Cognitive Impairments, Neuroinflammation and Blood–Brain Barrier Permeability in Mice Exposed to Chronic Sleep Fragmentation during the Daylight Period
Abstract
:1. Introduction
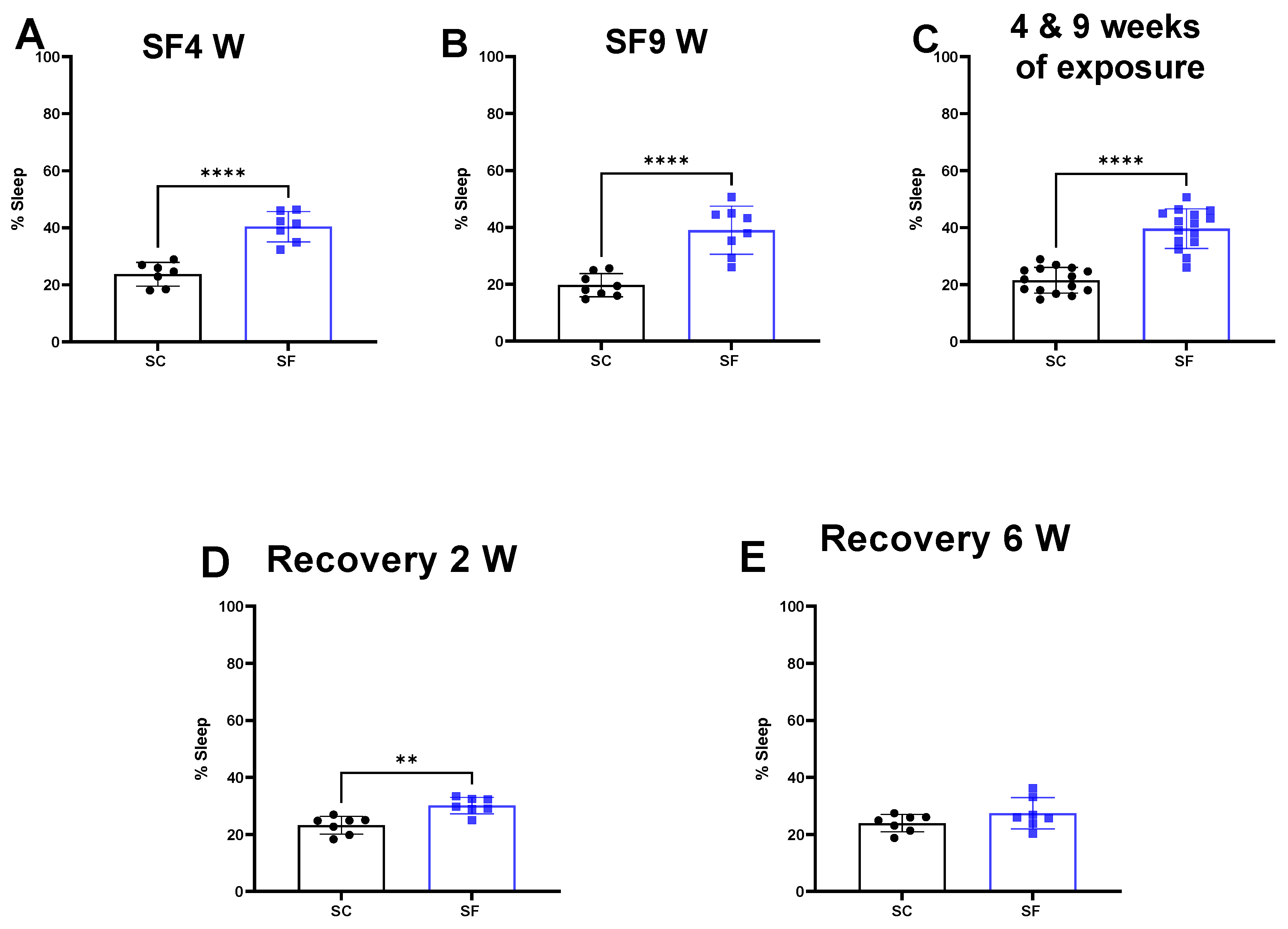
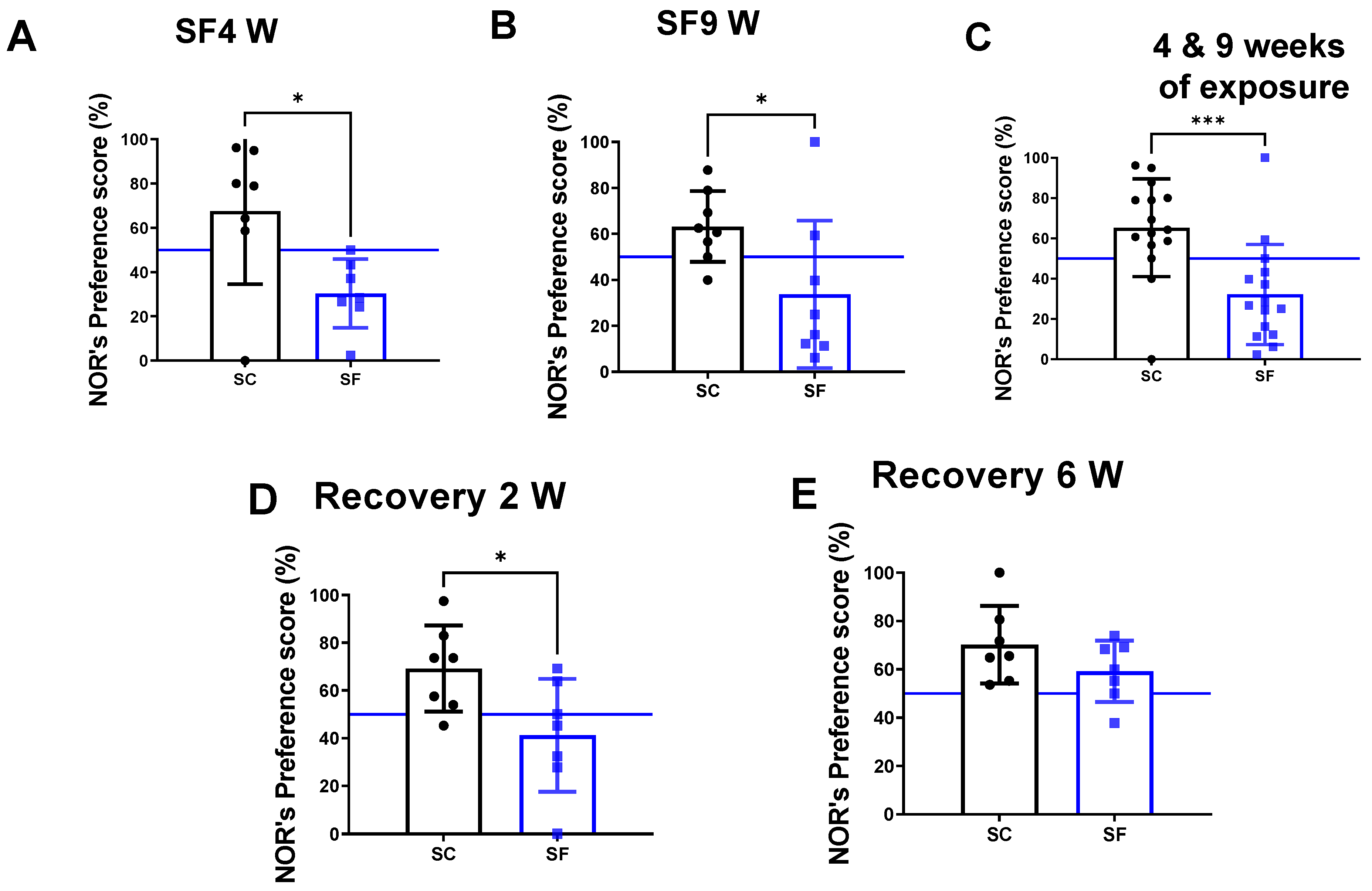
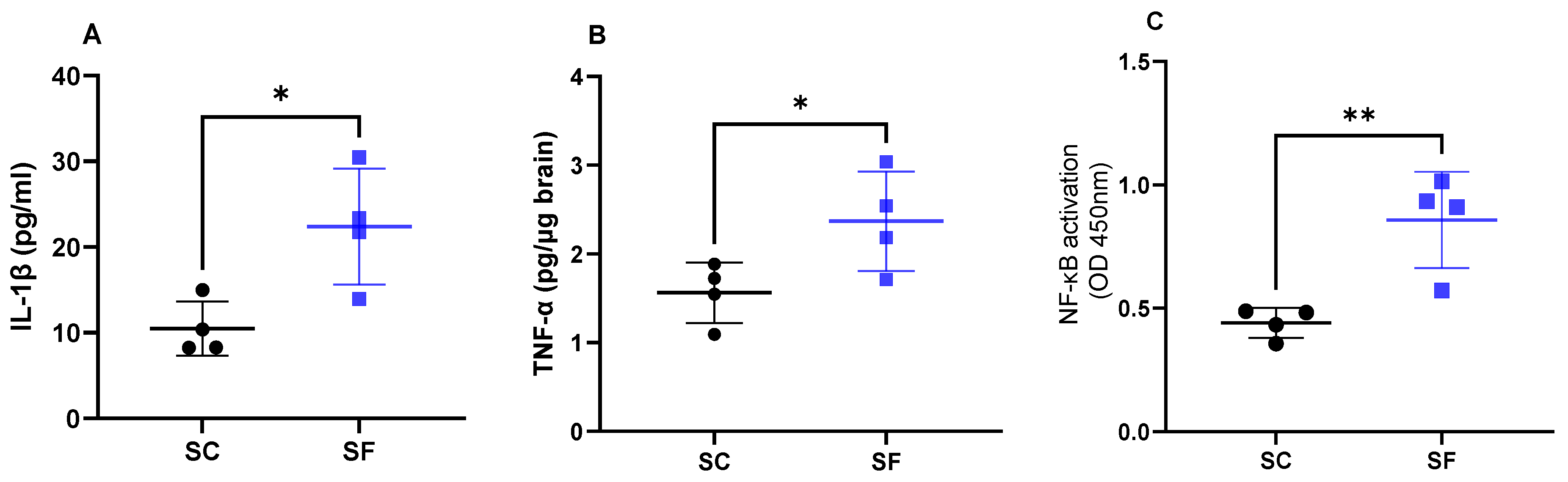
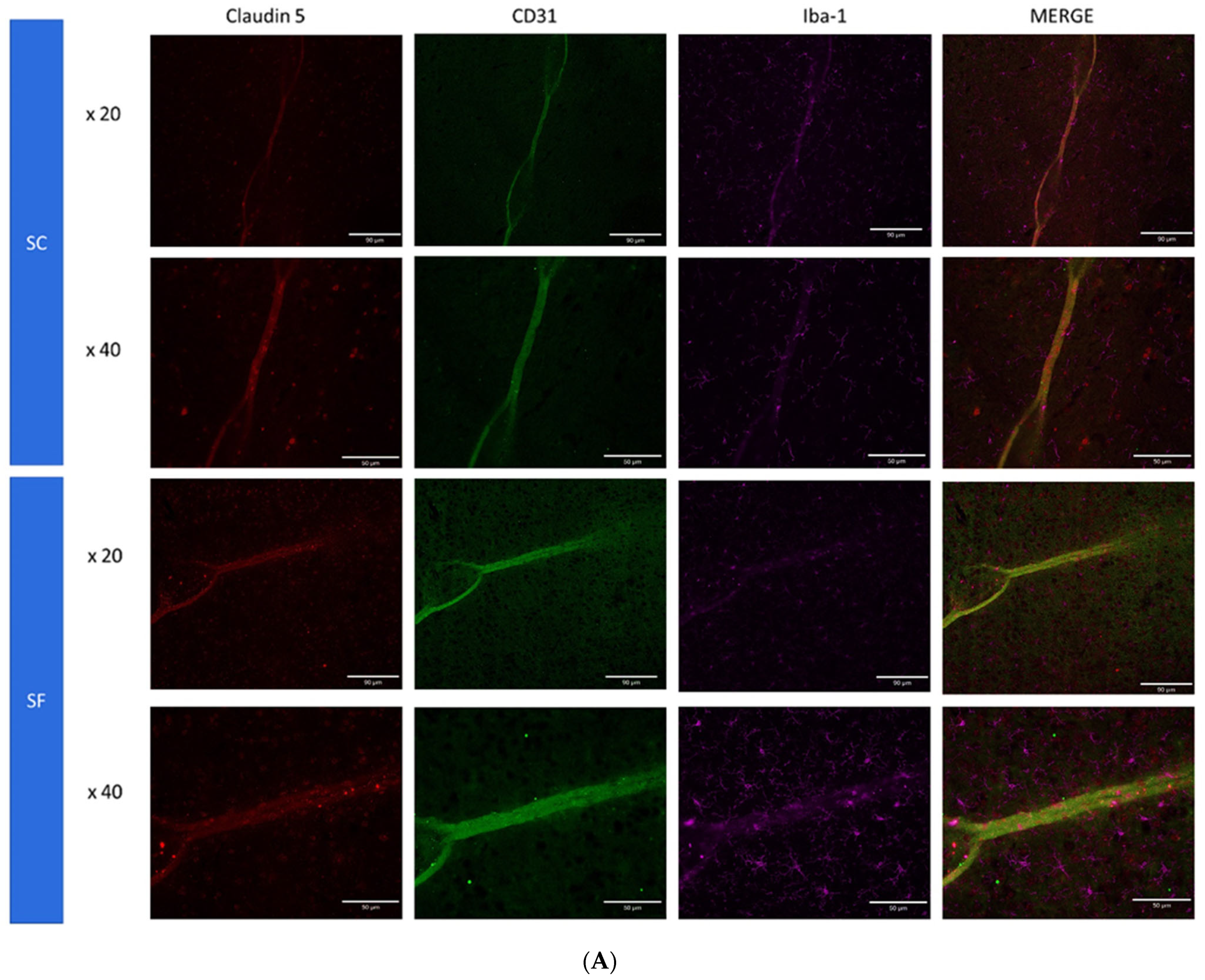
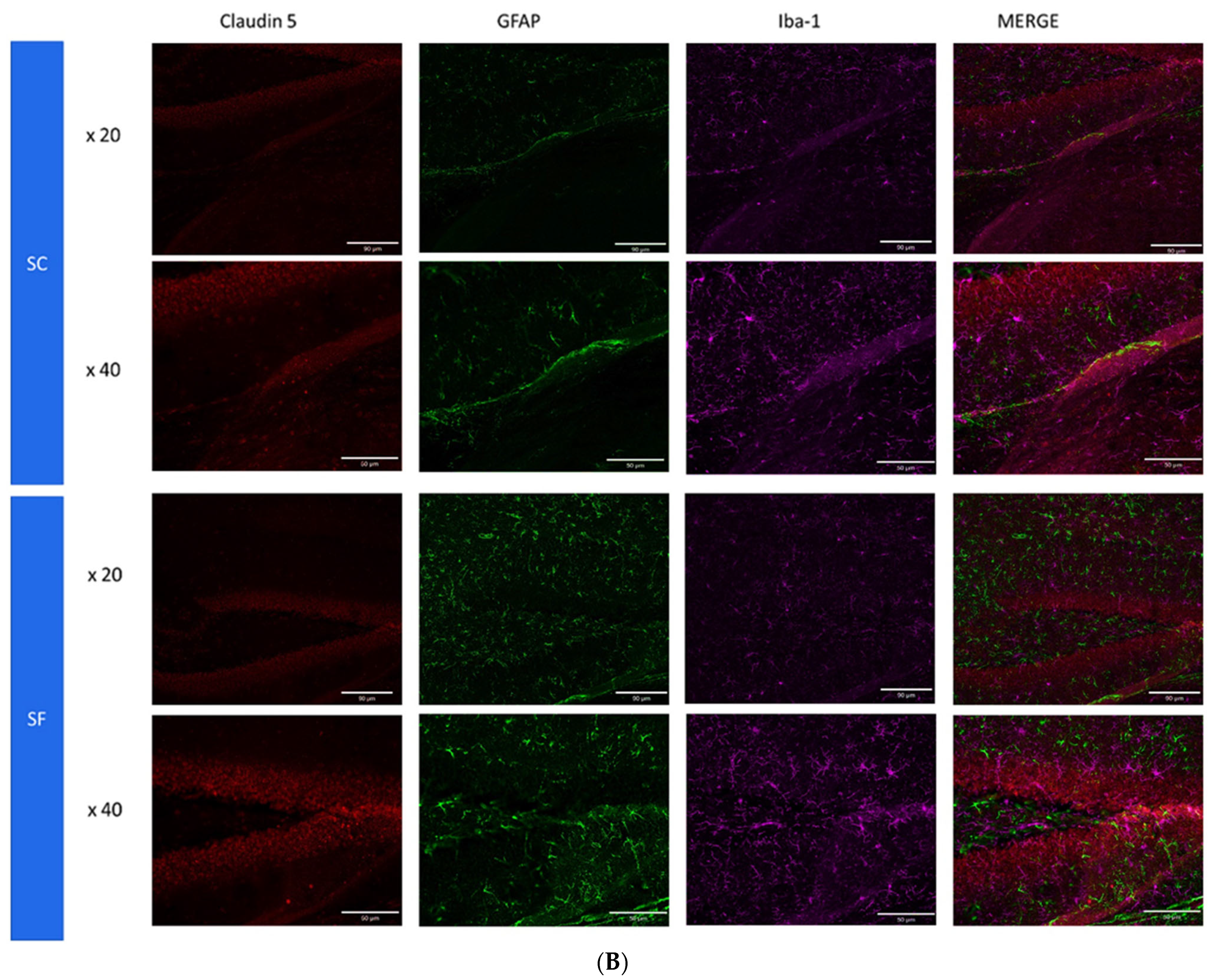
2. Results
3. Discussion
4. Materials and Methods
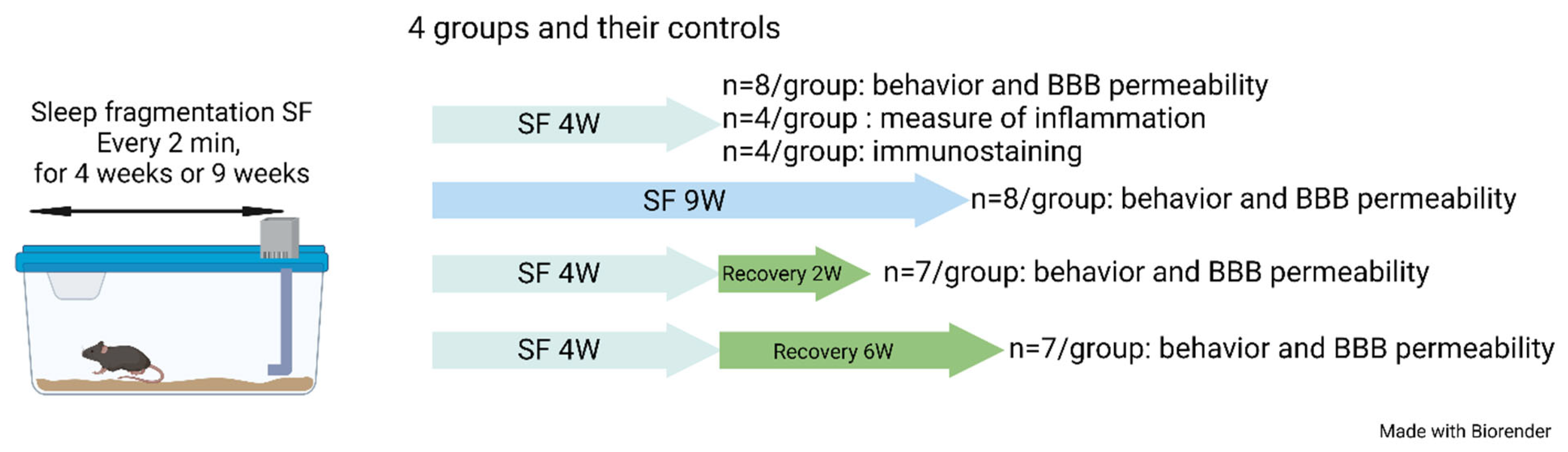
4.1. Sleep Fragmentation (SF)
4.2. Sleep Recording
4.3. Novel Object Recognition (NOR) Test
4.4. Inflammatory Markers
4.5. Blood Brain Permeability
4.6. Immunohistochemistry
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almendros, I.; Martinez-Garcia, M.A.; Farré, R.; Gozal, D. Obesity, Sleep Apnea, and Cancer. Int. J. Obes. 2020, 44, 1653–1667. [Google Scholar] [CrossRef]
- Badran, M.; Khalyfa, A.; Ericsson, A.; Gozal, D. Fecal Microbiota Transplantation from Mice Exposed to Chronic Intermittent Hypoxia Elicits Sleep Disturbances in Naïve Mice. Exp. Neurol. 2020, 334, 113439. [Google Scholar] [CrossRef]
- Stranks, E.K.; Crowe, S.F. The Cognitive Effects of Obstructive Sleep Apnea: An Updated Meta-Analysis. Arch. Clin. Neuropsychol. 2016, 31, 186–193. [Google Scholar] [CrossRef]
- Krysta, K.; Bratek, A.; Zawada, K.; Stepańczak, R. Cognitive Deficits in Adults with Obstructive Sleep Apnea Compared to Children and Adolescents. J. Neural Transm. 2017, 124, 187–201. [Google Scholar] [CrossRef] [Green Version]
- Lajoie, A.C.; Lafontaine, A.-L.; Kimoff, R.J.; Kaminska, M. Obstructive Sleep Apnea in Neurodegenerative Disorders: Current Evidence in Support of Benefit from Sleep Apnea Treatment. J. Clin. Med. 2020, 9, 297. [Google Scholar] [CrossRef] [Green Version]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of Obstructive Sleep Apnea in the General Population: A Systematic Review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Gileles-Hillel, A.; Kheirandish-Gozal, L.; Gozal, D. Biological Plausibility Linking Sleep Apnoea and Metabolic Dysfunction. Nat. Rev. Endocrinol. 2016, 12, 290–298. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef]
- Abad, V.C. Profile of Solriamfetol in the Management of Excessive Daytime Sleepiness Associated with Narcolepsy or Obstructive Sleep Apnea: Focus on Patient Selection and Perspectives. Nat. Sci. Sleep 2021, 13, 75–91. [Google Scholar] [CrossRef]
- Bucks, R.S.; Olaithe, M.; Rosenzweig, I.; Morrell, M.J. Reviewing the Relationship between OSA and Cognition: Where Do We Go from Here? Respirology 2017, 22, 1253–1261. [Google Scholar] [CrossRef] [Green Version]
- Hirsch Allen, A.J.M.; Bansback, N.; Ayas, N.T. The Effect of OSA on Work Disability and Work-Related Injuries. Chest 2015, 147, 1422–1428. [Google Scholar] [CrossRef]
- Vanek, J.; Prasko, J.; Genzor, S.; Ociskova, M.; Kantor, K.; Holubova, M.; Slepecky, M.; Nesnidal, V.; Kolek, A.; Sova, M. Obstructive Sleep Apnea, Depression and Cognitive Impairment. Sleep Med. 2020, 72, 50–58. [Google Scholar] [CrossRef]
- Lal, C.; Ayappa, I.; Ayas, N.; Beaudin, A.E.; Hoyos, C.; Kushida, C.A.; Kaminska, M.; Mullins, A.; Naismith, S.L.; Osorio, R.S.; et al. The Link between Obstructive Sleep Apnea and Neurocognitive Impairment: An Official American Thoracic Society Workshop Report. Ann. ATS 2022, 19, 1245–1256. [Google Scholar] [CrossRef]
- Gozal, D.; Kheirandish-Gozal, L.; Bhattacharjee, R.; Spruyt, K. Neurocognitive and Endothelial Dysfunction in Children with Obstructive Sleep Apnea. Pediatrics 2010, 126, e1161–e1167. [Google Scholar] [CrossRef]
- Cistulli, P.A.; Celermajer, D.S. Endothelial Dysfunction and Obstructive Sleep Apnea: The Jury Is Still Out! Am. J. Respir. Crit. Care Med. 2017, 195, 1135–1137. [Google Scholar] [CrossRef]
- Budhiraja, R.; Parthasarathy, S.; Quan, S.F. Endothelial Dysfunction in Obstructive Sleep Apnea. J. Clin. Sleep Med. 2007, 3, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Atkeson, A.; Yeh, S.Y.; Malhotra, A.; Jelic, S. Endothelial Function in Obstructive Sleep Apnea. Prog. Cardiovasc. Dis. 2009, 51, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Lal, C.; Strange, C.; Bachman, D. Neurocognitive Impairment in Obstructive Sleep Apnea. Chest 2012, 141, 1601–1610. [Google Scholar] [CrossRef]
- Hoyos, C.M.; Melehan, K.L.; Liu, P.Y.; Grunstein, R.R.; Phillips, C.L. Does Obstructive Sleep Apnea Cause Endothelial Dysfunction? A Critical Review of the Literature. Sleep Med. Rev. 2015, 20, 15–26. [Google Scholar] [CrossRef]
- Schwarz, E.I.; Puhan, M.A.; Schlatzer, C.; Stradling, J.R.; Kohler, M. Effect of CPAP Therapy on Endothelial Function in Obstructive Sleep Apnoea: A Systematic Review and Meta-Analysis. Respirology 2015, 20, 889–895. [Google Scholar] [CrossRef]
- Kerner, N.A.; Roose, S.P. Obstructive Sleep Apnea Is Linked to Depression and Cognitive Impairment: Evidence and Potential Mechanisms. Am. J. Geriatr. Psychiatry 2016, 24, 496–508. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.C.; Pack, A.I. Obstructive Sleep Apnea and Cognitive Impairment: Addressing the Blood-Brain Barrier. Sleep Med. Rev. 2014, 18, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Kim, L.J.; Martinez, D.; Fiori, C.Z.; Baronio, D.; Kretzmann, N.A.; Barros, H.M.T. Hypomyelination, Memory Impairment, and Blood–Brain Barrier Permeability in a Model of Sleep Apnea. Brain Res. 2015, 1597, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Zolotoff, C.; Voirin, A.-C.; Puech, C.; Roche, F.; Perek, N. Intermittent Hypoxia and Its Impact on Nrf2/HIF-1α Expression and ABC Transporters: An in Vitro Human Blood-Brain Barrier Model Study. Cell Physiol. Biochem. 2020, 54, 1231–1248. [Google Scholar] [CrossRef]
- Kilicarslan, R.; Alkan, A.; Sharifov, R.; Akkoyunlu, M.E.; Aralasmak, A.; Kocer, A.; Kart, L. The Effect of Obesity on Brain Diffusion Alteration in Patients with Obstructive Sleep Apnea. Sci. World J. 2014, 2014, 768415. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Khalyfa, A.; Gozal, D. Exosomes, Blood–Brain Barrier, and Cognitive Dysfunction in Pediatric Sleep Apnea. Sleep Biol. Rhythm. 2017, 15, 261–267. [Google Scholar] [CrossRef]
- Voirin, A.-C.; Celle, S.; Perek, N.; Roche, F. Sera of Elderly Obstructive Sleep Apnea Patients Alter Blood-Brain Barrier Integrity in Vitro: A Pilot Study. Sci. Rep. 2020, 10, 11309. [Google Scholar] [CrossRef]
- Khalyfa, A.; Gozal, D.; Kheirandish-Gozal, L. Plasma Extracellular Vesicles in Children with OSA Disrupt Blood–Brain Barrier Integrity and Endothelial Cell Wound Healing In Vitro. Int. J. Mol. Sci. 2019, 20, 6233. [Google Scholar] [CrossRef] [Green Version]
- Khalyfa, A.; Gozal, D.; Kheirandish-Gozal, L. Plasma Exosomes Disrupt the Blood–Brain Barrier in Children with Obstructive Sleep Apnea and Neurocognitive Deficits. Am. J. Respir. Crit. Care Med. 2018, 197, 1073–1076. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [Green Version]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood-Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, T.; Yang, X.; Fan, M.; Zhu, L.; Liu, S.; Wu, L.; Sun, C. Cryptotanshinone Attenuates Oxygen-Glucose Deprivation/ Recovery-Induced Injury in an in Vitro Model of Neurovascular Unit. Front. Neurol. 2019, 10, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, L.S.; Foster, C.G.; Courtney, J.-M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and Neurovascular Function in the Healthy and Diseased Brain. Front. Cell Neurosci. 2019, 13, 282. [Google Scholar] [CrossRef] [Green Version]
- Cuddapah, V.A.; Zhang, S.L.; Sehgal, A. Regulation of the Blood-Brain Barrier by Circadian Rhythms and Sleep. Trends Neurosci. 2019, 42, 500–510. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Hsuchou, H.; He, Y.; Kastin, A.J.; Wang, Y.; Pan, W. Sleep Restriction Impairs Blood–Brain Barrier Function. J. Neurosci. 2014, 34, 14697–14706. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Gonzalez, B.; Hurtado-Alvarado, G.; Esqueda-Leon, E.; Santana-Miranda, R.; Rojas-Zamorano, J.A.; Velazquez-Moctezuma, J. REM Sleep Loss and Recovery Regulates Blood-Brain Barrier Function. Curr. Neurovasc. Res. 2013, 10, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, S.; Sonobe, Y.; Cheng, Y.; Horiuchi, H.; Parajuli, B.; Kawanokuchi, J.; Mizuno, T.; Takeuchi, H.; Suzumura, A. Interleukin-1β Induces Blood-Brain Barrier Disruption by Downregulating Sonic Hedgehog in Astrocytes. PLoS ONE 2014, 9, e110024. [Google Scholar] [CrossRef] [Green Version]
- Venancio, D.P.; Suchecki, D. Prolonged REM Sleep Restriction Induces Metabolic Syndrome-Related Changes: Mediation by pro-Inflammatory Cytokines. Brain Behav. Immun. 2015, 47, 109–117. [Google Scholar] [CrossRef]
- Yehuda, S.; Sredni, B.; Carasso, R.L.; Kenigsbuch-Sredni, D. REM Sleep Deprivation in Rats Results in Inflammation and Interleukin-17 Elevation. J. Interferon Cytokine Res. 2009, 29, 393–398. [Google Scholar] [CrossRef]
- Hurtado-Alvarado, G.; Domínguez-Salazar, E.; Pavon, L.; Velázquez-Moctezuma, J.; Gómez-González, B. Blood-Brain Barrier Disruption Induced by Chronic Sleep Loss: Low-Grade Inflammation May Be the Link. J. Immunol. Res. 2016, 2016, 4576012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurtado-Alvarado, G.; Becerril-Villanueva, E.; Contis-Montes de Oca, A.; Domínguez-Salazar, E.; Salinas-Jazmín, N.; Pérez-Tapia, S.M.; Pavon, L.; Velázquez-Moctezuma, J.; Gómez-González, B. The Yin/Yang of Inflammatory Status: Blood-Brain Barrier Regulation during Sleep. Brain Behav. Immun. 2018, 69, 154–166. [Google Scholar] [CrossRef]
- Opp, M.R.; George, A.; Ringgold, K.M.; Hansen, K.M.; Bullock, K.M.; Banks, W.A. Sleep Fragmentation and Sepsis Differentially Impact Blood–Brain Barrier Integrity and Transport of Tumor Necrosis Factor-α in Aging. Brain Behav. Immun. 2015, 50, 259–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, V.; Nair, D.; Zhang, S.X.L.; Hakim, F.; Kaushal, N.; Kayali, F.; Wang, Y.; Li, R.C.; Carreras, A.; Gozal, D. Disrupted Sleep without Sleep Curtailment Induces Sleepiness and Cognitive Dysfunction via the Tumor Necrosis Factor-α Pathway. J. Neuroinflamm. 2012, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Chennaoui, M.; Sauvet, F.; Drogou, C.; Van Beers, P.; Langrume, C.; Guillard, M.; Gourby, B.; Bourrilhon, C.; Florence, G.; Gomez-Merino, D. Effect of One Night of Sleep Loss on Changes in Tumor Necrosis Factor Alpha (TNF-α) Levels in Healthy Men. Cytokine 2011, 56, 318–324. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood–Brain Barrier Breakdown in Alzheimer’s Disease and Other Neurodegenerative Disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Fang, C.; Chang, J. Blood–Brain Barrier Breakdown: An Emerging Biomarker of Cognitive Impairment in Normal Aging and Dementia. Front. Neurosci. 2021, 15, 688090. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The Blood-Brain Barrier in Aging and Neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Bilyukov, R.G.; Nikolov, M.S.; Pencheva, V.P.; Petrova, D.S.; Georgiev, O.B.; Mondeshki, T.L.; Milanova, V.K. Cognitive Impairment and Affective Disorders in Patients With Obstructive Sleep Apnea Syndrome. Front. Psychiatry 2018, 9, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferini-Strambi, L.; Baietto, C.; Di Gioia, M.R.; Castaldi, P.; Castronovo, C.; Zucconi, M.; Cappa, S.F. Cognitive Dysfunction in Patients with Obstructive Sleep Apnea (OSA): Partial Reversibility after Continuous Positive Airway Pressure (CPAP). Brain Res. Bull. 2003, 61, 87–92. [Google Scholar] [CrossRef]
- Puech, C.; Badran, M.; Runion, A.R.; Barrow, M.B.; Qiao, Z.; Khalyfa, A.; Gozal, D. Explicit Memory, Anxiety and Depressive like Behavior in Mice Exposed to Chronic Intermittent Hypoxia, Sleep Fragmentation, or Both during the Daylight Period. Neurobiol. Sleep Circadian Rhythm. 2022, 13, 100084. [Google Scholar] [CrossRef]
- Xu, L.-H.; Xie, H.; Shi, Z.-H.; Du, L.-D.; Wing, Y.-K.; Li, A.M.; Ke, Y.; Yung, W.-H. Critical Role of Endoplasmic Reticulum Stress in Chronic Intermittent Hypoxia-Induced Deficits in Synaptic Plasticity and Long-Term Memory. Antioxid. Redox Signal. 2015, 23, 695–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushal, N.; Ramesh, V.; Gozal, D. Human Apolipoprotein E4 Targeted Replacement in Mice Reveals Increased Susceptibility to Sleep Disruption and Intermittent Hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R19–R29. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.P.; McCoy, J.G.; McKenna, J.T.; Connolly, N.P.; McCarley, R.W.; Strecker, R.E. Spatial Learning and Memory Deficits Following Exposure to 24 h of Sleep Fragmentation or Intermittent Hypoxia in a Rat Model of Obstructive Sleep Apnea. Brain Res. 2009, 1294, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Row, B.W. Intermittent Hypoxia and Cognitive Function: Implications from Chronic Animal Models. Adv. Exp. Med. Biol. 2007, 618, 51–67. [Google Scholar] [CrossRef]
- Nair, D.; Zhang, S.X.L.; Ramesh, V.; Hakim, F.; Kaushal, N.; Wang, Y.; Gozal, D. Sleep Fragmentation Induces Cognitive Deficits via Nicotinamide Adenine Dinucleotide Phosphate Oxidase-Dependent Pathways in Mouse. Am. J. Respir. Crit. Care Med. 2011, 184, 1305–1312. [Google Scholar] [CrossRef] [Green Version]
- Nair, D.; Ramesh, V.; Gozal, D. Cognitive Deficits Are Attenuated in Neuroglobin Overexpressing Mice Exposed to a Model of Obstructive Sleep Apnea. Front. Neurol. 2018, 9, 426. [Google Scholar] [CrossRef]
- Verstraeten, E. Neurocognitive Effects of Obstructive Sleep Apnea Syndrome. Curr. Neurol. Neurosci. Rep. 2007, 7, 161–166. [Google Scholar] [CrossRef]
- Puech, C.; Badran, M.; Barrow, M.B.; Runion, A.R.; Gozal, D. Solriamfetol Improves Chronic Sleep Fragmentation-Induced Increases in Sleep Propensity and Ameliorates Explicit Memory in Male Mice. Sleep 2023, 46, zsad057. [Google Scholar] [CrossRef]
- Ferrara, M.; De Gennaro, L.; Casagrande, M.; Bertini, M. Selective Slow-Wave Sleep Deprivation and Time-of-Night Effects on Cognitive Performance upon Awakening. Psychophysiology 2000, 37, 440–446. [Google Scholar] [CrossRef]
- Stewart, C.A.; Auger, R.R.; Enders, F.T.B.; Felmlee-Devine, D.; Smith, G.E. The Effects of Poor Sleep Quality on Cognitive Function of Patients with Cirrhosis. J. Clin. Sleep Med. 2014, 10, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Wu, J.; Hua, F.; Chen, Y.; Zhan, F.; Xu, G. Sleep Deprivation Induces Cognitive Impairment by Increasing Blood-Brain Barrier Permeability via CD44. Front. Neurol. 2020, 11, 563916. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Kim, Y.; Karpova, S.A.; McCarley, R.W.; Strecker, R.E.; Gerashchenko, D. Chronic Sleep Restriction Elevates Brain Interleukin-1 Beta and Tumor Necrosis Factor-Alpha and Attenuates Brain-Derived Neurotrophic Factor Expression. Neurosci. Lett. 2014, 580, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voirin, A.-C.; Perek, N.; Roche, F. Inflammatory Stress Induced by a Combination of Cytokines (IL-6, IL-17, TNF-α) Leads to a Loss of Integrity on BEnd.3 Endothelial Cells in Vitro BBB Model. Brain Res. 2020, 1730, 146647. [Google Scholar] [CrossRef] [PubMed]
- Jewett, K.A.; Krueger, J.M. Humoral Sleep Regulation; Interleukin-1 and Tumor Necrosis Factor. Vitam. Horm. 2012, 89, 241–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The Semantics of Microglia Activation: Neuroinflammation, Homeostasis, and Stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef]
- Kang, R.; Gamdzyk, M.; Lenahan, C.; Tang, J.; Tan, S.; Zhang, J.H. The Dual Role of Microglia in Blood-Brain Barrier Dysfunction after Stroke. Curr. Neuropharmacol. 2020, 18, 1237–1249. [Google Scholar] [CrossRef]
- Ronaldson, P.T.; Davis, T.P. Regulation of Blood–Brain Barrier Integrity by Microglia in Health and Disease: A Therapeutic Opportunity. J. Cereb. Blood Flow Metab. 2020, 40, S6–S24. [Google Scholar] [CrossRef] [PubMed]
- Al-Bachari, S.; Naish, J.H.; Parker, G.J.M.; Emsley, H.C.A.; Parkes, L.M. Blood–Brain Barrier Leakage Is Increased in Parkinson’s Disease. Front. Physiol. 2020, 11, 593026. [Google Scholar] [CrossRef]
- Lee, R.-L.; Funk, K.E. Imaging Blood–Brain Barrier Disruption in Neuroinflammation and Alzheimer’s Disease. Front. Aging Neurosci. 2023, 15, 1144036. [Google Scholar] [CrossRef]
- Lee, H.; Pienaar, I.S. Disruption of the Blood-Brain Barrier in Parkinson’s Disease: Curse or Route to a Cure? Front. Biosci.-Landmark Ed. 2014, 19, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Grubac, Z.; Sutulovic, N.; Ademovic, A.; Velimirovic, M.; Rasic-Markovic, A.; Macut, D.; Petronijevic, N.; Stanojlovic, O.; Hrncic, D. Short-Term Sleep Fragmentation Enhances Anxiety-Related Behavior: The Role of Hormonal Alterations. PLoS ONE 2019, 14, e0218920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manchanda, S.; Singh, H.; Kaur, T.; Kaur, G. Low-Grade Neuroinflammation Due to Chronic Sleep Deprivation Results in Anxiety and Learning and Memory Impairments. Mol. Cell Biochem. 2018, 449, 63–72. [Google Scholar] [CrossRef]
- Irwin, M.R.; Wang, M.; Ribeiro, D.; Cho, H.J.; Olmstead, R.; Breen, E.C.; Martinez-Maza, O.; Cole, S. Sleep Loss Activates Cellular Inflammatory Signaling. Biological. Psychiatry 2008, 64, 538–540. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Gardi, J.; Kushikata, T.; Fang, J.; Krueger, J.M. Nuclear Factor-KappaB-like Activity Increases in Murine Cerebral Cortex after Sleep Deprivation. Am. J. Physiol. 1999, 276, R1812–R1818. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Alvarado, G.; Velázquez-Moctezuma, J.; Gómez-González, B. Chronic Sleep Restriction Disrupts Interendothelial Junctions in the Hippocampus and Increases Blood-Brain Barrier Permeability. J. Microsc. 2017, 268, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Serna-Rodríguez, M.F.; Bernal-Vega, S.; de la Barquera, J.A.O.-S.; Camacho-Morales, A.; Pérez-Maya, A.A. The Role of Damage Associated Molecular Pattern Molecules (DAMPs) and Permeability of the Blood-Brain Barrier in Depression and Neuroinflammation. J. Neuroimmunol. 2022, 371, 577951. [Google Scholar] [CrossRef]
- Palomares, J.A.; Tummala, S.; Wang, D.J.J.; Park, B.; Woo, M.A.; Kang, D.W.; St Lawrence, K.S.; Harper, R.M.; Kumar, R. Water Exchange across the Blood-Brain Barrier in Obstructive Sleep Apnea: An MRI Diffusion-Weighted Pseudo-Continuous Arterial Spin Labeling Study. J. Neuroimaging 2015, 25, 900–905. [Google Scholar] [CrossRef] [Green Version]
- Dion-Albert, L.; Dudek, K.A.; Russo, S.J.; Campbell, M.; Menard, C. Neurovascular Adaptations Modulating Cognition, Mood, and Stress Responses. Trends Neurosci. 2023, 46, 276–292. [Google Scholar] [CrossRef]
- Hüls, A.; Robins, C.; Conneely, K.N.; Edgar, R.; De Jager, P.L.; Bennett, D.A.; Wingo, A.P.; Epstein, M.P.; Wingo, T.S. Brain DNA Methylation Patterns in CLDN5 Associated With Cognitive Decline. Biol. Psychiatry 2022, 91, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Ibrahim Verbaas, C.A.; Bressler, J.; Schuur, M.; Smith, A.; Bis, J.C.; Davies, G.; Wolf, C.; Gudnason, V.; Chibnik, L.B.; et al. Genome-Wide Studies of Verbal Declarative Memory in Nondemented Older People: The Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium. Biol. Psychiatry 2015, 77, 749–763. [Google Scholar] [CrossRef] [Green Version]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social Stress Induces Neurovascular Pathology Promoting Depression. Nat. Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, A.C.; Uhlig, F.; Einwag, Z.; Cataldo, N.; Erdos, B. The Neuroendocrine Stress Response Impairs Hippocampal Vascular Function and Memory in Male and Female Rats. Neurobiol. Dis. 2022, 168, 105717. [Google Scholar] [CrossRef] [PubMed]
- Minett, T.; Classey, J.; Matthews, F.E.; Fahrenhold, M.; Taga, M.; Brayne, C.; Ince, P.G.; Nicoll, J.A.R.; Boche, D. MRC CFAS Microglial Immunophenotype in Dementia with Alzheimer’s Pathology. J. Neuroinflamm. 2016, 13, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-Specific Localisation of a Novel Calcium Binding Protein, Iba1. Brain Res. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Lituma, P.J.; Woo, E.; O’Hara, B.F.; Castillo, P.E.; Sibinga, N.E.S.; Nandi, S. Altered Synaptic Connectivity and Brain Function in Mice Lacking Microglial Adapter Protein Iba1. Proc. Natl. Acad. Sci. USA 2021, 118, e2115539118. [Google Scholar] [CrossRef]
- Umpierre, A.D.; Wu, L.-J. How Microglia Sense and Regulate Neuronal Activity. Glia 2021, 69, 1637–1653. [Google Scholar] [CrossRef]
- Kopp, C.; Longordo, F.; Nicholson, J.R.; Lüthi, A. Insufficient Sleep Reversibly Alters Bidirectional Synaptic Plasticity and NMDA Receptor Function. J. Neurosci. 2006, 26, 12456–12465. [Google Scholar] [CrossRef] [Green Version]
- Huber, R.; Deboer, T.; Tobler, I. Effects of Sleep Deprivation on Sleep and Sleep EEG in Three Mouse Strains: Empirical Data and Simulations. Brain Res. 2000, 857, 8–19. [Google Scholar] [CrossRef]
- Rabat, A.; Gomez-Merino, D.; Roca-Paixao, L.; Bougard, C.; Van Beers, P.; Dispersyn, G.; Guillard, M.; Bourrilhon, C.; Drogou, C.; Arnal, P.J.; et al. Differential Kinetics in Alteration and Recovery of Cognitive Processes from a Chronic Sleep Restriction in Young Healthy Men. Front. Behav. Neurosci. 2016, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Seda, G.; Matwiyoff, G.; Parrish, J.S. Effects of Obstructive Sleep Apnea and CPAP on Cognitive Function. Curr. Neurol. Neurosci. Rep. 2021, 21, 32. [Google Scholar] [CrossRef]
- Chang, E.-T.; Wang, H.-M. Cognitive Function Was Improved after Continuous Positive Airway Pressure Treatment in Obstructive Sleep Apnea Syndrome. Eur. Resp. J. 2016, 48, PA2368. [Google Scholar] [CrossRef]
- D’Rozario, A.L.; Hoyos, C.M.; Wong, K.K.H.; Unger, G.; Kim, J.W.; Vakulin, A.; Kao, C.-H.; Naismith, S.L.; Bartlett, D.J.; Grunstein, R.R. Improvements in Cognitive Function and Quantitative Sleep Electroencephalogram in Obstructive Sleep Apnea after Six Months of Continuous Positive Airway Pressure Treatment. Sleep 2022, 45, zsac013. [Google Scholar] [CrossRef] [PubMed]
- Kushida, C.A.; Nichols, D.A.; Holmes, T.H.; Quan, S.F.; Walsh, J.K.; Gottlieb, D.J.; Simon, R.D.; Guilleminault, C.; White, D.P.; Goodwin, J.L.; et al. Effects of Continuous Positive Airway Pressure on Neurocognitive Function in Obstructive Sleep Apnea Patients: The Apnea Positive Pressure Long-Term Efficacy Study (APPLES). Sleep 2012, 35, 1593–1602. [Google Scholar] [CrossRef]
- Crawford-Achour, E.; Dauphinot, V.; Martin, M.S.; Tardy, M.; Gonthier, R.; Barthelemy, J.C.; Roche, F. Protective Effect of Long-Term CPAP Therapy on Cognitive Performance in Elderly Patients with Severe OSA: The PROOF Study. J. Clin. Sleep Med. 2015, 11, 519–524. [Google Scholar] [CrossRef]
- Zimmerman, M.E.; Arnedt, J.T.; Stanchina, M.; Millman, R.P.; Aloia, M.S. Normalization of Memory Performance and Positive Airway Pressure Adherence in Memory-Impaired Patients with Obstructive Sleep Apnea. Chest 2006, 130, 1772–1778. [Google Scholar] [CrossRef]
- Franks, K.H.; Rowsthorn, E.; Nicolazzo, J.; Boland, A.; Lavale, A.; Baker, J.; Rajaratnam, S.M.W.; Cavuoto, M.G.; Yiallourou, S.R.; Naughton, M.T.; et al. The Treatment of Sleep Dysfunction to Improve Cognitive Function: A Meta-Analysis of Randomized Controlled Trials. Sleep Med. 2023, 101, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.L.; Moore, R.H.; Rosen, C.L.; Giordani, B.; Garetz, S.L.; Taylor, H.G.; Mitchell, R.B.; Amin, R.; Katz, E.S.; Arens, R.; et al. A Randomized Trial of Adenotonsillectomy for Childhood Sleep Apnea. N. Engl. J. Med. 2013, 368, 2366–2376. [Google Scholar] [CrossRef] [Green Version]
- Felver-Gant, J.C.; Bruce, A.S.; Zimmerman, M.; Sweet, L.H.; Millman, R.P.; Aloia, M.S. Working Memory in Obstructive Sleep Apnea. J. Clin. Sleep Med. 2007, 3, 589–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozal, D.; Khalyfa, A.; Qiao, Z.; Almendros, I.; Farré, R. Temporal Trajectories of Novel Object Recognition Performance in Mice Exposed to Intermittent Hypoxia. Eur. Respir. J. 2017, 50, 17014560. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, N.; Nair, D.; Gozal, D.; Ramesh, V. Socially Isolated Mice Exhibit a Blunted Homeostatic Sleep Response to Acute Sleep Deprivation Compared to Socially Paired Mice. Brain Res. 2012, 1454, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, V.; Kaushal, N.; Gozal, D. Sleep fragmentation differentially modifies EEG delta power during slow wave sleep in socially isolated and paired mice. Sleep Sci. 2009, 2, 64–75. [Google Scholar]
- Flores, A.E.; Flores, J.E.; Deshpande, H.; Picazo, J.A.; Xie, X.; Franken, P.; Heller, H.C.; Grahn, D.A.; O’Hara, B.F. Pattern Recognition of Sleep in Rodents Using Piezoelectric Signals Generated by Gross Body Movements. IEEE Trans. Biomed. Eng. 2007, 54, 225–233. [Google Scholar] [CrossRef]
- Vanneau, T.; Quiquempoix, M.; Trignol, A.; Verdonk, C.; Van Beers, P.; Sauvet, F.; Gomez-Merino, D.; Chennaoui, M. Determination of the Sleep-Wake Pattern and Feasibility of NREM/REM Discrimination Using the Non-Invasive Piezoelectric System in Rats. J. Sleep Res. 2021, 30, e13373. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K.D.; Medonza, D.C.; Crane, E.R.; O’Hara, B.F. Assessment of a Non-Invasive High-Throughput Classifier for Behaviours Associated with Sleep and Wake in Mice. BioMed Eng. OnLine 2008, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Yaghouby, F.; Donohue, K.D.; O’Hara, B.F.; Sunderam, S. Noninvasive Dissection of Mouse Sleep Using a Piezoelectric Motion Sensor. J. Neurosci. Methods 2016, 259, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mang, G.M.; Nicod, J.; Emmenegger, Y.; Donohue, K.D.; O’Hara, B.F.; Franken, P. Evaluation of a Piezoelectric System as an Alternative to Electroencephalogram/ Electromyogram Recordings in Mouse Sleep Studies. Sleep 2014, 37, 1383–1392. [Google Scholar] [CrossRef] [Green Version]
- Badran, M.; Puech, C.; Barrow, M.B.; Runion, A.R.; Gozal, D. Solriamfetol Enhances Wakefulness and Improves Cognition and Anxiety in a Murine Model of OSA. Sleep Med. 2023, 107, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The Novel Object Recognition Memory: Neurobiology, Test Procedure, and Its Modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [Green Version]
- Hammond, R.S.; Tull, L.E.; Stackman, R.W. On the Delay-Dependent Involvement of the Hippocampus in Object Recognition Memory. Neurobiol. Learn. Mem. 2004, 82, 26–34. [Google Scholar] [CrossRef]
- Mazzucco, M.R.; Vartanain, T.; Linden, J.R. In Vivo Blood-Brain Barrier Permeability Assays Using Clostridium Perfringens Epsilon Toxin. Bio. Protoc. 2020, 10, e3709. [Google Scholar] [CrossRef]
- Baganha, F.; Ritsma, L.; Quax, P.H.A.; de Vries, M.R. Assessment of Microvessel Permeability in Murine Atherosclerotic Vein Grafts Using Two-Photon Intravital Microscopy. Int. J. Mol. Sci. 2020, 21, 9244. [Google Scholar] [CrossRef] [PubMed]
- Egawa, G.; Nakamizo, S.; Natsuaki, Y.; Doi, H.; Miyachi, Y.; Kabashima, K. Intravital Analysis of Vascular Permeability in Mice Using Two-Photon Microscopy. Sci. Rep. 2013, 3, 1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devraj, K.; Guérit, S.; Macas, J.; Reiss, Y. An In Vivo Blood-Brain Barrier Permeability Assay in Mice Using Fluorescently Labeled Tracers. J. Vis. Exp. 2018, 132, 57038. [Google Scholar] [CrossRef]

| Primary Antibodies | Dilution | Manufacturer | Order |
| Rabbit monoclonal anti Claudin 5 | 1/1000 | Abcam (Cambridge, UK) Ab131259 | 1 |
| Rat monoclonal anti Iba1 | 1/500 | Invitrogen (Waltham, MA, USA) MA5-38266 | 2 |
| Goat Polyclonal anti-CD31/PECAM | 10 μg/mL | Novus Biological (Englewood, CO, USA) AF3628 | 3 (panel 1) |
| Chicken polyclonal anti GFAP | 1/5000 | Invitrogen (Waltham, MA, USA) PA1-10004 | 3 (panel 2) |
| Secondary Antibodies | Dilution | Manufacturer | Order |
| Donkey anti-Rabbit Alexa fluor 555 | 1/1000 | Thermofischer (Waltham, MA, USA) A32794 | 1 |
| Donkey anti-Rat Alexa fluor 647 | 1/1000 | Thermofischer (Waltham, MA, USA) A48272 | 2 |
| Donkey anti-Goat Alexa fluor 488 | 1/1000 | Thermofischer (Waltham, MA, USA) A32814 | 3 (panel 1) |
| Donkey anti Chicken Alexa fluor 488 | 1/5000 | Jackson ImmunoResearch (West Grove, PA, USA) 703-545-155 | 3 (panel 2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puech, C.; Badran, M.; Runion, A.R.; Barrow, M.B.; Cataldo, K.; Gozal, D. Cognitive Impairments, Neuroinflammation and Blood–Brain Barrier Permeability in Mice Exposed to Chronic Sleep Fragmentation during the Daylight Period. Int. J. Mol. Sci. 2023, 24, 9880. https://doi.org/10.3390/ijms24129880
Puech C, Badran M, Runion AR, Barrow MB, Cataldo K, Gozal D. Cognitive Impairments, Neuroinflammation and Blood–Brain Barrier Permeability in Mice Exposed to Chronic Sleep Fragmentation during the Daylight Period. International Journal of Molecular Sciences. 2023; 24(12):9880. https://doi.org/10.3390/ijms24129880
Chicago/Turabian StylePuech, Clementine, Mohammad Badran, Alexandra R. Runion, Max B. Barrow, Kylie Cataldo, and David Gozal. 2023. "Cognitive Impairments, Neuroinflammation and Blood–Brain Barrier Permeability in Mice Exposed to Chronic Sleep Fragmentation during the Daylight Period" International Journal of Molecular Sciences 24, no. 12: 9880. https://doi.org/10.3390/ijms24129880
APA StylePuech, C., Badran, M., Runion, A. R., Barrow, M. B., Cataldo, K., & Gozal, D. (2023). Cognitive Impairments, Neuroinflammation and Blood–Brain Barrier Permeability in Mice Exposed to Chronic Sleep Fragmentation during the Daylight Period. International Journal of Molecular Sciences, 24(12), 9880. https://doi.org/10.3390/ijms24129880








