-Alifonso, D.; Watt, G.M. Carbohydrate chemistry in drug discovery. Org. Biomol. Chem. 2011, 9, 3598. [Google Scholar] [CrossRef]
- Khan, T.; Date, A.; Chawda, H.; Patel, K. Polysaccharides as potential anticancer agents—A review of their progress. Carbohydr. Polym. 2019, 210, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, M.; Wang, C.; Yang, Y.; Chen, J.; Ding, J.; Guo, W. Characterization and in vitro antitumor activity of polysaccharides from the mycelium of Sarcodon aspratus. Int. J. Biol. Macromol. 2013, 52, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhao, P.; Li, X.; Guo, L.; Gao, W. The potential roles of natural plant polysaccharides in inflammatory bowel disease: A review. Carbohydr. Polym. 2022, 277, 118821. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, B.; Wang, Z.; Li, M.; Zhao, W. Natural Polysaccharides with Immunomodulatory Activities. Mini-Rev. Med. Chem. 2020, 20, 96–106. [Google Scholar] [CrossRef]
- Xie, J.; Jin, M.; Morris, G.A.; Zha, X.; Chen, H.; Yi, Y.; Li, J.; Wang, Z.; Gao, J.; Nie, S.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. 1), S60–S84. [Google Scholar] [CrossRef]
- Kang, J.; Jia, X.; Wang, N.; Xiao, M.; Song, S.; Wu, S.; Li, Z.; Wang, S.; Cui, S.W.; Guo, Q. Insights into the structure-bioactivity relationships of marine sulfated polysaccharides: A review. Food Hydrocoll. 2022, 123, 107049. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Niu, F.-J.; Xie, Y.-X.; Xie, S.-M.; Liu, Y.-N.; Yang, Y.-Y.; Zhou, C.-Z.; Wan, X.-H. A review: Natural polysaccharides from medicinal plants and microorganisms and their anti-herpetic mechanism. Biomed. Pharmacother. 2020, 129, 110469. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Dang, P.-P.; Zhao, Z.; Yuan, L.-C.; Zhou, Z.-H.; Wolf, D.; Luo, Y.-B. An assessment of the Chinese medicinal Dendrobium industry: Supply, demand and sustainability. J. Ethnopharmacol. 2019, 229, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, J.; Li, X.; Lu, J.; Wu, W.; Sun, Y.; Zhu, B.; Qin, L. Isolation, structural properties, bioactivities of polysaccharides from Dendrobium officinale Kimura et. Migo: A review. Int. J. Biol. Macromol. 2021, 184, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shang, Z.Z.; Li, Q.M.; Zha, X.Q.; Wu, D.L.; Yu, N.J.; Han, L.; Peng, D.Y.; Luo, J.P. Structural features and anti-gastric cancer activity of polysaccharides from stem, root, leaf and flower of cultivated Dendrobium huoshanense. Int. J. Biol. Macromol. 2020, 143, 651–664. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, K.; Zhao, Z.; Zhang, P.; Liu, H.; Zhou, G.; Cheng, Y.; Wu, W.; Cai, Y.; Wu, B.; et al. A novel polysaccharide from Dendrobium devonianum serves as a TLR4 agonist for activating macrophages. Int. J. Biol. Macromol. 2019, 133, 564–574. [Google Scholar] [CrossRef]
- Paudel, M.R.; Chand, M.B.; Pant, B.; Pant, B. Assessment of Antioxidant and Cytotoxic Activities of Extracts of Dendrobium crepidatum. Biomolecules 2019, 9, 478. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.; Jiang, L.; Zhang, Y.; Tian, Y.; Li, L.; Lu, Y.; Yang, W.; Shi, J. Dendrobium nobile Lindl. Alkaloids Decreases the Level of Intracellular β-Amyloid by Improving Impaired Autolysosomal Proteolysis in APP/PS1 Mice. Front. Pharmacol. 2018, 9, 1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Li, Y.; Liu, W.; Qi, Q.; Hu, X.; Li, S.; Lei, J.; Rong, L. The Structure-Activity Relationship and Molecular Mechanism of Anti-tumor Polysaccharide Isolated from Dendrobium nobile Lindl. ACS Omega 2019, 4, 20586–20594. [Google Scholar] [CrossRef] [Green Version]
- Luo, A.; He, X.; Zhou, S.; Fan, Y.; He, T.; Chun, Z. In vitro antioxidant activities of a water-soluble polysaccharide derived from Dendrobium nobile Lindl. extracts. Int. J. Biol. Macromol. 2009, 45, 359–363. [Google Scholar] [CrossRef]
- Ye, G.; Zhang, J.; Li, j.; Liu, H.; Wang, Z. Comparison of polysaccharides and its hypoglycemic activity from three Dendrobium species. Nat. Prod. Res. Dev. 2020, 32, 7. [Google Scholar] [CrossRef]
- Yue, H.; Zeng, H.; Ding, K. A review of isolation methods, structure features and bioactivities of polysaccharides from Dendrobium species. Chin. J. Nat. Med. 2020, 18, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Zaihua, W.; Jie, L.; Jinhui, Z.; Gen-fa, Z.; Cuiling, L.; Qingsheng, Y. Comparison of Polysaccharide and Alkaloid Contents in Dendrobium. Chin. Agric. Sci. Bull. 2015, 31, 242–246. [Google Scholar]
- Molina, A.; O’Neill, M.A.; Darvill, A.G.; Etzler, M.E.; Mohnen, D.; Hahn, M.G.; Esko, J.D. Free Glycans as Bioactive Molecules. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; pp. 539–548. [Google Scholar]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Chokboribal, J.; Tachaboonyakiat, W.; Sangvanich, P.; Ruangpornvisuti, V.; Jettanacheawchankit, S.; Thunyakitpisal, P. Deacetylation affects the physical properties and bioactivity of acemannan, an extracted polysaccharide from Aloe vera. Carbohydr. Polym. 2015, 133, 556–566. [Google Scholar] [CrossRef]
- Lopez-Legarda, X.; Arboleda-Echavarria, C.; Parra-Saldivar, R.; Rostro-Alanis, M.; Alzate, J.F.; Villa-Pulgarin, J.A.; Segura-Sanchez, F. Biotechnological production, characterization and in vitro antitumor activity of polysaccharides from a native strain of Lentinus crinitus. Int. J. Biol. Macromol. 2020, 164, 3133–3144. [Google Scholar] [CrossRef]
- Wang, S.; Li, G.; Zhang, X.; Wang, Y.; Qiang, Y.; Wang, B.; Zou, J.; Niu, J.; Wang, Z. Structural characterization and antioxidant activity of Polygonatum sibiricum polysaccharides. Carbohydr. Polym. 2022, 291, 119524. [Google Scholar] [CrossRef]
- Ye, G.; Li, J.; Zhang, J.; Liu, H.; Ye, Q.; Wang, Z. Structural characterization and antitumor activity of a polysaccharide from Dendrobium wardianum. Carbohydr. Polym. 2021, 269, 118253. [Google Scholar] [CrossRef]
- van Halbeek, H. 1H nuclear magnetic resonance spectroscopy of carbohydrate chains of glycoproteins. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1994; Volume 230, pp. 132–168. [Google Scholar]
- Vliegenthart, J.F.G.; Dorland, L.; Halbeek, H.V. High-Resolution, 1H-Nuclear Magnetic Resonance Spectroscopy as a Tool in the Structural Analysis of Carbohydrates Related to Glycoproteins. In Advances in Carbohydrate Chemistry and Biochemistry; Tipson, R.S., Horton, D., Eds.; Academic Press: Cambridge, MA, USA, 1983; Volume 41, pp. 209–374. [Google Scholar]
- Kasaai, M.R. Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques: A review. Carbohydr. Polym. 2010, 79, 801–810. [Google Scholar] [CrossRef]
- Duus, J.; Gotfredsen, C.H.; Bock, K. Carbohydrate Structural Determination by NMR Spectroscopy: Modern Methods and Limitations. Chem. Rev. 2000, 100, 4589–4614. [Google Scholar] [CrossRef]
- Guo, Q.; Cui, S.W.; Kang, J.; Ding, H.; Wang, Q.; Wang, C. Non-starch polysaccharides from American ginseng: Physicochemical investigation and structural characterization. Food Hydrocoll. 2015, 44, 320–327. [Google Scholar] [CrossRef]
- Wong, T.-L.; Li, L.-F.; Zhang, J.-X.; Zhang, Q.-W.; Zhang, X.-T.; Zhou, L.-S.; Fung, H.-Y.; Feng, L.; Cheng, H.-Y.; Huo, C.-Y.; et al. Oligosaccharide analysis of the backbone structure of the characteristic polysaccharide of Dendrobium officinale. Food Hydrocoll. 2023, 134, 108038. [Google Scholar] [CrossRef]
- Yarley, O.P.N.; Kojo, A.B.; Zhou, C.; Yu, X.; Gideon, A.; Kwadwo, H.H.; Richard, O. Reviews on mechanisms of in vitro antioxidant, antibacterial and anticancer activities of water-soluble plant polysaccharides. Int. J. Biol. Macromol. 2021, 183, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, J.J.; Toone, E.J. The Cluster Glycoside Effect. Chem. Rev. 2002, 102, 555–578. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell. 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Yilu, G.; Di, C. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Sun, Y.; Wang, Z.; Ye, Q. Composition analysis and anti-proliferation activity of polysaccharides from Dendrobium chrysotoxum. Int. J. Biol. Macromol. 2013, 62, 291–295. [Google Scholar] [CrossRef]
- Furlani, F.; Sacco, P.; Decleva, E.; Menegazzi, R.; Donati, I.; Paoletti, S.; Marsich, E. Chitosan Acetylation Degree Influences the Physical Properties of Polysaccharide Nanoparticles: Implication for the Innate Immune Cells Response. ACS Appl. Mater. Interfaces 2019, 11, 9794–9803. [Google Scholar] [CrossRef]
- Vilkas, E.; Radjabi-Nassab, F. The glucomannan system from Aloe vahombe (liliaceae). III. Comparative studies on the glucomannan components isolated from the leaves. Biochimie 1986, 68, 1123–1127. [Google Scholar] [CrossRef]
- Zeng, C.; Ye, G.; Li, G.; Cao, H.; Wang, Z.; Ji, S. RID serve as a more appropriate measure than phenol sulfuric acid method for natural water-soluble polysaccharides quantification. Carbohydr. Polym. 2022, 278, 118928. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Fu, D.T.; Oneill, R.A. Monosaccharide Composition Analysis of Oligosaccharides and Glycoproteins by High-Performance Liquid Chromatography. Anal. Biochem. 1995, 227, 377–384. [Google Scholar] [CrossRef]
- Dai, J.; Wu, Y.; Chen, S.; Zhu, S.; Yin, H.; Wang, M.; Tang, J. Sugar compositional determination of polysaccharides from Dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydr. Polym. 2010, 82, 629–635. [Google Scholar] [CrossRef]
- Pettolino, F.A.; Walsh, C.; Fincher, G.B.; Bacic, A. Determining the polysaccharide composition of plant cell walls. Nat. Protoc. 2012, 7, 1590–1607. [Google Scholar] [CrossRef] [PubMed]
- Sims, I.M.; Carnachan, S.M.; Bell, T.J.; Hinkley, S.F.R. Methylation analysis of polysaccharides: Technical advice. Carbohydr. Polym. 2018, 188, 1–7. [Google Scholar] [CrossRef]
- Lundborg, M.; Widmalm, G. Structural Analysis of Glycans by NMR Chemical Shift Prediction. Anal. Chem. 2011, 83, 1514–1517. [Google Scholar] [CrossRef]
- Guo, Q.; Cui, S.W.; Wang, Q.; Hu, X.; Kang, J.; Yada, R.Y. Structural characterization of a low-molecular-weight heteropolysaccharide (glucomannan) isolated from Artemisia sphaerocephala Krasch. Carbohydr. Res. 2012, 350, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, S.; Li, H. An Improved System to Evaluate Superoxide-Scavenging Effects of Bioflavonoids. ChemistryOpen 2021, 10, 503–514. [Google Scholar] [CrossRef] [PubMed]
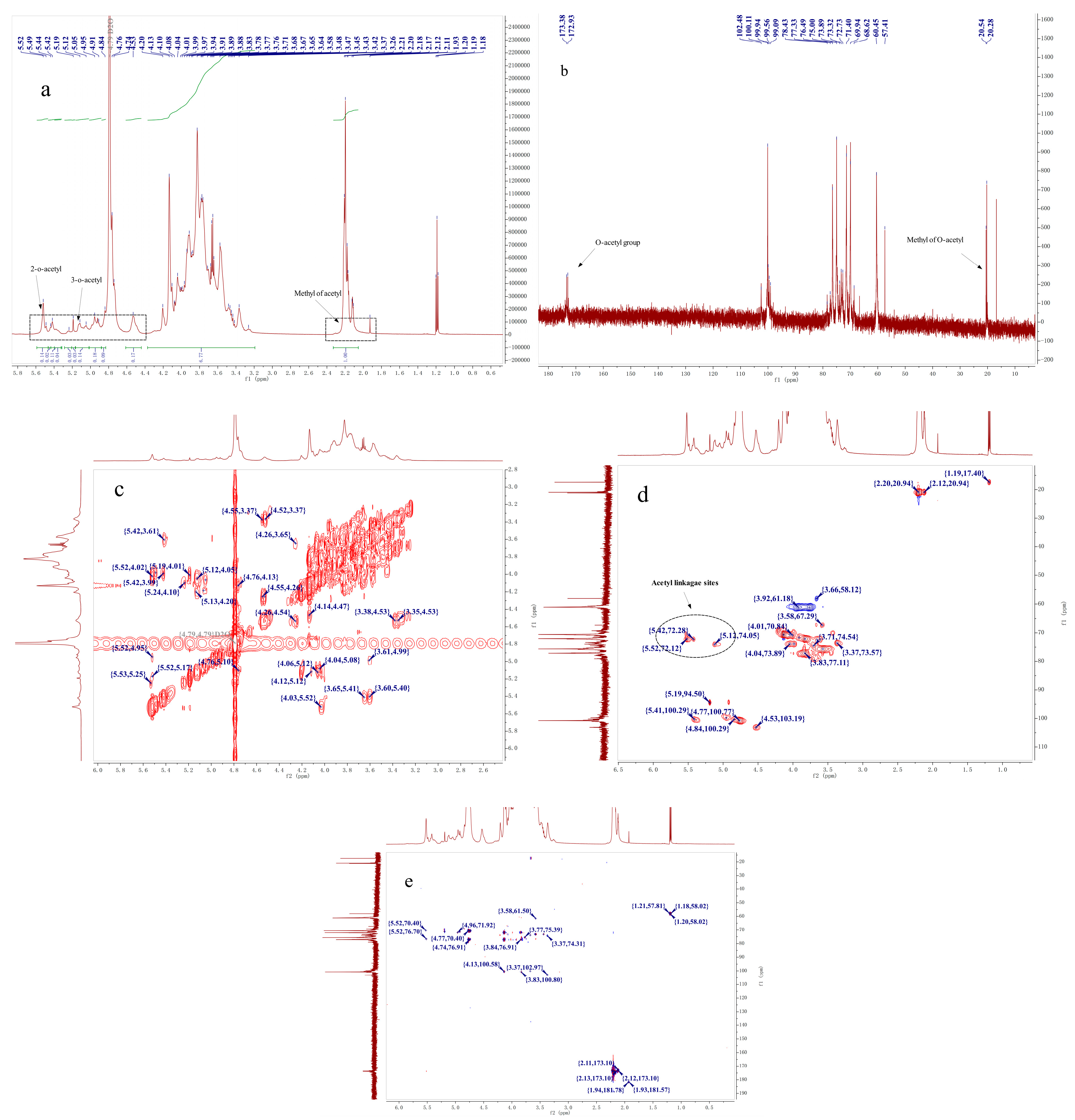

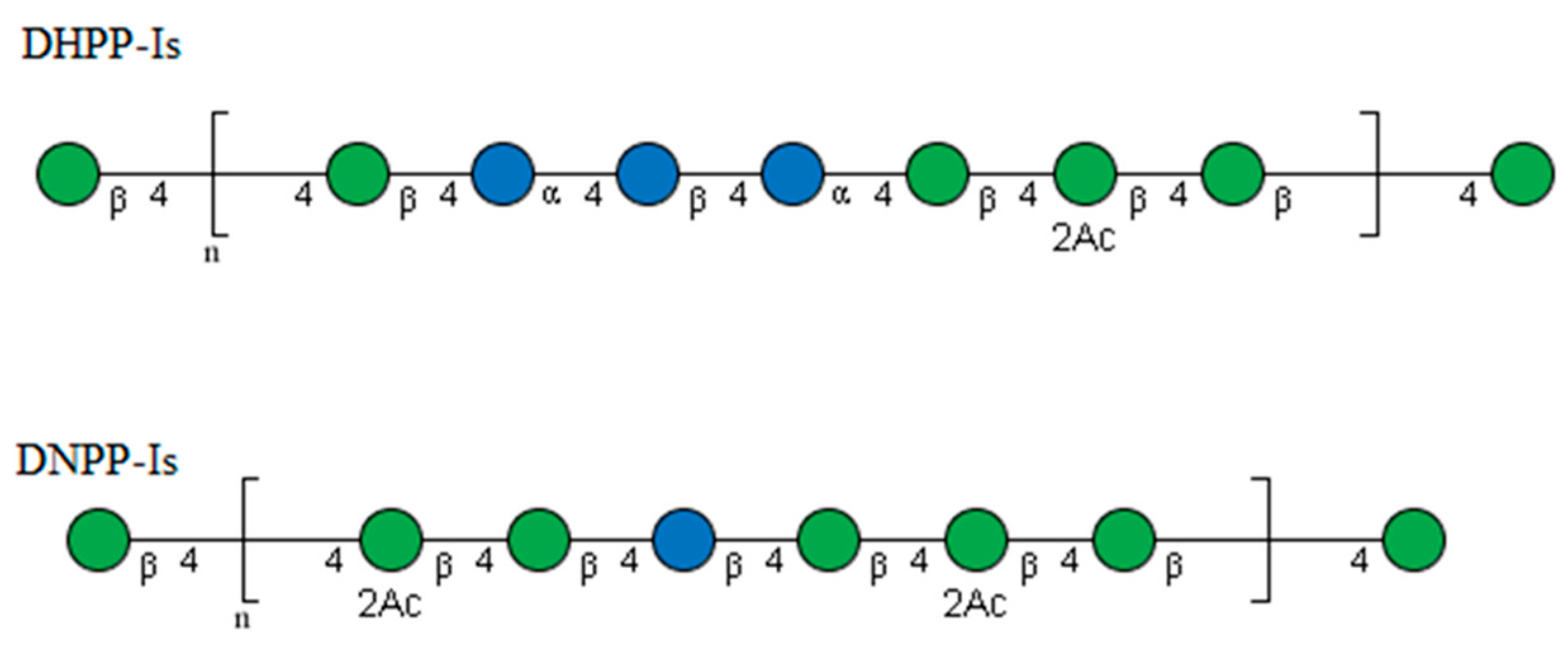

| Retention Time (min) | Mass Fraction (m/z) | Linkage Pattern | Molar Ratio | |
|---|---|---|---|---|
| DHPP-Is | DNPP-Is | |||
| 11.967 | 59, 71, 87, 102, 113, 118, 129, 145, 161, 162, 205 | T-Manp | 2.40 | 1.82 |
| 14.271 | 59, 71, 87, 99, 102, 113, 118, 129, 131, 162, 233 | 1,4-Linked Manp | 63.32 | 82.67 |
| 14.421 | 59, 71, 87, 99, 102, 113, 118, 129, 131, 162, 233 | 1,4-Linked Glcp | 35.99 | 15.34 |
| 16.200 | 59, 71, 85, 99, 102, 118, 127, 142, 162, 201, 261 | 1,4,6-Linked Manp | 0.28 | 0.15 |
| Sugar Residues | Ratio | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|---|
| →4)-β-D-Glcp-(1→ | 2.4 | δH | 4.53 | 3.36 | 3.61 | 3.71 | 3.65 | - |
| δC | 103.48 | 73.56 | 75.58 | 79.47 | 75.93 | - | ||
| →4)-α-D-Glcp-(1→ | 5.2 | δH | 5.42 | 3.65 | 3.98 | 3.86 | 3.68 | 3.78 |
| δC | 100.28 | 72.19 | 73.98 | 71.83 | 77.36 | 61.14 | ||
| →4)-β-D-Manp-(1→ | 8.2 | δH | 4.76 | 4.08 | 3.96 | 3.65 | 3.99 | 3.87, 3.84 |
| δC | 100.80 | 70.78 | 71.74 | 74.02 | 70.38 | 61.08 | ||
| →4)-2-O-acetyl-β-D-Manp-(1→ | 1.5 | δH | 4.98 | 5.51 | 4.02 | 3.98 | 3.67 | - |
| δC | 99.28 | 72.19 | 74.00 | 71.84 | 77.33 | - | ||
| →4)-3-O-acetyl-β-D-Manp-(1→ | 1.0 | δH | 5.41 | 4.21 | 5.12 | 4.05 | 3.62 | - |
| δC | 100.23 | 69.27 | 73.94 | 70.72 | 75.52 | - |
| Sugar Residues | Ratio | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|---|
| →4)-β-D-Glcp-(1→ | 1.2 | δH | 4.53 | 3.36 | 3.71 | 4.27 | 3.65 | 3.83, 4.00 |
| δC | 103.13 | 70.63 | 72.37 | 74.53 | 72.37 | 61.09 | ||
| →4)-α-D-Glcp-(1→ | 0.8 | δH | 5.42 | 3.65 | 3.98 | 3.86 | 3.68 | 3.78 |
| δC | 100.38 | 72.19 | 73.98 | 71.83 | 77.36 | 61.14 | ||
| →4)-β-D-Manp-(1→ | 3.6 | δH | 4.76 | 3.57 | 3.83 | 4.14 | 3.83 | 3.92, 3.77 |
| δC | 100.75 | 75.64 | 72.05 | 70.59 | 77.13 | 61.09 | ||
| →4)-2-O-acetyl-β-D-Manp-(1→ | 1.6 | δH | 4.96 | 5.52 | 4.03 | 3.82 | 3.83 | - |
| δC | 99.86 | 72.24 | 73.84 | 72.34 | 77.09 | 61.09 | ||
| →4)-3-O-acetyl-β-D-Manp-(1→ | 0.8 | δH | 5.41 | 4.21 | 5.12 | 4.04 | 3.82 | - |
| δC | 100.41 | 69.27 | 73.94 | 70.72 | 75.52 | 61.09 | ||
| →4)-2,3-O-acetyl-β-D-Manp-(1→ | 0.2 | δH | 5.42 | 5.53 | 5.24 | 4.10 | 3.77 | 3.94 |
| δC | 100.61 | 70.44 | 72.31 | 69.26 | 75.61 | 61.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, G.; Zhang, J.; Xu, X.; Zeng, C.; Ye, Q.; Wang, Z. Comparative Analysis of Water-Soluble Polysaccharides from Dendrobium Second Love ‘Tokimeki’ and Dendrobium nobile in Structure, Antioxidant, and Anti-Tumor Activity In Vitro. Int. J. Mol. Sci. 2023, 24, 10361. https://doi.org/10.3390/ijms241210361
Ye G, Zhang J, Xu X, Zeng C, Ye Q, Wang Z. Comparative Analysis of Water-Soluble Polysaccharides from Dendrobium Second Love ‘Tokimeki’ and Dendrobium nobile in Structure, Antioxidant, and Anti-Tumor Activity In Vitro. International Journal of Molecular Sciences. 2023; 24(12):10361. https://doi.org/10.3390/ijms241210361
Chicago/Turabian StyleYe, Guangying, Jinhui Zhang, Xiaoli Xu, Canbiao Zeng, Qingsheng Ye, and Zaihua Wang. 2023. "Comparative Analysis of Water-Soluble Polysaccharides from Dendrobium Second Love ‘Tokimeki’ and Dendrobium nobile in Structure, Antioxidant, and Anti-Tumor Activity In Vitro" International Journal of Molecular Sciences 24, no. 12: 10361. https://doi.org/10.3390/ijms241210361





