Reasons for the Sex Bias in Osteoarthritis Research: A Review of Preclinical Studies
Abstract
:1. Introduction
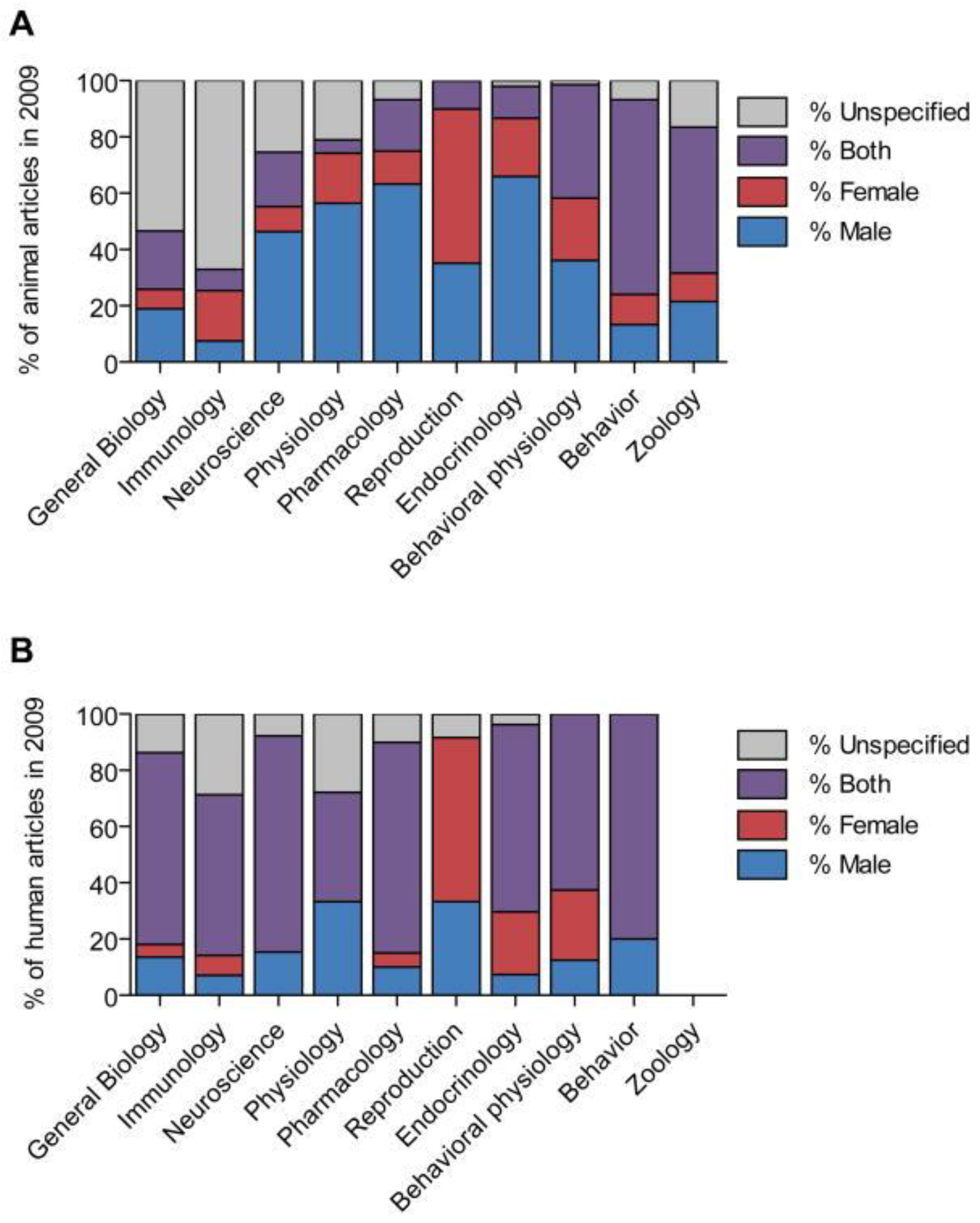
2. Sex Bias across the Disciplines
3. Sex Differences in Preclinical OA Research
3.1. The Role of Hormones
3.2. Sex Differences in Inflammation and Pain
3.3. Patterns of Onset and Progression of OA in Males and Females
3.4. Implications of Sex Bias and Current State of SABV in Preclinical Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Tschon, M.; Contartese, D.; Pagani, S.; Borsari, V.; Fini, M. Gender and Sex Are Key Determinants in Osteoarthritis not only Confounding Variables. A Systematic Review of Clinical Data. J. Clin. Med. 2021, 10, 3178. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Zhou, X.; Jin, M.; Nie, J.; Li, X. Molecular mechanisms of mechanical load-induced osteoarthritis. Int. Orthop. 2021, 45, 1125–1136. [Google Scholar] [CrossRef]
- Wood, M.J.; Miller, R.E.; Malfait, A.M. The Genesis of Pain in Osteoarthritis: Inflammation as a Mediator of Osteoarthritis Pain. Clin. Geriatr. Med. 2022, 38, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Mehana, E.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis from 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef]
- Overstreet, D.S.; Strath, L.J.; Jordan, M.; Jordan, I.A.; Hobson, J.M.; Owens, M.A.; Williams, A.C.; Edwards, R.R.; Meints, S.M. A Brief Overview: Sex Differences in Prevalent Chronic Musculoskeletal Conditions. Int. J. Environ. Res. Public Health 2023, 20, 4521. [Google Scholar] [CrossRef]
- Sacitharan, P.K. Ageing and Osteoarthritis. Subcell. Biochem. 2019, 91, 123–159. [Google Scholar] [CrossRef]
- Jang, S.; Lee, K.; Ju, J.H. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef]
- Maleki-Fischbach, M.; Jordan, J.M. New developments in osteoarthritis. Sex differences in magnetic resonance imaging-based biomarkers and in those of joint metabolism. Arthritis Res. Ther. 2010, 12, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawker, G.A.; King, L.K. The Burden of Osteoarthritis in Older Adults. Clin. Geriatr. Med. 2022, 38, 181–192. [Google Scholar] [CrossRef]
- Srikanth, V.K.; Fryer, J.L.; Zhai, G.; Winzenberg, T.M.; Hosmer, D.; Jones, G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr. Cartil. 2005, 13, 769–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Zheng, Z. Males and Females Have Distinct Molecular Events in the Articular Cartilage during Knee Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 7876. [Google Scholar] [CrossRef]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Borg, T.M.; Heidari, N.; Noorani, A.; Slevin, M.; Cullen, A.; Olgiati, S.; Zerbi, A.; Danovi, A.; Wilson, A. Gender-Specific Response in Pain and Function to Biologic Treatment of Knee Osteoarthritis: A Gender-Bias-Mitigated, Observational, Intention-to-Treat Study at Two Years. Stem. Cells Int. 2021, 2021, 6648437. [Google Scholar] [CrossRef]
- Peshkova, M.; Lychagin, A.; Lipina, M.; Di Matteo, B.; Anzillotti, G.; Ronzoni, F.; Kosheleva, N.; Shpichka, A.; Royuk, V.; Fomin, V.; et al. Gender-Related Aspects in Osteoarthritis Development and Progression: A Review. Int. J. Mol. Sci. 2022, 23, 2767. [Google Scholar] [CrossRef]
- Kim, J.R.; Kim, H.A. Molecular Mechanisms of Sex-Related Differences in Arthritis and Associated Pain. Int. J. Mol. Sci. 2020, 21, 7938. [Google Scholar] [CrossRef]
- Contartese, D.; Tschon, M.; De Mattei, M.; Fini, M. Sex Specific Determinants in Osteoarthritis: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2020, 21, 3696. [Google Scholar] [CrossRef]
- Karp, N.A.; Reavey, N. Sex bias in preclinical research and an exploration of how to change the status quo. Br. J. Pharmacol. 2019, 176, 4107–4118. [Google Scholar] [CrossRef]
- Csifo, E.N.; Nagy, E.E.; Horvath, E.M.; Farr, A.; Muntean, D.L. Mid-term effects of meloxicam on collagen type II degradation in a rat osteoarthritis model induced by iodoacetate. Farmacia 2015, 63, 556–560. [Google Scholar]
- Beery, A.K.; Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clayton, J.A.; Collins, F.S. Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Arnegard, M.E.; Whitten, L.A.; Hunter, C.; Clayton, J.A. Sex as a Biological Variable: A 5-Year Progress Report and Call to Action. J. Womens Health 2020, 29, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Correa-De-Araujo, R. Serious gaps: How the lack of sex/gender-based research impairs health. J. Womens Health 2006, 15, 1116–1122. [Google Scholar] [CrossRef]
- Reue, K.; Wiese, C.B. Illuminating the Mechanisms Underlying Sex Differences in Cardiovascular Disease. Circ Res. 2022, 130, 1747–1762. [Google Scholar] [CrossRef]
- Connelly, P.J.; Azizi, Z.; Alipour, P.; Delles, C.; Pilote, L.; Raparelli, V. The Importance of Gender to Understand Sex Differences in Cardiovascular Disease. Can. J. Cardiol. 2021, 37, 699–710. [Google Scholar] [CrossRef]
- Yoon, D.Y.; Mansukhani, N.A.; Stubbs, V.C.; Helenowski, I.B.; Woodruff, T.K.; Kibbe, M.R. Sex bias exists in basic science and translational surgical research. Surgery 2014, 156, 508–516. [Google Scholar] [CrossRef]
- Bind, R.H.; Minney, S.M.; Rosenfeld, S.; Hallock, R.M. The role of pheromonal responses in rodent behavior: Future directions for the development of laboratory protocols. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 124–129. [Google Scholar]
- Giles, J.M.; Whitaker, J.W.; Moy, S.S.; Fletcher, C.A. Effect of Environmental Enrichment on Aggression in BALB/cJ and BALB/cByJ Mice Monitored by Using an Automated System. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 236–243. [Google Scholar] [CrossRef]
- Beery, A.K. Inclusion of females does not increase variability in rodent research studies. Curr. Opin. Behav. Sci. 2018, 23, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Fitts, D.A. Minimizing animal numbers: The variable-criteria sequential stopping rule. Comp. Med. 2011, 61, 206–218. [Google Scholar] [PubMed]
- Becker, J.B.; Prendergast, B.J.; Liang, J.W. Female rats are not more variable than male rats: A meta-analysis of neuroscience studies. Biol. Sex Differ. 2016, 7, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prendergast, B.J.; Onishi, K.G.; Zucker, I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2014, 40, 1–5. [Google Scholar] [CrossRef]
- Itoh, Y.; Arnold, A.P. Are females more variable than males in gene expression? Meta-analysis of microarray datasets. Biol. Sex Differ. 2015, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Dayton, A.; Exner, E.C.; Bukowy, J.D.; Stodola, T.J.; Kurth, T.; Skelton, M.; Greene, A.S.; Cowley, A.W., Jr. Breaking the Cycle: Estrous Variation Does Not Require Increased Sample Size in the Study of Female Rats. Hypertension 2016, 68, 1139–1144. [Google Scholar] [CrossRef] [Green Version]
- Meziane, H.; Ouagazzal, A.M.; Aubert, L.; Wietrzych, M.; Krezel, W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: Implications for phenotyping strategies. Genes Brain Behav. 2007, 6, 192–200. [Google Scholar] [CrossRef]
- Shah, K.; McCormack, C.E.; Bradbury, N.A. Do you know the sex of your cells? Am. J. Physiol. Cell Physiol. 2014, 306, C3–C18. [Google Scholar] [CrossRef] [Green Version]
- Taylor, K.E.; Vallejo-Giraldo, C.; Schaible, N.S.; Zakeri, R.; Miller, V.M. Reporting of sex as a variable in cardiovascular studies using cultured cells. Biol. Sex Differ. 2011, 2, 11. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.K. Sex as an important biological variable in biomedical research. BMB Rep. 2018, 51, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Park, M.N.; Park, J.H.; Paik, H.Y.; Lee, S.K. Insufficient sex description of cells supplied by commercial vendors. Am. J. Physiol. Cell Physiol. 2015, 308, C578–C580. [Google Scholar] [CrossRef] [Green Version]
- Rosner, I.A.; Goldberg, V.M.; Getzy, L.; Moskowitz, R.W. Effects of estrogen on cartilage and experimentally induced osteoarthritis. Arthritis Rheum. 1979, 22, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Dennison, E.M. Osteoarthritis: The importance of hormonal status in midlife women. Maturitas 2022, 165, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; McAlindon, T.E.; Hannan, M.T.; Chaisson, C.E.; Klein, R.; Wilson, P.W.; Felson, D.T. Estrogen replacement therapy and worsening of radiographic knee osteoarthritis: The Framingham Study. Arthritis Rheum 1998, 41, 1867–1873. [Google Scholar] [CrossRef]
- Stevens-Lapsley, J.E.; Kohrt, W.M. Osteoarthritis in women: Effects of estrogen, obesity and physical activity. Womens Health 2010, 6, 601–615. [Google Scholar] [CrossRef]
- de Klerk, B.M.; Schiphof, D.; Groeneveld, F.P.; Koes, B.W.; van Osch, G.J.; van Meurs, J.B.; Bierma-Zeinstra, S.M. No clear association between female hormonal aspects and osteoarthritis of the hand, hip and knee: A systematic review. Rheumatology 2009, 48, 1160–1165. [Google Scholar] [CrossRef] [Green Version]
- Koelling, S.; Miosge, N. Sex differences of chondrogenic progenitor cells in late stages of osteoarthritis. Arthritis Rheum. 2010, 62, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, B.H.; Wang, X.; Antony, B.; Zhu, Z.; Han, W.; Cicuttini, F.; Wluka, A.E.; Winzenberg, T.; Blizzard, L.; et al. Associations between endogenous sex hormones and MRI structural changes in patients with symptomatic knee osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1100–1106. [Google Scholar] [CrossRef] [Green Version]
- Wardhana; Surachmanto, E.E.; Datau, E.A.; Ongkowijaya, J.; Karema-Kaparang, A.M. Transdermal bio-identical progesterone cream as hormonal treatment for osteoarthritis. Acta Med. Indones. 2013, 45, 224–232. [Google Scholar]
- Huang, K.; Wu, L.D. Dehydroepiandrosterone: Molecular mechanisms and therapeutic implications in osteoarthritis. J. Steroid. Biochem. Mol. Biol. 2018, 183, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Huan, Z.; Wang, Y.; Zhang, M.; Zhang, X.; Liu, Y.; Kong, L.; Xu, J. Follicle-stimulating hormone worsens osteoarthritis by causing inflammation and chondrocyte dedifferentiation. FEBS Open Bio 2021, 11, 2292–2303. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Huang, J.; Yang, F.; Hong, J.; Wang, W.; Yan, S. Sex hormone-binding globulin and arthritis: A Mendelian randomization study. Arthritis Res. Ther. 2020, 22, 118. [Google Scholar] [CrossRef] [PubMed]
- Ro, J.Y.; Zhang, Y.; Tricou, C.; Yang, D.; da Silva, J.T.; Zhang, R. Age and Sex Differences in Acute and Osteoarthritis-like Pain Responses in Rats. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1465–1472. [Google Scholar] [CrossRef]
- Sannajust, S.; Imbert, I.; Eaton, V.; Henderson, T.; Liaw, L.; May, M.; Barbe, M.F.; King, T. Females have greater susceptibility to develop ongoing pain and central sensitization in a rat model of temporomandibular joint pain. Pain 2019, 160, 2036–2049. [Google Scholar] [CrossRef]
- Gubbels Bupp, M.R. Sex, the aging immune system, and chronic disease. Cell. Immunol. 2015, 294, 102–110. [Google Scholar] [CrossRef]
- Maldonado, M.; Nam, J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed. Res. Int. 2013, 2013, 284873. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Sun, L.; Liu, L.; Jiao, K.; Wang, M. Differential expression of IGF1, IGFR1 and IGFBP3 in mandibular condylar cartilage between male and female rats applied with malocclusion. J. Oral. Rehabil. 2012, 39, 727–736. [Google Scholar] [CrossRef]
- Macrini, T.E.; Coan, H.B.; Levine, S.M.; Lerma, T.; Saks, C.D.; Araujo, D.J.; Bredbenner, T.L.; Coutts, R.D.; Nicolella, D.P.; Havill, L.M. Reproductive status and sex show strong effects on knee OA in a baboon model. Osteoarthr. Cartil. 2013, 21, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Boyan, B.D.; Hart, D.A.; Enoka, R.M.; Nicolella, D.P.; Resnick, E.; Berkley, K.J.; Sluka, K.A.; Kwoh, C.K.; Tosi, L.L.; O’Connor, M.I.; et al. Hormonal modulation of connective tissue homeostasis and sex differences in risk for osteoarthritis of the knee. Biol. Sex Differ. 2013, 4, 3. [Google Scholar] [CrossRef] [Green Version]
| Area of Focus | Objective | Main Conclusions | References |
|---|---|---|---|
| Differences in hormones | Role of estrogen | Estrogen is both protective and aggravating in OA pathogenesis. In females, estrogen helps to regenerate cartilage, thereby interfering with disease progression. The same restorative qualities of estrogen have not been seen in males. | [14,18,19,43,44,45,46,47] |
| Role of testosterone | Testosterone has been seen to have restorative qualities in males. In females, higher testosterone levels are associated with lower joint pain scores. Lower testosterone leads to increased pain sensitivity. | [48,49] | |
| Role of progesterone | Progesterone has been seen to have anti-inflammatory effects in OA due to its gene suppression of cytokine production. | [50] | |
| Role of dehydroepiandrosterone | Dehydroepiandrosterone (DHEA) has both protective and aggravating functions in OA progression. The overall effect of the hormone is not clear. It helps to modulate the balance between cartilage anabolism and catabolism. It can decrease remodeling of the subchondral bone to slow joint space deterioration. | [51] | |
| Role of follicle-stimulating hormone | Follicle-stimulating hormone, a hormone higher in women, works to promote the inflammatory response of chondrocytes. Introduction of FSH into the joint space can worsen OA. | [52] | |
| Role of sex hormone-binding globulin | Increased levels of sex hormone-binding globulin (SHBG) positively contribute to the development of OA. Additionally, high levels of this hormone could be considered a risk factor for OA. The exact mechanism of SHBG in OA is still being investigated. | [53] | |
| Differences in pain and inflammation | Pain response | Females tend to experience greater levels of pain than males and are more susceptible to developing chronic pain. | [54,55] |
| Inflammation and the immune system | Females have a heightened inflammatory response with higher levels of pro-inflammatory cytokines and macrophage stimulators. Males have increased anabolic growth factors, metalloproteinases, and glycosaminoglycans in their synovial fluid and less inflammatory mediators. In the same joint, females and males can experience different levels of inflammation which drives pain and debilitation. | [56,57] | |
| Differences in disease characteristics | Joint anatomical changes, biomechanical factors, disease progression | When controlling for time, females experience more cartilage loss, reduced bone volume, increased osteocyte formation, and a higher degree of OA progression and severity than males. This leads to greater functional disability in females in a shorter amount of time than males. | [58,59] |
| Gene expression, presence of growth factors | Females have reduced gene expression of growth factors (insulin-like growth factor-1, extracellular matrix proteins, IGF binding protein-3) compared to males. | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franke, M.; Mancino, C.; Taraballi, F. Reasons for the Sex Bias in Osteoarthritis Research: A Review of Preclinical Studies. Int. J. Mol. Sci. 2023, 24, 10386. https://doi.org/10.3390/ijms241210386
Franke M, Mancino C, Taraballi F. Reasons for the Sex Bias in Osteoarthritis Research: A Review of Preclinical Studies. International Journal of Molecular Sciences. 2023; 24(12):10386. https://doi.org/10.3390/ijms241210386
Chicago/Turabian StyleFranke, Madeline, Chiara Mancino, and Francesca Taraballi. 2023. "Reasons for the Sex Bias in Osteoarthritis Research: A Review of Preclinical Studies" International Journal of Molecular Sciences 24, no. 12: 10386. https://doi.org/10.3390/ijms241210386
APA StyleFranke, M., Mancino, C., & Taraballi, F. (2023). Reasons for the Sex Bias in Osteoarthritis Research: A Review of Preclinical Studies. International Journal of Molecular Sciences, 24(12), 10386. https://doi.org/10.3390/ijms241210386






