Lysophosphatidic Acid Receptor Signaling in the Human Breast Cancer Tumor Microenvironment Elicits Receptor-Dependent Effects on Tumor Progression
Abstract
1. Introduction
2. Results
2.1. Low LPAR1, LPAR4, and LPAR6 Gene Expression and High LPAR2 Gene Expression Correlate with a More Aggressive Breast Cancer Phenotype
2.2. Low LPAR1, LPAR4, and LPAR6 Gene Expression and High LPAR2 Gene Expression Are Particularly Correlated with Increased Cell Cycle Signaling
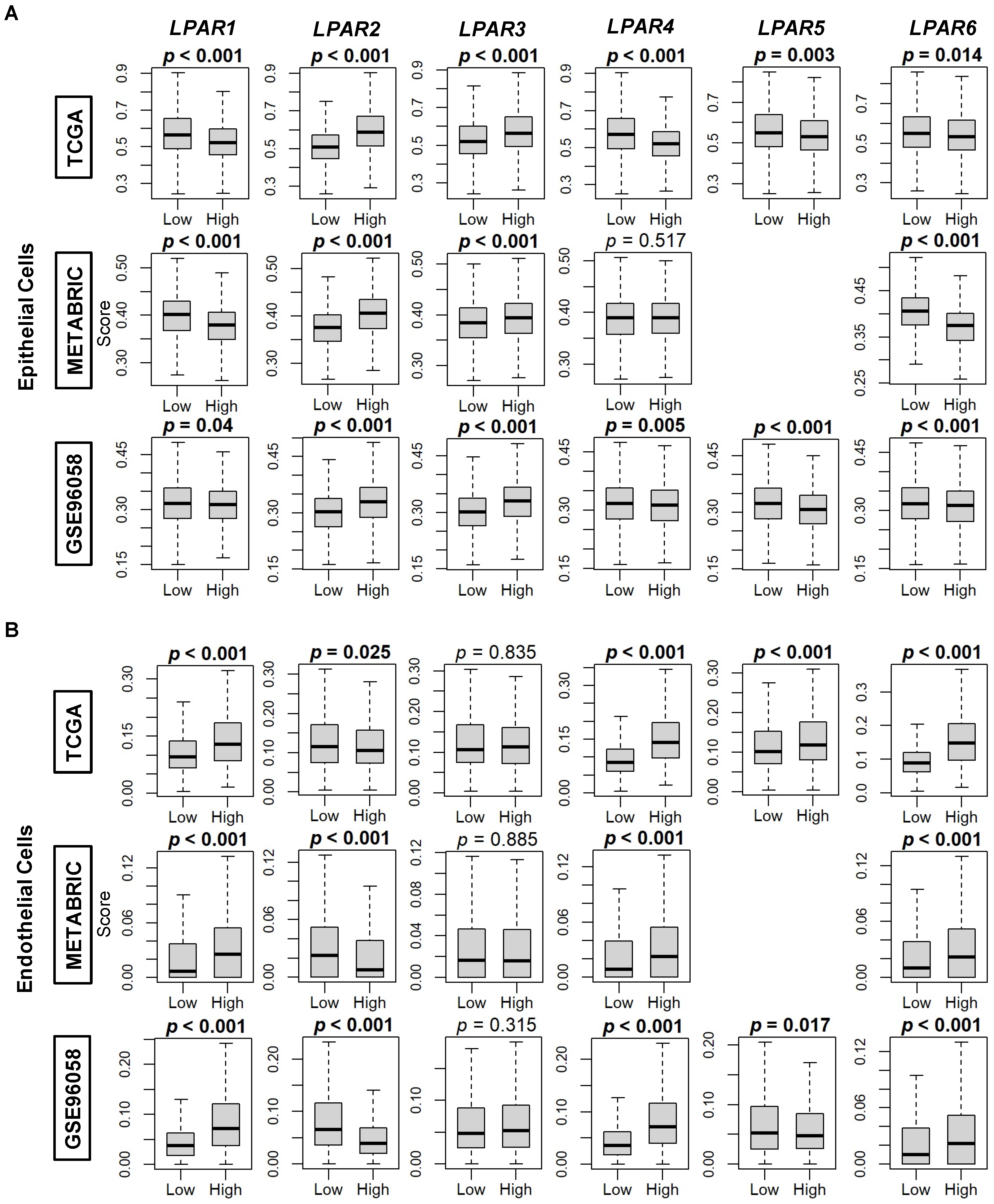
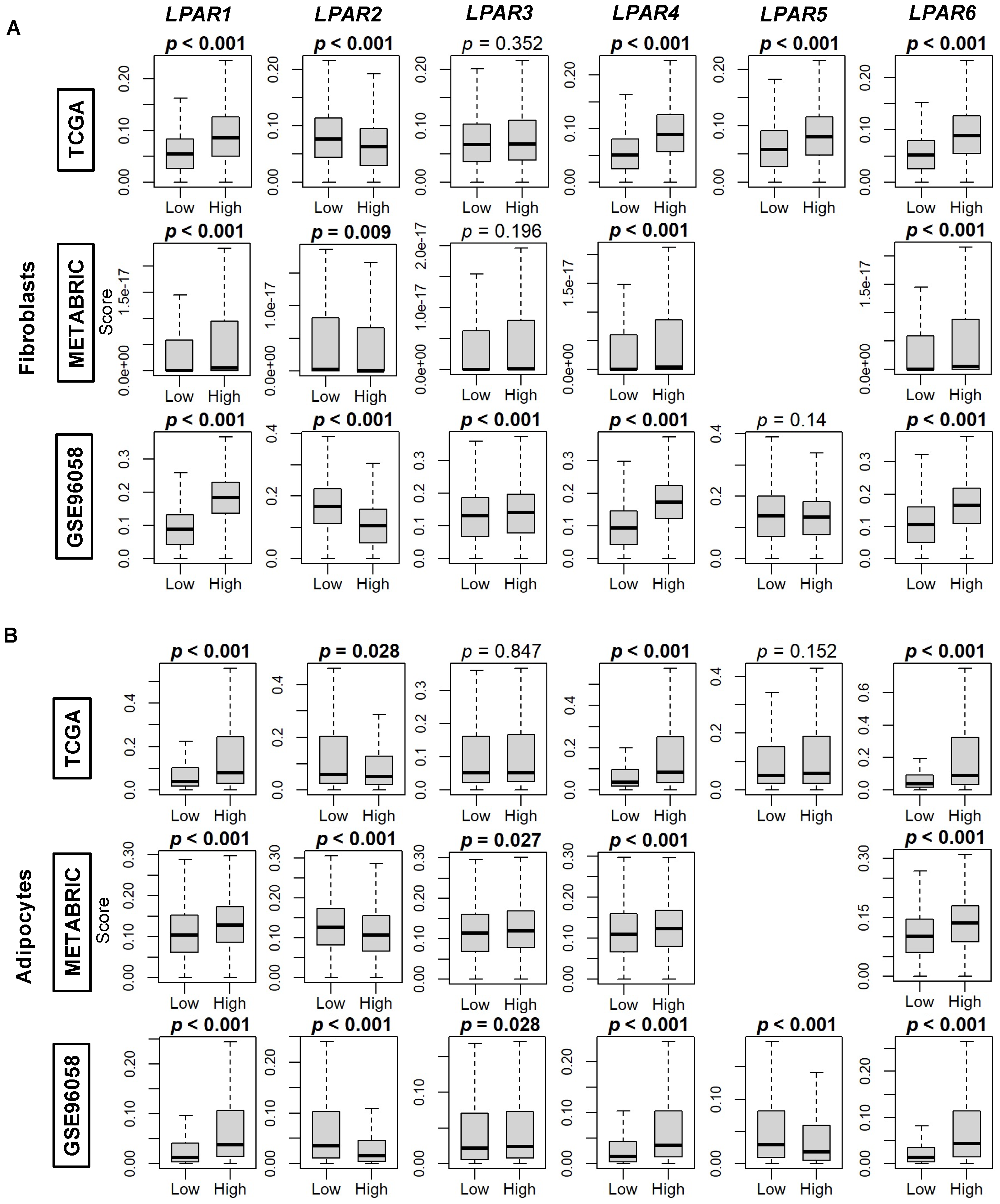
2.3. LPAR2 Is Predominantly Expressed in Cancers Cells, While the Other LPARs Are Expressed Primarily in the Stromal Cells in the Tumor Microenvironment
2.4. LPAR5- and LPAR6-High Tumors Correlate with Increased Tumor Immune Cell Infiltration and Decreased Immune System Evasion
3. Discussion
4. Materials and Methods
4.1. Data Acquisition
4.2. Gene Set Enrichment Analysis
4.3. Other Scores
4.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, Y.; Liu, X.; Liao, X.; He, J.; Niu, L. The clinicopathological features and survival outcomes of patients with different metastatic sites in stage iv breast cancer. BMC Cancer 2019, 19, 1091. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Cancer Statistics Female Breast Cancer Stat Bite; US Department of Health and Human Services: Atlanta, GA, USA, 2022.
- Benesch, M.G.K.; Tang, X.; Brindley, D.N. Autotaxin and breast cancer: Towards overcoming treatment barriers and sequelae. Cancers 2020, 12, 374. [Google Scholar] [CrossRef] [PubMed]
- Lainetti, P.F.; Leis-Filho, A.F.; Laufer-Amorim, R.; Battazza, A.; Fonseca-Alves, C.E. Mechanisms of resistance to chemotherapy in breast cancer and possible targets in drug delivery systems. Pharmaceutics 2020, 12, 1193. [Google Scholar] [CrossRef]
- Benesch, M.G.K.; Ko, Y.M.; McMullen, T.P.W.; Brindley, D.N. Autotaxin in the crosshairs: Taking aim at cancer and other inflammatory conditions. FEBS Lett. 2014, 588, 2712–2727. [Google Scholar] [CrossRef]
- Benesch, M.G.K.; MacIntyre, I.T.K.; McMullen, T.P.W.; Brindley, D.N. Coming of age for autotaxin and lysophosphatidate signaling: Clinical applications for preventing, detecting and targeting tumor-promoting inflammation. Cancers 2018, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, K.; Shimizu, T. Lysophosphatidic acid, a simple phospholipid with myriad functions. Pharmacol. Ther. 2023, 246, 108421. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Rives, S.A.; Gonzalez-Arenas, A. Autotaxin-lysophosphatidic acid: From inflammation to cancer development. Mediators Inflamm. 2017, 2017, 9173090. [Google Scholar] [CrossRef]
- Benesch, M.G.; Tang, X.; Venkatraman, G.; Bekele, R.T.; Brindley, D.N. Recent advances in targeting the autotaxin-lysophosphatidate-lipid phosphate phosphatase axis in vivo. J. Biomed. Res. 2016, 30, 272–284. [Google Scholar]
- Chun, J.; Hla, T.; Lynch, K.R.; Spiegel, S.; Moolenaar, W.H. International union of basic and clinical pharmacology. Lxxviii. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 2010, 62, 579–587. [Google Scholar] [CrossRef]
- van Meeteren, L.A.; Ruurs, P.; Stortelers, C.; Bouwman, P.; van Rooijen, M.A.; Pradere, J.P.; Pettit, T.R.; Wakelam, M.J.; Saulnier-Blache, J.S.; Mummery, C.L.; et al. Autotaxin, a secreted lysophospholipase d, is essential for blood vessel formation during development. Mol. Cell. Biol. 2006, 26, 5015–5022. [Google Scholar] [CrossRef]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. Lpa receptor signaling: Pharmacology, physiology, and pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.A.; Suárez-Pozos, E.; Soto-Verdugo, J.; Wang, H.; Afshari, F.S.; Li, G.; Manam, S.; Yasuda, D.; Ortega, A.; Lister, J.A.; et al. Lysophosphatidic acid signaling via lpa(6): A negative modulator of developmental oligodendrocyte maturation. J. Neurochem. 2022, 163, 478–499. [Google Scholar] [CrossRef] [PubMed]
- Nakanaga, K.; Hama, K.; Aoki, J. Autotaxin—An lpa producing enzyme with diverse functions. J. Biochem. 2010, 148, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Contos, J.J.A.; Ishii, I.; Fukushima, N.; Kingsbury, M.A.; Ye, X.; Kawamura, S.; Brown, J.H.; Chun, J. Characterization of lpa2 (edg4) and lpa1/lpa2 (edg2/edg4) lysophosphatidic acid receptor knockout mice: Signaling deficits without obvious phenotypic abnormality attributable to lpa2. Mol. Cell. Biol. 2002, 22, 6921–6929. [Google Scholar] [CrossRef]
- Yasuda, D.; Kobayashi, D.; Akahoshi, N.; Ohto-Nakanishi, T.; Yoshioka, K.; Takuwa, Y.; Mizuno, S.; Takahashi, S.; Ishii, S. Lysophosphatidic acid-induced yap/taz activation promotes developmental angiogenesis by repressing notch ligand dll4. J. Clin. Investig. 2019, 129, 4332–4349. [Google Scholar] [CrossRef]
- Brindley, D.N.; Tang, X.; Meng, G.; Benesch, M.G.K. Role of adipose tissue-derived autotaxin, lysophosphatidate signaling, and inflammation in the progression and treatment of breast cancer. Int. J. Mol. Sci. 2020, 21, 5938. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Study to Assess an Atx Inhibitor (ioa-289) in Patients with Metastatic Pancreatic Cancer; National Institute of Health: Bethesda, MD, USA, 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05586516 (accessed on 1 April 2023).
- Helmer, E.; Willson, A.; Brearley, C.; Westerhof, M.; Delage, S.; Shaw, I.; Cooke, R.; Sidhu, S. Pharmacokinetics and metabolism of ziritaxestat (glpg1690) in healthy male volunteers following intravenous and oral administration. Clin. Pharmacol. Drug Dev. 2022, 11, 246–256. [Google Scholar] [CrossRef]
- Decato, B.E.; Leeming, D.J.; Sand, J.M.B.; Fischer, A.; Du, S.; Palmer, S.M.; Karsdal, M.; Luo, Y.; Minnich, A. Lpa1 antagonist bms-986020 changes collagen dynamics and exerts antifibrotic effects in vitro and in patients with idiopathic pulmonary fibrosis. Respir. Res. 2022, 23, 61. [Google Scholar] [CrossRef]
- Llona-Minguez, S.; Ghassemian, A.; Helleday, T. Lysophosphatidic acid receptor (lpar) modulators: The current pharmacological toolbox. Prog. Lipid Res. 2015, 58, 51–75. [Google Scholar] [CrossRef]
- Gu, Z.; Yan, Y.; Yao, H.; Lin, K.; Li, X. Targeting the lpa1 signaling pathway for fibrosis therapy: A patent review (2010–present). Expert Opin. Ther. Pat. 2022, 32, 1097–1122. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hopkins, A.M.; Hou, J. The development of modulators for lysophosphatidic acid receptors: A comprehensive review. Bioorg. Chem. 2021, 117, 105386. [Google Scholar] [CrossRef] [PubMed]
- Balijepalli, P.; Sitton, C.C.; Meier, K.E. Lysophosphatidic acid signaling in cancer cells: What makes lpa so special? Cells 2021, 10, 2059. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, L.H.M.; Spohr, T.C.L.D.S.; Amaral, R.F.D.; Fonseca, A.C.C.D.; Garcia, C.; Mendes, F.D.A.; Freitas, C.; dos Santos, M.F.; Lima, F.R.S. Role of lysophosphatidic acid and its receptors in health and disease: Novel therapeutic strategies. Signal Trans. Target. Ther. 2021, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef]
- Benesch, M.G.; Wu, R.; Menon, G.; Takabe, K. High beta integrin expression is differentially associated with worsened pancreatic ductal adenocarcinoma outcomes. Am. J. Cancer Res. 2022, 12, 5403–5424. [Google Scholar] [PubMed]
- Almokadem, S.; Belani, C.P. Volociximab in cancer. Expert Opin. Biol. Ther. 2012, 12, 251–257. [Google Scholar] [CrossRef]
- Fusco, M.J.; West, H.J.; Walko, C.M. Tumor mutation burden and cancer treatment. JAMA Oncol. 2021, 7, 316. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The immune landscape of cancer. Immunity 2018, 48, 812–830.e814. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The molecular signatures database (msigdb) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Wu, S.Z.; Al-Eryani, G.; Roden, D.L.; Junankar, S.; Harvey, K.; Andersson, A.; Thennavan, A.; Wang, C.; Torpy, J.R.; Bartonicek, N.; et al. A single-cell and spatially resolved atlas of human breast cancers. Nat. Genet. 2021, 53, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Z.; Roden, D.L.; Wang, C.; Holliday, H.; Harvey, K.; Cazet, A.S.; Murphy, K.J.; Pereira, B.; Al-Eryani, G.; Bartonicek, N.; et al. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 2020, 39, e104063. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Benesch, M.G.K.; Brindley, D.N. Role of the autotaxin-lysophosphatidate axis in the development of resistance to cancer therapy. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158716. [Google Scholar] [CrossRef] [PubMed]
- Tigyi, G.J.; Yue, J.; Norman, D.D.; Szabo, E.; Balogh, A.; Balazs, L.; Zhao, G.; Lee, S.C. Regulation of tumor cell—Microenvironment interaction by the autotaxin-lysophosphatidic acid receptor axis. Adv. Biol. Regul. 2019, 71, 183–193. [Google Scholar] [CrossRef]
- Tan, Z.; Lei, H.; Guo, M.; Chen, Y.; Zhai, X. An updated patent review of autotaxin inhibitors (2017–present). Expert Opin. Ther. Pat. 2021, 31, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.K.; Tang, X.; Maeda, T.; Ohhata, A.; Zhao, Y.Y.; Kok, B.P.C.; Dewald, J.; Hitt, M.; Curtis, J.M.; McMullen, T.P.W.; et al. Inhibition of autotaxin delays breast tumor growth and lung metastasis in mice. FASEB J. 2014, 28, 2655–2666. [Google Scholar] [CrossRef]
- Banerjee, S.; Lee, S.; Norman, D.D.; Tigyi, G.J. Designing dual inhibitors of autotaxin-lpar gpcr axis. Molecules 2022, 27, 5487. [Google Scholar] [CrossRef]
- Meng, G.; Tang, X.; Yang, Z.; Benesch, M.G.K.; Marshall, A.; Murray, D.; Hemmings, D.G.; Wuest, F.; McMullen, T.P.W.; Brindley, D.N. Implications for breast cancer treatment from increased autotaxin production in adipose tissue after radiotherapy. FASEB J. 2017, 31, 4064–4077. [Google Scholar] [CrossRef]
- Abdel-Magid, A.F. Lysophosphatidic acid receptor 1 antagonists for the treatment of fibrosis. ACS Med. Chem. Lett. 2019, 10, 1378–1379. [Google Scholar] [CrossRef]
- Allanore, Y.; Distler, O.; Jagerschmidt, A.; Illiano, S.; Ledein, L.; Boitier, E.; Agueusop, I.; Denton, C.P.; Khanna, D. Lysophosphatidic acid receptor 1 antagonist sar100842 for patients with diffuse cutaneous systemic sclerosis: A double-blind, randomized, eight-week placebo-controlled study followed by a sixteen-week open-label extension study. Arthritis Rheumatol. 2018, 70, 1634–1643. [Google Scholar] [CrossRef]
- Hartman, Z.C.; Poage, G.M.; den Hollander, P.; Tsimelzon, A.; Hill, J.; Panupinthu, N.; Zhang, Y.; Mazumdar, A.; Hilsenbeck, S.G.; Mills, G.B.; et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines il-6 and il-8. Cancer Res. 2013, 73, 3470–3480. [Google Scholar] [CrossRef]
- Liu, S.; Umezu-Goto, M.; Murph, M.; Lu, Y.; Liu, W.; Zhang, F.; Yu, S.; Stephens, L.C.; Cui, X.; Murrow, G.; et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell 2009, 15, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Yu, I.; Tokumaru, Y.; Asaoka, M.; Oshi, M.; Yan, L.; Okuda, S.; Ishikawa, T.; Takabe, K. Elevated bile acid metabolism and microbiome are associated with suppressed cell proliferation and better survival in breast cancer. Am. J. Cancer Res. 2022, 12, 5271–5285. [Google Scholar]
- Oshi, M.; Tokumaru, Y.; Benesch, M.G.; Sugito, N.; Wu, R.; Yan, L.; Yamada, A.; Chishima, T.; Ishikawa, T.; Endo, I.; et al. High mir-99b expression is associated with cell proliferation and worse patient outcomes in breast cancer. Am. J. Cancer Res. 2022, 12, 4840–4852. [Google Scholar] [PubMed]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The genotype-tissue expression (gtex) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Aran, D.; Hu, Z.; Butte, A.J. Xcell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017, 18, 220. [Google Scholar] [CrossRef]
- Tokumaru, Y.; Oshi, M.; Murthy, V.; Tian, W.; Yan, L.; Angarita, F.A.; Nagahashi, M.; Matsuhashi, N.; Futamura, M.; Yoshida, K.; et al. Low intratumoral genetic neutrophil-to-lymphocyte ratio (nlr) is associated with favorable tumor immune microenvironment and with survival in triple negative breast cancer (tnbc). Am. J. Cancer Res. 2021, 11, 5743–5755. [Google Scholar]
- Chouliaras, K.; Oshi, M.; Asaoka, M.; Tokumaru, Y.; Khoury, T.; Endo, I.; Ishikawa, T.; Takabe, K. Increased intratumor heterogeneity, angiogenesis and epithelial to mesenchymal transition pathways in metaplastic breast cancer. Am. J. Cancer Res. 2021, 11, 4408–4420. [Google Scholar]
- Wu, R.; Oshi, M.; Asaoka, M.; Huyser, M.R.; Tokumaru, Y.; Yamada, A.; Yan, L.; Endo, I.; Ishikawa, T.; Takabe, K. Apobec3f expression in triple-negative breast cancer is associated with tumor microenvironment infiltration and activation of cancer immunity and improved survival. Am. J. Cancer Res. 2022, 12, 744–762. [Google Scholar]
- Wu, R.; Sarkar, J.; Tokumaru, Y.; Takabe, Y.; Oshi, M.; Asaoka, M.; Yan, L.; Ishikawa, T.; Takabe, K. Intratumoral lymphatic endothelial cell infiltration reflecting lymphangiogenesis is counterbalanced by immune responses and better cancer biology in the breast cancer tumor microenvironment. Am. J. Cancer Res. 2022, 12, 504–520. [Google Scholar] [PubMed]
- Oshi, M.; Roy, A.M.; Gandhi, S.; Tokumaru, Y.; Yan, L.; Yamada, A.; Endo, I.; Takabe, K. The clinical relevance of unfolded protein response signaling in breast cancer. Am. J. Cancer Res. 2022, 12, 2627–2640. [Google Scholar] [PubMed]
- Wakiyama, H.; Masuda, T.; Motomura, Y.; Hu, Q.; Tobo, T.; Eguchi, H.; Sakamoto, K.; Hirakawa, M.; Honda, H.; Mimori, K. Cytolytic activity (cyt) score is a prognostic biomarker reflecting host immune status in hepatocellular carcinoma (hcc). Anticancer Res. 2018, 38, 6631–6638. [Google Scholar] [CrossRef] [PubMed]







| LPAR1 | LPAR2 | LPAR3 | LPAR4 | LPAR5 | LPAR6 | |
|---|---|---|---|---|---|---|
| Most Common Subtype | ER+HER2– | TNBC | TNBC | – | – | ER+HER2– |
| Most Common Grade | Grade 1 | Grade 3 | Grade 3 | Grade 1 | – | Grade 1 |
| Highest Proliferation (Low vs. High LPAR Group) | Low | High | – | Low | – | Low |
| Highest Mutation Burden (Low vs. High LPAR Group) | Low | High | – | – | – | Low |
| Normal vs. Tumor (Highest Expression) | Normal | Tumor | Normal | Normal | Tumor | Normal |
| TME Cell Type (Highest Expression) | CAFs | Cancer Epithelial | – | CAFs | Myeloid | Endothelial |
| Highest Cytolytic Activity (Low vs. High LPAR Group) | – | – | High | – | High | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benesch, M.G.K.; Wu, R.; Tang, X.; Brindley, D.N.; Ishikawa, T.; Takabe, K. Lysophosphatidic Acid Receptor Signaling in the Human Breast Cancer Tumor Microenvironment Elicits Receptor-Dependent Effects on Tumor Progression. Int. J. Mol. Sci. 2023, 24, 9812. https://doi.org/10.3390/ijms24129812
Benesch MGK, Wu R, Tang X, Brindley DN, Ishikawa T, Takabe K. Lysophosphatidic Acid Receptor Signaling in the Human Breast Cancer Tumor Microenvironment Elicits Receptor-Dependent Effects on Tumor Progression. International Journal of Molecular Sciences. 2023; 24(12):9812. https://doi.org/10.3390/ijms24129812
Chicago/Turabian StyleBenesch, Matthew G. K., Rongrong Wu, Xiaoyun Tang, David N. Brindley, Takashi Ishikawa, and Kazuaki Takabe. 2023. "Lysophosphatidic Acid Receptor Signaling in the Human Breast Cancer Tumor Microenvironment Elicits Receptor-Dependent Effects on Tumor Progression" International Journal of Molecular Sciences 24, no. 12: 9812. https://doi.org/10.3390/ijms24129812
APA StyleBenesch, M. G. K., Wu, R., Tang, X., Brindley, D. N., Ishikawa, T., & Takabe, K. (2023). Lysophosphatidic Acid Receptor Signaling in the Human Breast Cancer Tumor Microenvironment Elicits Receptor-Dependent Effects on Tumor Progression. International Journal of Molecular Sciences, 24(12), 9812. https://doi.org/10.3390/ijms24129812








