Development of Phytochemical Delivery Systems by Nano-Suspension and Nano-Emulsion Techniques
Abstract
:1. Introduction
2. Phytochemicals (PHYs)
2.1. Phytochemicals and Nutraceuticals: Not Quite the Same
2.2. Phytochemicals: An Overview
2.2.1. Specific Sources and Benefits of the Main Types of PHYs
2.2.2. Let Us Eat in Color
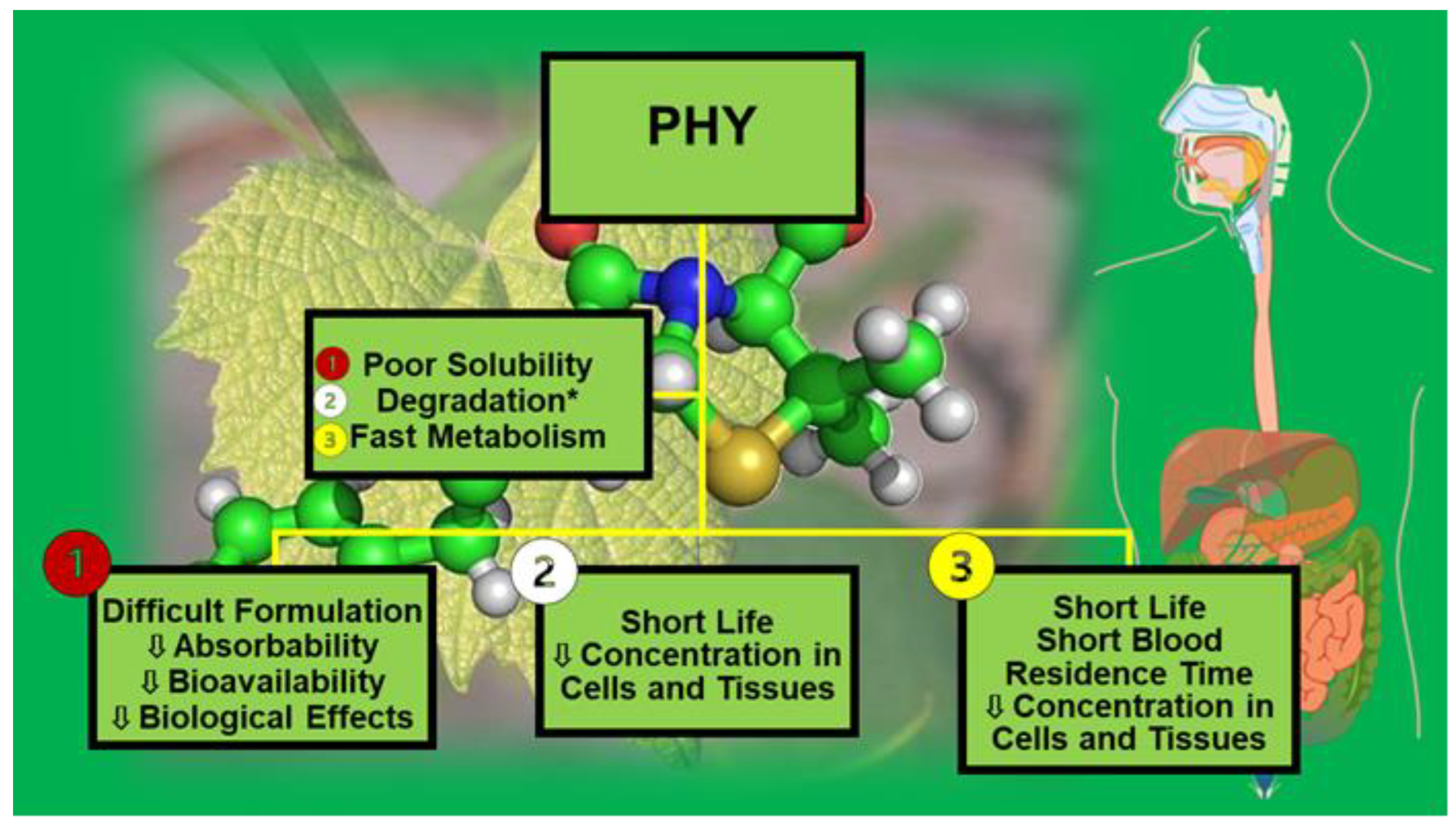
3. Physicochemical and Pharmacokinetic Drawbacks Limiting the Development of Phytochemicals as Clinically Administrable Therapeutics
3.1. Improving Solubility of Bioactive Compounds
3.1.1. Particle Engineering Techniques (PETs)
3.1.2. Formulation Approaches (FAs)
4. Nanotechnology
4.1. Physiological Barriers to Oral Drug Delivery
4.2. Advantages of Nanotechnology Application
4.3. Nanosuspension and Nanoemulsion Approaches
4.3.1. Nanosuspension Techniques
4.3.2. Manufacturing Methods
Antisolvent Precipitation Techniques
Homogenization
Wet or Media Milling
Dry Co-Grinding Method
Liquid Emulsion Evaporation Technique
Sonoprecipitation Method
4.3.3. Nanosuspension-Based Phytochemical Delivery Systems
4.3.4. Emulsion-Based Techniques
High-Energy and Low-Energy Methods
Microfluidization (MF)
Ultrasonication (US)
Phase Inversion Composition (PIC) and Phase Inversion Temperature (PIT)
Spontaneous Emulsification (SE)
Novel Nanoemulsion Preparation Techniques
4.3.5. NE-Based Phytochemical Delivery Systems
4.3.6. NS- and NE-Based Phytochemical Formulations: The State of the Art in Graphs
5. Nanomaterials and Nanoparticles: What We Know and What We Should Know
5.1. Ongoing Actions to Address Challenges Related to Nanotechnology
5.2. Providing Nanotechnology Guidance and Information
5.2.1. Safety of Nanocarriers
Titanium Dioxide: The European Case
5.2.2. Strategies to Reduce the Toxicity of Nanoparticles
6. Phytochemical-Loaded Nanomedicines: Where Are We and Where Are We Going?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| PHYs | Phytochemicals |
| NPs | Nanoparticles |
| DDSs | drug delivery systems |
| DSs | delivery systems |
| GIT | gastrointestinal tract |
| NSs | Nanosuspensions |
| NEs | Nanoemulsions |
| PETs | particle engineering techniques |
| CH | conventional homogenization |
| HPH | high-pressure homogenization |
| UHPH | ultra-high-pressure homogenization |
| FAs | formulation approaches |
| SD | spray drying |
| SFD | spray freeze-drying |
| SFL | spray freezing into liquid |
| TFF | thin film freezing |
| SFE | supercritical fluid extraction |
| EE | encapsulation efficiency |
| DCs | dendritic cells |
| ABC | ATP-binding cassette |
| N.A. | not applicable |
| N.R. | not reported |
| EA | ellagic acid |
| HLB | hydrophilic–lipophilic balance |
| GRAS | generally regarded as safe |
| B-U | bottom-up |
| T-D | top-down |
| CTNIs | combination techniques |
| CTNO | combination technology |
| MM | mechanical method |
| RES | Resveratrol |
| ATRA | all-trans retinoic acid |
| RSA | radical scavenging activity |
| ORAC | oxygen radical absorbance capacity |
| DL | drug loading |
| NRG | naringenin |
| ROS | reactive oxygen species |
| DLA | Dalton lymphoma ascites |
| GSH | glutathione reductase |
| OA | oleanolic acid |
| HPC-SSL | hydroxypropyl cellulose-SSL |
| PVP-K30 | polyinylpyrrolidone-K30 |
| HELF | human embryonic lung fibroblast |
| CSL | celastrol |
| SC | supercritical |
| SC | sodium cholate |
| SLS | sodium lauryl sulfate |
| PEG | polyethylene glycol |
| TPGS | D-α-tocopherol polyethylene glycolsuccinate |
| SGF | simulated gastric fluid |
| PB | phosphate buffer |
| GLA | glaucocalyxin A |
| SEOA | sucrose ester-stabilized oleanolic acid |
| SEDDSs | self-emulsifying drug delivery systems |
| SNEDDSs | self-nanoemulsifying drug delivery systems |
| SMEDDSs | self-microemulsifying drug delivery systems |
| SDEDDSs | self-double-emulsifying drug delivery systems |
| LET | low-energy techniques |
| HET | high-energy techniques |
| MF | microfluidization |
| US | ultrasonication |
| PIT | phase inversion temperature |
| PIC | phase inversion composition |
| SE | spontaneous emulsification |
| EOs | essential oils |
| RO | rosmarinic acid |
| LDL | low-density lipoprotein |
| HDL | high-density lipoprotein |
| PNEs | pickering nanoemulsions |
| CmLN | curcumin-loaded lipid NEs |
| PUFA | polyunsaturated fatty acid |
| OSCC | oral squamous cell carcinoma |
| CUR-NEs | curcumin-loaded lipid NEs |
| EGCG | epigallocatechin-3-gallate |
| ALA | α-lipoic acid |
| PGPR | polyglycerol polyricinoleate |
| TAC | total antioxidant capacity |
| FRAP | ferric-reducing antioxidant power |
| ORAC | oxygen radical absorbance capacity |
| 3GIO | tri-glycerol monooleate |
| SML | sucrose monooleate |
| PGPR | polyglycerol polyricinoleate |
| BC | bacterial cellulose |
| WPI | whey protein isolate |
| ESE | ethoxylated sorbitan esters |
| PEGM | poly(ethylene glycol) monooleate |
| 3,3-DODOXAP | 3,3-dioctadecyloxacarbocyanine perchlorate |
| DHA | docosahexaenoic acid |
| HSS | high-speed shearing |
| SEO | Satureja montana essential oil |
| PPG | polypropylene glycol |
| MDA-MB-231 | cell model of late-stage breast cancer |
| SMP | sucrose palmitate |
| BCL | baicalein |
| M | myricitrin |
| ITCs | isothiocyanates |
| SFN | sulforaphane |
| AITC | allyl isothiocyanate |
| BITC | benzyl isothiocyanate |
| PEITC | phenethyl isothiocyanate |
| PLGA | poly (lactic-glycolic acid) |
| EFFR | epidermal growth factor receptor |
| CO | cerium oxide |
| MICs | minimal inhibitory concentrations |
| MBCs | minimal bactericidal concentrations |
| KPF | kaempferol |
| KPF-NE | KPF-loaded NE |
| KPF-MNE | KPF-loaded mucoadhesive NE |
| FDA | Food and Drug Administration |
| CDER | Center for Drug Evaluation and Research |
| OTR | Office of Testing and Research |
| GDUFA | Generic Drug User Fee Amendments |
| NRAWG | Nanotechnology Risk Assessment Working Group |
| SLNs | solid lipid NPs |
| JNK | c-Jun N-terminal kinase |
| [P(b-Asp-co-APIA)-PEG] | pH-sensitive poly{(benzyl-L-aspartate)-co-[N-(3-aminopropyl) imidazole-L-aspartamide]}-poly(ethylene glycol) |
| IBD | inflammatory bowel disease |
| RA | rheumatoid arthritis |
| PS-NPs | polysaccharide-based nanocarriers |
| [P(Asp-g-Im)-PEG] | poly(aspartic acid-graft-imidazole)-poly(ethylene glycol) |
| mPEG-PCL | mono methoxy poly (ethylene glycol)-poly (e-caprolactone) di-block copolymers |
| CNTs | carbon nanotubes |
| OS | oxidative stress |
| MWCNTs | multiwalled CNTs |
| EFSA | European Food Safety Authority |
| EMA | European Medicine Agency |
| EEA | European Economic Area |
| QWP | Quality Working Party |
| SLNPs | solid lipid NPs |
| PVA | polyvinyl alcohol |
| PNIPAM | poly(N-isopropylacrylamide) |
| PCB | poly(carboxybetaine) |
| PSB | poly(sulfo-betaine) |
| SWCNTs | single-walled carbon nanotubes |
| NC | nanocomposite |
| FSP | flame spray pyrolysis |
| CAPs | clinically applied phytochemicals |
References
- Alfei, S.; Schito, A.M.; Zuccari, G. Nanotechnological Manipulation of Nutraceuticals and Phytochemicals for Healthy Purposes: Established Advantages vs. Still Undefined Risks. Polymers 2021, 13, 2262. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Garcia, S.N.; Vazquez-Cruz, M.A.; Garcia-Mier, L.; Contreras-Medina, L.M.; Guevara-González, R.G.; Garcia-Trejo, J.F.; Feregrino-Perez, A.A. Phytochemical and Pharmacological Properties of Secondary Metabolites in Berries. In Therapeutic Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 397–427. [Google Scholar] [CrossRef]
- Phytochemical. Available online: https://en.wikipedia.org/wiki/Phytochemical (accessed on 6 April 2023).
- Higdon, J.; Drake, V.J. An Evidence-Based Approach to Phytochemicals and Other Dietary Factors, 2nd ed.; Thieme: Rio de Janeiro, Brazil, 2012; p. 3682013. ISBN 978-3-13-169712-7. [Google Scholar]
- Rutz, A.; Sorokina, M.; Galgonek, J.; Mietchen, D.; Willighagen, E.; Gaudry, A.; Graham, J.G.; Stephan, R.; Page, R.; Vondrášek, J.; et al. The LOTUS Initiative for Open Knowledge Management in Natural Products Research. eLife 2022, 11, e70780. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef] [PubMed]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-Inflammatory Potential of Ellagic Acid, Gallic Acid and Punicalagin A&B Isolated from Punica Granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.-S.; Yang, S.-F.; Sethi, G.; Hu, D.-N. Natural Bioactives in Cancer Treatment and Prevention. BioMed. Res. Int. 2015, 2015, 182835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imm, B.-Y.; Kim, C.H.; Imm, J.-Y. Effects of Partial Substitution of Lean Meat with Pork Backfat or Canola Oil on Sensory Properties of Korean Traditional Meat Patties (Tteokgalbi). Food Sci. Anim. Resour. 2014, 34, 496–499. [Google Scholar] [CrossRef] [Green Version]
- Bisio, A.; Milella, L.; Parricchi, A.; Alfei, S.; Vignola, L.; De Mieri, M.; Schito, A.; Hamburger, M.; De Tommasi, N. Biological activity of constituents of Salvia chamaedryoides. Plant. Med. 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Bisio, A.; De Mieri, M.; Milella, L.; Schito, A.M.; Parricchi, A.; Russo, D.; Alfei, S.; Lapillo, M.; Tuccinardi, T.; Hamburger, M.; et al. Antibacterial and Hypoglycemic Diterpenoids from Salvia chamaedryoides. J. Nat. Prod. 2017, 80, 503–514. [Google Scholar] [CrossRef]
- Ting, Y.; Jiang, Y.; Ho, C.-T.; Huang, Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Xiao, J.; Cao, Y.; Huang, Q. Edible Nanoencapsulation Vehicles for Oral Delivery of Phytochemicals: A Perspective Paper. J. Agric. Food Chem. 2017, 65, 6727–6735. [Google Scholar] [CrossRef]
- Zuccari, G.; Alfei, S.; Zorzoli, A.; Marimpietri, D.; Turrini, F.; Baldassari, S.; Marchitto, L.; Caviglioli, G. Increased Water-Solubility and Maintained Antioxidant Power of Resveratrol by Its Encapsulation in Vitamin E TPGS Micelles: A Potential Nutritional Supplement for Chronic Liver Disease. Pharmaceutics 2021, 13, 1128. [Google Scholar] [CrossRef]
- Kumar, G.; Virmani, T.; Sharma, A.; Pathak, K. Codelivery of Phytochemicals with Conventional Anticancer Drugs in Form of Nanocarriers. Pharmaceutics 2023, 15, 889. [Google Scholar] [CrossRef]
- Li, J.J.; Ge, Z.S.; Toh, K.; Liu, X.Y.; Dirisala, A.; Ke, W.D.; Wen, P.Y.; Zhou, H.; Wang, Z.; Xiao, S.Y.; et al. Enzymatically Transformable Polymersome-Based Nanotherapeutics to Eliminate Minimal Relapsable Cancer. Adv. Mater. 2021, 33, 2105254. [Google Scholar] [CrossRef]
- Alfei, S. Nanotechnology Applications to Improve Solubility of Bioactive Constituents of Foods for Health-Promoting Purposes. In Nanofood Engineering, 1st ed.; Hebbar, U., Ranjan, S., Dasgupta, N., Mishra, R.K., Eds.; Springer: Cham, Switzerland, 2020; Volume 1, pp. 189–258. [Google Scholar]
- Phytonutrients: What Are They & Why Are They Important? Available online: https://www.cancernutrition.org/resources/phytonutrients-what-are-they-why-are-they-important/#:~:text=Phytonutrients%20are%20natural%20chemicals%20produced%20by%20plant%20foods.,over%2025%2C000%20%E2%80%93%20phytonutrients%20found%20in%20plant%20foods (accessed on 27 April 2023).
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [Green Version]
- Westergaard, D.; Li, J.; Jensen, K.; Kouskoumvekaki, I.; Panagiotou, G. Exploring Mechanisms of Diet-Colon Cancer Associations through Candidate Molecular Interaction Networks. BMC Genom. 2014, 15, 380. [Google Scholar] [CrossRef] [Green Version]
- Budisan, L.; Gulei, D.; Zanoaga, O.M.; Irimie, A.I.; Sergiu, C.; Braicu, C.; Gherman, C.D.; Berindan-Neagoe, I. Dietary Intervention by Phytochemicals and Their Role in Modulating Coding and Non-Coding Genes in Cancer. Int. J. Mol. Sci. 2017, 18, 1178. [Google Scholar] [CrossRef] [Green Version]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.Y.; Blumberg, J.B. Phytochemical composition of nuts. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 1), 329–332. [Google Scholar]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef] [Green Version]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and Dark Chocolate Polyphenols: From Biology to Clinical Applications. Front. Immunol. 2017, 8, 677. [Google Scholar] [CrossRef] [Green Version]
- Tümen, İ.; Akkol, E.K.; Taştan, H.; Süntar, I.; Kurtca, M. Research on the antioxidant, wound healing, and anti-inflammatory activities and the phytochemical composition of maritime pine (Pinus pinaster Ait). J. Ethnopharmacol. 2018, 211, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Saab, A.M.; Gambari, R.; Sacchetti, G.; Guerrini, A.; Lampronti, I.; Tacchini, M.; El Samrani, A.; Medawar, S.; Makhlouf, H.; Tannoury, M.; et al. Phytochemical and pharmacological properties of essential oils from Cedrus species. Nat. Prod. Res. 2018, 32, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, B.J.; Tallian, K. Essential oil of lavender in anxiety disorders: Ready for prime time? Ment. Health Clin. 2018, 7, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Prakash, D. Phytonutrients as therapeutic agents. J. Complement. Integr. Med. 2014, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxidative Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metab. Clin. Exp. 2008, 57, 712–717. [Google Scholar] [CrossRef] [Green Version]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Phytochemicals in cancer prevention and therapy: Truth or dare? Toxins 2010, 2, 517–551. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med. Res. Rev. 2018, 38, 951–976. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Ahmad, Z.; Hassan, S.S.; Azim, S. A Therapeutic Connection between Dietary Phytochemicals and ATP Synthase. Curr. Med. Chem. 2017, 24, 3894–3906. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I. Functional Foods for Health: The Interrelated Antioxidant and Anti-Inflammatory Role of Fruits, Vegetables, Herbs, Spices and Cocoa in Humans. Curr. Pharm. Des. 2016, 22, 6701–6715. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [Green Version]
- Jianbo, X.; Bai, W. Bioactive phytochemicals. Crit. Rev. Food Sci. Nutr. 2019, 59, 827–829. [Google Scholar] [CrossRef] [Green Version]
- Prakash, B. Functional and Preservative Properties of Phytochemicals; Elsevier Science & Technology: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-818593-3. [Google Scholar]
- Food Source of Carotenoids and Why They Are So Important. Available online: https://nutritionyoucanuse.com/food-sources-of-carotenoids (accessed on 28 April 2023).
- List of Phytochemicals in Food. Available online: https://en.wikipedia.org/wiki/List_of_phytochemicals_in_food (accessed on 28 April 2023).
- A List of Food High in Phytosterols. Available online: https://lazyplant.com/foods-with-phytosterols/ (accessed on 28 April 2023).
- Gutiérrez-Grijalva, E.P.; López-Martínez, L.X.; Contreras-Angulo, L.A.; Elizalde-Romero, C.A.; Heredia, J.B. Plant Alkaloids: Structures and Bioactive Properties. In Plant-Derived Bioactives; Swamy, M., Ed.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Abellán, Á.; León, D.; Ferreres, F.; Guy, A.; Oger, C.; Galano, J.M.; Durand, T.; Gil-Izquierdo, Á. Sorting out the phytoprostane and phytofuran profile in vegetable oils. Food Res. Int. 2018, 107, 619–628. [Google Scholar] [CrossRef]
- Bakuradze, T.; Tausend, A.; Galan, J.; Groh, I.A.M.; Berry, D.; Tur, J.A.; Marko, D.; Richling, E. Antioxidative activity and health benefits of anthocyanin-rich fruit juice in healthy volunteers. Free Radic. Res. 2019, 53 (Suppl. 1), 1045–1055. [Google Scholar] [CrossRef]
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.-F.; Shi, J.; Yan, N. Anthocyanins and Proanthocyanidins: Chemical Structures, Food Sources, Bioactivities, and Product Development. Food Rev. Int. 2022, 1–29. [Google Scholar] [CrossRef]
- Aboonabi, A.; Aboonabi, A. Anthocyanins reduce inflammation and improve glucose and lipid metabolism associated with inhibiting nuclear factor-kappaB activation and increasing PPAR-γ gene expression in metabolic syndrome subjects. Free Radic. Biol. Med. 2020, 150, 30–39. [Google Scholar] [CrossRef]
- What Are High Fiber Foods? Chart, Fiber Needs, and More. Available online: https://www.healthline.com/nutrition/high-fiber-foods-chart (accessed on 19 May 2023).
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Chou, C.-W.; Kumar, K.S.; Fu, K.-T.; Wang, H.-M.; Hsu, L.-S.; Kuo, Y.-H.; Wu, C.-R.; Chen, S.-C.; Yang, H.-L. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem. Toxicol. 2012, 50, 1245–1255. [Google Scholar] [CrossRef]
- Ahire, V.; Mishra, K.P. Ellagic Acid as a Potential Anti-Cancer Drug. Int. J. Radiol. Radiat. Ther. 2017, 3, 1–3. [Google Scholar]
- Larrosa, M.; Conesa, M.T.G.; Espín, J.C.; Tomás-Barberán, F.A. Ellagitannins, ellagic acid and vascular health. Mol. Asp. Med. 2010, 31, 513–539. [Google Scholar] [CrossRef] [PubMed]
- Nejad, K.H.; Dianat, M.; Sarkaki, A. Ellagic acid improves electrocardiogram waves and blood pressure against global cerebral ischemia rat experimental models. Electron. Phys. 2015, 7, 1153–1162. [Google Scholar]
- Hoseinynejad, K.; Gharib-Naseri, M.K.; Sarkaki, A.; Dianat, M.; Badavi, M.; Farbood, Y. Effects of ellagic acid pretreatment on renal functions disturbances induced by global cerebral ischemic-reperfusion in rat. Iran J. Basic Med. Sci. 2017, 20, 75–82. [Google Scholar]
- Firdaus, F.; Zafeer, M.F.; Waseem, M.; Anis, E.; Hossain, M.M.; Afzal, M. Ellagic acid mitigates arsenic-trioxide-induced mitochondrial dysfunction and cytotoxicity in SH-SY5Y cells. J. Biochem. Mol. Toxicol. 2018, 32, e22024. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and Challenges of Tannins as an Alternative to In-Feed Antibiotics for Farm Animal Production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and Hydrolysable Tannins: Occurrence, Dietary Intake and Pharmacological Effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [Green Version]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration—Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef]
- Zuniga, K.E.; Bishop, N.J.; Turner, A.S. Dietary Lutein and Zeaxanthin Are Associated with Working Memory in an Older Population. Public Health Nutr. 2021, 24, 1708–1715. [Google Scholar] [CrossRef]
- Healthline. Nutrition. Lutein and Zeaxanthin: Benefits, Dosage and Food Sources. Available online: https://www.healthline.com/nutrition/lutein-and-zeaxanthin (accessed on 6 April 2023).
- Healthline. Nutrition. Sulforaphane: Benefits, Dosage and Food Sources. Available online: https://www.healthline.com/nutrition/sulforaphane (accessed on 6 April 2023).
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An Overview on the Anti-inflammatory Potential and Antioxidant Profile of Eugenol. Oxidative Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef]
- Palmer-Young, E.C.; Calhoun, A.C.; Mirzayeva, A.; Sadd, B.M. Effects of the Floral Phytochemical Eugenol on Parasite Evolution and Bumble Bee Infection and Preference. Sci. Rep. 2018, 8, 2074. [Google Scholar] [CrossRef] [Green Version]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological Properties and Health Benefits of Eugenol: A Comprehensive Review. Oxidative Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef]
- Minich, D.M. A Review of the Science of Colorful, Plant-Based Food and Practical Strategies for “Eating the Rainbow”. J. Nutr. Metab. 2019, 2, 2125070. [Google Scholar] [CrossRef]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef]
- Sivakumar, M.; Tang, S.Y.; Tan, K.W. Cavitation technology—A greener processing technique for the generation of pharmaceutical nanoemulsions. Ultrason. Sonochem. 2014, 21, 2069–2083. [Google Scholar] [CrossRef]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef] [Green Version]
- Alfei, S.; Taptue, G.B.; Catena, S.; Bisio, A. Synthesis of Water-soluble, Polyester-based Dendrimer Prodrugs for Exploiting Therapeutic Properties of Two Triterpenoid Acids. Chin. J. Polym. Sci. 2018, 36, 999–1010. [Google Scholar] [CrossRef]
- Schito, A.M.; Schito, G.C.; Alfei, S. Synthesis and Antibacterial Activity of Cationic Amino Acid-Conjugated Dendrimers Loaded with a Mixture of Two Triterpenoid Acids. Polymers 2021, 13, 521. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M.; Zuccari, G. Considerable Improvement of Ursolic Acid Water Solubility by Its Encapsulation in Dendrimer Nanoparticles: Design, Synthesis and Physicochemical Characterization. Nanomaterials 2021, 11, 2196. [Google Scholar] [CrossRef]
- Schito, A.M.; Caviglia, D.; Piatti, G.; Zorzoli, A.; Marimpietri, D.; Zuccari, G.; Schito, G.C.; Alfei, S. Efficacy of Ursolic Acid-Enriched Water-Soluble and Not Cytotoxic Nanoparticles against Enterococci. Pharmaceutics 2021, 13, 1976. [Google Scholar] [CrossRef]
- Kale, B.B.; Aloorkar, N.H.; Deshmukh, S.M.; Sulake, S.P.; Humbe, P.V.; Mane, P.P. Recent advancements in particle engi-neering techniques for pharmaceutical applications. Indo. Am. J. Pharm. Res. 2014, 4, 2027–2049. [Google Scholar]
- Koshy, P.; Pacharane, S.; Chaudhry, A.; Jadhav, K.; Kadam, V. Drug particle engineering of poorly water-soluble drugs. Der. Pharm. Lett. 2010, 2, 65–76. [Google Scholar]
- Morales, J.O.; Watts, A.B.; McConville, J.T. Mechanical particle-size reduction techniques. In Formulating Poorly Water-Soluble Drugs; Williams, R.O., III, Watts, A.B., Miller, D.A., Eds.; Springer: New York, NY, USA, 2016; pp. 133–170. ISBN 9781461411444. [Google Scholar]
- Yang, W.; Owens, D.E.; Williams, R.O. Pharmaceutical Cryogenic Technologies. In Formulating Poorly Water-Soluble Drugs; Williams, R., III, Watts, A., Miller, D., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer: New York, NY, USA, 2012; Volume 3, pp. 443–500. [Google Scholar] [CrossRef]
- Li, J.; Kataoka, K. Chemo-Physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Deshpande, D.; Korde, A.; Amiji, M. A review of multifunctional nanoemulsion systems to overcome oral and CNS drug delivery barriers. Mol. Membr. Biol. 2010, 27, 260–273. [Google Scholar] [CrossRef]
- Moreno Raja, M.; Lim, P.Q.; Wong, Y.S.; Xiong, G.M.; Zhang, Y.; Venkatraman, S.; Huang, Y. Chapter 18—Polymeric Nanomaterials: Methods of Preparation and Characterization. In Nanocarriers for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 557–653. ISBN 978-0-12-814033-8. [Google Scholar]
- Brown, T.D.; Whitehead, K.A.; Mitragotri, S.S. Materials for Oral Delivery of Proteins and Peptides. Nat. Rev. Mater. 2019, 5, 127–148. [Google Scholar] [CrossRef]
- Durán-Lobato, M.; Niu, Z.; Alonso, M.J. Oral Delivery of Biologics for Precision Medicine. Adv. Mater. 2020, 32, 1901935. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral Drug Delivery with Polymeric Nanoparticles: The Gastrointestinal Mucus Barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Kamada, N.; Moon, J.J. Oral Nanomedicine for Modulating Immunity, Intestinal Barrier Functions, and Gut Microbiome. Adv. Drug Deliv. Rev. 2021, 179, 114021. [Google Scholar] [CrossRef]
- Han, Y.; Gao, Z.; Chen, L.; Kang, L.; Huang, W.; Jin, M.; Wang, Q.; Bae, Y.H. Multifunctional Oral Delivery Systems for Enhanced Bioavailability of Therapeutic Peptides/Proteins. Acta Pharm. Sin. B 2019, 9, 902–922. [Google Scholar] [CrossRef]
- Fox, C.B.; Kim, J.; Le, L.V.; Nemeth, C.L.; Chirra, H.D.; Desai, T.A. Micro/Nanofabricated Platforms for Oral Drug Delivery. J. Control. Release 2015, 219, 431–444. [Google Scholar] [CrossRef] [Green Version]
- Zuccari, G.; Baldassari, S.; Ailuno, G.; Turrini, F.; Alfei, S.; Caviglioli, G. Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid. Appl. Sci. 2020, 10, 3353. [Google Scholar] [CrossRef]
- Avachat, A.M.; Patel, V.G. Self nanoemulsifying drug delivery system of stabilized ellagic acid–phospholipid complex with improved dissolution and permeability. Saudi Pharm. J. 2015, 23, 276–289. [Google Scholar] [CrossRef] [Green Version]
- Madrigal-Carballo, S.; Lim, S.; Rodriguez, G.; Vila, A.O.; Krueger, C.G.; Gunasekaran, S.; Reed, J.D. Biopolymer coating of soybean lecithin liposomes via layer-by-layer self-assembly as novel delivery system for ellagic acid. J. Funct. Foods 2010, 2, 99–106. [Google Scholar] [CrossRef]
- Hajipour, H.; Hamishehkar, H.; Rahmati-yamchi, M.; Shanehbandi, D.; Nazari Soltan Ahmad, S.; Hasani, A. Enhanced Anti-Cancer Capability of Ellagic Acid Using Solid Lipid Nanoparticles (SLNs). Int. J. Cancer Manag. 2018, 11, e9402. [Google Scholar] [CrossRef]
- Bulani, V.D.; Kothavade, P.S.; Kundaikar, H.; Gawali, N.B.; Chowdhury, A.A.; Degani, M.S.; Juvekar, A.R. Inclusion complex of ellagic acid with β-cyclodextrin: Characterization and in vitro anti-inflammatory evaluation. J. Mol. Struct. 2016, 1105, 308–315. [Google Scholar] [CrossRef]
- Mady, F.; Ibrahim, S.R.-M. Cyclodextrin-based nanosponge for improvement of solubility and oral bioavailability of Ellagic acid. Pak. J. Pharm. Sci. 2018, 31, 2069–2076. [Google Scholar]
- Alfei, S.; Turrini, F.; Catena, S.; Zunin, P.; Parodi, B.; Zuccari, G.; Pittaluga, A.M.; Boggia, R. Preparation of ellagic acid micro and nano formulations with amazingly increased water solubility by its entrapment in pectin or non-PAMAM dendrimers suitable for clinical applications. New J. Chem. 2019, 43, 2438–2448. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Liu, B.; Feng, S.-S. A strategy for precision engineering of nanoparticles of biodegradable copolymers for quantitative control of targeted drug delivery. Biomaterials 2010, 31, 9145–9155. [Google Scholar] [CrossRef]
- Sha, X.; Guo, J.; Chen, Y.; Fang, X. Effect of phospholipid composition on pharmacokinetics and biodistribution of epirubicin liposomes. J. Liposome Res. 2011, 22, 80–88. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Drechsler, M.; Lesieur, S. Neurotrophin delivery using nanotechnology. Drug Discov. Today 2013, 18, 1263–1271. [Google Scholar] [CrossRef]
- Han, H.S.; Koo, S.Y.; Choi, K.Y. Emerging Nanoformulation Strategies for Phytocompounds and Applications from Drug Delivery to Phototherapy to Imaging. Bioact. Mater. 2022, 14, 182–205. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zu, Y.; Wang, L.; Wang, L.; Wang, H.; Li, Y.; Wu, M.; Zhao, X.; Fu, Y. Preparation, Characterization and Antitumor Activity Evaluation of Apigenin Nanoparticles by the Liquid Antisolvent Precipitation Technique. Drug Deliv. 2017, 24, 1713–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Shaal, L.; Müller, R.H.; Shegokar, R. SmartCrystal Combination Technology–Scale up from Lab to Pilot Scale and Long Term Stability. Die Pharm. Int. J. Pharm. Sci. 2010, 65, 877–884. [Google Scholar]
- Patel, V.R.; Agrawal, Y. Nanosuspension: An Approach to Enhance Solubility of Drugs. J. Adv. Pharm. Technol. Amp. Res. 2011, 2, 81–87. [Google Scholar] [CrossRef]
- Tehrani, A.A.; Omranpoor, M.M.; Vatanara, A.; Seyedabadi, M.; Ramezani, V. Formation of nanosuspensions in bottom-up approach: Theories and optimization. DARU J. Pharm. Sci. 2019, 27, 451. [Google Scholar] [CrossRef]
- Liedtke, S.; Wissing, S.; Müller, R.H.; Mäder, K. Influence of High Pressure Homogenisation Equipment on Nanodispersions Characteristics. Int. J. Pharm. 2000, 196, 183–185. [Google Scholar] [CrossRef]
- Levy, R.; Okun, Z.; Shpigelman, A. High-Pressure Homogenization: Principles and Applications Beyond Microbial Inactivation. Food Eng. Rev. 2020, 13, 490–508. [Google Scholar] [CrossRef]
- Chavan, R.B.; Thipparaboina, R.; Kumar, D.; Shastri, N.R. Evaluation of the Inhibitory Potential of HPMC, PVP and HPC Polymers on Nucleation and Crystal Growth. RSC Adv. 2016, 6, 77569–77576. [Google Scholar] [CrossRef]
- Niwa, T.; Miura, S.; Danjo, K. Universal Wet-Milling Technique to Prepare Oral Nanosuspension Focused on Discovery and Preclinical Animal Studies—Development of Particle Design Method. Int. J. Pharm. 2011, 405, 218–227. [Google Scholar] [CrossRef]
- Wongmekiat, A.; Tozuka, Y.; Oguchi, T.; Yamamoto, K. Formation of Fine Drug Particles by Cogrinding with Cyclodextrins. I. The Use of β-Cyclodextrin Anhydrate and Hydrate. Pharm. Res. 2002, 19, 1867–1872. [Google Scholar] [CrossRef]
- Watanabe, T.; Ohno, I.; Wakiyama, N.; Kusai, A.; Senna, M. Stabilization of Amorphous Indomethacin by Co-Grinding in a Ternary Mixture. Int. J. Pharm. 2002, 241, 103–111. [Google Scholar] [CrossRef]
- Tran, T.T.-D.; Tran, P.H.-L.; Nguyen, M.N.U.; Tran, K.T.M.; Pham, M.N.; Tran, P.C.; Vo, T.V. Amorphous Isradipine Nanosuspension by the Sonoprecipitation Method. Int. J. Pharm. 2014, 474, 146–150. [Google Scholar] [CrossRef]
- De Paz, E.; Martín, Á.; Estrella, A.; Rodríguez-Rojo, S.; Matias, A.A.; Duarte, C.M.; Cocero, M.J. Formulation of β-carotene by precipitation from pressurized ethyl acetate-on-water emulsions for application as natural colorant. Food Hydrocoll. 2012, 26, 17–27. [Google Scholar] [CrossRef]
- Lai, F.; Franceschini, I.; Corrias, F.; Sala, M.C.; Cilurzo, F.; Sinico, C.; Pini, E. Maltodextrin Fast Dissolving Films for Quercetin Nanocrystal Delivery. A Feasibility Study. Carbohydr. Polym. 2015, 121, 217–223. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Huang, Q. Quercetin Nanosuspensions Produced by High-Pressure Homogenization. J. Agric. Food Chem. 2014, 62, 1852–1859. [Google Scholar] [CrossRef]
- Campardelli, R.; Reverchon, E. α-Tocopherol nanosuspensions produced using a supercritical assisted process. J. Food Eng. 2015, 149, 131–136. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, Q.; Gu, N. Preparation of All-Trans Retinoic Acid Nanosuspensions Using a Modified Precipitation Method. Drug Dev. Ind. Pharm. 2006, 32, 857–863. [Google Scholar] [CrossRef]
- Sinha, B.; Müller, R.H.; Möschwitzer, J.P. Precipitation followed by high pressure homogenization as a combinative approach to prepare drug nanocrystals. In Tag der Pharmazie; Abstract V2, Booklet Page 6; FU Berlin: Berlin, Germany, 2012. [Google Scholar]
- Qiao, F.; Zhao, Y.; Mai, Y.; Guo, J.; Dong, L.; Zhang, W.; Yang, J. Isoliquiritigenin Nanosuspension Enhances Cytostatic Effects in A549 Lung Cancer Cells. Planta Med. 2020, 86, 538–547. [Google Scholar] [CrossRef]
- Huang, T.; Wang, Y.; Shen, Y.; Ao, H.; Guo, Y.; Han, M.; Wang, X. Preparation of High Drug-Loading Celastrol Nanosuspensions and Their Anti-Breast Cancer Activities in Vitro and in Vivo. Sci. Rep. 2020, 10, 8851. [Google Scholar] [CrossRef]
- Momenkiaei, F.; Raofie, F. Preparation of Curcuma longa L. Extract Nanoparticles Using Supercritical Solution Expansion. J. Pharm. Sci. 2019, 108, 1581–1589. [Google Scholar] [CrossRef]
- Gera, S.; Talluri, S.; Rangaraj, N.; Sampathi, S. Formulation and Evaluation of Naringenin Nanosuspensions for Bioavailability Enhancement. AAPS PharmSciTech 2017, 18, 3151–3162. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Pooja, D.; Ravuri, H.G.; Gunukula, A.; Kulhari, H.; Sistla, R. Fabrication of Surfactant-Stabilized Nanosuspension of Naringenin to Surpass Its Poor Physiochemical Properties and Low Oral Bioavailability. Phytomedicine 2018, 40, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, S.; Radhakrishnan, A.; Sengodan, T.; Thangavelu, S. Augmented Anticancer Activity of Naringenin-Loaded TPGS Polymeric Nanosuspension for Drug Resistive MCF-7 Human Breast Cancer Cells. Drug Dev. Ind. Pharm. 2018, 44, 1752–1761. [Google Scholar] [CrossRef]
- Han, M.; Li, Z.; Guo, Y.; Zhang, J.; Wang, X. A Nanoparticulate Drug-Delivery System for Glaucocalyxin A: Formulation, Characterization, Increased in Vitro, and Vivo Antitumor Activity. Drug Deliv. 2016, 23, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Das, S.; Ng, K.; Heng, P.W.S. Formulation, Biological and Pharmacokinetic Studies of Sucrose Ester-Stabilized Nanosuspensions of Oleanolic Acid. Pharm. Res. 2011, 28, 2020–2033. [Google Scholar] [CrossRef]
- Gaur, P.K. Nanosuspension of Flavonoid-Rich Fraction from Psidium Guajava Linn for Improved Type 2-Diabetes Potential. J. Drug Deliv. Sci. Technol. 2021, 62, 102358. [Google Scholar] [CrossRef]
- Ali, T.; Hussain, F.; Naeem, M.; Khan, A.; Al-Harrasi, A. Nanotechnology Approach for Exploring the Enhanced Bioactivities and Biochemical Characterization of Freshly Prepared Nigella sativa L. Nanosuspensions and Their Phytochemical Profile. Front. Bioeng. Biotechnol. 2022, 10, 888177. [Google Scholar] [CrossRef]
- Sindhoor, S.M.; Naveen, N.R.; Rao, G.S.N.K.; Gopan, G.; Chopra, H.; Park, M.N.; Alshahrani, M.M.; Jose, J.; Emran, T.B.; Kim, B. A Spotlight on Alkaloid Nanoformulations for the Treatment of Lung Cancer. Front. Oncol. 2022, 12, 994155. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A Review on Polymer and Lipid-Based Nanocarriers and Its Application to Nano-Pharmaceutical and Food-Based Systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef]
- Chime, S.A.; Kenechukwu, F.C.; Attama, A.A. Nanoemulsions–Advances in Formulation, Characterization and Applications in Drug Delivery; IntechOpen: London, UK, 2014; ISBN 9789535116288. [Google Scholar]
- Odriozola-Serrano, I.; Oms-Oliu, G.; Martã n-Belloso, O. Nanoemulsion-Based Delivery Systems to Improve Functionality of Lipophilic Components. Front. Nutr. 2014, 1, 24. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.R.; Ho, M.J.; Choi, Y.W.; Kang, M.J. A Polyvinylpyrrolidone-Based Supersaturable Self-Emulsifying Drug Delivery System for Enhanced Dissolution of Cyclosporine A. Polymers 2017, 9, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Beg, S.; Khurana, R.K.; Sandhu, P.S.; Kaur, R.; Katare, O.P. Recent advances in self-emulsifying drug delivery systems (SEDDS). Crit. Rev. Ther. Drug Carr. Syst. 2014, 31, 121–185. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Montañez, G.; Ragazzo-Sánchez, J.A.; Picart-Palmade, L.; Calderón-Santoyo, M.; Chevalier-Lucia, D. Optimization of Nanoemulsions Processed by High-Pressure Homogenization to Protect a Bioactive Extract of Jackfruit (Artocarpus heterophyllus Lam). Innov. Food Sci. Emerg. Technol. 2017, 40, 35–41. [Google Scholar] [CrossRef]
- Shariffa, Y.N.; Tan, T.B.; Uthumporn, U.; Abas, F.; Mirhosseini, H.; Nehdi, I.A.; Wang, Y.-H.; Tan, C.P. Producing a Lycopene Nanodispersion: Formulation Development and the Effects of High Pressure Homogenization. Food Res. Int. 2017, 101, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Tabilo-Munizaga, G.; Villalobos-Carvajal, R.; Herrera-Lavados, C.; Moreno-Osorio, L.; Jarpa-Parra, M.; Pérez-Won, M. Physicochemical Properties of High-Pressure Treated Lentil Protein-Based Nanoemulsions. LWT 2019, 101, 590–598. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, Y.; Bai, L.; Liu, F.; Deng, Y.; McClements, D.J. Fabrication of β-Carotene Nanoemulsion-Based Delivery Systems Using Dual-Channel Microfluidization: Physical and Chemical Stability. J. Colloid Interface Sci. 2017, 490, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef] [Green Version]
- Gahruie, H.H.; Ziaee, E.; Eskandari, M.H.; Hosseini, S.M.H. Characterization of Basil Seed Gum-Based Edible Films Incorporated with Zataria Multiflora Essential Oil Nanoemulsion. Carbohydr. Polym. 2017, 166, 93–103. [Google Scholar] [CrossRef]
- Kumar, R.; Kaur, K.; Uppal, S.; Mehta, S.K. Ultrasound Processed Nanoemulsion: A Comparative Approach between Resveratrol and Resveratrol Cyclodextrin Inclusion Complex to Study Its Binding Interactions, Antioxidant Activity and UV Light Stability. Ultrason. Sonochem. 2017, 37, 478–489. [Google Scholar] [CrossRef]
- Chuesiang, P.; Siripatrawan, U.; Sanguandeekul, R.; Mcclements, D.J.; McLandsborough, L. Antimicrobial Activity of PIT-Fabricated Cinnamon Oil Nanoemulsions: Effect of Surfactant Concentration on Morphology of Foodborne Pathogens. Food Control 2019, 98, 405–411. [Google Scholar] [CrossRef]
- García-Melero, J.; López-Mitjavila, J.-J.; García-Celma, M.J.; Rodríguez-Abreu, C.; Grijalvo, S. Rosmarinic Acid-Loaded Polymeric Nanoparticles Prepared by Low-Energy Nano-Emulsion Templating: Formulation, Biophysical Characterization, and In Vitro Studies. Materials 2022, 15, 4572. [Google Scholar] [CrossRef]
- Liew, S.N.; Utra, U.; Alias, A.K.; Tan, T.B.; Tan, C.P.; Yussof, N.S. Physical, Morphological and Antibacterial Properties of Lime Essential Oil Nanoemulsions Prepared via Spontaneous Emulsification Method. LWT 2020, 128, 109388. [Google Scholar] [CrossRef]
- Kumar, A.; Dhiman, A.; Suhag, R.; Sehrawat, R.; Upadhyay, A.; McClements, D.J. Comprehensive review on potential applications of microfluidization in food processing. Food Sci. Biotechnol. 2022, 31, 17–36. [Google Scholar] [CrossRef]
- Ozturk, O.K.; Turasan, H. Latest developments in the applications of microfluidization to modify the structure of macromolecules leading to improved physicochemical and functional properties. Crit. Rev. Food Sci. Nutr. 2021, 62, 4481–4503. [Google Scholar] [CrossRef]
- Dhiman, A.; Prabhakar, P.K. Micronization in food processing: A comprehensive review of mechanistic approach, physicochemical, functional properties and self-stability of micronized food materials. J. Food Eng. 2021, 292, 110248. [Google Scholar] [CrossRef]
- Pratap-Singh, A.; Guo, Y.; Lara Ochoa, S.; Fathordoobady, F.; Singh, A. Optimal ultrasonication process time remains constant for a specific nanoemulsion size reduction system. Sci. Rep. 2021, 11, 9241. [Google Scholar] [CrossRef]
- Perazzo, A.; Preziosi, V.; Guido, S. Phase inversion emulsification: Current understanding and applications. Adv. Colloid Interface Sci. 2015, 222, 581–599. [Google Scholar] [CrossRef]
- Miller, C.A. Spontaneous Emulsification: Recent Developments with Emphasis on Self-Emulsification. In Emulsions and Emulsion Stability: Surfactant Science Series/61, 2nd ed.; Sjoblom, J., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 107–126. [Google Scholar] [CrossRef]
- Solans, C.; Morales, D.; Homs, M. Spontaneous Emulsification. Curr. Opin. Colloid Interface Sci. 2016, 22, 88–93. [Google Scholar] [CrossRef]
- Guha, I.F.; Anand, S.; Varanasi, K.K. Creating Nanoscale Emulsions Using Condensation. Nat. Commun. 2017, 8, 1371. [Google Scholar] [CrossRef] [Green Version]
- Pickering Emulsions. Available online: https://en.wikipedia.org/wiki/Pickering_emulsion (accessed on 6 April 2023).
- Kang, D.J.; Bararnia, H.; Anand, S. Synthesizing Pickering Nanoemulsions by Vapor Condensation. ACS Appl. Mater. Interfaces 2018, 10, 21746–21754. [Google Scholar] [CrossRef]
- Simion, V.; Stan, D.; Constantinescu, C.A.; Deleanu, M.; Dragan, E.; Tucureanu, M.M.; Gan, A.M.; Butoi, E.; Constantin, A.; Manduteanu, I.; et al. Conjugation of curcumin-loaded lipid nanoemulsions with cell-penetrating peptides increases their cellular uptake and enhances the anti-inflammatory effects in endothelial cells. J. Pharm. Pharmacol. 2016, 68, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Nazari-Vanani, R.; Moezi, L.; Heli, H. In Vivo Evaluation of a Self-Nanoemulsifying Drug Delivery System for Curcumin. Biomed. Pharmacother. 2017, 88, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Sugasini, D.; Lokesh, B.R. Curcumin and Linseed Oil Co-Delivered in Phospholipid Nanoemulsions Enhances the Levels of Docosahexaenoic Acid in Serum and Tissue Lipids of Rats. Prostaglandins Leukot. Essent. Fat. Acids 2017, 119, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gouda, W.; Hafiz, N.A.; Mageed, L.; Alazzouni, A.S.; Khalil, W.K.; Afify, M.; Abdelmaksoud, M.D. Effects of nano-curcumin on gene expression of insulin and insulin receptor. Bull. Natl. Res. Cent. 2019, 43, 128. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wang, J.; Zhang, C.; Bao, Z.; Wu, L. Curcumin Nanoemulsions Inhibit Oral Squamous Cell Carcinoma Cell Proliferation by PI3K/Akt/MTOR Suppression and MiR-199a Upregulation: A Preliminary Study. Oral Dis. 2022. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, G.; Xia, Q.; Sun, R. Development and characterization of a self-double-emulsifying drug delivery system containing both epigallocatechin-3-gallate and α-lipoic acid. J. Mater. Sci. 2015, 50, 6567–6577. [Google Scholar] [CrossRef]
- Koutelidakis, A.E.; Argyri, K.; Sevastou, Z.; Lamprinaki, D.; Panagopoulou, E.; Paximada, E.; Sali, A.; Papalazarou, V.; Mallouchos, A.; Evageliou, V.; et al. Bioactivity of Epigallocatechin Gallate Nanoemulsions Evaluated in Mice Model. J. Med. Food 2017, 20, 923–931. [Google Scholar] [CrossRef]
- Chow, P.Y.; Gue, S.Z.; Leow, S.K.; Goh, L.B. Solid self-microemulsifying system (S-SMECS) for enhanced bioavailability and pigmentation of highly lipophilic bioactive carotenoid. Powder Technol. 2015, 274, 199–204. [Google Scholar] [CrossRef]
- Yoo, J.H.; Shanmugam, S.; Thapa, P.; Lee, E.-S.; Balakrishnan, P.; Baskaran, R.; Yoon, S.-K.; Choi, H.-G.; Yong, C.S.; Yoo, B.K.; et al. Novel self-nanoemulsifying drug delivery system for enhanced solubility and dissolution of lutein. Arch. Pharm. Res. 2010, 33, 417–426. [Google Scholar] [CrossRef]
- Shanmugam, S.; Baskaran, R.; Balakrishnan, P.; Thapa, P.; Yong, C.S.; Yoo, B.K. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) containing phosphatidylcholine for enhanced bioavailability of highly lipophilic bioactive carotenoid lutein. Eur. J. Pharm. Biopharm. 2011, 79, 250–257. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, J.; Xiao, H.; Mcclements, D. Nanoemulsion-Based Delivery Systems for Poorly Water-Soluble Bioactive Compounds: Influence of Formulation Parameters on Polymethoxyflavone Crystallization. Food Hydrocoll. 2012, 272, 517–528. [Google Scholar] [CrossRef] [Green Version]
- Qian, C.; Decker, E.; Xiao, H.; McClements, D.J. Nanoemulsion delivery systems: Influence of carrier oil on β-carotene bioaccessibility. Food Chem. 2012, 135, 1440–1447. [Google Scholar] [CrossRef]
- Ha, T.V.A.; Kim, S.; Choi, Y.; Kwak, H.-S.; Lee, S.J.; Wen, J.; Oey, I.; Ko, S. Antioxidant activity and bioaccessibility of size-different nanoemulsions for lycopene-enriched tomato extract. Food Chem. 2015, 178, 115–121. [Google Scholar] [CrossRef]
- Tran, T.H.; Guo, Y.; Song, D.; Bruno, R.; Lu, X. Quercetin-Containing Self-Nanoemulsifying Drug Delivery System for Improving Oral Bioavailability. J. Pharm. Sci. 2014, 103, 840–852. [Google Scholar] [CrossRef]
- Fuior, E.V.; Deleanu, M.; Constantinescu, C.A.; Rebleanu, D.; Voicu, G.; Simionescu, M.; Calin, M. Functional Role of VCAM-1 Targeted Flavonoid-Loaded Lipid Nanoemulsions in Reducing Endothelium Inflammation. Pharmaceutics 2019, 11, 391. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Xiang, C.; Wang, P.; Yin, Y.; Hou, Y. Biocompatible nanoemulsions based on hemp oil and less surfactants for oral delivery of baicalein with enhanced bioavailability. Int. J. Nanomed. 2017, 12, 2923–2931. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Chen, X.; Zhao, G.; Tang, T.; Dong, W.; Wang, C.; Zhang, J.; Liao, Z. Preparation, Characterization, and Pharmacokinetic Evaluation of Imperatorin Lipid Microspheres and Their Effect on the Proliferation of MDA-MB-231 Cells. Pharmaceutics 2018, 10, 236. [Google Scholar] [CrossRef] [Green Version]
- Setiawan, S.D.; Ramadhani, C.C.; Veronika, A.; Ningrum, A.D.K.; Nugroho, B.H.; Syukri, Y. Study of Self Nano-Emulsifying Drug Delivery System (SNEDDS) Loaded Red Fruit Oil (Pandanus conoideus Lamk.) As an Eliminated Cancer Cell MCF-7. J. Drug Deliv. 2018, 8, 229–232. [Google Scholar]
- Jufri, M.; Iswandana, R.; Wardani, D.A.; Malik, S.F. Formulation of red fruit oil nanoemulsion using sucrose palmitate. Int. J. Appl. Pharm. 2022, 14, 175–180. [Google Scholar] [CrossRef]
- Kalantari, A.; Kósa, D.; Nemes, D.; Ujhelyi, Z.; Fehér, P.; Vecsernyés, M.; Váradi, J.; Fenyvesi, F.; Kuki, Á.; Gonda, S.; et al. Self-Nanoemulsifying Drug Delivery Systems Containing Plantago lanceolata—An Assessment of Their Antioxidant and Antiinflammatory Effects. Molecules 2017, 22, 1773. [Google Scholar] [CrossRef] [Green Version]
- Prihapsara, F.; Harini, M.; Widiyani, T.; Artanti, A.N.; Ani, I.L. Antidiabetic Activity of Self Nanoemulsifying Drug Delivery System from Bay Leaves (Eugenia polyantha Wight) Ethyl Acetate Fraction. IOP Conf. Ser. Mater. Sci. Eng. 2017, 176, 012004. [Google Scholar] [CrossRef]
- Qian, J.; Meng, H.; Xin, L.; Xia, M.; Shen, H.; Li, G.; Xie, Y. Self-Nanoemulsifying Drug Delivery Systems of Myricetin: Formulation Development, Characterization, and in Vitro and in Vivo Evaluation. Colloids Surf. B Biointerfaces 2017, 160, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Man, N.; Wang, Q.; Li, H.; Adu-Frimpong, M.; Sun, C.; Zhang, K.; Yang, Q.; Wei, Q.; Ji, H.; Toreniyazov, E.; et al. Improved oral bioavailability of myricitrin by liquid self-microemulsifying drug delivery systems. J. Drug Deliv. Sci. Technol. 2019, 52, 597–606. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Naqvi, A.A.; Alam, M.A.; Rub, R.A.; Ahmad, F.J. Enhancement of Quercetin Oral Bioavailability by Self-Nanoemulsifying Drug Delivery System and Their Quantification Through Ultra High Performance Liquid Chromatography and Mass Spectrometry in Cerebral Ischemia. Drug Res. 2017, 67, 564–575. [Google Scholar] [CrossRef]
- Jakab, G.; Fülöp, V.; Bozó, T.; Balogh, E.; Kellermayer, M.; Antal, I. Optimization of Quality Attributes and Atomic Force Microscopy Imaging of Reconstituted Nanodroplets in Baicalin Loaded Self-Nanoemulsifying Formulations. Pharmaceutics 2018, 10, 275. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Bao, Y. Nanodelivery of Natural Isothiocyanates as a Cancer Therapeutic. Free Radic. Biol. Med. 2021, 167, 125–140. [Google Scholar] [CrossRef]
- Encinas-Basurto, D.; Ibarra, J.; Juárez, J.; Burboa, M.G.; Barbosa, S.; Taboada, P.; Troncoso-Rojas, R.; Valdez, M.A. Poly(Lactic-Co-Glycolic Acid) Nanoparticles for Sustained Release of Allyl Isothiocyanate: Characterization, in Vitro Release and Biological Activity. J. Microencapsul. 2017, 34, 231–242. [Google Scholar] [CrossRef]
- Encinas-Basurto, D.; Juarez, J.; Valdez, A.M.; Burboa, G.M.; Barbosa, S.; Taboada, P. Targeted Drug Delivery via Human Epidermal Growth Factor Receptor for Sustained Release of Allyl Isothiocyanate. Curr. Top. Med. Chem. 2018, 18, 1252–1260. [Google Scholar] [CrossRef]
- Kumar, R.; Kaur, K.; Pandey, S.K.; Kumar, R.; Uppal, S.; Mehta, S.K. Fabrication of Benzylisothiocynate Encapsulated Nanoemulsion through Ultrasonication: Augmentation of Anticancer and Antimicrobial Attributes. J. Mol. Liq. 2018, 263, 324–333. [Google Scholar] [CrossRef]
- Uppal, S.; Aashima; Kumar, R.; Sareen, S.; Kaur, K.; Mehta, S.K. Biofabrication of Cerium Oxide Nanoparticles Using Emulsification for an Efficient Delivery of Benzyl Isothiocyanate. Appl. Surf. Sci. 2020, 510, 145011. [Google Scholar] [CrossRef]
- Uppal, S.; Sharma, P.; Kumar, R.; Kaur, K.; Bhatia, A.; Mehta, S.K. Effect of Benzyl Isothiocyanate Encapsulated Biocompatible Nanoemulsion Prepared via Ultrasonication on Microbial Strains and Breast Cancer Cell Line MDA MB 231. Colloids Surf. A Physicochem. Eng. Asp. 2020, 596, 124732. [Google Scholar] [CrossRef]
- Maccelli, A.; Vitanza, L.; Imbriano, A.; Fraschetti, C.; Filippi, A.; Goldoni, P.; Maurizi, L.; Ammendolia, M.G.; Crestoni, M.E.; Fornarini, S.; et al. Satureja montana L. Essential Oils: Chemical Profiles/Phytochemical Screening, Antimicrobial Activity and O/W NanoEmulsion Formulations. Pharmaceutics 2020, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, F.; Maurizi, L.; Conte, A.L.; Marazzato, M.; Maccelli, A.; Crestoni, M.E.; Hanieh, P.N.; Forte, J.; Conte, M.P.; Zagaglia, C.; et al. Nanoemulsions of Satureja montana Essential Oil: Antimicrobial and Antibiofilm Activity against Avian Escherichia coli Strains. Pharmaceutics 2021, 13, 134. [Google Scholar] [CrossRef]
- Yen, C.C.; Chang, C.W.; Hsu, M.C.; Wu, Y.T. Self-nanoemulsifying drug delivery system for resveratrol: Enhanced oral bioavailability and reduced physical fatigue in rats. Int. J. Mol. Sci. 2017, 18, 1853. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.F.; Zhou, J.; Hu, X.; Cong, Z.Q.; Liu, C.Y.; Pan, R.L.; Chang, Q.; Liu, X.M.; Liao, Y.H. Improving oral bioavailability of resveratrol by a udp-glucuronosyltransferase inhibitory excipient-based self-microemulsion. Eur. J. Pharm. Sci. 2018, 114, 303–309. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. A new alternative insight of nanoemulsion conjugated with k-carrageenan for wound healing study in diabetic mice: In vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2019, 133, 236–250. [Google Scholar] [CrossRef]
- Nirmala, M.J.; Durai, L.; Gopakumar, V.; Nagarajan, R. Anticancer and antibacterial effects of a clove bud essential oil-based nanoscale emulsion system. Int. J. Nanomed. 2019, 14, 6439–6450. [Google Scholar] [CrossRef] [Green Version]
- Abadi, A.V.M.; Karimi, E.; Oskoueian, E.; Mohammad, G.R.K.S.; Shafaei, N. Chemical investigation and screening of anti-cancer potential of Syzygium aromaticum L. bud (clove) essential oil nanoemulsion. 3 Biotech 2022, 12, 49. [Google Scholar] [CrossRef]
- Colombo, M.; Melchiades, G.L.; Figueiró, F.; Battastini, A.M.O.; Teixeira, H.F.; Koester, L.S. Validation of an HPLC-UV method for analysis of Kaempferol-loaded nanoemulsion and its application to in vitro and in vivo tests. J. Pharm. Biomed. Anal. 2017, 145, 831–837. [Google Scholar] [CrossRef]
- Colombo, M.; Figueiró, F.; de Fraga Dias, A.; Teixeira, H.F.; Battastini, A.M.O.; Koester, L.S. Kaempferol-Loaded Mucoadhesive Nanoemulsion for Intranasal Administration Reduces Glioma Growth in Vitro. Int. J. Pharm. 2018, 543, 214–223. [Google Scholar] [CrossRef]
- Chen, J.; Li, F.; Li, Z.; McClements, D.J.; Xiao, H. Encapsulation of Carotenoids in Emulsion-Based Delivery Systems: Enhancement of β-Carotene Water-Dispersibility and Chemical Stability. Food Hydrocoll. 2017, 69, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, A.K.; de Carvalho Gomes, C.; de Araújo Amaral, M.L.; de Medeiros, L.D.; Medeiros, I.; Porto, D.L.; Aragão, C.F.; Maciel, B.L.; de Araújo Morais, A.H.; Passos, T.S. Nanoencapsulation Improved Water Solubility and Color Stability of Carotenoids Extracted from Cantaloupe Melon (Cucumis melo L.). Food Chem. 2019, 270, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Alarcón, C.; Inostroza-Riquelme, M.; Torres-Gallegos, C.; Araya, C.; Miranda, M.; Sánchez-Caamaño, J.C.; Moreno-Villoslada, I.; Oyarzun-Ampuero, F.A. Protection of Astaxanthin from Photodegradation by Its Inclusion in Hierarchically Assembled Nano and Microstructures with Potential as Food. Food Hydrocoll. 2018, 83, 36–44. [Google Scholar] [CrossRef]
- Silva, H.D.; Poejo, J.; Pinheiro, A.C.; Donsì, F.; Serra, A.T.; Duarte, C.M.M.; Ferrari, G.; Cerqueira, M.A.; Vicente, A.A. Evaluating the Behaviour of Curcumin Nanoemulsions and Multilayer Nanoemulsions during Dynamic in Vitro Digestion. J. Funct. Foods 2018, 48, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Artiga-Artigas, M.; Lanjari-Pérez, Y.; Martín-Belloso, O. Curcumin-Loaded Nanoemulsions Stability as Affected by the Nature and Concentration of Surfactant. Food Chem. 2018, 266, 466–474. [Google Scholar] [CrossRef]
- Abdou, E.S.; Galhoum, G.F.; Mohamed, E.N. Curcumin Loaded Nanoemulsions/Pectin Coatings for Refrigerated Chicken Fillets. Food Hydrocoll. 2018, 83, 445–453. [Google Scholar] [CrossRef]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and Antioxidant Efficiency of Nanoemulsion-Based Edible Coating Containing Ginger (Zingiber officinale) Essential Oil and Its Effect on Safety and Quality Attributes of Chicken Breast Fillets. Food Control 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Akbas, E.; Soyler, B.; Oztop, M.H. Formation of Capsaicin Loaded Nanoemulsions with High Pressure Homogenization and Ultrasonication. LWT 2018, 96, 266–273. [Google Scholar] [CrossRef]
- Advancing the Science of Nanotechnology in Drug Development. Available online: https://www.fda.gov/drugs/news-events-human-drugs/advancing-science-nanotechnology-drug-development (accessed on 28 April 2023).
- Drug Products, Including Biological Products, That Contain Nanomaterials. Available online: https://www.fda.gov/media/109910/download (accessed on 6 April 2023).
- D’Mello, S.R.; Cruz, C.N.; Chen, M.L.; Kapoor, M.; Lee, S.L.; Tyner, K.M. The evolving landscape of drug products containing nanomaterials in the United States. Nat. Nanotechnol. 2017, 12, 523–529. [Google Scholar] [CrossRef]
- Cruz, C.N.; Tyner, K.M.; Velazquez, L.; Hyams, K.C.; Jacobs, A.; Shaw, A.B.; Jiang, W.; Lionberger, R.; Hinderling, P.; Kong, Y.; et al. CDER risk assessment exercise to evaluate potential risks from the use of nanomaterials in drug products. AAPS J. 2013, 15, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Tyner, K.M.; Zou, P.; Yang, X.; Zhang, H.; Cruz, C.N.; Lee, S.L. Product quality for nanomaterials: Current U.S. experience and perspective. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 640–654. [Google Scholar] [CrossRef]
- Chen, M.L.; John, M.; Lee, S.L.; Tyner, K.M. Development Considerations for Nanocrystal Drug Products. AAPS J. 2017, 19, 642–651. [Google Scholar] [CrossRef]
- Kapoor, M.; Lee, S.L.; Tyner, K.M. Liposomal Drug Product Development and Quality: Current US Experience and Perspective. AAPS J. 2017, 19, 632–641. [Google Scholar] [CrossRef]
- Zou, P.; Tyner, K.; Raw, A.; Lee, S. Physicochemical Characterization of Iron Carbohydrate Colloid Drug Products. AAPS J. 2017, 19, 1359–1376. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2021, 22, 385. [Google Scholar] [CrossRef]
- Toragall, V.; Jayapala, N.; Muthukumar, S.P.; Vallikanan, B. Biodegradable Chitosan-Sodium Alginate-Oleic Acid Nanocarrier Promotes Bioavailability and Target Delivery of Lutein in Rat Model with No Toxicity. Food Chem. 2020, 330, 127195. [Google Scholar] [CrossRef]
- Missaoui, W.N.; Arnold, R.D.; Cummings, B.S. Toxicological status of nanoparticles: What we know and what we don’t know. Chem. Biol. Interact. 2018, 295, 1–12. [Google Scholar] [CrossRef]
- Freyre-Fonseca, V.; Delgado-Buenrostro, N.L.; Gutiérrez-Cirlos, E.B.; Calderón-Torres, C.M.; Cabellos-Avelar, T.; Sánchez-Pérez, Y.; Pinzón, E.; Torres, I.; Molina-Jijón, E.; Zazueta, C.; et al. Titanium Dioxide Nanoparticles Impair Lung Mitochondrial Function. Toxicol. Lett. 2011, 202, 111–119. [Google Scholar] [CrossRef]
- Yu, Y.; Duan, J.; Yu, Y.; Li, Y.; Sun, Z. Silica nanoparticle-induced blockage of autophagy leads to autophagic cell death in HepG2 cells. J. Biomed. Nanotechnol. 2017, 13, 485–499. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Zhang, C.; Cui, X.; Zhai, S.; Liu, Y.; Li, C.; Zhu, H.; Qu, G.; Jiang, G.; et al. Tuning Cell Autophagy by Diversifying Carbon Nanotube Surface Chemistry. ACS Nano 2014, 8, 2087–2099. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Wang, L.; Shao, P.; Sun, P.; Yang, C.S. A Review on Chemical and Physical Modifications of Phytosterols and Their Influence on Bioavailability and Safety. Crit. Rev. Food Sci. Nutr. 2022, 62, 5638–5657. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.T.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and Microparticles for Skin Drug Delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Mohammed, Y.; Pastore, M.N.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.N.; Abd, E.; Leite-Silva, V.R.; Benson, H.A.E.; et al. Topical and Cutaneous Delivery Using Nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Din, F.U.; Choi, J.Y.; Kim, D.W.; Mustapha, O.; Kim, D.S.; Thapa, R.K.; Ku, S.K.; Youn, Y.S.; Oh, K.T.; Yong, C.S.; et al. Irinotecan-Encapsulated Double-Reverse Thermosensitive Nanocarrier System for Rectal Administration. Drug Deliv. 2017, 24, 502–510. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, K.; Aoki, H.; Teruyama, C.; Iijima, M.; Tsutsumi, H.; Kuroda, S.; Hamano, K. A Novel Hybrid Drug Delivery System for Treatment of Aortic Aneurysms. Int. J. Mol. Sci. 2020, 21, 5538. [Google Scholar] [CrossRef]
- Sim, T.; Han, S.M.; Lim, C.; Won, W.R.; Lee, E.S.; Youn, Y.S.; Oh, K.T. A PH-Sensitive Polymer for Cancer Targeting Prepared by One-Step Modulation of Functional Side Groups. Macromol. Res. 2019, 27, 795–802. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, M.; Liu, K. Colon-Targeted Drug Delivery of Polysaccharide-Based Nanocarriers for Synergistic Treatment of Inflammatory Bowel Disease: A Review. Carbohydr. Polym. 2021, 272, 118530. [Google Scholar] [CrossRef]
- Sim, T.; Lim, C.; Hoang, N.H.; Kim, J.E.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Synergistic Photodynamic Therapeutic Effect of Indole-3-Acetic Acid Using a PH Sensitive Nano-Carrier Based on Poly(Aspartic Acid-Graft-Imidazole)-Poly(Ethylene Glycol). J. Mater. Chem. B 2017, 5, 8498–8505. [Google Scholar] [CrossRef]
- Manjili, H.K.; Ghasemi, P.; Malvandi, H.; Mousavi, M.S.; Attari, E.; Danafar, H. Pharmacokinetics and in vivo delivery of curcumin by copolymeric mPEG-PCL micelles. Eur. J. Pharm. Biopharm. 2017, 116, 17–30. [Google Scholar] [CrossRef]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]
- Sayes, C.M.; Gobin, A.M.; Ausman, K.D.; Mendez, J.; West, J.L.; Colvin, V.L. Nano-C60 cytotoxicity is due to lipid peroxidation. Biomaterials 2005, 26, 7587–7595. [Google Scholar] [CrossRef]
- Long, T.C.; Saleh, N.; Tilton, R.D.; Lowry, G.V.; Veronesi, B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): Implications for nanoparticle neurotoxicity. Environ. Sci. Technol. 2006, 40, 4346–4352. [Google Scholar] [CrossRef]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.; Seaton, A.; Stone, V.; Brown, S.; Macnee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef]
- Kane, A.B.; Hurt, R.H. Nanotoxicology: The asbestos analogy revisited. Nat. Nanotechnol. 2008, 3, 378–379. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, Y.; Bai, R.; Wu, Z.; Qian, W.; Yang, L.; Cai, R.; Yan, H.; Li, T.; Pandey, V.; et al. Long-term pulmonary exposure to multi-walled carbon nanotubes promotes breast cancer metastatic cascades. Nat. Nanotechnol. 2019, 14, 719–727. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Dobrovolskaia, M.A.; Cheney, D.L.; Greish, K.F.; Ghandehari, H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2019, 144, 112–132. [Google Scholar] [CrossRef]
- Yousef, M.I.; Mutar, T.F.; Kamel, M.A.E. Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicol. Rep. 2019, 6, 336–346. [Google Scholar] [CrossRef]
- Pacheco, A.; Martins, A.; Guilhermino, L. Toxicological interactions induced by chronic exposure to gold nanoparticles and microplastics mixtures in Daphnia magna. Sci. Total Environ. 2018, 628–629, 474–483. [Google Scholar] [CrossRef]
- Li, J.; Schiavo, S.; Xiangli, D.; Rametta, G.; Miglietta, M.L.; Oliviero, M.; Changwen, W.; Manzo, S. Early ecotoxic effects of ZnO nanoparticle chronic exposure in Mytilus galloprovincialis revealed by transcription of apoptosis and antioxidant-related genes. Ecotoxicology 2018, 27, 369–384. [Google Scholar] [CrossRef]
- Oberdörster, G.; Ferin, J.; Lehnert, B.E. Correlation between particle size, in vivo particle persistence, and lung injury. Environ. Health Perspect. 1994, 102 (Suppl. 5), 173–179. [Google Scholar] [PubMed] [Green Version]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Liong, M.; Madler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadrieh, N.; Wokovich, A.M.; Gopee, N.V.; Zheng, J.; Haines, D.; Parmiter, D.; Siitonen, P.H.; Cozart, C.R.; Patri, A.K.; McNeil, S.E.; et al. Lack of Significant Dermal Penetration of Titanium Dioxide from Sunscreen Formulations Containing Nano- and Submicron-Size TiO2 Particles. Toxicol. Sci. 2010, 115, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Bevacqua, E.; Occhiuzzi, M.A.; Grande, F.; Tucci, P. TiO2-NPs toxicity and safety: An update of the findings published over the last six years. Mini Rev. Med. Chem. 2022; epub ahead of print. [Google Scholar] [CrossRef]
- Boutillier, S.; Fourmentin, S.; Laperche, B. History of titanium dioxide regulation as a food additive: A review. Environ. Chem. Lett. 2022, 20, 1017–1033. [Google Scholar] [CrossRef]
- European Medicine Agency (EMA). Available online: https://www.ema.europa.eu/en/documents/report/final-feedback-european-medicine-agency-ema-eu-commission-request-evaluate-impact-removal-titanium_en.pdf (accessed on 6 April 2023).
- McClements, D.J. Emulsion design to improve the delivery of functional lipophilic components. Annu. Rev. Food Sci. Technol. 2010, 1, 241–269. [Google Scholar] [CrossRef]
- Maier, M.A.; Jayaraman, M.; Matsuda, S.; Liu, J.; Barros, S.; Querbes, W.; Tam, Y.K.; Ansell, S.M.; Kumar, V.; Qin, J.; et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 2013, 21, 1570–1578. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xia, T.; Duch, M.C.; Ji, Z.; Zhang, H.; Li, R.; Sun, B.; Lin, S.; Meng, H.; Liao, Y.P.; et al. Pluronic F108 coating decreases the lung fibrosis potential of multiwall carbon nanotubes by reducing lysosomal injury. Nano Lett. 2012, 12, 3050–3061. [Google Scholar] [CrossRef]
- Mutlu, G.M.; Budinger, G.R.; Green, A.A.; Urich, D.; Soberanes, S.; Chiarella, S.E.; Alheid, G.F.; McCrimmon, D.R.; Szleifer, I.; Hersam, M.C. Biocompatible nanoscale dispersion of single-walled carbon nanotubes minimizes in vivo pulmonary toxicity. Nano Lett. 2010, 10, 1664–1670. [Google Scholar] [CrossRef] [Green Version]
- Del Rosso, T.; Louro, S.R.W.; Deepak, F.L.; Romani, E.C.; Zaman, Q.; Tahir; Pandoli, O.; Cremona, M.; Freire Junior, F.L.; De Beule, P.A.A.; et al. Biocompatible Au@Carbynoid/Pluronic-F127 Nanocomposites Synthesized by Pulsed Laser Ablation Assisted CO2 Recycling. Appl. Surf. Sci. 2018, 441, 347–355. [Google Scholar] [CrossRef]
- Yuan, Y.; Ding, J.; Xu, J.; Deng, J.; Guo, J. TiO2 nanoparticles co-doped with silver and nitrogen for antibacterial application. J. Nanosci. Nanotechnol. 2010, 10, 4868–4874. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Madler, L. Flame spray pyrolysis: An enabling technology for nanoparticles design and fabrication. Nanoscale 2010, 2, 1324–1347. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Ji, Z.; Sun, B.; Zhang, H.; Chang, C.H.; Lin, S.; Meng, H.; Liao, Y.P.; Wang, M.; et al. Surface charge and cellular processing of covalently functionalized multiwall carbon nanotubes determine pulmonary toxicity. ACS Nano 2013, 7, 2352–2368. [Google Scholar] [CrossRef] [Green Version]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug. Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Thomas, R.; Park, I.K.; Jeong, Y.Y. Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer. Int. J. Mol. Sci. 2013, 14, 15910–15930. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Banobre-Lopez, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Health Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef]
- Schubert, J.; Chanana, M. Coating Matters: Review on Colloidal Stability of Nanoparticles with Biocompatible Coatings in Biological Media, Living Cells and Organisms. Curr. Med. Chem. 2018, 25, 4553–4586. [Google Scholar] [CrossRef]
- Wang, X.; Xia, T.; Ntim, S.A.; Ji, Z.; Lin, S.; Meng, H.; Chung, C.H.; George, S.; Zhang, H.; Wang, M.; et al. Dispersal state of multiwalled carbon nanotubes elicits profibrogenic cellular responses that correlate with fibrogenesis biomarkers and fibrosis in the murine lung. ACS Nano 2011, 5, 9772–9787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlenoff, J.B. Zwitteration: Coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef] [PubMed]
- Pombo García, K.; Zarschler, K.; Barbaro, L.; Barreto, J.A.; O’Malley, W.; Spiccia, L.; Stephan, H.; Graham, B. Zwitterionic-coated “stealth” nanoparticles for biomedical applications: Recent advances in countering biomolecular corona formation and uptake by the mononuclear phagocyte system. Small 2014, 10, 2516–2529. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.R.; Wu, S.T.; Tsai, Z.T.; Wang, J.J.; Yen, T.C.; Tsai, J.S.; Shih, M.F.; Liu, C.L. Characterization of quaternized chitosantem. ionic-coated “stealth” nanoparticles forel potential magnetic resonance imaging contrast agent for cell tracking. Polym. Int. 2011, 60, 945–950. [Google Scholar] [CrossRef]
- Tang, S.; Tang, Y.; Zhong, L.; Murat, K.; Asan, G.; Yu, J.; Jian, R.; Wang, C.; Zhou, P. Short- and long-term toxicities of multi-walled carbon nanotubes in vivo and in vitro. J. Appl. Toxicol. 2012, 32, 900–912. [Google Scholar] [CrossRef]
- Boyles, M.S.; Young, L.; Brown, D.M.; MacCalman, L.; Cowie, H.; Moisala, A.; Smail, F.; Smith, P.J.; Proudfoot, L.; Windle, A.H.; et al. Multi-walled carbon nanotube induced frustrated phagocytosis, cytotoxicity and pro-inflammatory conditions in macrophages are length dependent and greater than that of asbestos. Toxicol. Vitr. 2015, 29, 1513–1528. [Google Scholar] [CrossRef]
- Rao, G.T.; Babu, B.; Stella, R.J.; Manjari, V.P.; Ravikumar, R.V. Spectral investigations on undoped and Cu(2)(+) doped ZnO-CdS composite nanopowders. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 139, 86–93. [Google Scholar]
- Adeleye, A.S.; Pokhrel, S.; Madler, L.; Keller, A.A. Influence of nanoparticle doping on the colloidal stability and toxicity of copper oxide nanoparticles in synthetic and natural waters. Water Res. 2018, 132, 12–22. [Google Scholar] [CrossRef]
- Ahmad, J.; Siddiqui, M.A.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A.; Khan, S.T.; Wahab, R.; Al-Khedhairy, A.A.; Al-Salim, A.; Musarrat, J.; et al. Copper doping enhanced the oxidative stress-mediated cytotoxicity of TiO2 nanoparticles in A549 cells. Hum. Exp. Toxicol. 2018, 37, 496–507. [Google Scholar] [CrossRef]
- George, S.; Pokhrel, S.; Xia, T.; Gilbert, B.; Ji, Z.; Schowalter, M.; Rosenauer, A.; Damoiseaux, R.; Bradley, K.A.; Madler, L.; et al. Use of a rapid cytotoxicity screening approach to engineer a safer zinc oxide nanoparticle through iron doping. ACS Nano 2010, 4, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Xia, T.; Zhao, Y.; Sager, T.; George, S.; Pokhrel, S.; Li, N.; Schoenfeld, D.; Meng, H.; Lin, S.; Wang, X.; et al. Decreased dissolution of ZnO by iron doping yields nanoparticles with reduced toxicity in the rodent lung and zebrafish embryos. ACS Nano 2011, 5, 1223–1235. [Google Scholar] [CrossRef] [Green Version]
- More, M.P.; Pardeshi, S.R.; Pardeshi, C.V.; Sonawane, G.A.; Shinde, M.N.; Deshmukh, P.K.; Naik, J.B.; Kulkarni, A.D. Recent Advances in Phytochemical-Based Nano-Formulation for Drug-Resistant Cancer. Med. Drug Discov. 2021, 10, 100082. [Google Scholar] [CrossRef]

















| Edible Plants | Essential Oils | Benefits | |
|---|---|---|---|
| Brightly Colored | Not Brightly Colored | ||
| Berries | Potatoes Almonds Pecans Pistachios Cauliflower Walnuts Cashews Hazelnuts Tea Dark chocolate Cacao beans Barley Beans Lentils Rice Coffee Mung beans Soybeans Cloves Cinnamon Cumin Nutmeg | Pine needles Cedar Lavender | Boost the immune system [29] Combat OS and FR [30] ⇓ Blood sugar levels [30,31] ⇓ Blood pressure [29] ⇓ Diabetes risk [30,32] ⇓ Serious health issues [30,32] Prevent chronic disease [29,30] Protect from pathogens [33,34,35] Protect brain and liver [29] ⇓ Cholesterol [29] ⇓ Inflammation [30,36,37] Support detoxification [30,38] Ward off osteoporosis [29] |
| Cranberries | |||
| Blackberries | |||
| Strawberries | |||
| Cherries | |||
| Currants | |||
| Grapes | |||
| Plums | |||
| Purple potatoes | |||
| Red Cabbage | |||
| Cabbage | |||
| Kohlrabi | |||
| Broccoli sprouts | |||
| Apples | |||
| Bananas | |||
| Peaches | |||
| Antiparasitic herbs | |||
| Egg yolks | |||
| Orange peppers | |||
| Oranges | |||
| Pumpkins | |||
| Yellow corn | |||
| Kale | |||
| Parsley | |||
| Romain lettuce | |||
| Spinach | |||
| Olive oil | |||
| Melons | |||
| PHYs | Sources | Health Benefits |
|---|---|---|
| Carotenoids [41,42] | Carrots, tomatoes, parsley Orange and green leafy vegetables Chenopods, fenugreek Spinach, cabbage, radish, turnips | Act as antioxidants Protect against uterine, prostate, colorectal, lung digestive tract cancers |
| Phytosterols [42,43] | Vegetables, nuts, fruits, seeds | Suppress the growth of diverse tumor cell lines |
| Limonoids [42] | Citrus fruits | Inhibit phase I enzymes and induce phase II detoxification enzymes in liver Provide protection to lung tissue, detoxify enzymes |
| Curcuminoids [42] | Turmeric, curry powder, mango, ginger | Analgesic, anti-inflammatory, anticancer, antioxidative Anti-depressive Protective against hay fever and depression, ⇓ cholesterol and itching risk |
| Indole compounds [42] (indole-3-carbinol) | Cabbage, cauliflower, broccoli, kale Brussels sprouts | Strong antioxidant, DNA protector, chemo-preventive, anticancer ⇑ Heart health |
| Alkaloids [44] | Plants (also animals and bacteria) | Antimalarial, antiasthma, anticancer, cholinomimetics Vasodilatory, antiarrhythmic Analgesic, antibacterial, antihyperglycemic, psychotropic, stimulant |
| Phytoprostanes [45] Phytofurans [45] | Almonds, vegetal oils, olives, algae Passion fruit, nut kernels, rice | Immunomodulators, anti-inflammatory, antitumor |
| Polyphenols [42] | Fruits, vegetables, cereals, beverages, legumes Chocolates, oilseeds | Action against free radicals, anti-inflammatory, anti-allergenic Inhibition of platelet aggregation, protective against hepatotoxins |
| Flavonoids * [42] | Fruits, vegetables, cereals, beverages, legumes Chocolates, oilseeds | Action against free radicals, anti-inflammatory, anti-allergenic Inhibition of platelet aggregation, protective against hepatotoxins |
| Iso-flavonoids ** [42] | Fruits, vegetables, cereals, beverages, legumes Chocolates, oilseeds | Action against free radicals, anti-inflammatory, anti-allergenic Inhibition of platelet aggregation, protective against hepatotoxins |
| Anthocyanidins ** [42,46,47,48] Anthocyanins ** [42,46,47,48] | Fruits, vegetables, cereals, beverages, legumes Chocolates, oilseeds | Act against free radicals, anti-inflammatory, anti-allergenic Inhibit platelet aggregation, protect against hepatotoxins Help control weight, prevent heart disease Increase insulin sensitivity Reduce inflammation, decrease diabetic complications, protect DNA Protect the brain Boost other PHYs and phytonutrients |
| Glucosinolates [42] | Cruciferous vegetables | Protection against cancer of colon, rectum, stomach |
| Phytoestrogens [42] | Legumes, berries, whole grains, cereals Red wine, peanuts, red grapes | Protection against bone loss, heart disease, cardiovascular diseases Protection against breast and uterine cancers |
| Terpenoids [42] Isoprenoids [42] | Mosses, liverworts, algae, lichens, mushrooms | Antimicrobial, antiparasitic, antiviral, antiallergic, anti-inflammatory Chemotherapeutic, antihyperglycemic, antispasmodic |
| Fibers [49] | Fruits and vegetables (green leafy), oats | ⇓ Blood cholesterol, ⇓ cardiovascular disease |
| Polysaccharides [42] | Fruits and vegetables | Antimicrobial, antiparasitic, antiviral, antiallergic, anti-inflammatory ⇓ Serum, ⇑ defense mechanisms |
| Saponins [42] | Oats, leaves, flowers, green fruits of tomato | Protection against pathogens, antimicrobial, anti-inflammatory Antiulcer agent |
| Tannins [42] | Cranberries, currants, blackberries, apples, grapes, peaches Strawberries, almonds Hazelnuts, pecans, pistachios, walnuts, barley beans, lentils, rice Tea, cacao beans Dark chocolate, antiparasitic herbs | Act as antioxidants, fight pathogens, ⇓ blood pressure, ⇓ inflammation ⇓ Serious health risks Regulate the immune system |
| Lutein [42] Zeaxanthin [42] | Egg yolks, orange peppers, oranges, pumpkins, yellow corn, kale Parsley, romaine lettuce, spinach, pistachios, olive oil | Protect retina from damage, ⇑ eye function ⇑ Memory and brain function Promote the body’s use of insulin, ⇑ skin health, ⇓ blood pressure ⇓ Inflammation Support heart health |
| Eugenol [50] | Cloves, cinnamon, cumin, nutmeg, coffee Mung beans, soybeans, bananas, melons Strawberries, tomatoes | Acts as anti-inflammatory and antioxidant, eliminates parasites, fights fungi Inhibits serious health concerns, protects the brain and the liver, ⇓ Bacterial biofilm Supports heart and stomach health |
| Color Group | Foods | PHYs | Properties | Refs. |
|---|---|---|---|---|
| Green | Asparagus, avocados, celery, cucumbers Green beans, green peppers, kale, kiwi Spinach, zucchini | EGCG, glucosinolates Indoles, isoflavones Isothiocyanates, lutein, and zeaxanthin Sulforaphane | Promote wound healing and healthy gums Support arteries, blood cells, eyes, liver, and lungs | [18,66] |
| Purple | Black beans, blackberries Eggplants, elderberries, plums Purple cabbage, purple grapes, raisins | Anthocyanins, flavonoids, phenols Tannins, RES | Protect against serious health issues Support arteries, bones, brain, cognition, healthy aging, and heart | [18,66] |
| Red | Cherries, cranberries, kidney beans Red beans, strawberries, tomatoes Watermelon | Anthocyanins, ellagic acid, eugenol Hesperidin Lycopene, tannins, quercetin | Protect against heart disease and other serious health issues Support prostate, urinary tract, and DNA health | [18,66] |
| Yellow | Apricots, cantaloupe, carrots, grapefruit Yellow pears, yellow peppers Yellow winter squash | α-Carotene, β-carotene, β-cryptoxanthin, Lutein, zeaxanthin, hesperidin | Boost the immune system, support heart and vision health | [18,66] |
| White | Apples, cauliflower Great northern beans Mushrooms, onions | Allicin, ECGC, glucosinolates, indoles Tannins, quercetin | Protect against heart disease and other serious health issues Support arteries, bones, and circulation | [18] |
| Methods | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Emulsion solvent extraction Double-emulsion solvent extraction | Low cost | ⇑ Residual solvent ⇓ Encapsulation efficiency (EE) Thermal degradation Multi-step processes Micronization step needed | [17] |
| SD | Improved bioavailability Taste masking Modified release achievable Aseptic manufacturing Fine powders Improved stability | High overhead Possible thermal degradation Not for every compound Not widely available Multi-step processes Micronization step needed | [17] |
| Liquid antisolvent technique | Low cost | ⇑ Residual solvent ⇓ EE Thermal degradation Multi-step processes Micronization step needed | [17] |
| Spray freeze-drying (SFD) * | Higher rate of freezing Independent control over particle size | ⇓ Biological activity Possible protein denaturation Excipients required | [17] |
| Spray freezing into liquid (SFL) * | Higher level of biological activity High degree of atomization Ultra-rapid freezing (URF) Formation of highly porous amorphous NPs | Possible ⇑ viscosity of the feed liquid Limited applications ⇑ Cost of equipment Time- and energy-intensive | [17] |
| Thin-film freezing (TFF) ** | High-yield products Flexibility for processable drugs Large-scale production Simple, efficient, robust process ⇑ Stability of the protein product | ⇑ Cost of equipment | [17] |
| Supercritical fluid extraction (SFE) | Single-step process Controllable particle size Controllable morphology Controllable crystallinity Monitorable residual solvent | ⇑ Cost of equipment | [17] |
| Solvent evaporation technique | Low cost | ⇑ Residual solvent ⇓ EE Thermal degradation Multi-step processes Micronization step needed | [17] |
| Barrier | Region | Event | Factors | Drug Loss | Processes | Refs. |
|---|---|---|---|---|---|---|
| Biochemical | Stomach | Enzymatic degradation pH degradation ⇓ Interaction of drug with epithelial cells | HCl Pepsin * Mucus Lipases | 94–98% | Deamidation Oxidation Hydrolysis | [80,81] |
| Small intestine | Enzymatic degradation | Trypsins Chymotrypsins Carboxypeptidases Elastases | ||||
| Colon | Long residence time (20 h) | ⇓ Concentrations of digestive enzymes Neutral pH (6–6.7) ⇓ Fluid volumes/drug | N.A. | N.R. | [82] | |
| Mucosal | Small intestine | ⇓ Interaction of drug with epithelial cells ⇓ Penetration of drugs ⇓ Absorption | Two mucus ** layers with viscoelastic and hydrogel-like structure | N.R. | ⇓ Transport ⇓ Absorption | [81,83] |
| Microbial degradation | Gut Microbiota | N.R. | ⇓ Transport ⇓ Absorption Degradation | [84] | ||
| Cellular | Intestinal epithelium *** | Governs the absorption of drugs | Epithelial 1 TJs 2 M-cells 3 Vitamin B12 4 Hydrogen-coupled peptide transporters 4 Transferring receptors 5 IgG neonatal receptors 5 ABC | N.R. | ⇓ Transport ⇓ Absorption Higher efflux | [81,85,86] |
| Immunosuppression | Activated T-cell antibody- secreting plasma cells Macrophages Dendritic cells (DCs) | N.R. | Elimination | [84] | ||
| Enzymatic degradation | Cytochrome P450 P-glycoprotein | N.R. | Oxidation | [79] |
| Nanosuspension Techniques | ||
|---|---|---|
| Conventional Techniques | Combined Techniques (CTNIs) | |
| Bottom-up (B-U) | Top-down (T-D) | Nanoedge™ Technique (Baxter Healthcare, Baxter Manufacturing, Firenze, Italy) H 69 Technology (SmartCrystal® technology group, Pharmasol, Kaiserslautern, Germany) H 42 Technology (SmartCrystal® technology, Pharmasol, Kaiserslautern, Germany) H 96 Technology (SmartCrystal®, Abbott/Soliqs, Ludwigshafen, Germany) Combination Technology (CTNO) (Eurofins CDMO Alphora Inc., Mississauga, ON, Canada) |
| PHY | NPs/Mechanical Method (MM)/ Combined Techniques (CTNIs) | Particle Size | Characteristics |
|---|---|---|---|
| β-Carotene [109] (B-U) | n-Octenyl succinate-modified starch (NPs) | 300–600 nm sized particles | ⇑ Dispersibility ⇑ Coloring strength ⇑ Bioavailability |
| Quercetin [110] (B-U) | Maltodextrin (NPs) | 753 nm sized particles | Water re-dispersible ⇑ RSA ⇑ ORAC |
| Quercetin [111] (T-D) | HPH (MM) + spray drying (MM) | 400 nm sized particles | ⇑ Antioxidant ⇑ Anti-inflammatory ⇑ Anticancer properties ⇑ Water solubility ⇑ Oral bioavailability |
| α-Tocopherol [112] (B-U) | Supercritical assisted process | 150 nm sized particles | ⇑ Solubility ⇑ Bioavailability |
| All-trans retinoic acid (ATRA) [113] | Nanoedge™ Technique | 155 nm sized particles | Orally administrable 30′ Operation time |
| Resveratrol (RES) [114] | H 69 Technology | 150 nm sized NPs | Orally administrable 10 cycles HPH/1200 bar |
| RES [17] | H 42 Technology | 200 nm sized NPs | Orally administrable 1 HPH cycle at 1500 bar |
| Hesperidin [17] * | CTNO | 599 nm sized NPs | ⇑ Solubility Long-term stability Orally administrable Topically applicable Five homogenization cycles (1000 bar) |
| Rutin [17] ** | CTNO | 600 nm sized NPs | ⇑ Solubility Orally administrable Topically applicable 1 Cycle of HPH at 100 bar |
| Apigenin [17] | CTNO | 275 nm sized NPs | 1 Cycle HPH at 300 bar |
| Isoliquiritigenin [115] T-D | Wet media milling (MM) | 238 nm sized NPs (HPC-SSL) 354 nm sized NPs (PVP-K30) | ⇑ Solubility ⇑ Cytotoxicity ⇑ Cellular uptake ⇑ Apoptosis induction ⇓ Toxicity |
| Celastrol [116] B-U | Antisolvent precipitation method | 148 nm sized NPs | Stable in plasma Orally administrable ⇑ EE% ⇑ DL% ⇑ Solubility (vitro) ⇑ Cytotoxicity (vivo) ⇑ Cumulative release (48 h) |
| Curcuma longa L. extracts [117] B-U | Supercritical fluid expansion | 47 nm sized NPs | ⇑ Solubility |
| Naringenin (NRG) [118] B-U | Antisolvent sonoprecipitation | 117 nm sized NPs | ⇑ Absorption in GIT ⇑ Dissolution ⇑ Oral bioavailability |
| NRG [119] B-U | Precipitation–ultrasonication | 118 nm sized NPs | ⇑ Drug dissolution ⇑ Pharmacokinetic profile ⇑ Stability |
| NRG [120] B-U | HPH | 81 nm sized NPs | ⇑ Intracellular ROS level ⇑ Mitochondrial membrane potential ⇑ Caspase-3 activity ⇑ Lipid peroxidation status ⇓ GSH levels ⇑ Antitumor activity on DLA cells ⇑ Life span ⇓ Cancer cell number ⇓ Tumor weight |
| Glaucocalyxin A [121] B-U | Precipitation–ultrasonication | 143 nm sized NPs | ⇑ In vitro antitumor activity ⇑ In vivo anticancer efficacy |
| Oleanolic acid (OA) [122] B-U | Organic solvent evaporation | 100 nm sized NPs | ⇑ Stability ⇑ Saturation solubility ⇑ Dissolution rate ⇑ Cytotoxicity ⇑ Bio-efficacy ⇑ Bioavailability |
| P. guajava L. extracts [123] B-U | Nanoprecipitation | 241–327 nm sized NPs | ⇑ Antihyperglycemic activity ⇑ Physical parameters ⇑ Hepatic parameters ⇑ Renal parameters ⇑ Absorption ⇓ Metabolism ⇑ Stability |
| Nigella sativa L. [124] B-U | Nanoprecipitation | N.R. | ⇑ Total phenolic content ⇑ Total flavonoid contents ⇑ Antioxidant activity ⇑ Antidiabetic activity ⇑ Antibiofilm activity ⇑ Bioavailability |
| Type of NEs | Oily Phase | Advantages | Drawbacks | Ref. | |
|---|---|---|---|---|---|
| NEs o/w or w/o (100–500 nm) | Captex 355 Captex 8000 Witepsol Myritol 318 Isopropyl myristate Capryol 90 Sefsol-218 Triacetin Isopropyl myristate Castor oil Olive oil | PHY protection Controlled release Sustained release ⇑ DL% | No high-melting PHYs 5–10% additives * required | [1] | |
| SEDDSs o/w ** | ⇑ Oral bioavailability Possibility of easy scale-up ⇑ DL% Allow delivery of peptides/lipids without the risk of digestion | 5–10% additives * required For low-therapeutic-dose PHYs Many parameters The physicochemical properties of PHYs can influence the efficiency of oral absorption and the performances of SEDDSs | [1] | ||
| SNEDDSs ** (<50 nm) | SMEDDSs ** (100–200 nm) | ||||
| SDEDDSs ** (w/o/w) or (o/w/oil) | |||||
| Technique | Method | PHYs | Activity | Particle Size (nm) | Ref. |
|---|---|---|---|---|---|
| HET | High-pressure homogenization (HPH) | Jackfruit pulp extract * rich in carotenoids | Antioxidant | 166 | [131] |
| Lycopene | Radical scavenging activity | 92.6 | [132] | ||
| Lentil | ⇓ Blood pressure ⇓ Bad cholesterol (LDL) ⇑ Good cholesterol (HDL) ⇓ Heart disease risk | 149 | [133] | ||
| Microfluidization (MF) | β-Carotene | Antioxidant | 140–160 | [134,135] | |
| Ultrasonication (US) | Essential oils (EOs) (Zataria multiflora) | Antibacterial | 91–211 | [136] | |
| RES | ⇓ Diseases by oxidative stress | 20.4 **; 24.5 *** | [137] | ||
| LET | Phase inversion temperature (PIT) | Cinnamon EO | Antioxidant Antimicrobial | 101 | [138] |
| Phase inversion composition (PIC) | Rosmarinic acid (RA) | Antiviral Anti-inflammatory Antioxidant | 70–100 | [139] | |
| Spontaneous emulsification (SE) | Key lime (Citrus aurantifolia) EO | Antibacterial | 21 | [140] | |
| Kaffir lime (Citrus hystrix) EO | 28 | ||||
| Calamansi lime (Citrofortunella microcarpa) EO | 60 |
| PHY | Emulsion Type/Method | Additives | Results | Ref. |
|---|---|---|---|---|
| Curcumin | NE | Tween 20 | ⇓ Toxicity ⇑ Bioavailability ⇑ Bioactivity ⇑ Anti-inflammatory | [151] |
| o/w SNEDDS Mild agitation | Tween 80 PEG 600 | ⇑ Oral bioavailability ⇑ C max | [152] | |
| NE/HPH | PEG (3%) | ⇑ Oral bioavailability ⇓ DHA levels | [153] | |
| Emulsion–diffusion evaporation | N.R. | ⇓ Blood glucose levels ⇑ Insulin | [154] | |
| Interfacial prepolymer deposition and SE | Lipoid 100 | Inhibition of OSCC cells ⇓ PI3K/Akt/mTOR ⇑ miR-199a | [155] | |
| EGCG (E) + ALA (A) | SDEDDS | PGPR S721, P10, L23, and S40 | ⇑Photo-stability Antioxidant ⇑ EE | [156] |
| EGCG | NE (o/w) | BC WPI | No toxicity ⇑ Antioxidant | [157] |
| Carotenoids (Paprika Oleoresin) | SMEDDS | Tween 80 | ⇑ Solubility | [158] |
| Lutein | SMEDDS | Tween 80 Labrasol Transcutol HP/Lutro-E400 1 | ⇑ Solubility ⇑ Bioavailability | [159,160] |
| Polymethoxyflavones (PMFs) | NE | Tween 20 Tween 85 | ⇑ Dissolution rate | [161] |
| β-Carotene | o/w NE | Tween 20 | ⇑ Emulsion stability ⇑ Solubility ⇑ Bio accessibility | [162] |
| Lycopene | Microemulsion (ME) | ESE 3GIO SML | ⇑ Solubility | [163] |
| Quercetin | SNEDDS | Tween 80 PEG 400 | ⇑ Solubility | [164] |
| Naringenin Hesperetin | NE | Glycerin | ⇑ Solubility ⇑ In vitro stability No cytotoxicity (in vitro) No hemolysis (in vivo) Anti-inflammatory | [165] |
| Baicalein | NE/HPH | PEGM Sodium oleate Hoechst 33,258 3,3-DODOXAP | ⇑ Oral bioavailability ⇑ GIT permeability ⇑ Transcellular transport ability ⇓ Cytotoxicity | [166] |
| Imperatorin | NE/HSS NE/HPH | Polaxamer 188 | ⇑ Bioavailability Antiproliferative (MDA-MB-231) | [167] |
| Pandanus conoideus Lamk (red fruit) | SNEDDS | PPG Tween 20 | ⇑ Cytotoxic activity | [168] |
| Pandanus conoideus Lamk (red fruit) | NE/high-speed mixer | PPG SEPIGEL 305™ | Antioxidant | [169] |
| Plantago lanceolata L. | SNEDDS | Labrasol or Kolliphor RH 40 Transcutol HP | ⇑ Solubility ⇑ Permeability ⇑ Bioavailability ⇑ Pharmacological effects | [170] |
| Bay Leaves extracts (Eugenia polyantha Wight) | Tween 80 PEG 400 | [171] | ||
| Myricitrin | Capryol 90 Cremophor RH 40 PEG 400 Cremophor EL Transcutol HP | [172] | ||
| Myricitrin | Cremophor EL35 Dimethyl carbinol | [173] | ||
| Quercetin | PEG 200 Tween 40 Tween 60 Tween 80 PEG 400 Transcutol HP | [174] | ||
| Baicalin | Peceol® (14.3%, w/w) Kolliphor® EL (57.1%, w/w) Transcutol® P (28.6%, w/w) | [175] | ||
| AITC | Emulsion solvent evaporation | Polyvinyl alcohol (PVA) (3%) | ⇓ Degradation ⇓ Volatility ⇑ Shelf life Sustained release ⇑ Toxicity to tumor | [176,177] |
| ⇑⇑ Anticancer activity ⇓ Toxicity | [178] | |||
| BITC | US | Tween 80 Decyl-β-d-glucopyranoside | ⇑ EE% | [179] |
| BITC | US | Tween 80 | ⇑ EE% Good DL% MDA-MB-231 cell inhibition | [180] |
| Heating stirring/sonication | Good long-term stability ⇑⇑⇑ EE Sustained release ⇑ Cytotoxicity (MDA-MB-231) | [181] | ||
| SEO | o/w NEs | Tween 20 Tween 80 | ⇑ Antibacterial effects | [182] |
| o/w NEs Votexed/sonication | Tween 80 | ⇑ Antibacterial effects ⇓ Biofilm formation | [183] | |
| RES | SNEDDS | Capryol 90 Cremophor EL Tween 20 | ⇑ Oral bioavailability ⇑ Anti-fatigue effect | [184] |
| SMEDDS | Labrafil Labrasol RH40 | ⇑ Oral bioavailability | [185] | |
| Astaxanthin + α-tocopherol | κ-Carrageenan o/w NE/SE | Span® 80 (1%) PEG (1%) Tween® 20 (1%) | ⇓ Toxicity ⇓ Hyperglycemia ⇓ Diabetes complications ⇑ Wound healing | [186] |
| κ-Carrageenan o/w NE Ultrasonication | None | |||
| Cloves | SMEDDS | Tween 20 Tween 80 | ⇑ Anticancer effects ⇑ Antibacterial effects | [187] |
| Cloves | US | Tween 20 Tween 80 PEG | Cytoprotective effects ⇑ Anticancer effects ⇓ Toxicity | [188] |
| Kaempferol | HPH | Egg lecithin Tween 80 | ⇑ Permeation Safe Antioxidant capability ⇑ Drug delivery into rat’s brain ⇓ C6 cell viability | [189,190] |
| β-Carotene | o/w NE/US and MF | Casein | ⇑ Water dispersibility ⇑ Chemical stability | [191] |
| o/w NE/HPH | Porcine gelatin | ⇑ Water dispersibility ⇑ Stability ⇑ Dispersibility in foods | [192] | |
| Astaxanthin | o/w NE/SE | Lecithin | ⇑ Stability ⇓ Photodegradation | [193] |
| Curcumin | o/w NE/HPH | SDS | ⇑ Bioavailability ⇑ Antioxidant effects ⇑ Lipids digestion | [194] |
| o/w NE/MF | Lecithin Tween 20 SMP | ⇑ Stability ⇑ Antioxidant effects | [195] | |
| o/w NE/SE | Tween 80 | ⇑ Antimicrobial effects | [196] | |
| Ginger EO | o/w NE/US | Tween 80 | ⇑ Antimicrobial effects ⇑ Antioxidant effects | [197] |
| Capsaicin | o/w NE/HPH+US | Tween 80 | ⇑ Antimicrobial effects ⇑ Physical properties | [198] |
| Post-Administration Event | NNDF | NDF | Result | Advantages | Ref. |
|---|---|---|---|---|---|
| Interaction with specialized immune cells | NO | SI | Easier and faster macrophage-mediated targeting | ⇓ Risk of side effects ⇓ Toxicity to nontarget organs ⇑ Effectiveness | [199] |
| Possible presence of a special coating | NO | SI | Capability to bypass immune cell attack | ||
| SI | Prolonged residence time in bloodstream |
| Formulation | Cargo | Administration Route | Animal Model Disease | Tests | Results | Ref. |
|---|---|---|---|---|---|---|
| SLN | Irinotecan | Rectal | Mice Cancer | Gel properties Pharmacokinetics Morphology Anticancer activity Immunohistopathology | Easily administered to the anus Rapid and strong gelling No damage to the rat rectum No body weight loss | [216] |
| Liposome | SP60015 (JNK inhibitor) Pitavastatin | Intravenous (iliac vein) | Male mice Aneurysm | Binding in vitro Binding in vivo Charging capacity Recharging capacity Drug release | Good drug transport Targeted drug release Repeatable drug release Safe | [217] |
| [P(bAsp-co-APIA)-PEG] | Docetaxel | N.A. | N.A. Cancer RA | DL% EE% Drug release | Biodegradable Biocompatible ⇓ Toxicity pH-sensitive | [218] |
| PS-NPs | Dictyophora indusiata | Gavage | Male mice IBD Colitis | Disease activity index Histological analysis Myeloperoxidase activity Goblet cells Mucous thickness Nitrogen oxide Cytokines Proteins | Effect against colitis Ameliorated intestinal injury ⇓ Oxidative stress ⇓ Proinflammatory cytokine ⇓ Inflammation ⇑ Mucins ⇑ Tight junction proteins (TJs) Restored intestinal microbiome ⇓ Harmful bacterial flora ⇑ Beneficial bacterial flora | [219] |
| [P(Asp-g-Im)-PEG] | Indole-3-acetic acid | Subcutaneous injection | Female nude mice Skin cancer | DL% EE% Morphology Drug release Cell viability Hemolysis Anticancer activity | ⇓ Systemic toxicity (physiologic pH) ⇑ Antitumor efficacy ⇑ Accumulation in cancer cells ⇑ Release in cancer cells | [220] |
| mPEG-PCL micelles | Curcumin | Intravenous injection | Rats */Mice ** Breast cancer | DL% EE% Size Drug release Hemolysis In vivo organ toxicity In vivo anticancer effects | ⇓ Systemic toxicity (physiologic pH) ⇑ Antitumor efficacy ⇑ Accumulation in cancer cells ⇑ Circulation time No mortality No organ toxicity No organ degeneration No necrosis No neutrophils No activation of immune response | [221] |
| Acute Toxicity | |||
|---|---|---|---|
| NP Type | Animal/Cells | Toxicity | Ref. |
| Fe2O3 NPs ** ZnO NPs ** | Human mesothelioma cells Rodent mesothelioma cells | ⇓ Overall cell culture activity ⇓⇓⇓ DNA content | [222] |
| CuO, TiO2 ZnO, CuZnFe2O4 | A549 cells | Cytotoxicity DNA damage ⇑ OS by ROS Oxidative lesions | [223] |
| Nano-C60 fullerene aggregate | Human dermal fibroblasts Human liver carcinoma cells (HepG2) Neuronal human astrocytes | ⇓ Normal cellular function Lipid peroxidation ⇑ ROS Membrane damage | [224] |
| TiO2 NPs | Brain microglia (BV2) | ⇑ ROS Neurotoxicity | [225] |
| Chronic toxicity | |||
| NP Type | Animal/Cells | Toxicity | Ref. |
| CNTs | Mice * | Asbestos-like pathogenicity | [226] |
| [227] | |||
| MWCNTs | Female mice | Breast cancer metastasis | [228] |
| Inorganic NPs | Rodents Non-rodents | Genotoxicity Carcinogenesis Embryotoxicity | [229] |
| Al2O3 NPs ZnO NPs | Rats# | Hepato-renal toxicities ⇓ Hepatic expression of mtTFA and PGC-1α proteins | [230] |
| Au NPs | Daphnia magna | Mortality ⇓ Reproductive development ⇓ Reproductive fitness ⇓ Total eggs and offspring Aborted eggs | [231] |
| ZnO NPs | Mytilus galloprovincialis | ⇓ Transcription of key genes involved in DNA damage/repair, antioxidation, and apoptosis | [232] |
| TiO2 NPs | Rats | Inflammation Lung injury ⇓ Alveolar macrophage function | [233] |
| ZnO NPs ** ZnCl2 NPs ** | P. subcapitata | Cytotoxicity | [234] |
| ZnO NPs | RAW 264.7 cells BEAS-2B cells | Cytotoxicity OS | [235] |
| Strategy | Features | Type of NPs | Results | Ref. |
|---|---|---|---|---|
| Use of next generation lipids * | ⇑ High potency Biodegradability | SLNPs | Rapid elimination from plasma ⇑ Tolerability in preclinical studies ⇑ In vivo potency | [241] |
| Surface coating strategies * | Biocompatibility ⇑ Colloidal stability ⇓ Degradation Faster excretion ⇓ Accumulation Reversible coating Altered dispersion state | Polymeric SLNPs Inorganic NPs | ⇑ Dispersion state ⇓ Agglomeration ⇓ Cellular uptake ⇓ Pro-fibrogenic effects | [242] |
| ⇓ Lung toxicity | [243] | |||
| AuC/PF127 NCs | ⇑ Stability ⇑ Biocompatibility Photodynamic therapeutic | [244] | ||
| Doping | Altered density of surface reactive chemicals ⇓ Binding energy of metal ions to oxygen ⇓ NP dissolution ⇓ Toxic ion release ⇓ ROS generation | Inorganic NPs | ⇓ Dissolution ⇓ Toxicity | [245] |
| ⇓ Dissolution ⇓ Toxicity | [246] | |||
| Surface chemistry properties modifications | Altered charge density Altered hydrophobicity | CNTs | ⇓ Pro-fibrogenic effects ⇓ Uptake in THP-1 and BEAS-2B cells | [247] |
| AuNPs | ⇓ Toxicity ⇓ Uptake in cells | [248] | ||
| Fe2O3 NPs | ⇑ Stability ⇓ Toxicity ⇑ Biocompatibility | [249,250,251,252] |
| Compound | Status * | Source | Activity |
|---|---|---|---|
| Artemisinin | Phase III | Artemisia annua | Anticancer |
| Ursolic acid | Phase II | Fruits (waxes of apples, pears) | Antioxidant |
| Thymoquinone | Phase II | Herbs Spices | Hepatoprotective Antioxidant Anticancer |
| Sulforaphane | Phase II | Brassica vegetables | Anticancer Antioxidant Antimicrobial Anti-inflammatory |
| PEITC | Phase II | Watercress | Anticancer (lung, oral) |
| Not specified ITC | Phase I | Cruciferous vegetables | Bladder cancer |
| Sinomenine | Phase III | Roots of Sinomenium acutum | Anti-inflammatory Anti-rheumatic |
| Silibinin | Phase IV | Milk thistle Coffee | Hepatoprotective Anticancer |
| Catechin | Phase IV | Green tea Beans | Antioxidant |
| Salvianolic acid B | Phase II | Red sage | Antioxidant Angiogenetic |
| RES | Phase IV | Grapes Blueberries Raspberries Mulberries | Antioxidant Anti-inflammatory Cardioprotective Anti-carcinogenic |
| Quercetin | Phase III | Fruits Red onions Kale | Anti-inflammatory Anticancer |
| Paclitaxel | Approved (Taxol®) FDA (1998) | Bark of Pacific yew tree | Mitotic inhibitor in cancer Chemotherapy |
| Genistein | Phase III | Plants (lupins, fava beans, soybeans) | Anticancer Anti-inflammatory |
| Lycopene | Phase IV | Tomato | Antioxidant Anticancer |
| EGCG | Phase III | Green tea White tea Black tea | Antioxidant Chemo-preventive |
| Epicatechin | Phase II | Woody plants | Antioxidant |
| Caffeic acid | Phase III | Coffee Eucalyptus | Anticancer Antioxidant Anti-inflammatory |
| Camptothecin | Phase I | Stem wood of the Chinese tree Camptotheca acuminate | Anticancer |
| Combretastatin | Phase II | Bark of Combretum caffrum | Anticancer |
| Curcumin | Phase IV | Tumeric | Inhibition of tumor cell proliferation Anti-inflammatory |
| Compound | Status * | Formulation Type | Indication |
|---|---|---|---|
| Camptothecin | Phase II | PEG–drug conjugate | Gastric cancer |
| PhaseI/II | Polyglutamic acid–drug conjugate | Colon cancer Ovarian cancer | |
| Phase I/II | Cyclodextrin NP | Solid tumors, renal cell carcinoma, rectal cancer, non-small-cell lung cancer | |
| Phase I | HPMA–drug conjugate | Solid tumors | |
| Phase I | Fleximer–drug conjugate | Gastric cancer Lung cancer | |
| Curcumin | Phase I | Liposome | Advanced cancer |
| Irinotecan | Onivyde® Approved: FDA (2015) | Liposome | Metastatic pancreatic cancer |
| Paclitaxel | Abraxane® Approved: FDA (2005, 2012, 2013) | NPs, albumin-bound | Breast cancer Non-small-cell lung cancer Pancreatic cancer |
| Phase I | Polymeric micelle | Ovarian cancer | |
| Phase I | Polymeric NPs | Peritoneal neoplasms | |
| Phase I/II | Liposome | Ovarian cancer Breast cancer Lung cancer | |
| Phase II | Liposome | Triple-negative breast cancer | |
| Phase II | Liposome | Solid tumors Gastric cancer Metastatic breast cancer | |
| Phase II/III | PEG-PAA polymeric micelle | Gastric cancer Breast cancer | |
| Phase II/III | DHA–drug conjugate | Melanoma Liver cancer Adenocarcinoma Kidney cancer Non-small-cell lung cancer | |
| Phase III | Polyglutamic acid–drug conjugate | Lung cancer Ovarian cancer | |
| Phase III | Polymeric micelle | Advanced breast cancer | |
| Apealea® Approved: EMA (2018) | Micelle | Ovarian cancer Primary peritoneal cancer | |
| Genexol-PM® Approved: marketed in Korea and Europe (2007) | PEG-PLA polymeric micelle | Breast cancer Lung cancer | |
| Phase I | HPMA–drug conjugate | Solid tumor | |
| Vincristine | Marqibo® Approved: FDA (2012) | Liposome | Acute lymphoid leukemia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuccari, G.; Alfei, S. Development of Phytochemical Delivery Systems by Nano-Suspension and Nano-Emulsion Techniques. Int. J. Mol. Sci. 2023, 24, 9824. https://doi.org/10.3390/ijms24129824
Zuccari G, Alfei S. Development of Phytochemical Delivery Systems by Nano-Suspension and Nano-Emulsion Techniques. International Journal of Molecular Sciences. 2023; 24(12):9824. https://doi.org/10.3390/ijms24129824
Chicago/Turabian StyleZuccari, Guendalina, and Silvana Alfei. 2023. "Development of Phytochemical Delivery Systems by Nano-Suspension and Nano-Emulsion Techniques" International Journal of Molecular Sciences 24, no. 12: 9824. https://doi.org/10.3390/ijms24129824
APA StyleZuccari, G., & Alfei, S. (2023). Development of Phytochemical Delivery Systems by Nano-Suspension and Nano-Emulsion Techniques. International Journal of Molecular Sciences, 24(12), 9824. https://doi.org/10.3390/ijms24129824








