Dendritic Cell Subpopulations Are Associated with Morphological Features of Breast Ductal Carcinoma In Situ
Abstract
:1. Introduction
2. Results
2.1. Description of Study Group
| Characteristic | Missing | ||
|---|---|---|---|
| Age (years) | 56 [31–85] | ||
| Maximal tumor foci size (mm) | 13.00 [0.25–75.00] | 3 | |
| Architectural pattern (N, %) | |||
| Solid | 69 | 75.0 | |
| Cribriform | 51 | 55.4 | |
| Micropapillary | 31 | 34.7 | |
| Papillary | 16 | 17.4 | |
| Comedo | 15 | 16.3 | |
| Apocrine | 7 | 7.6 | |
| Clinging | 1 | 1.1 | |
| Spindle cell | 1 | 1.1 | |
| Highest nuclear grade (N, %) | |||
| G1 | 4 | 4.0 | |
| G2 | 52 | 57.0 | |
| G3 | 36 | 39.0 | |
| ER (%) | 53.94 ± 40.63 | 15 | |
| PR (%) | 31.58 ± 36.85 | 15 | |
| Histological features (N, %) | |||
| Comedo necrosis | 60 | 65.2 | |
| Ductal spread | 70 | 77.9 | 1 |
| Lobular cancerization | 48 | 52.7 | 1 |
| Microinvasion | 10 | 10.9 | |
| Microcalcifications | 72 | 78.3 | |
| Neoductgenesis (N, %) | |||
| Neoductgenesis | 26 | 28.0 | |
| Other clinical features (N, %) | |||
| Family history of BC | 13 | 17.3 | 17 |
| The palpability of the lesions | 18 | 25.4 | 21 |
| Paget disease | 10 | 10.9 | |
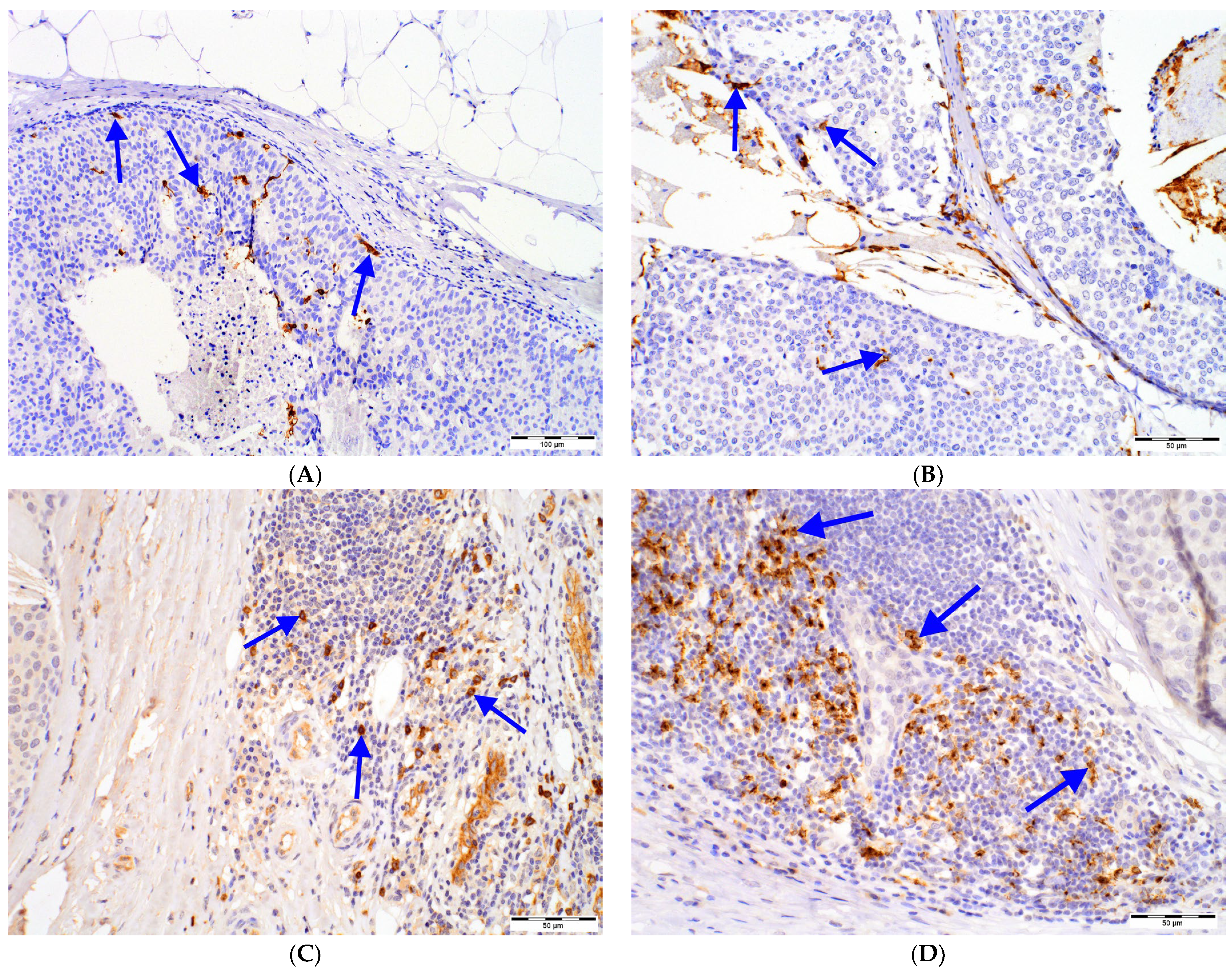
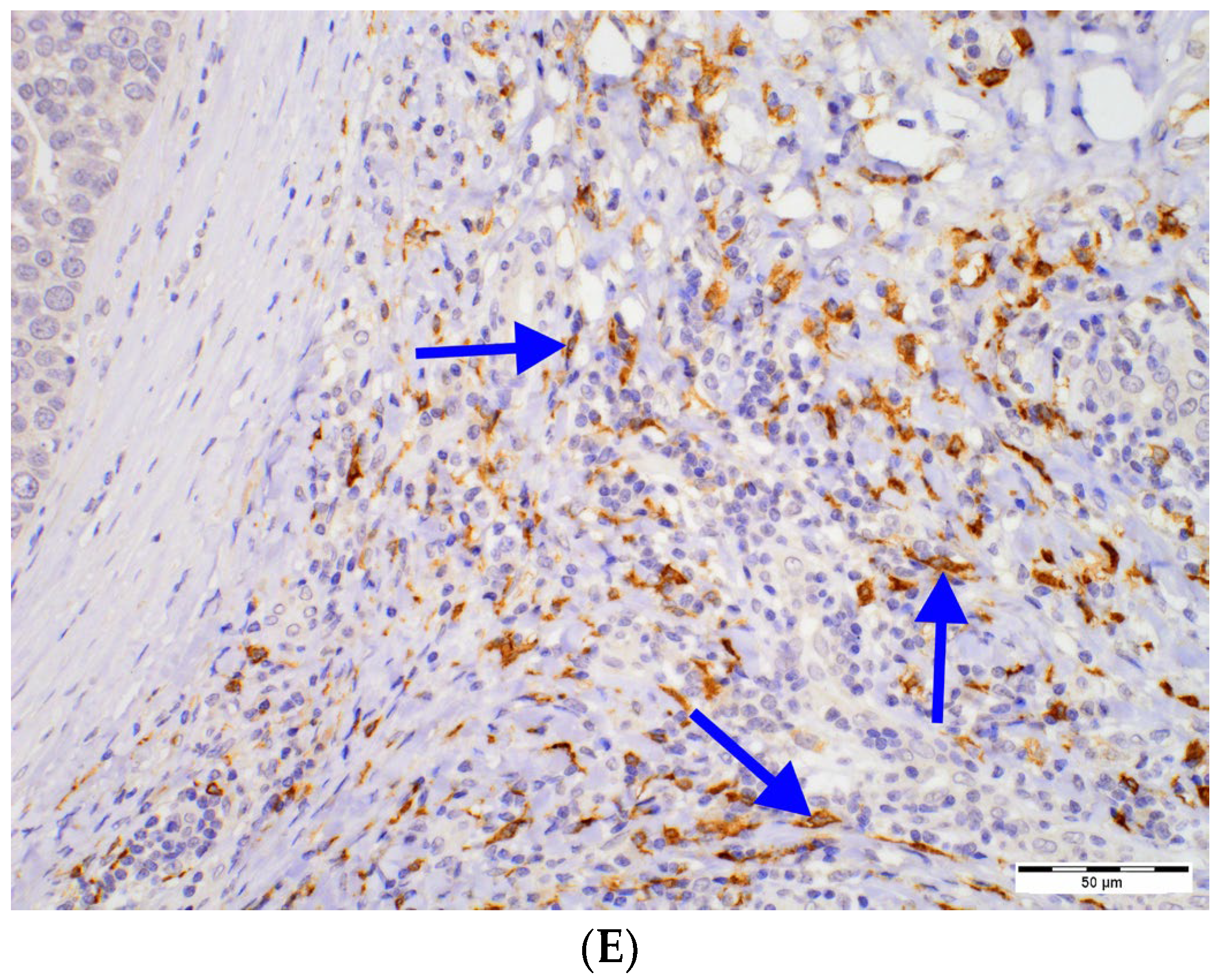
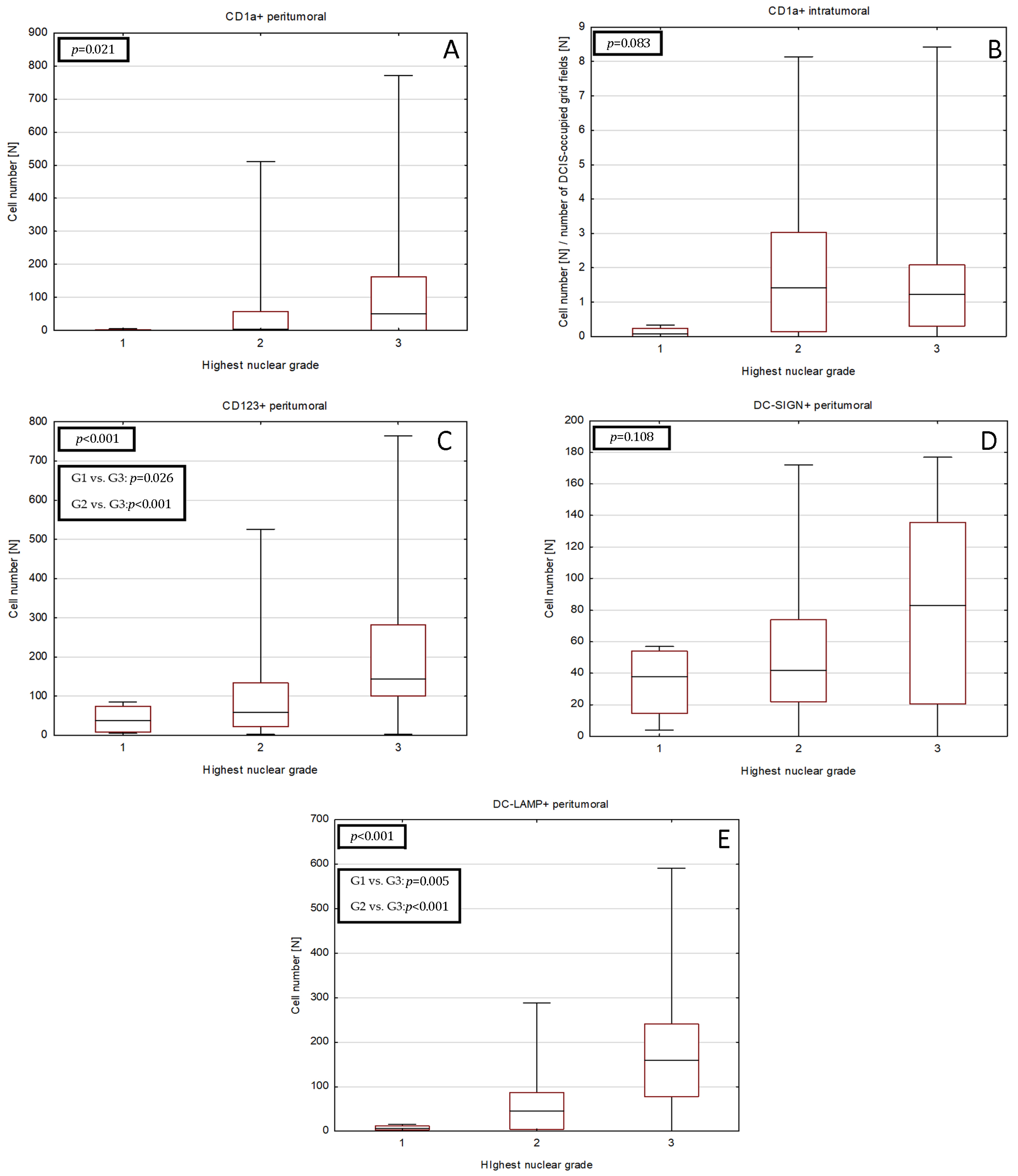
2.2. Relationships between DCs Densities and Histopathological Features of DCIS
| CD1a+ Peritumoral Cell Number [N] | Paget Disease | ||
| Absent | Present | p/pBH | |
| 4 [0–57] | 218 [91–311] | <0.001/<0.001 | |
| DC-LAMP+ peritumoral cell number [N] | Cribriform architectural type | ||
| Absent | Present | p/pBH | |
| 123 [65–176] | 30 [4–107] | 0.002/0.009 | |
| Comedo architectural type | |||
| Absent | Present | p/pBH | |
| 61 [6–119] | 177 [154–351] | <0.001/<0.001 | |
| Comedo necrosis | |||
| Absent | Present | p/pBH | |
| 28 [6–103] | 87 [24–177] | 0.007/0.02 | |
| Ductal spread | |||
| Absent | Present | p/pBH | |
| 7 [2–18] | 107 [45–172] | <0.001/<0.001 | |
| Lobular cancerization | |||
| Absent | Present | p/pBH | |
| 37 [3–119] | 107 [48–193] | 0.002/0.009 | |
2.3. Relationships between DCs Densities and Neoductgenesis
2.4. Correlations between DCs Densities and Tumor Size or HR Expression
2.5. Univariate Logistic Regression Analysis of Relationship between DCs Subpopulations and DCIS Histological Features
| Neoductgenesis Feature Score | CD1a+ Intratumoral Cell Number $ [N] | p/pBH | CD1a+ Peritumoral Cell Number [N] | p/pBH | CD123+ Peritumoral Cell Number [N] | p/pBH | DC-SIGN+ Peritumoral Cell Number [N] | p/pBH | DC-LAMP+ Peritumoral Cell Number [N] | p/pBH |
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration of ducts (score) | ||||||||||
| 0 | 1.7 [0.1–5.0] | 0.672/- | 116 [3–235] * | 0.032/ 0.048 | 115.5 [45–203] | 0.064/- | 38 [27–105] | 0.909/- | 64 [13–154] | 0.305/- |
| 1 | 1.2 [0.1–2.3] | 4 [0–81] * | 97 [40–191] | 46 [20–94] | 76 [15–176] | |||||
| 2 | 1.6 [0.1–1.9] | 5 [0–57] | 51 [10–84] | 46 [22–96] | 9 [5–87] | |||||
| Lymphocytic infiltration (score) | ||||||||||
| 0 | 0.1 [0–1.6] *,# | 0.002/ 0.005 | 0 [0–4] *,# | <0.001/ <0.001 | 51 [15–85] *,# | <0.001/ <0.001 | 45 [25–58] | 0.132/- | 7 [2–18] *,# | <0.001/ <0.001 |
| 1 | 1.7 [0.4–3.5] * | 14 [2–91] * | 87 [40–199] *,#,† | 42 [13–104] | 87 [45–167] *,#,† | |||||
| 2 | 1.7 [1.1–2.5] # | 86 [18–164] # | 184 [139–455] #,† | 94 [16–136] | 169 [128–316] #,† | |||||
| Fibrosis (score) | ||||||||||
| 0 | 1.2 [0–2.7] | 0.176/- | 3 [0–57] | 0.312/- | 59 [15–134] * | 0.012/ 0.018 | 40 [19–75] | 0.575/- | 14 [3–76] *,# | <0.001/ <0.001 |
| 1 | 2.2 [1–3] | 21 [2–81] | 102 [61–176] | 50 [31–94] | 87 [37–164] * | |||||
| 2 | 1.4 [0–1.7] | 50 [0–155] | 158 [52–302] * | 53 [9–130] | 174 [86–248] # | |||||
| CD1a+ intratumoral cell number $ [N] | p-value | CD1a+ peritumoral cell number [N] | p-value | CD123+ peritumoral cell number [N] | p-value | DC-SIGN+ peritumoral cell number [N] | p-value | DC-LAMP+ peritumoral cell number [N] | p-value | |
| Neoductgenesis | ||||||||||
| present | 1.6 [0.2–1.9] | 0.857 | 50 [0–102] | 0.185 | 158 [80–339] | 0.002 | 53 [13–124] | 0.728 | 174 [87–248] | <0.001 |
| absent | 1.3 [0.1–2.7] | 4 [0–76] | 69 [23–134] | 45 [22–76] | 41 [4–107] | |||||
| Estrogen Receptor Expression (%) | ||
|---|---|---|
| Spearman R | p/pBH | |
| CD1a+ peritumoral | −0.38 | <0.001/0.002 |
| CD123+ peritumoral | −0.43 | <0.001/<0.001 |
| DC-LAMP+ peritumoral | −0.62 | <0.001/<0.001 |
| Progesterone receptor expression (%) | ||
| Spearman R | p/pBH | |
| CD1a+ peritumoral | −0.42 | <0.001/<0.001 |
| CD123+ peritumoral | −0.48 | <0.001/0.001 |
| DC-LAMP+ peritumoral | −0.59 | <0.001/<0.001 |
| Maximal tumor size in histological examination (mm) | ||
| Spearman R | p/pBH | |
| CD123+ peritumoral | 0.29 | 0.007/0.01 |
| DC-LAMP+ peritumoral | 0.42 | <0.001/<0.001 |

| Number of Peripheral CD1a+ Cells >100 | Paget Disease | |
| OR (95%CI) | p/pBH | |
| 13.22 (2.99–58.39) | <0.001/<0.001 | |
| per 10 DC-LAMP+ cells increase | Cribriform architectural type | |
| OR (95%CI) | p/pBH | |
| 0.95 (0.91–0.99) | 0.017/0.017 | |
| Comedo architectural type | ||
| OR (95%CI) | p/pBH | |
| 1.13 (1.06–1.20) | <0.001/<0.001 | |
| Comedo necrosis | ||
| OR (95%CI) | p/pBH | |
| 1.08 (1.02–1.14) | 0.010/0.012 | |
| Ductal spread | ||
| OR (95%CI) | p/pBH | |
| 1.21 (1.08–1.36) | 0.001/0.003 | |
| Lobular cancerization | ||
| OR (95%CI) | p/pBH | |
| 1.08 (1.03–1.14) | 0.003/0.005 | |
3. Discussion
4. Material and Methods
4.1. Patient Selection
4.2. Immunohistochemical Techniques
| Antibody | Clone | Dilution | Antigen Retrieval | Incubation Time | Manufacturer | Detection System |
|---|---|---|---|---|---|---|
| CD1a | MTB1 | 8:100 | Citrate | 60 min | Novocastra | Immunologic |
| CD123 | BR4MS | 1:100 | EDTA | 30 min | Novocastra | Immunologic |
| DC-LAMP | polyclonal | 1:50 | EDTA | 30 min | Novus | Immunologic |
| DC-SIGN | 5D7 | 1:50 | EDTA | 60 min | Abcam | Immunologic |
| ER | SP1 | RTU | Citrate | 30 min | Roche | Ultra Vision Dab Detection Kit |
| PR | 1E2 | RTU | Citrate | 60 min | Roche | Ultra Vision Dab Detection Kit |
4.3. Histologic Evaluation, DCs Scoring and Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BC | breast cancer |
| CD1a | cluster of differentiation 1a |
| CD83 | cluster of differentiation 83 |
| CD123 | cluster of differentiation 123 |
| DCIS | ductal carcinoma in situ |
| DCs | dendritic cells |
| DC-LAMP | dendritic-cell-lysosome-associated membrane glycoprotein |
| DC-SIGN | dendritic-cell-specific intercellular-adhesion-molecule-3-grabbing non-integrin |
| DNA | deoxyribonucleic acid |
| ER | estrogen receptor |
| HBV | hepatitis B virus |
| HER2 | human epidermal growth factor receptor 2 |
| HPFs | high-power fields |
| IBC | invasive breast cancer |
| LI | lymphocytic infiltration |
| PR | progesterone receptor |
| TDLU | terminal ductal lobular unit |
| TGF-β | transforming growth factor β |
References
- Martínez-Pérez, C.; Turnbull, A.K.; Ekatah, G.E.; Arthur, L.M.; Sims, A.H.; Thomas, J.S.; Dixon, J.M. Current treatment trends and the need for better predictive tools in the management of ductal carcinoma in situ of the breast. Cancer Treat Rev. 2017, 55, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dettogni, R.S.; Stur, E.; Laus, A.C.; da Costa Vieira, R.A.; Marques, M.M.C.; Santana, I.V.V.; Pulido, J.Z.; Ribeiro, L.F.; de Jesus Parmanhani, N.; Agostini, L.P.; et al. Potential biomarkers of ductal carcinoma in situ progression. BMC Cancer 2020, 20, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCormick, B.; Winter, K.; Hudis, C.; Kuerer, H.M.; Rakovitch, E.; Smith, B.L.; Sneige, N.; Moughan, J.; Shah, A.; Germain, I.; et al. RTOG 9804: A prospective randomized trial for goodrisk ductal carcinoma in situ comparing radiotherapy with observation. J. Clin. Oncol. 2015, 33, 709–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Sollie, T.; Tot, T.; Pinder, S.E.; Amini, R.-M.; Blomqvist, C.; Fjällskog, M.-L.; Christensson, G.; Abdsaleh, S.; Wärnberg, F. Breast Cancer with Neoductgenesis: Histopathological Criteria and Its Correlation with Mammographic and Tumour Features. Int. J. Breast Cancer. 2014, 2014, 581706. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Sollie, T.; Tot, T.; Blomqvist, C.; Abdsaleh, S.; Liljegren, G.; Wärnberg, F. Ductal Breast Carcinoma in Situ: Mammographic Features and Its Relation to Prognosis and Tumour Biology in a Population Based Cohort. Int. J. Breast Cancer 2017, 2017, 4351319. [Google Scholar] [CrossRef] [Green Version]
- Łazarczyk, A.; Streb, J.; Hałubiec, P.; Streb-Smoleń, A.; Jach, R.; Hodorowicz-Zaniewska, D.; Łuczyńska, E.; Szpor, J. Ne-oductgenesis in Ductal Carcinoma In Situ Coexists with Morphological Abnormalities Characteristic for More Aggressive Tumor Biology. Diagnostics 2023, 13, 787. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Cheng, Y.; Cao, X. Dendritic cell migration in inflammation and immunity. Cell Mol. Immunol. 2021, 18, 2461–2471. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Heikkila, P.; von Smitten, K.; Vakkila, J.; Leidenius, M. Metastasis to sentinel lymph nodes in breast cancer is associated with maturation arrest of dendritic cells and poor co-localization of dendritic cells and CD8+ T cells. Virchows Arch. 2011, 459, 391–398. [Google Scholar] [CrossRef]
- Szpor, J.; Streb, J.; Glajcar, A.; Sadowski, P.; Streb-Smoleń, A.; Jach, R.; Hodorowicz-Zaniewska, D. Presence of Dendritic Cell Subsets in Sentinel Nodes of Breast Cancer Patients Is Related to Nodal Burden. Int. J. Mol. Sci. 2022, 23, 8461. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Jin, R.; Liu, F.; Rao, H.; Wei, L.; Chen, H.; Feng, B. LAMP3 expression in the liver is involved in T cell activation and adaptive immune regulation in hepatitis B virus infection. Front. Immunol. 2023, 14, 1127572. [Google Scholar] [CrossRef]
- Szpor, J.; Streb, J.; Glajcar, A.; Frączek, P.; Winiarska, A.; Tyrak, K.E.; Basta, P.; Okoń, K.; Jach, R.; Hodorowicz-Zaniewska, D. Dendritic cells are associated with prognosis and survival in breast cancer. Diagnostics 2021, 11, 702. [Google Scholar] [CrossRef] [PubMed]
- Merlotti, A.; Dantas, E.; Remes Lenicov, F.; Ceballos, A.; Jancic, C.; Varese, A.; Rubione, J.; Stover, S.; Geffner, J.; Sabatté, J. Fucosylated clusterin in semen promotes the uptake of stress-damaged proteins by dendritic cells via DC-SIGN. Hum. Reprod. 2015, 30, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Goff, S.L.; Danforth, D.N. The Role of Immune Cells in Breast Tissue and Immunotherapy for the Treatment of Breast Cancer. Clin. Breast Cancer. 2021, 21, e63–e73. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, N.N.; Basu, A.; Gautam, N.; Ramamoorthi, G.; Kodumudi, K.N.; Kumer, N.B.; Loftus, L.; Czerniecki, B.J. Intercepting Premalignant, Preinvasive Breast Lesions Through Vaccination. Front. Immunol. 2021, 12, 4864. [Google Scholar] [CrossRef] [PubMed]
- Stovgaard, E.S.; Nielsen, D.; Hogdall, E.; Balslev, E. Triple negative breast cancer—Prognostic role of immune-related factors: A systematic review. Acta Oncol. 2018, 57, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.P.; Derakhshandeh, R.; Jones, L.; Webb, T.J. Mechanisms of immune evasion in breast cancer. BMC Cancer 2018, 18, 556. [Google Scholar] [CrossRef] [Green Version]
- Anstine, L.J.; Keri, R. A new view of the mammary epithelial hierarchy and its implications for breast cancer initiation and metastasis. J. Cancer Metastasis Treat. 2019, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Gatti-Mays, M.E.; Balko, J.M.; Gameiro, S.R.; Bear, H.D.; Prabhakaran, S.; Fukui, J.; Disis, M.L.; Nanda, R.; Gulley, J.L.; Kalinsky, J.; et al. If we build it they will come: Targeting the immune response to breast cancer. npj Breast Cancer 2019, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Shihab, I.; Khalil, B.A.; Elemam, N.M.; Hachim, I.Y.; Hachim, M.Y.; Hamoudi, R.A.; Maghazachi, A.A. Understanding the role of innate immune cells and identifying genes in breast cancer microenvironment. Cancers 2020, 12, 2226. [Google Scholar] [CrossRef]
- Nelson, A.C.; Machado, H.L.; Schwertfeger, K.L. Breaking through to the Other Side: Microenvironment Contributions to DCIS Initiation and Progression. J. Mammary Gland Biol. Neoplasia 2018, 23, 207–221. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours, 5th ed.; International Agency for Pesearch on Cancer: Lyon, France, 2019; Volume 2. [Google Scholar]
- Martinet, L.; Filleron, T.; le Guellec, S.; Rochaix, P.; Garrido, I.; Girard, J.-P. High Endothelial Venule Blood Vessels for Tumor-Infiltrating Lymphocytes Are Associated with Lymphotoxin β–Producing Dendritic Cells in Human Breast Cancer. J. Immunol. 2013, 191, 2001–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gervais, A.; Levêque, J.; Bouet-Toussaint, F.; Burtin, F.; Lesimple, T.; Sulpice, L.; Patard, J.-J.; Genetet, N.; Catros-Quemener, V. Dendritic cells are defective in breast cancer patients: A potential role for polyamine in this immunodeficiency. Breast Cancer Res. 2005, 7, R326–R335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Cunha, A.; Michelin, M.A.; Murta, E.F.C. Pattern response of dendritic cells in the tumor microenvironment and breast cancer. World J. Clin. Oncol. 2014, 5, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Jia, Z.; Zhang, H.; Gong, Z.; Feng, J.; Xu, H. Identification and validation of tumor microenvironment-related prognostic biomarkers in breast cancer. Transl. Cancer Res. TCR 2021, 10, 4355–4364. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Wu, W.; Amei, A.; Yan, X.; Lu, L.; Wang, Z. Regulation and characterization of tumor-infiltrating immune cells in breast cancer. Int. Immunopharmacol. 2021, 90, 107167. [Google Scholar] [CrossRef]
- El Deeb, N.M.F.; Mehanna, R.A. Assessment of Maturation Status of Tumor-Infiltrating Dendritic Cells in Invasive Ductal Carcinoma of the Breast: Relation with Vascular Endothelial Growth Factor Expression. Turk Patoloji Derg. Turk. J. Pathol. 2013, 29, 193–200. [Google Scholar] [CrossRef]
- Sisirak, V.; Vey, N.; Goutagny, N.; Renaudineau, S.; Malrfroy, M.; Thys, S.; Treilleux, I.; Intidhar Labidi-Galy, S.; Bachelot, T.; Dezutter-Dambuyant, C.; et al. Breast cancer-derived transforming growth factor-β and tumor necrosis factor-α compromise interferon-α production by tumor-associated plasmacytoid dendritic cells. Int. J. Cancer 2013, 133, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Giorello, M.B.; Matas, A.; Marenco, P.; Davies, K.M.; Borzone, F.R.; de Luján Calcagno, M.; García-Rivello, H.; Wernicke, A.; Martinez, L.M.; Labovsky, V.; et al. CD1a- and CD83-positive dendritic cells as prognostic markers of metastasis development in early breast cancer patients. Breast Cancer 2021, 28, 1328–1339. [Google Scholar] [CrossRef]
- Arantes Perez, A.; Balabram, D.; De Almeida Salles, M.; Gobbi, H. Ductal Carcinoma In Situ of the Breast: Correlation between Histopathological Features and Age of Patients. Diagn. Pathol. 2014, 9, 227. Available online: http://www.diagnosticpathology.org/content/9/1/227 (accessed on 30 January 2023). [CrossRef] [Green Version]
- Coventry, B.J.; Lee, P.L.; Gibbs, D.; Hart, D. Dendritic cell density and activation status in human breast cancer-CD1a, CMRF-44, CMRF-56 and CD-83 expression. Br. J. Cancer 2002, 86, 546–551. [Google Scholar] [CrossRef] [Green Version]
- López, C.; Gibert-Ramos, A.; Bosch, R.; Korzynska, A.; García-Rojo, M.; Bueno, G.; García-Fontgivell, J.F.; Martínez-González, S.; Fontoura, L.; Gras Navarro, A.; et al. Differences in the Immune Response of the Nonmetastatic Axillary Lymph Nodes between Triple-Negative and Luminal a Breast Cancer Surrogate Subtypes. Am. J. Pathol. 2021, 191, 545–554. [Google Scholar] [CrossRef]
- Ozkan-Gurdal, S.; Cabioglu, N.; Ozcinar, B.; Muslumanoglu, M.; Ozmen, V.; Kecer, M.; Yavuz, E.; Igci, A. Factors predicting microinvasion in ductal carcinoma in situ. Asian Pac. J. Cancer Prev. 2014, 15, 55–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, T.; Yanai, K.; Tokuda, S.; Nakazawa, Y.; Kurozumi, S.; Obayashi, S.; Yajima, R.; Hirakata, T.; Kuwano, H. Clinicopathological features of ductal carcinoma in situ from 18F-FDG-PET findings. Anticancer Res. 2017, 37, 5053–5056. [Google Scholar] [CrossRef] [PubMed]
- al Nemer, A.M. Histologic factors predicting invasion in patients with ductal carcinoma in situ (DCIS) in the preoperative core biopsy. Pathol. Res. Pract. 2017, 213, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, M.; Reverté, L.; Sauras, E.; Gallardo, N.; Bosch, R.; Roso, A.; Petit, A.; Peg, V.; Riu, F.; García-Fontgivell, J.; et al. Prognostic Implications of the Residual Tumor Microenvironment after Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer Patients without Pathological Complete Response. Cancers 2023, 15, 597. [Google Scholar] [CrossRef]
- Argenziano, M.; Occhipinti, S.; Scomparin, A.; Angelini, C.; Novelli, F.; Soster, M.; Giovarelli, M.; Cavalli, R. Exploring chi-tosan-shelled nanobubbles to improve HER2 + immunotherapy via dendritic cell targeting. Drug Deliv. Transl. Res. 2022, 12, 2007–2018. [Google Scholar] [CrossRef]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Tabar, L.; Chen, H.H.T.; Yen, M.F.A.; Tot, T.; Tung, T.H.; Chen, L.S.; Chiu, Y.H.; Duffy, S.W.; Smith, R.A. Mammographic tumor features can predict long-term outcomes reliably in women with 1-14-mm invasive breast carcinoma: Suggestions for the reconsideration of current therapeutic practice and the TNM classification system. Cancer 2004, 101, 1745–1759. [Google Scholar] [CrossRef]
- Valenzi, E.; Tabib, T.; Papazoglou, A.; Sembrat, J.; Trejo Bittar, H.E.; Rojas, M.; Lafyatis, R. Disparate Interferon Signaling and Shared Aberrant Basaloid Cells in Single-Cell Profiling of Idiopathic Pulmonary Fibrosis and Systemic Sclerosis-Associated Interstitial Lung Disease. Front. Immunol. 2021, 12, 595811. [Google Scholar] [CrossRef]
- Kafaja, S.; Valera, I.; Divekar, A.A.; Saggar, R.; Abtin, F.; Furst, D.E.; Khanna, D.; Singh, R.R. pDCs in lung and skin fibrosis in a bleomycin-induced model and patients with systemic sclerosis. JCI Insight 2018, 3, e98380. [Google Scholar] [CrossRef] [Green Version]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 23, 104. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Rong, Y.; Tang, X.; Yi, K.; Qi, P.; Hou, J.; Liu, W.; He, Y.; Gao, X.; Yuan, C.; et al. Engineered exosomes as an In Situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol. Cancer 2022, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Jugniot, N.; Dahl, J.J.; Paulmurugan, R. Immunotheranostic microbubbles (iMBs)—A modular platform for dendritic cell vaccine delivery applied to breast cancer immunotherapy. J. Exp. Clin. Cancer Res. 2022, 41, 299. [Google Scholar] [CrossRef]
- Bulgarelli, J.; Tazzari, M.; Granato, A.M.; Ridolfi, L.; Maiocchi, S.; de Rosa, F.; Petrini, M.; Pancisi, E.; Gentili, G.; Vergani, B.; et al. Dendritic Cell Vaccination in Metastatic Melanoma Turns “Non-T Cell Inflamed” Into “T-Cell Inflamed” Tumors. Front. Immunol. 2019, 9, 2353. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Li, J.; Huang, M.; Cui, Q.; Liu, X.; Sun, K. Dendritic cell vaccines in breast cancer: Immune modulation and immunotherapy. Biomed. Pharm. 2023, 162, 114685. [Google Scholar] [CrossRef] [PubMed]
- Ridolfi, L.; de Rosa, F.; Fiammenghi, L.; Petrini, M.; Granato, A.M.; Ancarani, V.; Pancisi, E.; Soldati, V.; Cassan, S.; Bulgarelli, J.; et al. Complementary vaccination protocol with dendritic cells pulsed with autologous tumour lysate in patients with resected stage III or IV mela-noma: Protocol for a phase II randomised trial (ACDC Adjuvant Trial). BMJ Open 2018, 8, e021701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lester, S.C.; Bose, S.; Chen, Y.-Y.; Connolly, J.L.; de Baca, M.E.; Fitzgibbons, P.L.; Hayes, D.F.; Kleer, C.; O’Malley, F.P.; Page, D.L.; et al. CAP Laboratory Improvement Programs Protocol for the Examination of Specimens from Patients with Invasive Carcinoma of the Breast. Arch. Pathol. Lab. Med. 2009, 133, 1515–1538. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpor, J.; Streb, J.; Glajcar, A.; Streb-Smoleń, A.; Łazarczyk, A.; Korta, P.; Brzuszkiewicz, K.; Jach, R.; Hodorowicz-Zaniewska, D. Dendritic Cell Subpopulations Are Associated with Morphological Features of Breast Ductal Carcinoma In Situ. Int. J. Mol. Sci. 2023, 24, 9918. https://doi.org/10.3390/ijms24129918
Szpor J, Streb J, Glajcar A, Streb-Smoleń A, Łazarczyk A, Korta P, Brzuszkiewicz K, Jach R, Hodorowicz-Zaniewska D. Dendritic Cell Subpopulations Are Associated with Morphological Features of Breast Ductal Carcinoma In Situ. International Journal of Molecular Sciences. 2023; 24(12):9918. https://doi.org/10.3390/ijms24129918
Chicago/Turabian StyleSzpor, Joanna, Joanna Streb, Anna Glajcar, Anna Streb-Smoleń, Agnieszka Łazarczyk, Paulina Korta, Karolina Brzuszkiewicz, Robert Jach, and Diana Hodorowicz-Zaniewska. 2023. "Dendritic Cell Subpopulations Are Associated with Morphological Features of Breast Ductal Carcinoma In Situ" International Journal of Molecular Sciences 24, no. 12: 9918. https://doi.org/10.3390/ijms24129918






