Effect of Hesperidin on Barrier Function and Reactive Oxygen Species Production in an Oral Epithelial Cell Model, and on Secretion of Macrophage-Derived Inflammatory Mediators during Porphyromonas gingivalis Infection
Abstract
1. Introduction
2. Results
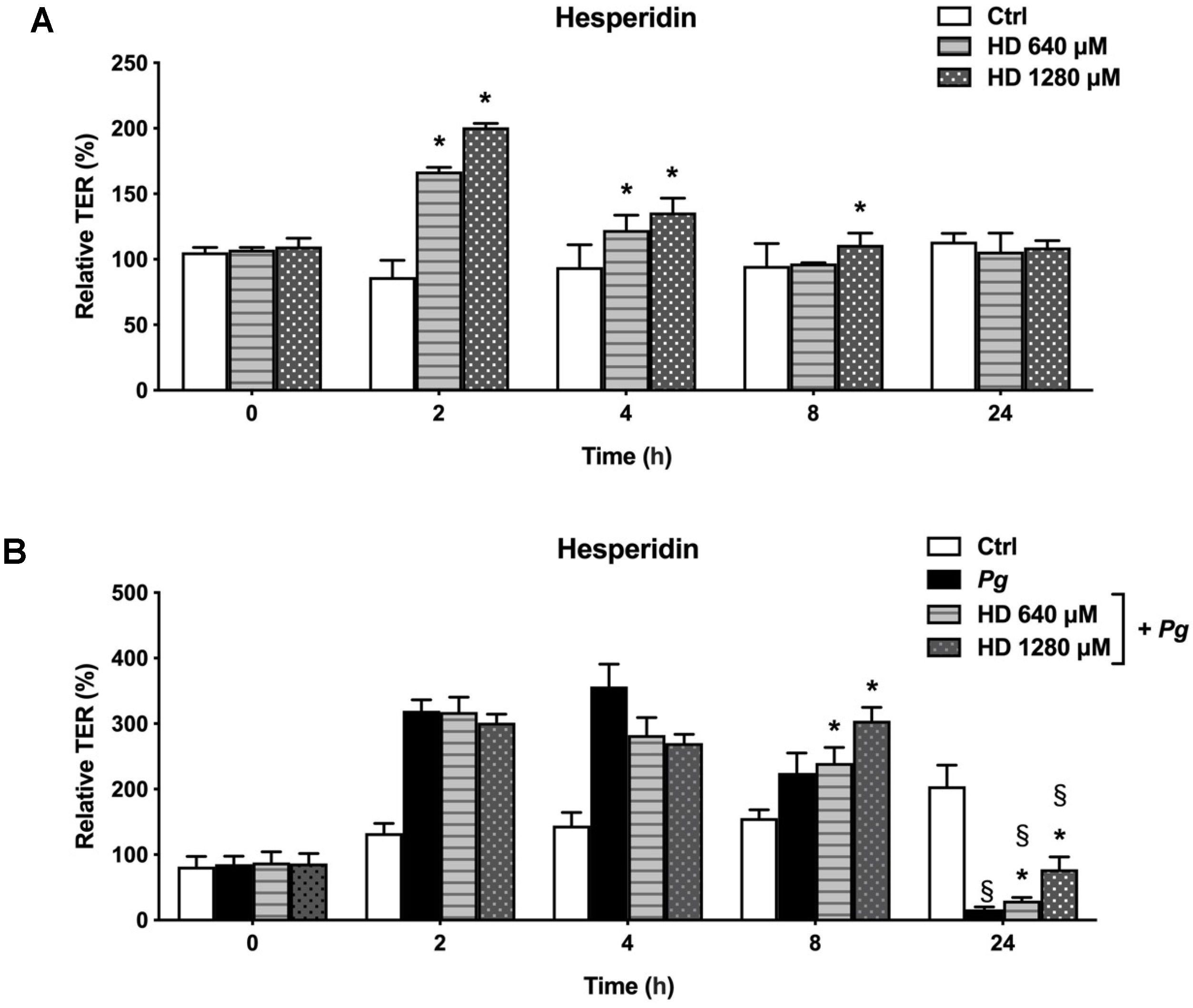
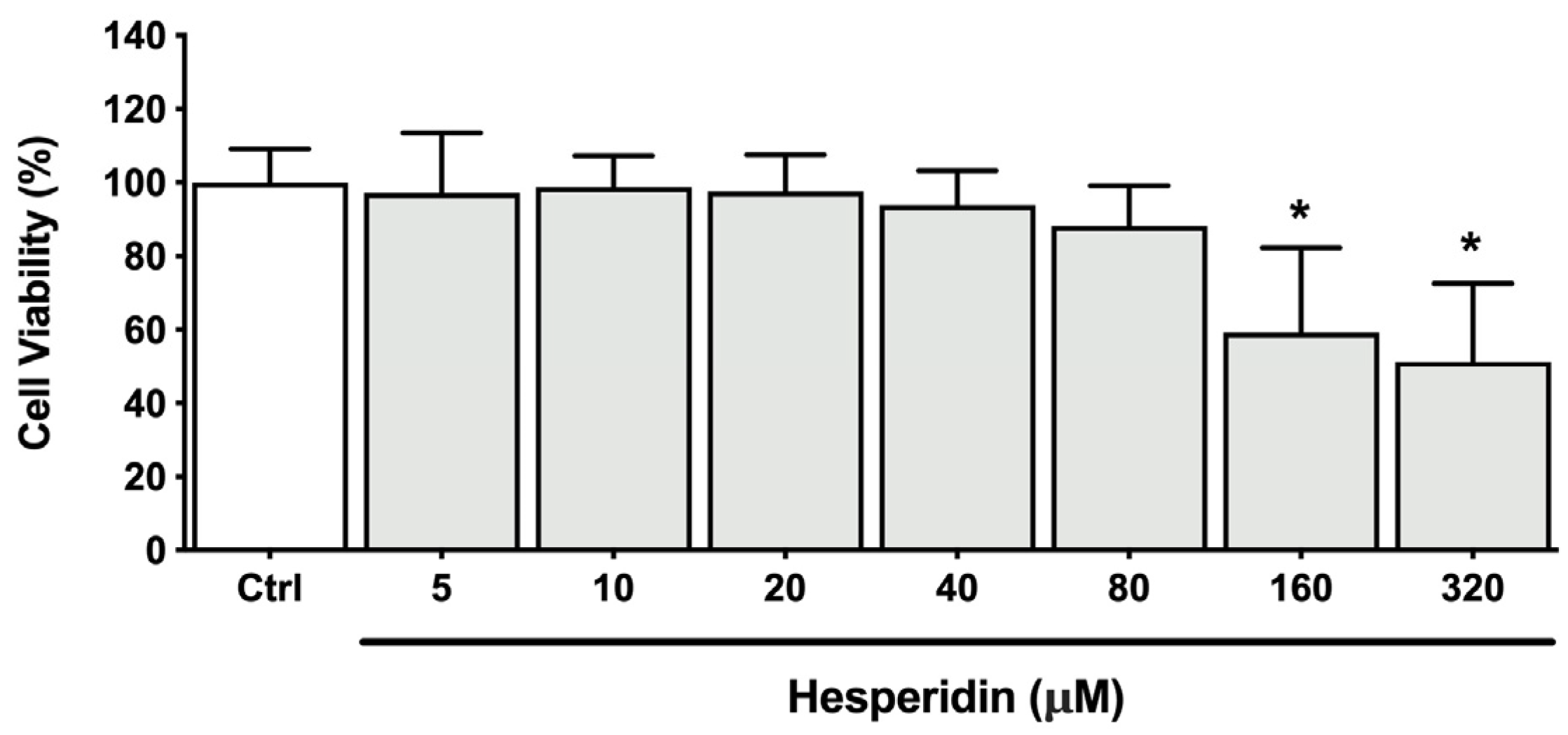
2.1. Effect of Hesperidin on the Disruption of Epithelial Barrier Integrity
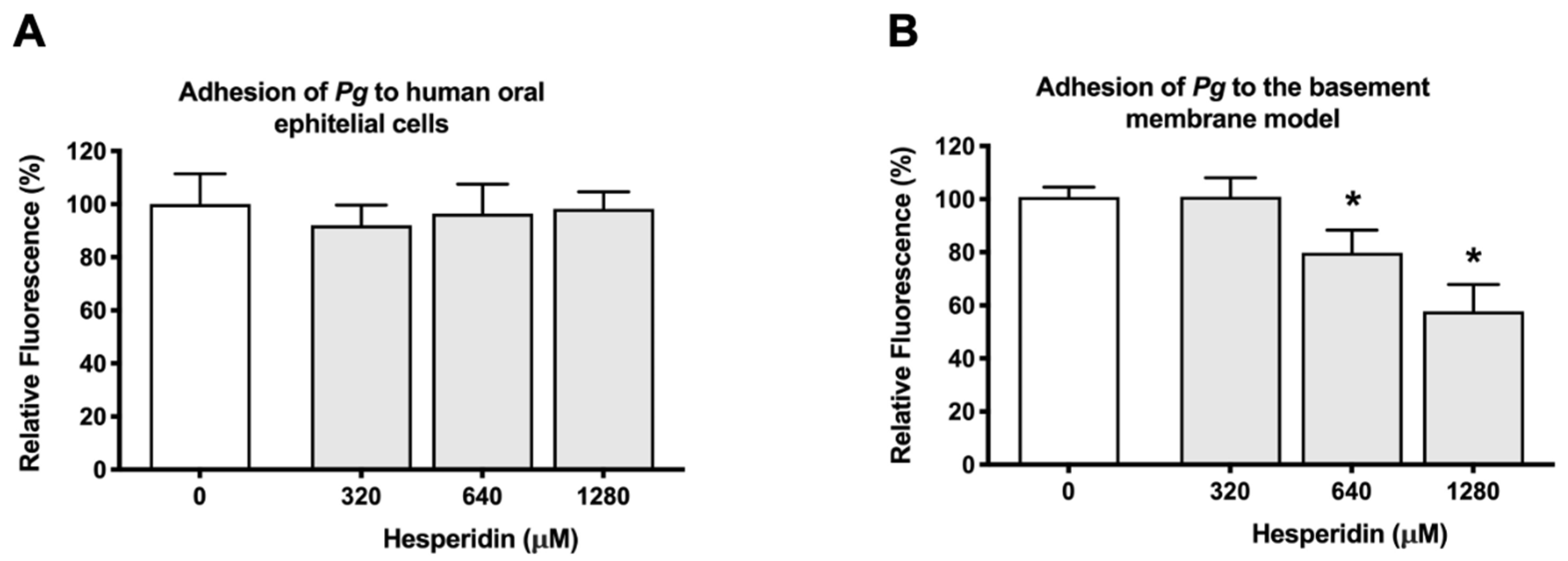
2.2. Effect of Hesperidin on the Adherence of P. gingivalis to Human Oral Epithelial Cells and a Basement Membrane Model
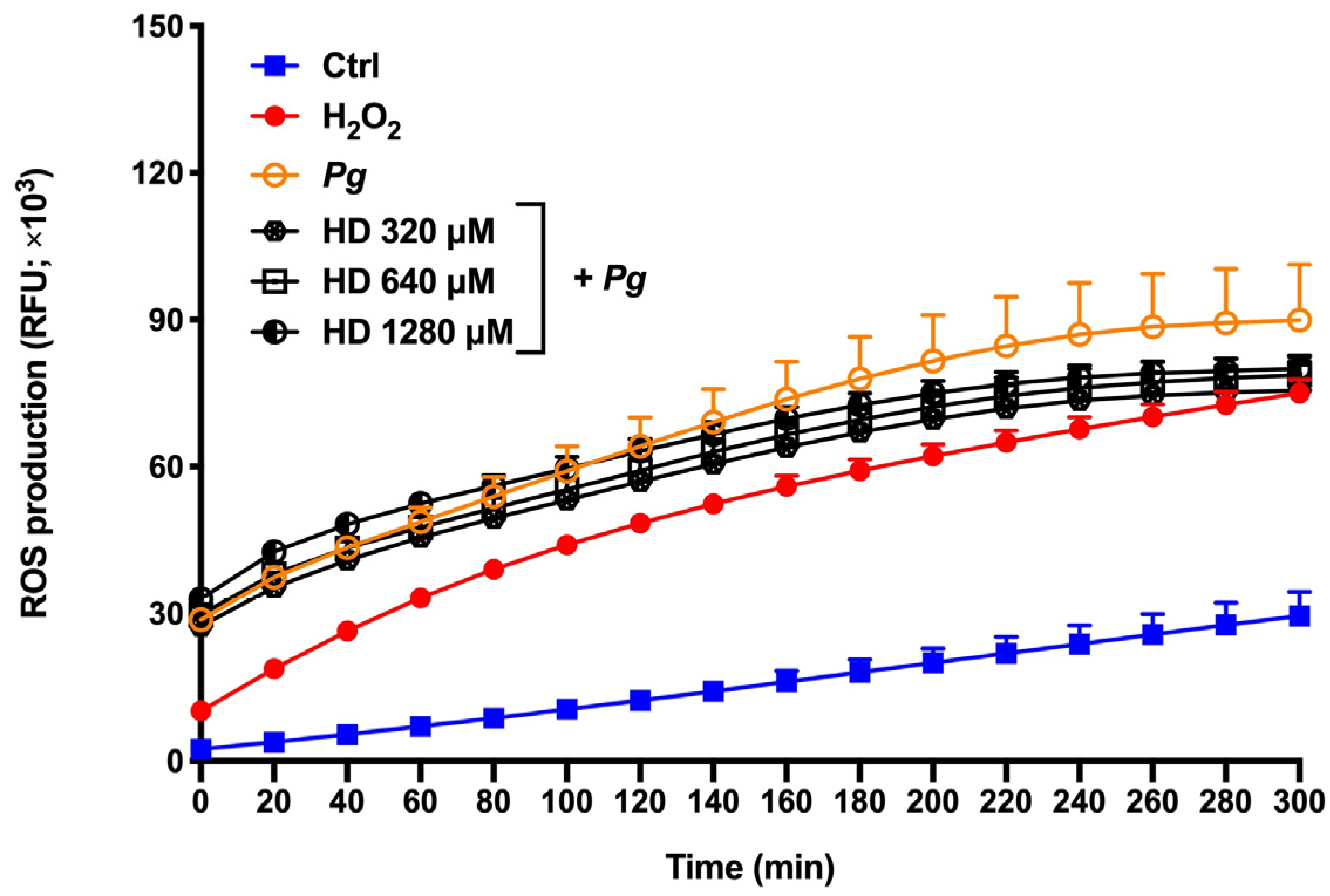
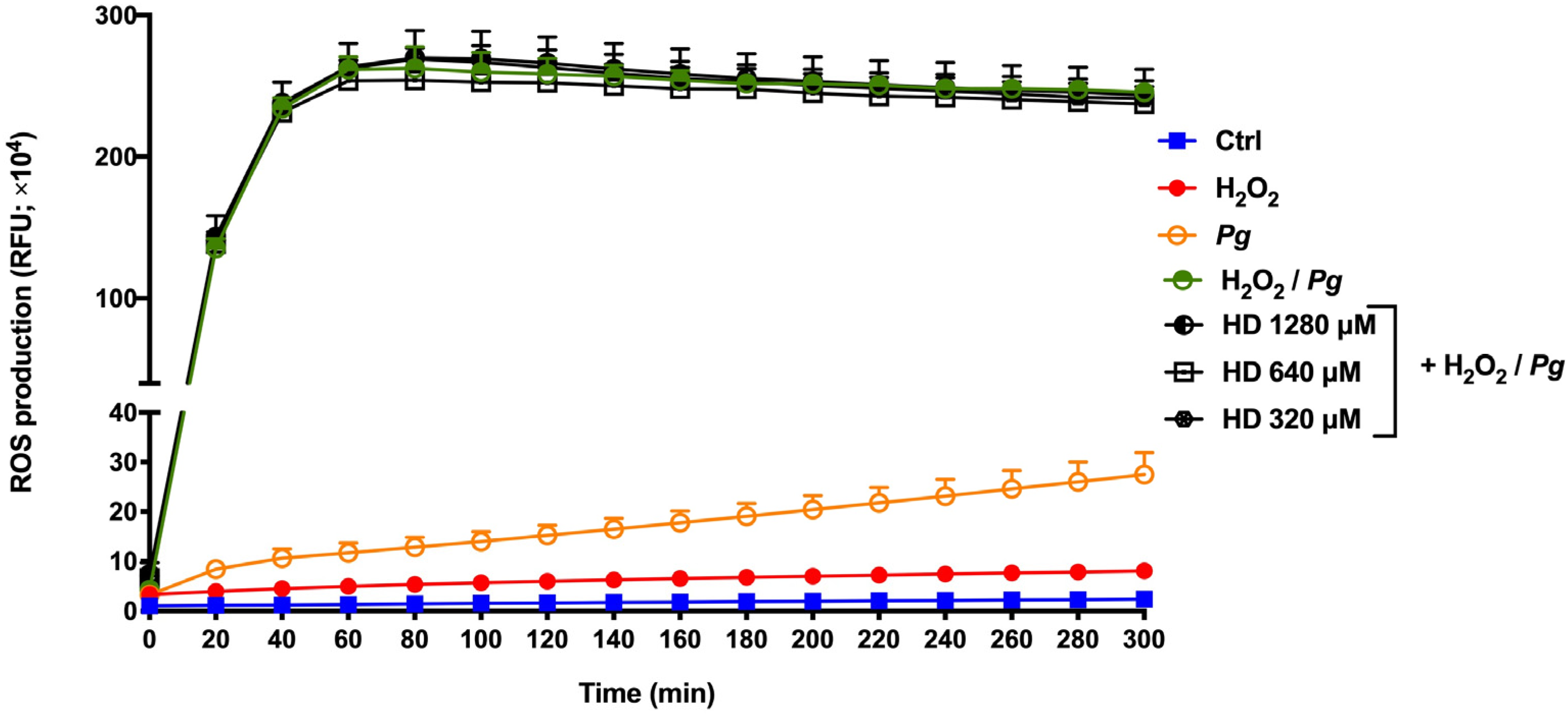
2.3. Effect of Hesperidin on ROS Production
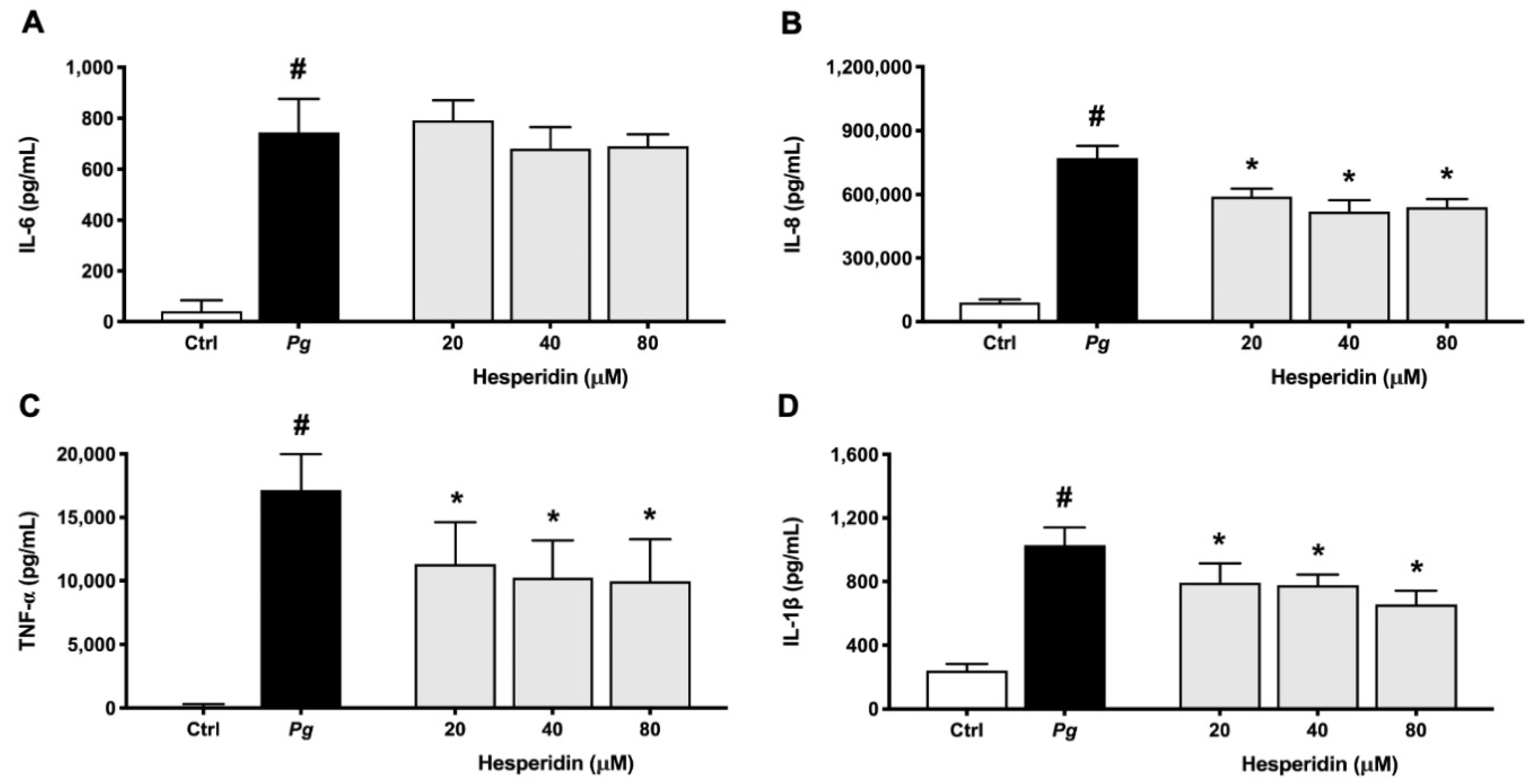
2.4. Effect of Hesperidin on Cytokine and MMP Expression
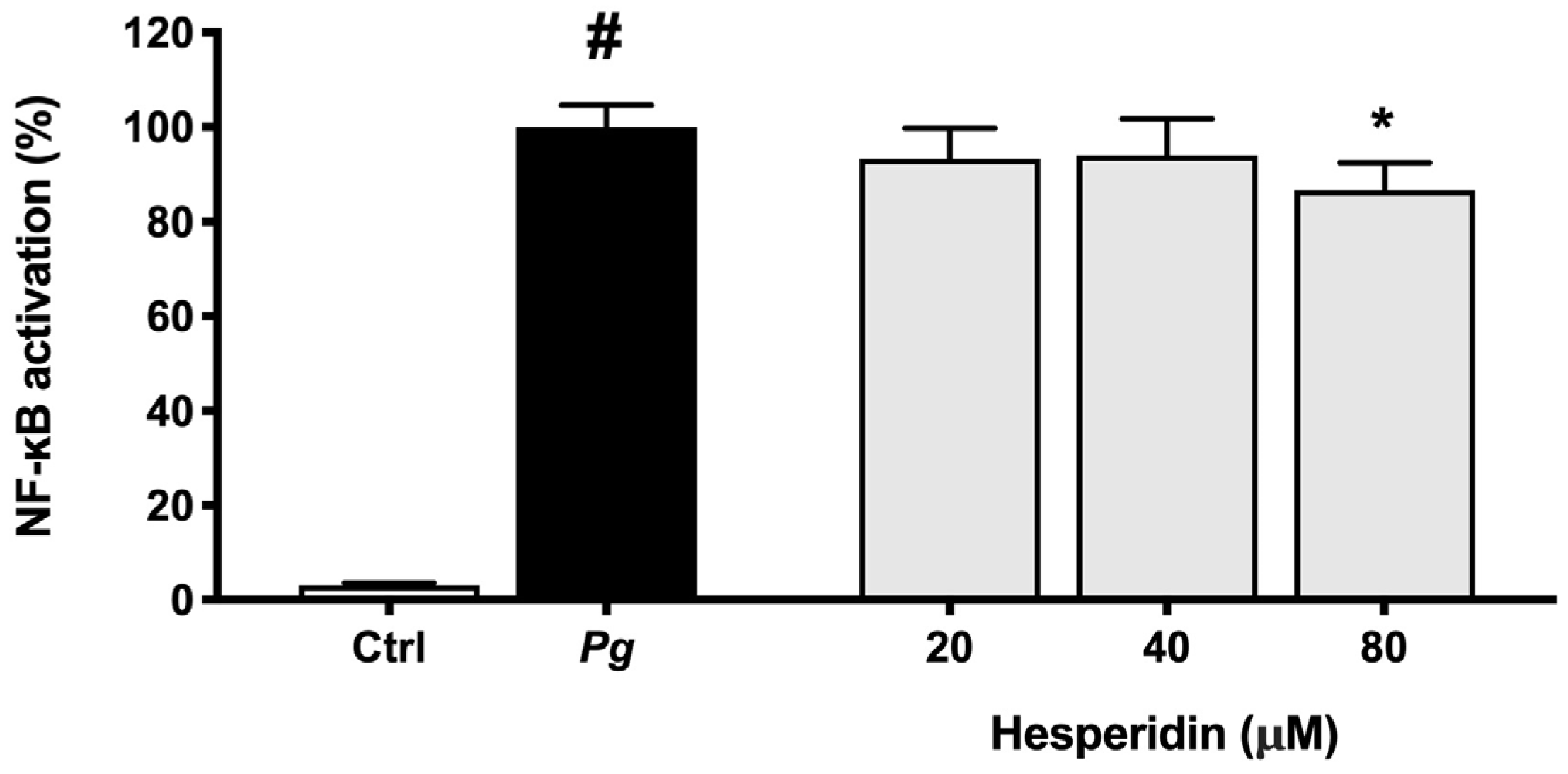
2.5. Effect of Hesperidin on NF-κB Activation
3. Discussion
4. Materials and Methods
4.1. Hesperidin
4.2. Bacteria and Growth Conditions
4.3. Cell Cultures
4.4. Oral Epithelial Barrier Integrity
4.5. Adherence of P. gingivalis to Human Oral Epithelial Cells and a Basement Membrane Model
4.6. Reactive Oxygen Species Production by Human Oral Epithelial Cells
4.7. Cytokine and Matrix Metalloproteinases (MMPs) Secretion by Macrophages
4.8. Activation of NF-κB in P. gingivalis-Stimulated Monocytes
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Roberts, F.A.; Darveau, R.P. Microbial protection and virulence in periodontal tissue as a function of polymicrobial communities: Symbiosis and dysbiosis. Periodontology 2000 2015, 69, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.L.; Dawson, D.R., 3rd; Morford, L.A.; Peyyala, R.; Miller, C.S.; Gonzaléz, O.A. Periodontal disease immunology: ‘Double indemnity’ in protecting the host. Periodontology 2000 2013, 62, 163–202. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cai, W.; Zhao, S.; Shi, L.; Chen, Y.; Li, X.; Sun, X.; Mao, Y.; He, B.; Hou, Y.; et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 608–622. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Liu, S.; Zhang, S.; Pan, Y. The Role of Porphyromonas gingivalis Outer Membrane Vesicles in Periodontal Disease and Related Systemic Diseases. Front. Cell. Infect. Microbiol. 2021, 10, 585917. [Google Scholar] [CrossRef]
- Fujita, T.; Yoshimoto, T.; Kajiya, M.; Ouhara, K.; Matsuda, S.; Takemura, T.; Akutagawa, K.; Takeda, K.; Mizuno, N.; Kurihara, H. Regulation of defensive function on gingival epithelial cells can prevent periodontal disease. Jpn. Dent. Sci. Rev. 2018, 54, 66–75. [Google Scholar] [CrossRef]
- Ji, S.; Choi, Y.S.; Choi, Y. Bacterial invasion and persistence: Critical events in the pathogenesis of periodontitis? J. Periodontal Res. 2015, 50, 570–585. [Google Scholar] [CrossRef]
- Holt, S.C.; Ebersole, J.L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontology 2000 2005, 38, 72–122. [Google Scholar] [CrossRef]
- Groeger, S.E.; Meyle, J. Epithelial barrier and oral bacterial infection. Periodontology 2000 2015, 69, 46–67. [Google Scholar] [CrossRef] [PubMed]
- Grenier, D.; La, V.D. Proteases of Porphyromonas gingivalis as important virulence factors in periodontal disease and potential targets for plant-derived compounds: A review article. Curr. Drug. Targets 2011, 12, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Amano, A. Disruption of epithelial barrier and impairment of cellular function by Porphyromonas gingivalis. Front. Biosci. 2007, 12, 3965–3974. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Calder, P.C. Effects of citrus fruit juices and their bioactive components on inflammation and immunity: A narrative review. Front. Immunol. 2021, 12, 712608. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef]
- Kuo, P.J.; Fu, E.; Lin, C.Y.; Ku, C.T.; Chiang, C.Y.; Fu, M.M.; Fu, M.W.; Tu, H.P.; Chiu, H.C. Ameliorative effect of hesperidin on ligation-induced periodontitis in rats. J. Periodontol. 2019, 90, 271–280. [Google Scholar] [CrossRef]
- Gonçalves, V.P.; Musskopf, M.L.; Rivera-Concepcion, A.; Yu, C.; Wong, S.W.; Tuin, S.A.; Jiao, Y.; Susin, C.; Spolidorio, L.C.; Miguez, P.A. Systemic dietary hesperidin modulation of osteoclastogenesis, bone homeostasis and periodontal disease in mice. Int. J. Mol. Sci. 2022, 13, 7100. [Google Scholar] [CrossRef]
- Canakçi, C.F.; Ciçek, Y.; Canakçi, V. Reactive oxygen species and human inflammatory periodontal diseases. Biochemistry 2005, 70, 619–628. [Google Scholar] [CrossRef]
- Groeger, S.; Doman, E.; Chakraborty, T.; Meyle, J. Effects of Porphyromonas gingivalis infection on human gingival epithelial barrier function in vitro. Eur. J. Oral. Sci. 2010, 118, 582–589. [Google Scholar] [CrossRef]
- Gibbons, R.J. Bacterial adhesion to oral tissues: A model for infectious diseases. J. Dent. Res. 1989, 68, 750–760. [Google Scholar] [CrossRef]
- Guo, K.; Ren, J.; Gu, G.; Wang, G.; Gong, W.; Wu, X.; Ren, H.; Hong, Z.; Li, J. Hesperidin protects against intestinal inflammation by restoring intestinal barrier function and up-regulating Treg cells. Mol. Nutr. Food Res. 2019, 63, e1800975. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, N.; Belibasakis, G.N. Porphyromonas gingivalis: An invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 2012, 333, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Groeger, S.; Meyle, J. Oral mucosal epithelial cells. Front. Immunol. 2019, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Andrian, E.; Grenier, D.; Rouabhia, M. In vitro models of tissue penetration and destruction by Porphyromonas gingivalis. Infect. Immun. 2004, 72, 4689–4698. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mo, L.; Niu, Y.; Li, X.; Zhou, X.; Xu, X. The role of reactive oxygen species and autophagy in periodontitis and their potential linkage. Front. Physiol. 2017, 8, 439. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell. Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Kalpana, K.B.; Srinivasan, M.; Menon, V.P. Evaluation of antioxidant activity of hesperidin and its protective effect on H2O2 induced oxidative damage on pBR322 DNA and RBC cellular membrane. Mol. Cell. Biochem. 2009, 323, 21–29. [Google Scholar] [CrossRef]
- Chen, M.; Gu, H.; Ye, Y.; Lin, B.; Sun, L.; Deng, W.; Zhang, J.; Liu, J. Protective effects of hesperidin against oxidative stress of tert-butyl hydroperoxide in human hepatocytes. Food Chem. Toxicol. 2010, 48, 2980–2987. [Google Scholar] [CrossRef]
- de Souza, A.B.F.; de Matos, N.A.; Castro, T.F.; Costa, G.P.; Oliveira, L.A.M.; Nogueira, K.O.P.C.; Ribeiro, I.M.L.; Talvani, A.; Cangussú, S.D.; de Menezes, R.C.A.; et al. Effects in vitro and in vivo of hesperidin administration in an experimental model of acute lung inflammation. Free. Radic. Biol. Med. 2022, 180, 253–262. [Google Scholar] [CrossRef]
- El-Sayed, e.-S.M.; Abo-Salem, O.M.; Abd-Ellah, M.F.; Abd-Alla, G.M. Hesperidin, an antioxidant flavonoid, prevents acrylonitrile-induced oxidative stress in rat brain. J. Biochem. Mol. Toxicol. 2008, 22, 268–273. [Google Scholar] [CrossRef]
- Sahu, B.D.; Kuncha, M.; Sindhura, G.J.; Sistla, R. Hesperidin attenuates cisplatin-induced acute renal injury by decreasing oxidative stress, inflammation and DNA damage. Phytomedicine 2013, 20, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Simitzis, P.E.; Ilias-Dimopoulos, V.; Charismiadou, M.A.; Biniari, E.E.; Deligeorgis, S.G. Effects of dietary hesperidin supplementation on lamb performance and meat characteristics. Anim. Sci. J. 2013, 84, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Zhen, A.X.; Ok, S.; Fernando, P.D.S.M.; Herath, H.M.U.L.; Piao, M.J.; Kang, K.A.; Hyun, J.W. Hesperidin protects SH-SY5Y neuronal cells against high glucose-induced apoptosis via regulation of MAPK signaling. Antioxidants 2022, 11, 1707. [Google Scholar] [CrossRef] [PubMed]
- Smalley, J.W.; Birss, A.J.; Silver, J. The periodontal pathogen Porphyromonas gingivalis harnesses the chemistry of the mu-oxo bishaem of iron protoporphyrin IX to protect against hydrogen peroxide. FEMS Microbiol. Lett. 2000, 183, 159–164. [Google Scholar] [PubMed]
- Choi, I.Y.; Kim, S.J.; Jeong, H.J.; Park, S.H.; Song, Y.S.; Lee, J.H.; Kang, T.H.; Park, J.H.; Hwang, G.S.; Lee, E.J.; et al. Hesperidin inhibits expression of hypoxia inducible factor-1 alpha and inflammatory cytokine production from mast cells. Mol. Cell. Biochem. 2007, 305, 153–161. [Google Scholar] [CrossRef]
- Nizamutdinova, I.T.; Jeong, J.J.; Xu, G.H.; Lee, S.H.; Kang, S.S.; Kim, Y.S.; Chang, K.C.; Kim, H.J. Hesperidin, hesperidin methyl chalone and phellopterin from Poncirus trifoliata (Rutaceae) differentially regulate the expression of adhesion molecules in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells. Int. Immunopharmacol. 2008, 8, 670–678. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Cai, L.; Hu, C.M.; Zhang, L. Suppression of adjuvant arthritis by hesperidin in rats and its mechanisms. J. Pharm. Pharmacol. 2008, 60, 221–228. [Google Scholar] [CrossRef]
- Tervahartiala, T.; Pirilä, E.; Ceponis, A.; Maisi, P.; Salo, T.; Tuter, G.; Kallio, P.; Törnwall, J.; Srinivas, R.; Konttinen, Y.T.; et al. The in vivo expression of the collagenolytic matrix metalloproteinases (MMP-2, -8, -13, and -14) and matrilysin (MMP-7) in adult and localized juvenile periodontitis. J. Dent. Res. 2000, 79, 1969–1977. [Google Scholar] [CrossRef]
- Uitto, V.J.; Overall, C.M.; McCulloch, C. Proteolytic host cell enzymes in gingival crevice fluid. Periodontology 2000 2003, 31, 77–104. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Menon, V.P. Effect of hesperidin on matrix metalloproteinases and antioxidant status during nicotine-induced toxicity. Toxicology 2007, 238, 90–98. [Google Scholar] [CrossRef]
- Kamaraj, S.; Anandakumar, P.; Jagan, S.; Ramakrishnan, G.; Devaki, T. Modulatory effect of hesperidin on benzo(a)pyrene induced experimental lung carcinogenesis with reference to COX-2, MMP-2 and MMP-9. Eur. J. Pharmacol. 2010, 649, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Chen, L.; Liu, M.; Tan, H.; Zheng, L. Hesperidin reduces adverse symptomatic intracerebral hemorrhage by promoting TGF-β1 for treating ischemic stroke using tissue plasminogen activator. Neurol. Sci. 2020, 41, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Milward, M.R.; Chapple, I.L.; Wright, H.J.; Millard, J.L.; Matthews, J.B.; Cooper, P.R. Differential activation of NF-kappaB and gene expression in oral epithelial cells by periodontal pathogens. Clin. Exp. Immunol. 2007, 148, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Sato, T.; Ohno-Oishi, M.; Ozawa, M.; Maekawa, S.; Shiga, Y.; Yabana, T.; Yasuda, M.; Himori, N.; Omodaka, K.; et al. CHOP deletion and anti-neuroinflammation treatment with hesperidin synergistically attenuate NMDA retinal injury in mice. Exp. Eye Res. 2021, 213, 108826. [Google Scholar] [CrossRef] [PubMed]
- Gröger, S.; Michel, J.; Meyle, J. Establishment and characterization of immortalized human gingival keratinocyte cell lines. J. Periodontal Res. 2008, 43, 604–614. [Google Scholar] [CrossRef]
- Carlsen, H.; Moskaug, J.Ø.; Fromm, S.H.; Blomhoff, R. In vivo imaging of NF-kappa B activity. J. Immunol. 2002, 168, 1441–1446. [Google Scholar] [CrossRef]
- Lagha, A.B.; Huacho, P.M.; Grenier, D. A cocoa (Theobroma cacao L.) extract impairs the growth, virulence properties, and inflammatory potential of Fusobacterium nucleatum and improves oral epithelial barrier function. PLoS ONE 2021, 16, e0252029. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maquera-Huacho, P.M.; Spolidorio, D.P.; Manthey, J.; Grenier, D. Effect of Hesperidin on Barrier Function and Reactive Oxygen Species Production in an Oral Epithelial Cell Model, and on Secretion of Macrophage-Derived Inflammatory Mediators during Porphyromonas gingivalis Infection. Int. J. Mol. Sci. 2023, 24, 10389. https://doi.org/10.3390/ijms241210389
Maquera-Huacho PM, Spolidorio DP, Manthey J, Grenier D. Effect of Hesperidin on Barrier Function and Reactive Oxygen Species Production in an Oral Epithelial Cell Model, and on Secretion of Macrophage-Derived Inflammatory Mediators during Porphyromonas gingivalis Infection. International Journal of Molecular Sciences. 2023; 24(12):10389. https://doi.org/10.3390/ijms241210389
Chicago/Turabian StyleMaquera-Huacho, Patricia Milagros, Denise Palomari Spolidorio, John Manthey, and Daniel Grenier. 2023. "Effect of Hesperidin on Barrier Function and Reactive Oxygen Species Production in an Oral Epithelial Cell Model, and on Secretion of Macrophage-Derived Inflammatory Mediators during Porphyromonas gingivalis Infection" International Journal of Molecular Sciences 24, no. 12: 10389. https://doi.org/10.3390/ijms241210389
APA StyleMaquera-Huacho, P. M., Spolidorio, D. P., Manthey, J., & Grenier, D. (2023). Effect of Hesperidin on Barrier Function and Reactive Oxygen Species Production in an Oral Epithelial Cell Model, and on Secretion of Macrophage-Derived Inflammatory Mediators during Porphyromonas gingivalis Infection. International Journal of Molecular Sciences, 24(12), 10389. https://doi.org/10.3390/ijms241210389




