Mild Cognitive Impairment Is Associated with Enhanced Activation of Th17 Lymphocytes in Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
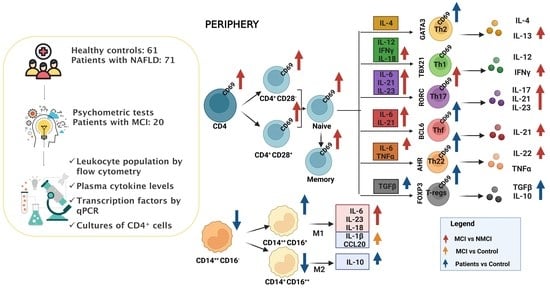
2. Results
2.1. Patient Characteristics
2.2. Monocyte Populations in Peripheral Blood
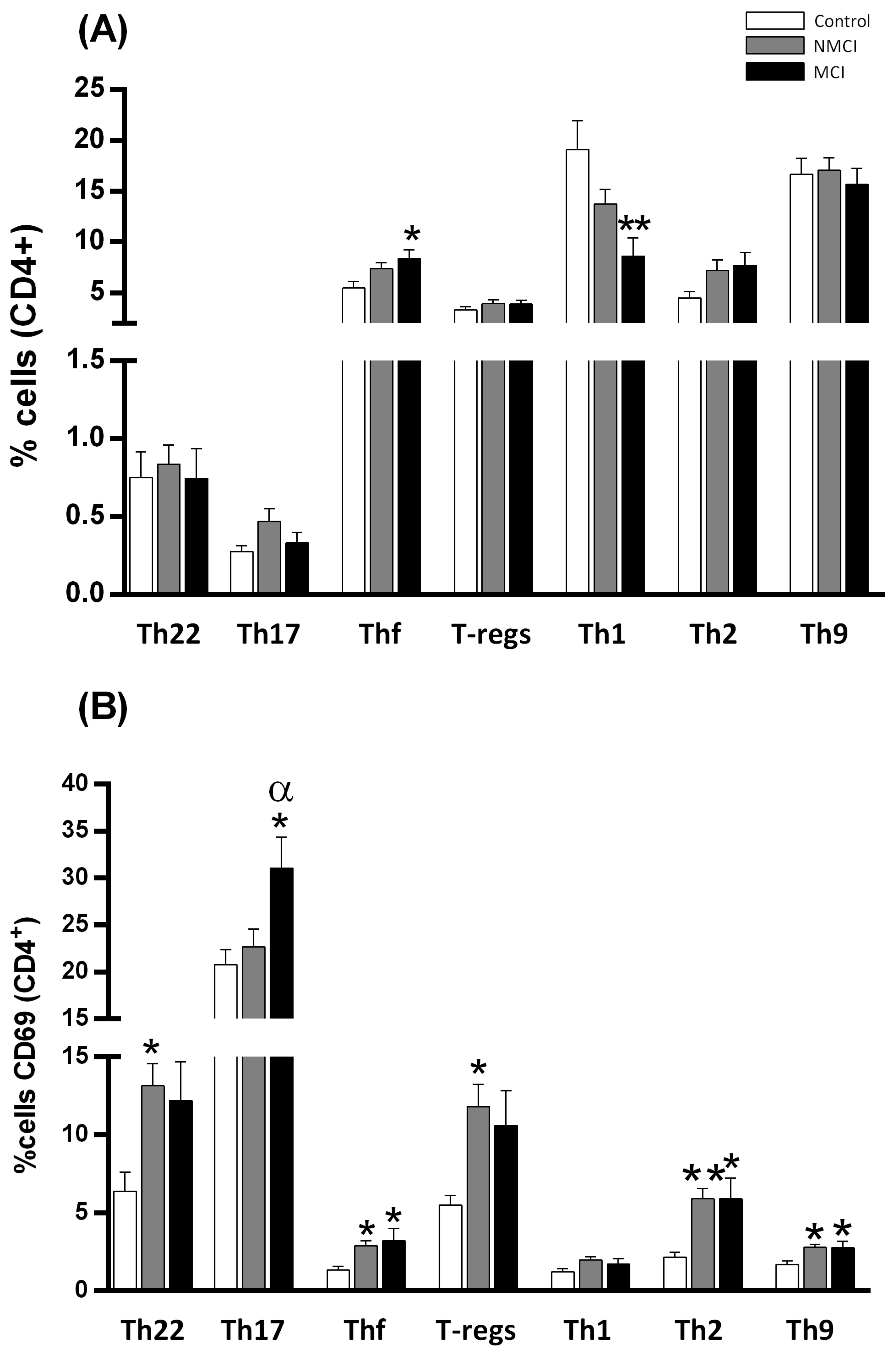
2.3. MCI Association with the Induction of the Early Activation Marker CD69 in CD4+ T Lymphocytes in Peripheral Blood
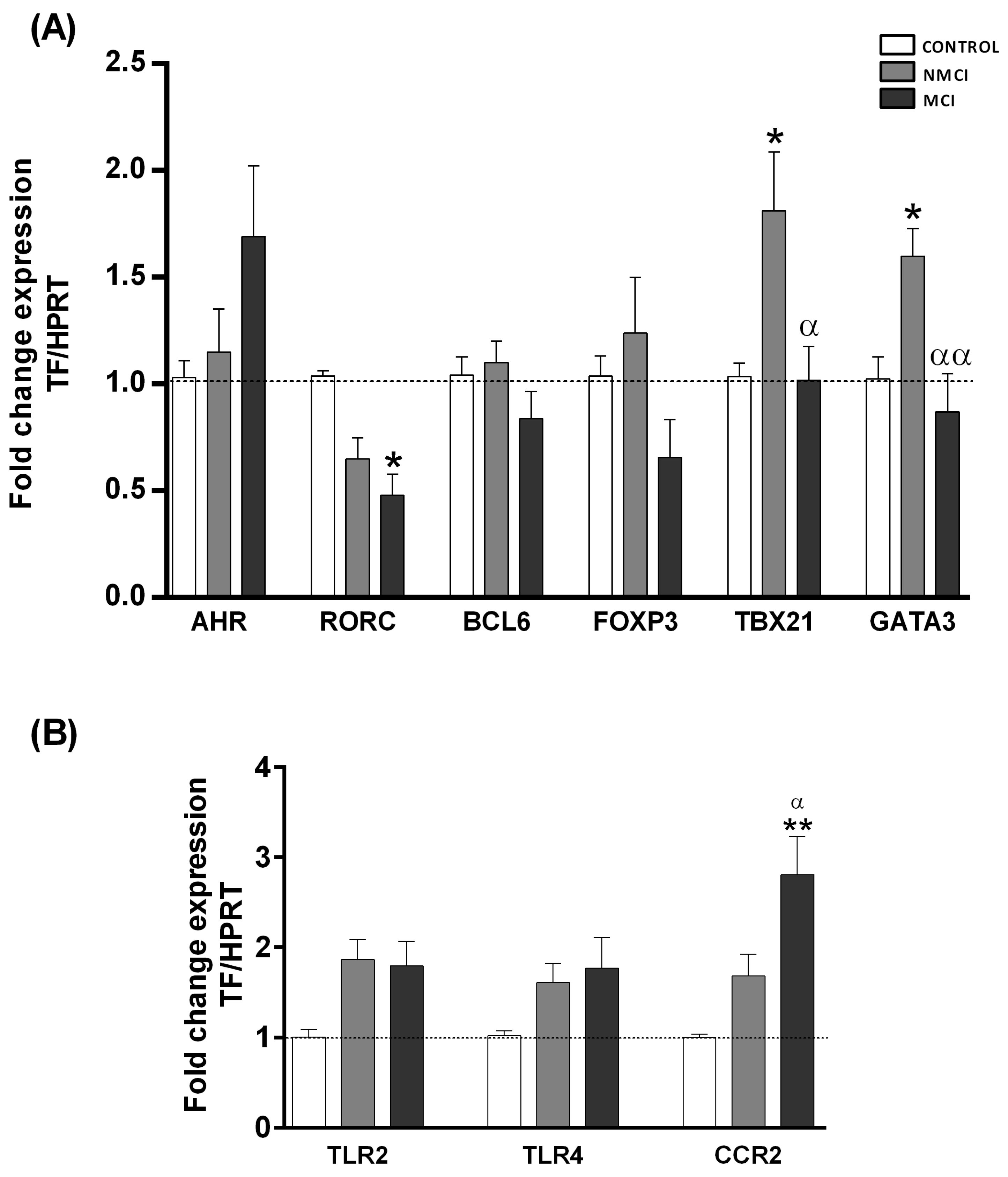
2.4. Expression Analysis of Transcription Factors Characteristic of Different CD4+ T-Lymphocyte Subsets
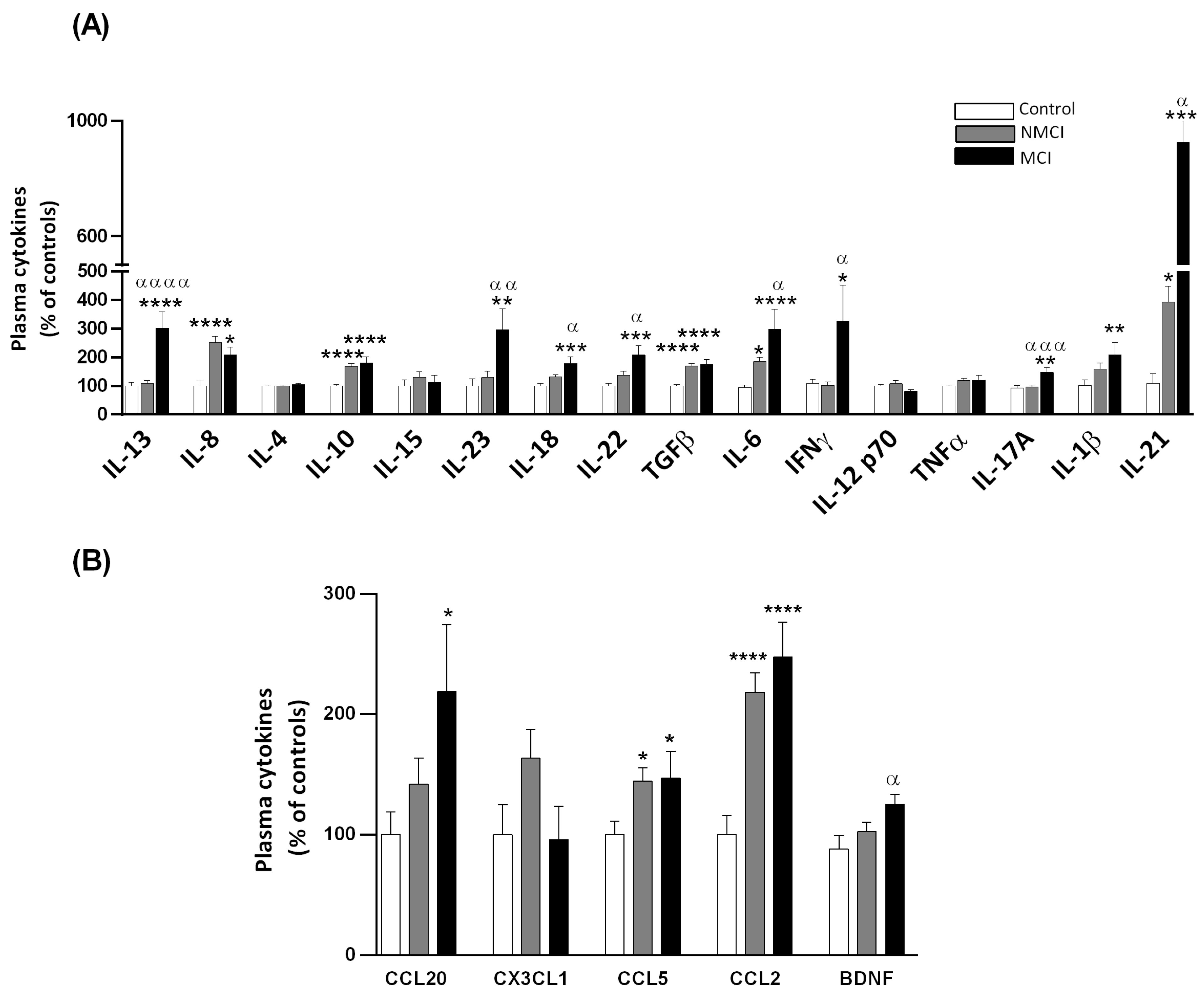
2.5. Plasma Levels of Different Cytokines and Chemokines
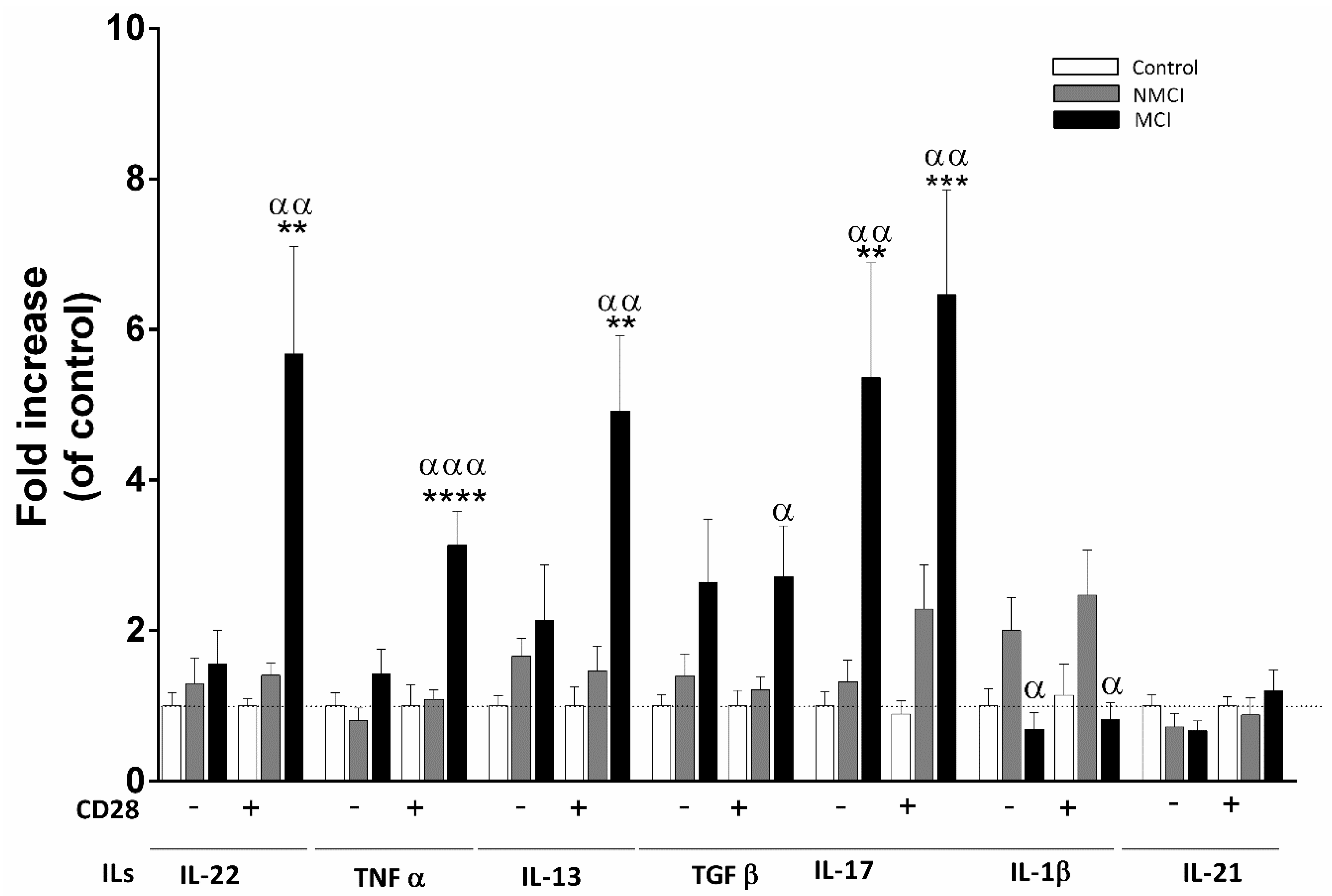
2.6. Analysis of Cytokines Released by CD4+ T Cell Cultures
2.7. Logistic Regression Analyses of Predictors of the Presence of MCI in NAFLD Patients
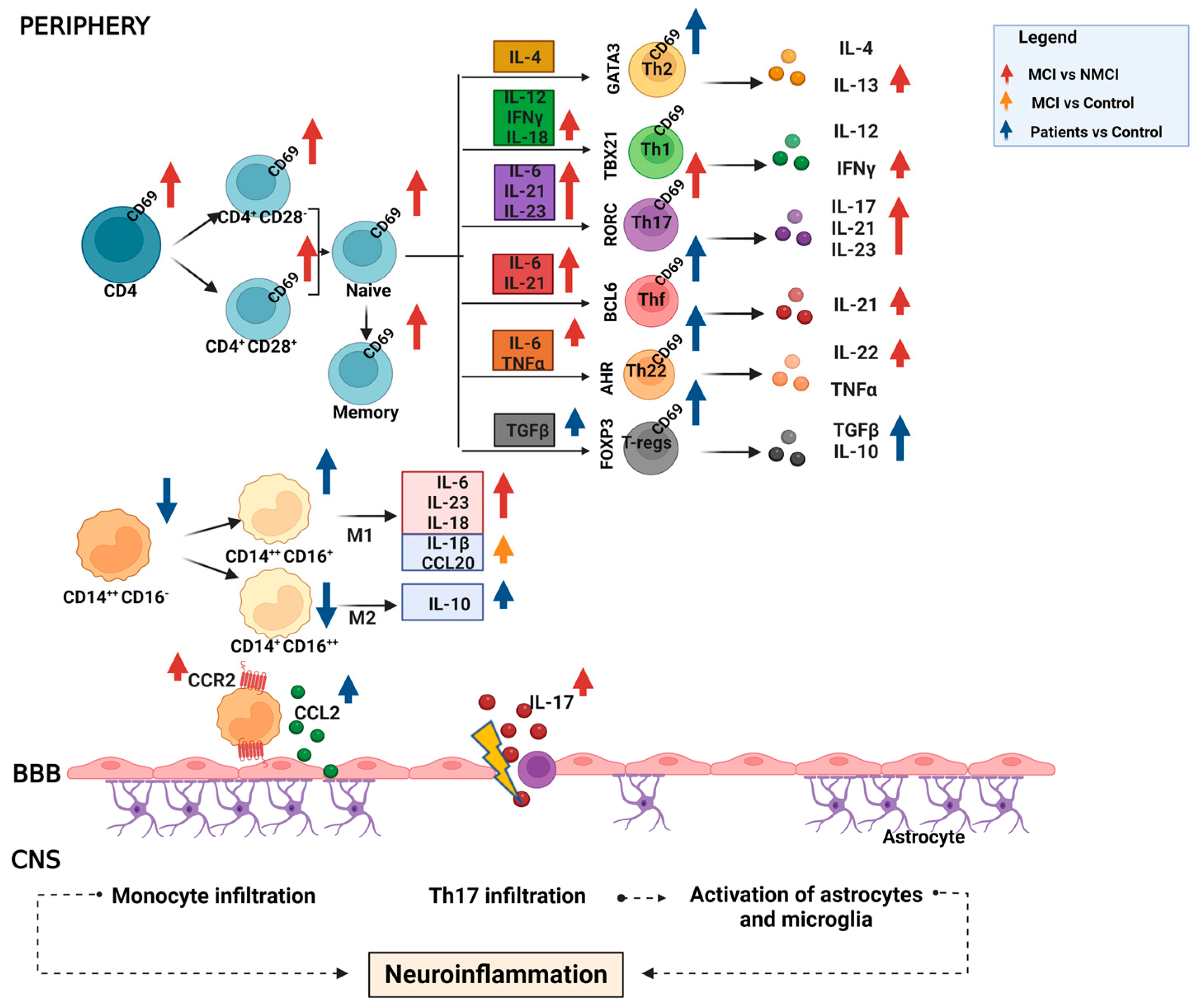
3. Discussion
4. Materials and Methods
4.1. Patients and Controls
4.2. NAFLD Patient Classification into NAFL or NASH
4.3. MCI Diagnosis
4.4. Characterization of Leukocyte Populations by Flow Cytometry
4.5. Monoclonal Antibodies
4.6. Determination of Plasma Cytokine Levels
4.7. Analysis of Transcription Factors by Quantitative PCR
4.8. Analysis of Cytokines Released by CD4+ Lymphocyte Cultures
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serfaty, L.; Lemoine, M. Definition and natural history of metabolic steatosis: Clinical aspects of NAFLD, NASH and cirrhosis. Diabetes Metab. 2008, 34 Pt 2, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Bugianesi, E.; Forlani, G.; Cerrelli, F.; Lenzi, M.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; Melchionda, N. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003, 37, 917–923, Erratum in Hepatology 2003, 38, 536. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence; incidence; and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Celikbilek, A.; Celikbilek, M.; Bozkurt, G. Cognitive assessment of patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2018, 30, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Kjærgaard, K.; Mikkelsen, A.C.D.; Wernberg, C.W.; Grønkjær, L.L.; Eriksen, P.L.; Damholdt, M.F.; Mookerjee, R.P.; Vilstrup, H.; Lauridsen, M.M.; Thomsen, K.L. Cognitive Dysfunction in Non-Alcoholic Fatty Liver Disease-Current Knowledge; Mechanisms and Perspectives. J. Clin. Med. 2021, 10, 673. [Google Scholar] [CrossRef]
- Giménez-Garzó, C.; Fiorillo, A.; Ballester-Ferré, M.P.; Gallego, J.J.; Casanova-Ferrer, F.; Urios, A.; Benlloch, S.; Martí-Aguado, D.; San-Miguel, T.; Tosca, J.; et al. A new score unveils a high prevalence of mild cognitive impairment in patients with nonalcoholic fatty liver disease. J. Clin. Med. 2021, 10, 2806. [Google Scholar] [CrossRef]
- Montoliu, C.; Piedrafita, B.; Serra, M.A.; del Olmo, J.A.; Urios, A.; Rodrigo, J.M.; Felipo, V. IL-6 and IL-18 in blood may discriminate cirrhotic patients with and without minimal hepatic encephalopathy. J. Clin. Gastroenterol. 2009, 43, 272–279. [Google Scholar] [CrossRef]
- Díaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Cognitive and motor perturbations in elderly with longstanding diabetes mellitus. Nutrition 2014, 30, 628–635. [Google Scholar] [CrossRef]
- Shin, S.Y.; Julian, L.; Katz, P. The relationship between cognitive function and physical function in rheumatoid arthritis. J. Rheumatol. 2013, 40, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Cabrera-Pastor, A.; Llansola, M.; Montoliu, C.; Malaguarnera, M.; Balzano, T.; Taoro-Gonzalez, L.; García-García, R.; Mangas-Losada, A.; Izquierdo-Altarejos, P.; Arenas, Y.M.; et al. Peripheral inflammation induces neuroinflammation that alters neurotransmission and cognitive and motor function in hepatic encephalopathy: Underlying mechanisms and therapeutic implications. Acta Physiol. 2019, 226, e13270. [Google Scholar] [CrossRef]
- Mangas-Losada, A.; García-García, R.; Leone, P.; Ballester, M.P.; Cabrera-Pastor, A.; Urios, A.; Gallego, J.J.; Martínez-Pretel, J.J.; Giménez-Garzó, C.; Revert, F.; et al. Selective improvement by rifaximin of changes in the immunophenotype in patients who improve minimal hepatic encephalopathy. J. Transl. Med. 2019, 17, 293. [Google Scholar] [CrossRef] [Green Version]
- Magaki, S.; Yellon, S.M.; Mueller, C.; Kirsch, W.M. Immunophenotypes in the circulation of patients with mild cognitive impairment. J. Psychiatr. Res. 2008, 42, 240–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saresella, M.; Calabrese, E.; Marventano, I.; Piancone, F.; Gatti, A.; Alberoni, M.; Nemni, R.; Clerici, M. Increased activity of Th-17 and Th-9 lymphocytes and a skewing of the post-thymic differentiation pathway are seen in Alzheimer’s disease. Brain Behav. Immun. 2011, 25, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Mangas-Losada, A.; García-García, R.; Urios, A.; Escudero-García, D.; Tosca, J.; Giner-Durán, R.; Serra, M.A.; Montoliu, C.; Felipo, V. Minimal hepatic encephalopathy is associated with expansion and activation of CD4+CD28−; Th22 and Tfh and B lymphocytes. Sci. Rep. 2017, 7, 6683. [Google Scholar] [CrossRef] [PubMed]
- Petranovic, D.; Pilcic, G.; Valkovic, T.; Sotosek Tokmadzic, V.; Laskarin, G. Perforin- and granulysin-mediated cytotoxicity and interleukin 15 play roles in neurocognitive impairment in patients with acute lymphoblastic leukaemia. Med. Hypotheses 2014, 83, 122–126. [Google Scholar] [CrossRef]
- Zindler, E.; Zipp, F. Neuronal injury in chronic CNS inflammation. Best Pract. Res. Clin. Anaesthesiol. 2010, 24, 551–562. [Google Scholar] [CrossRef]
- Tosello-Trampont, A.C.; Landes, S.G.; Nguyen, V.; Novobrantseva, T.I.; Hahn, Y.S. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-production. J. Biol. Chem. 2012, 287, 40161–40172. [Google Scholar] [CrossRef] [Green Version]
- Lefere, S.; Tacke, F. Macrophages in obesity and non-alcoholic fatty liver disease: Crosstalk with metabolism. JHEP Rep. 2019, 1, 30–43. [Google Scholar] [CrossRef] [Green Version]
- Van Herck, M.A.; Weyler, J.; Kwanten, W.J.; Dirinck, E.L.; De Winter, B.Y.; Francque, S.M.; Vonghia, L. The Differential Roles of T Cells in Non-alcoholic Fatty Liver Disease and Obesity. Front. Immunol. 2019, 10, 82. [Google Scholar] [CrossRef] [Green Version]
- Chackelevicius, C.M.; Gambaro, S.E.; Tiribelli, C.; Rosso, N. Th17 involvement in nonalcoholic fatty liver disease progression to non-alcoholic steatohepatitis. World J. Gastroenterol. 2016, 2, 9096–9103. [Google Scholar] [CrossRef]
- Shawcross, D.L.; Davies, N.A.; Williams, R.; Jalan, R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J. Hepatol. 2004, 40, 247–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felipo, V.; Urios, A.; Montesinos, E.; Molina, I.; Garcia-Torres, M.L.; Civera, M.; Olmo, J.A.; Ortega, J.; Martinez-Valls, J.; Serra, M.A.; et al. Contribution of hyperammonemia and inflammatory factors to cognitive impairment in minimal hepatic encephalopathy. Metab. Brain Dis. 2012, 27, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Civera, M.; Urios, A.; Garcia-Torres, M.L.; Real, J.T.; Ortega, J.; Martinez-Valls, J.; Cassinello, N.; del Olmo, J.A.; Rodrigo, J.M.; Montoliu, C. Relationship between insulin resistance, inflammation and liver cell apoptosis in patients with severe obesity. Diabetes Metab. Res. Rev. 2010, 26, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Felipo, V.; Urios, A.; García-Torres, M.L.; El Mlili, N.; del Olmo, J.A.; Civera, M.; Ortega, J.; Ferrandez, A.; Martínez-Valls, J.; Cassinello, N.; et al. Alterations in adipocytokines and cGMP homeostasis in morbid obesity patients reverse after bariatric surgery. Obesity 2013, 21, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Higarza, S.G.; Arboleya, S.; Gueimonde, M.; Gomez-Lazaro, E.; Arias, J.L.; Arias, N. Neurobehavioral dysfunction in non-alcoholic steatohepatitis is associated with hyperammonemia; gut dysbiosis; and metabolic and functional brain regional deficits. PLoS ONE 2019, 14, e0223019. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, G.; Zelber-Sagi, S.; Preis, S.R.; Beiser, A.S.; DeCarli, C.; Speliotes, E.K.; Satizabal, C.L.; Vasan, R.S.; Seshadri, S. Association of Nonalcoholic Fatty Liver Disease With Lower Brain Volume in Healthy Middle-aged Adults in the Framingham Study. JAMA Neurol. 2018, 75, 97–104. [Google Scholar] [CrossRef]
- Balzano, T.; Forteza, J.; Molina, P.; Giner, J.; Monzó, A.; Sancho-Jiménez, J.; Urios, A.; Montoliu, C.; Felipo, V. The Cerebellum of Patients with Steatohepatitis Shows Lymphocyte Infiltration; Microglial Activation and Loss of Purkinje and Granular Neurons. Sci. Rep. 2018, 8, 3004. [Google Scholar] [CrossRef] [Green Version]
- Balzano, T.; Forteza, J.; Borreda, I.; Molina, P.; Giner, J.; Leone, P.; Urios, A.; Montoliu, C.; Felipo, V. Histological Features of Cerebellar Neuropathology in Patients With Alcoholic and Nonalcoholic Steatohepatitis. J. Neuropathol. Exp. Neurol. 2018, 77, 837–845. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.G.; Krenz, A.; Toussaint, L.E.; Maurer, K.J.; Robinson, S.A.; Yan, A.; Torres, L.; Bynoe, M.S. Non-alcoholic fatty liver disease induces signs of Alzheimer’s disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. J. Neuroinflamm. 2016, 13, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilanova, M.; Tavares, D.; Ferreira, P.; Oliveira, L.; Nóbrega, A.; Appelberg, R.; Arala-Chaves, M. Role of monocytes in the up-regulation of the early activation marker CD69 on B and T murine lymphocytes induced by microbial mitogens. Scand. J. Immunol. 1996, 43, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Afeltra, A.; Galeazzi, M.; Ferri, G.M.; Amoroso, A.; De Pità, O.; Porzio, F.; Bonomo, L. Expression of CD69 antigen on synovial fluid T cells in patients with rheumatoid arthritis and other chronic synovitis. Ann. Rheum. Dis. 1993, 52, 457–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crispin, J.C.; Martinez, A.; de Pablo, P.; Velasquillo, C.; Alcocer-Varela, J. Participation of the CD69 antigen in the T-cell activation process of patients with systemic lupus erythematosus. Scand. J. Immunol. 1998, 48, 196–200. [Google Scholar] [CrossRef]
- Cibrián, D.; Sánchez-Madrid, F. CD69: From activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, W.; Kolls, J.K.; Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008, 28, 454–467. [Google Scholar] [CrossRef] [Green Version]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007, 10, 1173–1175. [Google Scholar] [CrossRef] [Green Version]
- Oberstein, T.J.; Taha, L.; Spitzer, P.; Hellstern, J.; Herrmann, M.; Kornhuber, J.; Maler, J.M. Imbalance of circulating Th17 and regulatory T cells in Alzheimer’s disease: A case control study. Front. Immunol. 2018, 9, 1213. [Google Scholar] [CrossRef] [Green Version]
- Dolati, S.; Ahmadi, M.; Khalili, M.; Taheraghdam, A.A.; Siahmansouri, H.; Babaloo, Z.; Aghebati-Maleki, L.; Jadidi-Niaragh, F.; Younesi, V.; Yousefi, M. Peripheral Th17/Treg imbalance in elderly patients with ischemic stroke. Neurol. Sci. 2018, 39, 647–654. [Google Scholar] [CrossRef]
- Cipollini, V.; Anrather, J.; Orzi, F.; Iadecola, C. Th17 and Cognitive Impairment: Possible Mechanisms of Action. Front. Neuroanat. 2019, 13, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarantino, G.; Costantini, S.; Finelli, C.; Capone, F.; Guerriero, E.; La Sala, N.; Gioia, S.; Castello, G. Is serum Interleukin-17 associated with early atherosclerosis in obese patients? J. Transl. Med. 2014, 12, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Munker, S.; Müllenbach, R.; Weng, H.L. IL-13 Signaling in Liver Fibrogenesis. Front. Immunol. 2012, 3, 116. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.G.; Homer, R.J.; Zhu, Z.; Lanone, S.; Wang, X.; Koteliansky, V.; Shipley, J.M.; Gotwals, P.; Noble, P.; Chen, Q.; et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J. Exp. Med. 2001, 194, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Miossec, P.; van den Berg, W. Th1/Th2 cytokine balance in arthritis. Arthritis Rheum. 1997, 40, 2105–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolosowska, N.; Keuters, M.H.; Wojciechowski, S.; Keksa-Goldsteine, V.; Laine, M.; Malm, T.; Goldsteins, G.; Koistinaho, J.; Dhungana, H. Peripheral administration of IL -13 induces anti-inflammatory microglial/macrophage responses and provides neuroprotection in ischemic stroke. Neurotherapeutics 2019, 16, 1304–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamzei Taj, S.; Le Blon, D.; Hoornaert, C.; Daans, J.; Quarta, A.; Praet, J.; Van der Linden, A.; Ponsaerts, P.; Hoehn, M. Targeted intracerebral delivery of the anti-inflammatory cytokine IL13 promotes alternative activation of both microglia and macrophages after stroke. J. Neuroinflamm. 2018, 15, 174. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Newcomb, D.C.; Boswell, M.G.; Zhou, W.; Huckabee, M.M.; Goleniewska, K.; Sevin, C.M.; Hershey, G.K.; Kolls, J.K.; Peebles, R.S., Jr. Human TH17 cells express a functional IL-13 receptor and IL-13 attenuates IL-17A production. J. Allergy Clin. Immunol. 2011, 127, 1006–1013.e134. [Google Scholar] [CrossRef] [Green Version]
- Sozzani, S.; Zhou, D.; Locati, M.; Rieppi, M.; Proost, P.; Magazin, M.; Vita, N.; van Damme, J.; Mantovani, A. Receptors and transduction pathways for monocyte chemotactic protein-2 and monocyte chemotactic protein-3. Similarities and differences with MCP-1. J. Immunol. 1994, 152, 3615–3622. [Google Scholar] [CrossRef]
- Reale, M.; Iarlori, C.; Feliciani, C.; Gambi, D. Peripheral chemokine receptors; their ligands; cytokines and Alzheimer’s disease. J. Alzheimers Dis. 2008, 14, 147–159. [Google Scholar] [CrossRef]
- Fantuzzi, L.; Tagliamonte, M.; Gauzzi, M.C.; Lopalco, L. Dual CCR5/CCR2 targeting: Opportunities for the cure of complex disorders. Cell Mol. Life Sci. 2019, 76, 4869–4886. [Google Scholar] [CrossRef] [Green Version]
- Britschgi, M.; Wyss-Coray, T. Systemic and acquired immune responses in Alzheimer’s disease. Int. Rev. Neurobiol. 2007, 82, 205–233. [Google Scholar] [CrossRef]
- Galimberti, D.; Fenoglio, C.; Lovati, C.; Venturelli, E.; Guidi, I.; Corrà, B.; Scalabrini, D.; Clerici, F.; Mariani, C.; Bresolin, N.; et al. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiol. Aging 2006, 27, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, C.; Le, T.; Swain, M.G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factor signaling during peripheral organ inflammation. J. Neurosci. 2009, 29, 2089–2102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Non alcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Newsome, P.N.; Sasso, M.; Deeks, J.J.; Paredes, A.; Boursier, J.; Chan, W.K.; Yilmaz, Y.; Czernichow, S.; Zheng, M.H.; Wong, V.W.; et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020, 5, 362–373, Erratum in Lancet Gastroenterol. Hepatol. 2020, 5, e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, R.D.; Foley, J.M. The neurological disorders associated with liver disease. Proc. Assoc. Res. Nerv. Ment. Dis. 1953, 32, 198–237. [Google Scholar]

| Parameter | Controls | NAFLD Patients without MCI | NAFLD Patients with MCI | p Value |
|---|---|---|---|---|
| Number of subjects [n (% of total patients)] | 61 | 51 (71) | 20 (29) | |
| Age a | 58 ± 1.0 | 58 ± 1.1 | 59 ± 2.4 | 0.966 |
| Sex [n (%)] | 0.314 | |||
| Male | 34 (53) | 26 (51) | 8 (40) | |
| Female | 30 (47) | 25 (49) | 12 (60) | |
| Education (years of schooling) a | 14 ± 0.5 | 14 ± 0.6 | 12 ± 1.0 | 0.181 |
| Comorbidity [n (%)] | ||||
| Diabetes mellitus | 18 (35) | 12 (60) | 0.060 | |
| Dyslipidemia | 30 (59) | 12 (60) | 0.928 | |
| Arterial Hypertension | 25 (49) | 13 (65) | 0.228 | |
| Metabolic Syndrome | 21 (41) | 12 (60) | 0.156 | |
| Laboratory Parameters a | ||||
| AST (U/L) | 25.8 ± 1.2 | 37.8 ± 3.0 ** | 36.5 ± 3.6 * | 0.0012 |
| ALT (U/L) | 25.7 ± 1.7 | 46.5 ± 3.6 **** | 45.4 ± 5.1 ** | <0.0001 |
| Albumin (g/dL) | 4.4 ± 0.04 | 4.4 ± 0.04 | 4.3 ± 0.09 | 0.205 |
| Bilirubin (mg/dL) | 0.5 ± 0.03 | 0.8 ± 0.07 | 0.6 ± 0.12 | 0.067 |
| Creatinine (mg/dL) | 0.8 ± 0.02 | 0.8 ± 0.03 | 0.8 ± 0.07 | 0.845 |
| Platelets (×109/L) | 242.3 ± 9.0 | 227.0 ± 10.3 | 292.1 ± 22.8 */bb | 0.005 |
| INR | 1.01 ± 0.00 | 1.02 ± 0.01 | 1.2 ± 0.19 | 0.099 |
| Ammonia (µM) | 9.9 ± 0.5 | 15.1 ± 1.3 ** | 18.2 ± 3.1 *** | <0.0001 |
| BMI (Body Mass Index) a | 29 ± 1.8 | 32 ± 0.7 | 32.4 ± 1.5 | 0.415 |
| MCI Score a | −0.8 ± 0.3 | −1.0 ± 0.3 | −7.2 ± 0.5 ****/bbbb | <0.0001 |
| Diagnosis [n (%)] | ||||
| NAFL | 27 (53) | 12 (60) | 0.593 | |
| NASH | 24 (47) | 8 (40) |
| Correlations with MCI Score | ||
| Parameter | Correlation Coefficient | p Value |
| IL-10 | −0.342 | 0.001 |
| IL-6 | −0.318 | 0.007 |
| IL-13 | −0.417 | <0.0001 |
| IL-17A | −0.323 | 0.001 |
| CCL2 | −0.244 | 0.035 |
| RORC | 0.419 | 0.012 |
| CCR2 | −0.410 | 0.011 |
| CD4+ (CD69+) | −0.254 | 0.039 |
| CD4+ naïve (CD69+) | −0.246 | 0.046 |
| CD4+CD28− (CD69+) | −0.309 | 0.012 |
| CD4+CD28+ (CD69+) | −0.290 | 0.022 |
| Univariate Logistic Regression Analyses | ||
| Independent Variables | OR (95% CI) | p |
| IL-6 | 1.06 (0.87–1.29) | 0.560 |
| IL-13 | 1.37 (1.12–1.67) | 0.002 |
| IL-17A | 110.6 (0.65–18,952) | 0.07 |
| IL18 | 1.002 (0.999–1.005) | 0.280 |
| IL21 | 1.026 (0.993–1.060) | 0.128 |
| IL22 | 1.000 (1.000–1.001) | 0.439 |
| IL23 | 1.024 (1.000–1.048) | 0.051 |
| INFγ | 3.95 (0.75–20.7) | 0.105 |
| BDNF | 1.000 (1.000–1.001) | 0.077 |
| CD4+ memory | 1.037 (0.986–1.090) | 0.156 |
| CD4+ (CD69+) | 1.172 (0.997–1.377) | 0.055 |
| CD4+ naïve (CD69+) | 1.116 (0.917–1.359) | 0.272 |
| CD4+ memory (CD69+) | 1.118 (0.987–1.266) | 0.080 |
| CD4+CD28− (CD69+) | 1.084 (1.002–1.173) | 0.044 |
| CD4+CD28+ (CD69+) | 1.142 (0.987–1.322) | 0.075 |
| Th17 (CD69+) | 0.960 (0.848–1.088) | 0.525 |
| Multivariate Logistic Regression Analyses | ||
| Predictor Variables | OR (95% CI) | p |
| IL-13 | 1.459 (1.111–1.916) | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorillo, A.; Gallego, J.-J.; Casanova-Ferrer, F.; Giménez-Garzó, C.; Urios, A.; Ballester, M.-P.; Durbán, L.; Rios, M.-P.; Megías, J.; San Miguel, T.; et al. Mild Cognitive Impairment Is Associated with Enhanced Activation of Th17 Lymphocytes in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 10407. https://doi.org/10.3390/ijms241210407
Fiorillo A, Gallego J-J, Casanova-Ferrer F, Giménez-Garzó C, Urios A, Ballester M-P, Durbán L, Rios M-P, Megías J, San Miguel T, et al. Mild Cognitive Impairment Is Associated with Enhanced Activation of Th17 Lymphocytes in Non-Alcoholic Fatty Liver Disease. International Journal of Molecular Sciences. 2023; 24(12):10407. https://doi.org/10.3390/ijms241210407
Chicago/Turabian StyleFiorillo, Alessandra, Juan-José Gallego, Franc Casanova-Ferrer, Carla Giménez-Garzó, Amparo Urios, Maria-Pilar Ballester, Lucia Durbán, Maria-Pilar Rios, Javier Megías, Teresa San Miguel, and et al. 2023. "Mild Cognitive Impairment Is Associated with Enhanced Activation of Th17 Lymphocytes in Non-Alcoholic Fatty Liver Disease" International Journal of Molecular Sciences 24, no. 12: 10407. https://doi.org/10.3390/ijms241210407