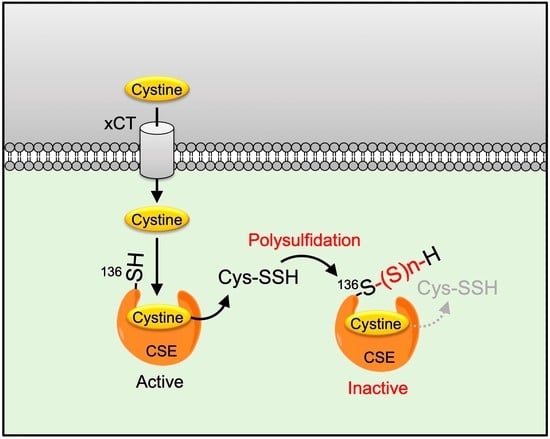
Cystathionine γ-Lyase Self-Inactivates by Polysulfidation during Cystine Metabolism
Abstract
:1. Introduction
2. Results
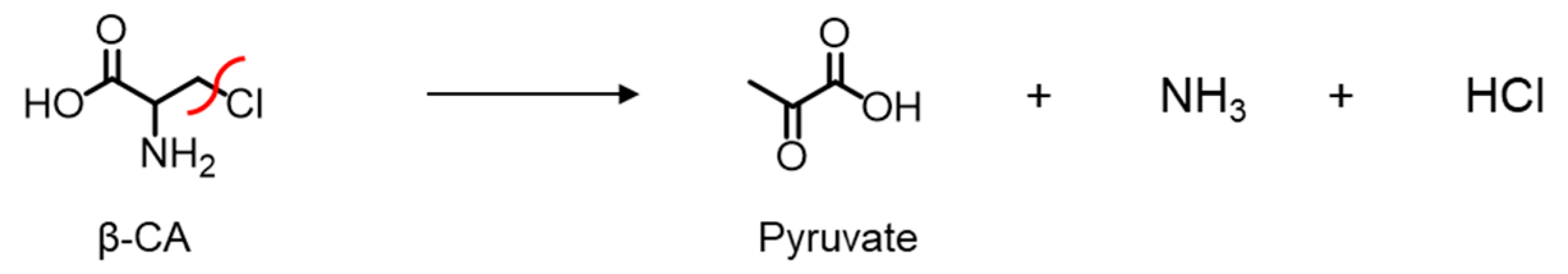
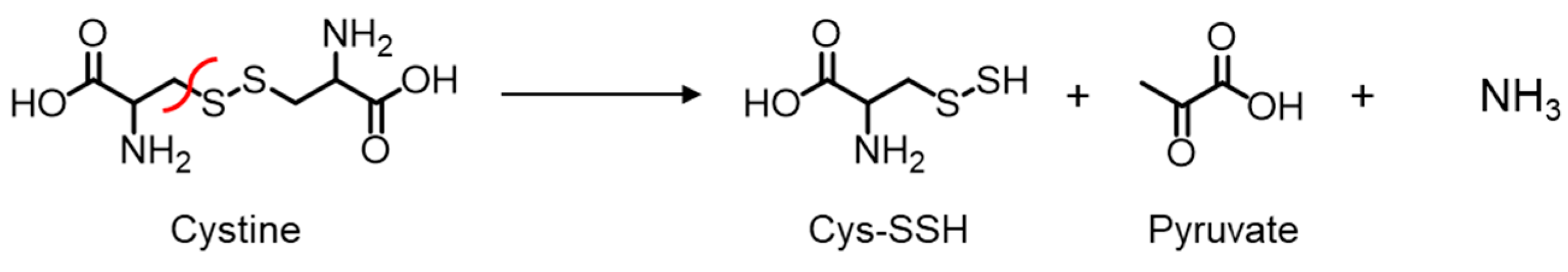
2.1. Cys136 and/or Cys171 Are Redox Sensors of CSE during the β-Lyase Activity toward Cystine to Generate Cys-SSH
2.2. Exogeneous Cys-SSH and Polysulfides Inhibit CSE β-Lyase Activity toward Cystine
2.3. Cys136 Is an Essential Site for the Inactivation of CSE by Polysulfides
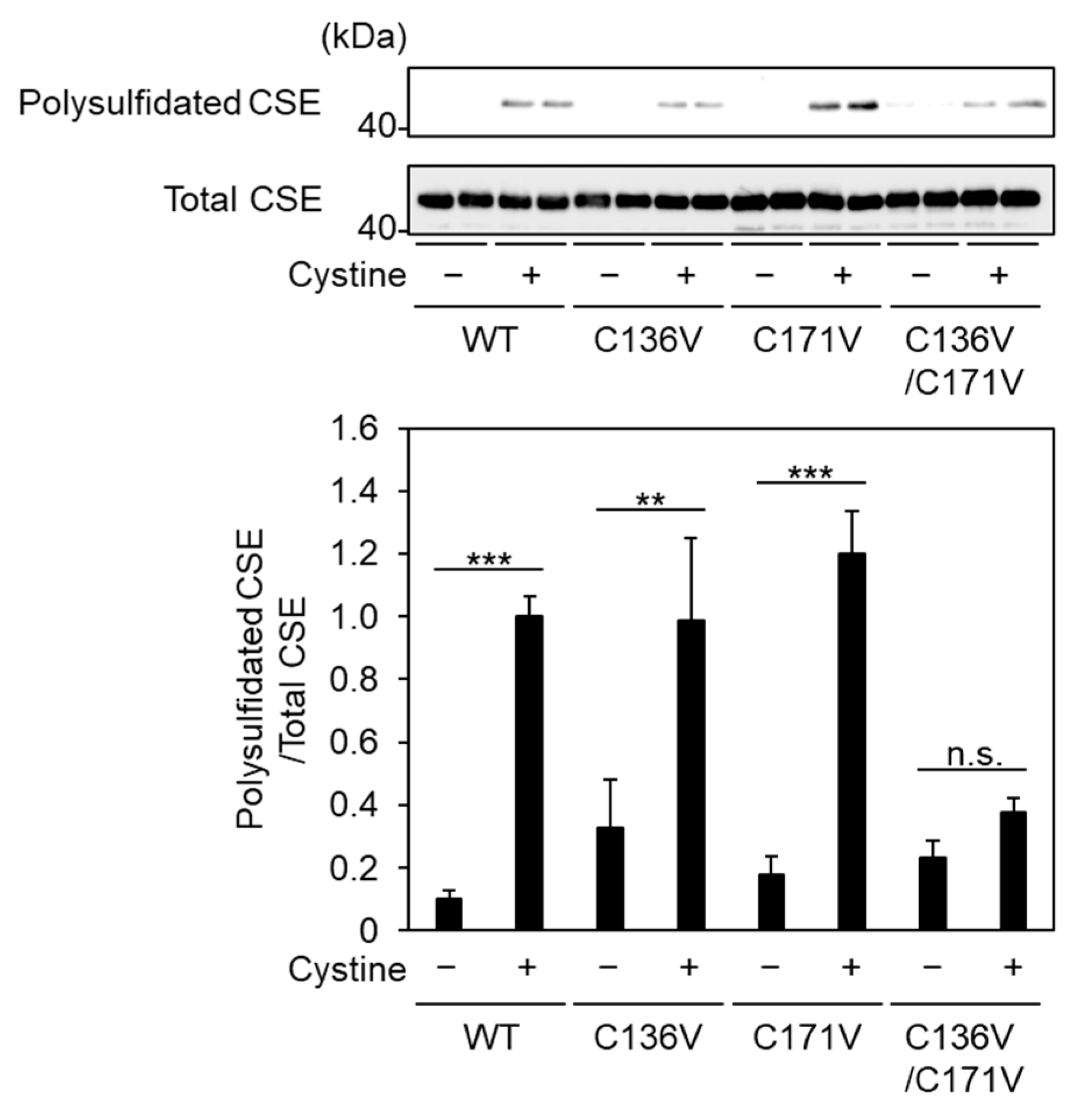
2.4. Cys136 and/or Cys 171 Represents the Sites of Polysulfidation on CSE
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plasmid Construction
4.3. CSE Purification
4.4. Measurement of CSE Activity
4.5. Live-Cell Fluorescence Imaging of Cys-SSH
4.6. Detection of Polysulfidated CSE Using a Biotin-Polyethyleneglycol-Conjugated Maleimide Capture Assay
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| β-CA | β-chloro-L-alanine |
| biotin-PEG-MAL | biotin-polyethylene glycol-conjugated maleimide |
| CBS | cystathionine β-synthase |
| CSE | cystathionine-γ-lyase |
| Cys-SSH | cysteine hydropersulfide |
| DTT | dithiothreitol |
| ECL | enhanced chemiluminescence |
| NO | nitric oxide |
| PAGE | polyacrylamide gel electrophoresis |
| PLP | pyridoxal 5′-phosphate |
| SSP4 | sulfane sulfur probe 4 |
References
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matoba, Y.; Noda, M.; Yoshida, T.; Oda, K.; Ezumi, Y.; Yasutake, C.; Izuhara-Kihara, H.; Danshiitsoodol, N.; Kumagai, T.; Sugiyama, M. Catalytic specificity of the Lactobacillus plantarum cystathionine γ-lyase presumed by the crystallographic analysis. Sci. Rep. 2020, 10, 14886. [Google Scholar] [CrossRef] [PubMed]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akaike, T.; Ida, T.; Wei, F.Y.; Nishida, M.; Kumagai, Y.; Alam, M.M.; Ihara, H.; Sawa, T.; Matsunaga, T.; Kasamatsu, S.; et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017, 8, 1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabil, O.; Yadav, V.; Banerjee, R. Heme-dependent Metabolite Switching Regulates H2S Synthesis in Response to Endoplasmic Reticulum (ER) Stress. J. Biol. Chem. 2016, 291, 16418–16423. [Google Scholar] [CrossRef] [Green Version]
- Hine, C.; Harputlugil, E.; Zhang, Y.; Ruckenstuhl, C.; Lee, B.C.; Brace, L.; Longchamp, A.; Treviño-Villarreal, J.H.; Mejia, P.; Ozaki, C.K.; et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 2015, 160, 132–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, R. Catalytic promiscuity and heme-dependent redox regulation of H(2)S synthesis. Curr. Opin. Chem. Biol. 2017, 37, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Doulias, P.T.; Tenopoulou, M.; Greene, J.L.; Raju, K.; Ischiropoulos, H. Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Sci. Signal. 2013, 6, rs1. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, D.G.F.; Nunes, J.; Tomé, C.S.; Zuhra, K.; Costa, J.M.F.; Antunes, A.M.M.; Giuffrè, A.; Vicente, J.B. Human Cystathionine γ-Lyase Is Inhibited by s-Nitrosation: A New Crosstalk Mechanism between NO and H2S. Antioxidants 2021, 10, 1391. [Google Scholar] [CrossRef]
- Luo, C.; Ji, D.; Li, Y.; Cao, Y.; Zhang, S.; Yan, W.; Xue, K.; Chai, J.; Wu, Y.; Liu, H.; et al. Abnormal nitration and S-sulfhydration modification of Sp1-CSE-H2S pathway trap the progress of hyperhomocysteinemia into a vicious cycle. Free Radic. Biol. Med. 2021, 164, 20–33. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef] [Green Version]
- Ogasawara, Y.; Ishii, K.; Tanabe, S. Enzymatic assay of gamma-cystathionase activity using pyruvate oxidase-peroxidase sequential reaction. J. Biochem. Biophys. Methods 2002, 51, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Shieh, M.; Ni, X.; Xu, S.; Lindahl, S.P.; Yang, M.; Matsunaga, T.; Flaumenhaft, R.; Akaike, T.; Xian, M. Shining a light on SSP4: A comprehensive analysis and biological applications for the detection of sulfane sulfurs. Redox Biol. 2022, 56, 102433. [Google Scholar] [CrossRef]
- Jung, M.; Kasamatsu, S.; Matsunaga, T.; Akashi, S.; Ono, K.; Nishimura, A.; Morita, M.; Abdul Hamid, H.; Fujii, S.; Kitamura, H.; et al. Protein polysulfidation-dependent persulfide dioxygenase activity of ethylmalonic encephalopathy protein 1. Biochem. Biophys. Res. Commun. 2016, 480, 180–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakanyan, V. Reactive Chemicals and Electrophilic Stress in Cancer: A Minireview. High. Throughput 2018, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Araki, S.; Takata, T.; Tsuchiya, Y.; Watanabe, Y. Reactive sulfur species impair Ca2+/calmodulin-dependent protein kinase II via polysulfidation. Biochem. Biophys. Res. Commun. 2019, 508, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Ihara, H.; Hatano, N.; Tsuchiya, Y.; Akaike, T.; Watanabe, Y. Reactive sulfur species inactivate Ca2+/calmodulin-dependent protein kinase IV via S-polysulfidation of its active-site cysteine residue. Biochem. J. 2017, 474, 2547–2562. [Google Scholar] [CrossRef]
- Ono, K.; Akaike, T.; Sawa, T.; Kumagai, Y.; Wink, D.A.; Tantillo, D.J.; Hobbs, A.J.; Nagy, P.; Xian, M.; Lin, J.; et al. Redox chemistry and chemical biology of H2S, hydropersulfides, and derived species: Implications of their possible biological activity and utility. Free Radic. Biol. Med. 2014, 77, 82–94. [Google Scholar] [CrossRef] [Green Version]
- Dóka, É.; Ida, T.; Dagnell, M.; Abiko, Y.; Luong, N.C.; Balog, N.; Takata, T.; Espinosa, B.; Nishimura, A.; Cheng, Q.; et al. Control of protein function through oxidation and reduction of persulfidated states. Sci. Adv. 2020, 6, eaax8358. [Google Scholar] [CrossRef] [Green Version]
- Filipovic, M.R. Persulfidation (S-sulfhydration) and H2S. Handb. Exp. Pharmacol. 2015, 230, 29–59. [Google Scholar] [CrossRef]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol. Cell 2012, 45, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altaany, Z.; Ju, Y.; Yang, G.; Wang, R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci. Signal. 2014, 7, ra87. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Hatano, N.; Kambe, T.; Miyamoto, Y.; Ihara, H.; Yamamoto, H.; Sugimoto, K.; Kume, K.; Yamaguchi, F.; Tokuda, M.; et al. Nitric oxide-mediated modulation of calcium/calmodulin-dependent protein kinase II. Biochem. J. 2008, 412, 223–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Soud, H.M.; Wang, J.; Rousseau, D.L.; Fukuto, J.M.; Ignarro, L.J.; Stuehr, D.J. Neuronal nitric oxide synthase self-inactivates by forming a ferrous-nitrosyl complex during aerobic catalysis. J. Biol. Chem. 1995, 270, 22997–23006. [Google Scholar] [CrossRef] [Green Version]
- Takata, T.; Araki, S.; Tsuchiya, Y.; Watanabe, Y. Persulfide Signaling in Stress-Initiated Calmodulin Kinase Response. Antioxid. Redox Signal. 2020, 33, 1308–1319. [Google Scholar] [CrossRef]
- Akiyama, M.; Unoki, T.; Aoki, H.; Nishimura, A.; Shinkai, Y.; Warabi, E.; Nishiyama, K.; Furumoto, Y.; Anzai, N.; Akaike, T.; et al. Cystine-dependent antiporters buffer against excess intracellular reactive sulfur species-induced stress. Redox Biol. 2022, 57, 102514. [Google Scholar] [CrossRef]
- Nishi, N.; Tanabe, H.; Oya, H.; Urushihara, M.; Miyanaka, H.; Wada, F. Identification of probasin-related antigen as cystathionine gamma-lyase by molecular cloning. J. Biol. Chem. 1994, 269, 1015–1019. [Google Scholar] [CrossRef]
- Shinkai, Y.; Masuda, A.; Akiyama, M.; Xian, M.; Kumagai, Y. Cadmium-Mediated Activation of the HSP90/HSF1 Pathway Regulated by Reactive Persulfides/Polysulfides. Toxicol. Sci. 2017, 156, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Steegborn, C.; Clausen, T.; Sondermann, P.; Jacob, U.; Worbs, M.; Marinkovic, S.; Huber, R.; Wahl, M.C. Kinetics and inhibition of recombinant human cystathionine gamma-lyase. Toward the rational control of transsulfuration. J. Biol. Chem. 1999, 274, 12675–12684. [Google Scholar] [CrossRef] [Green Version]
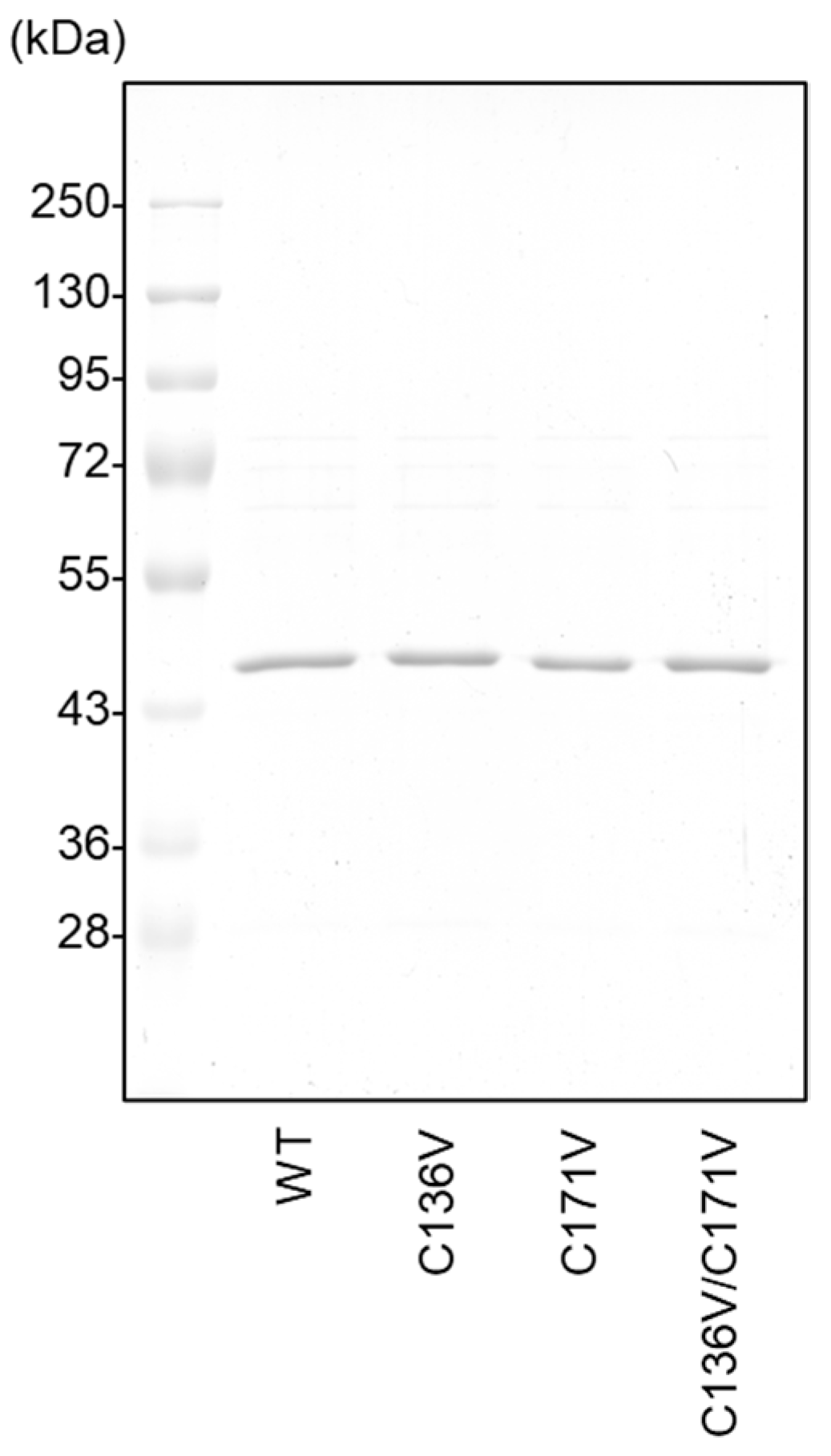
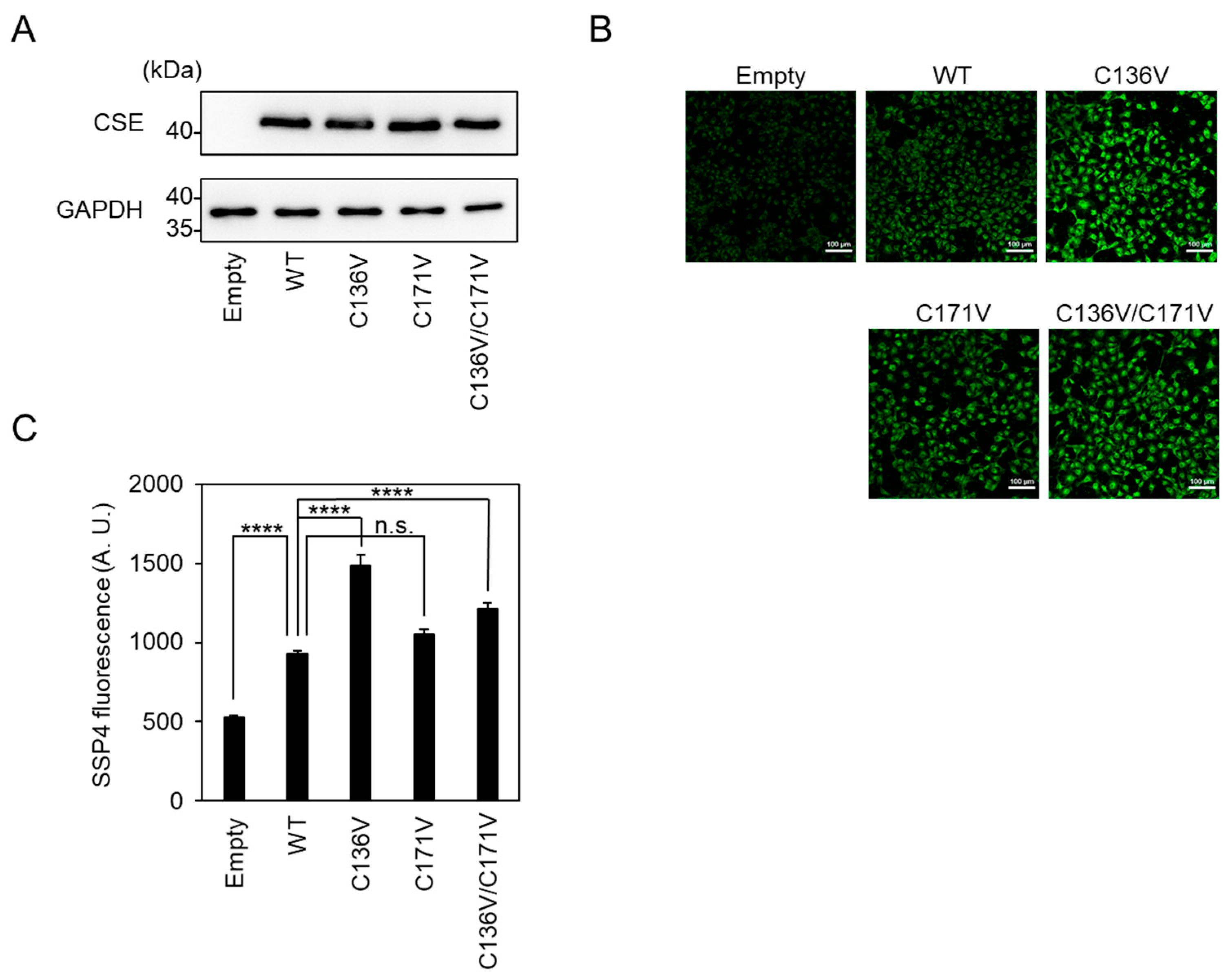
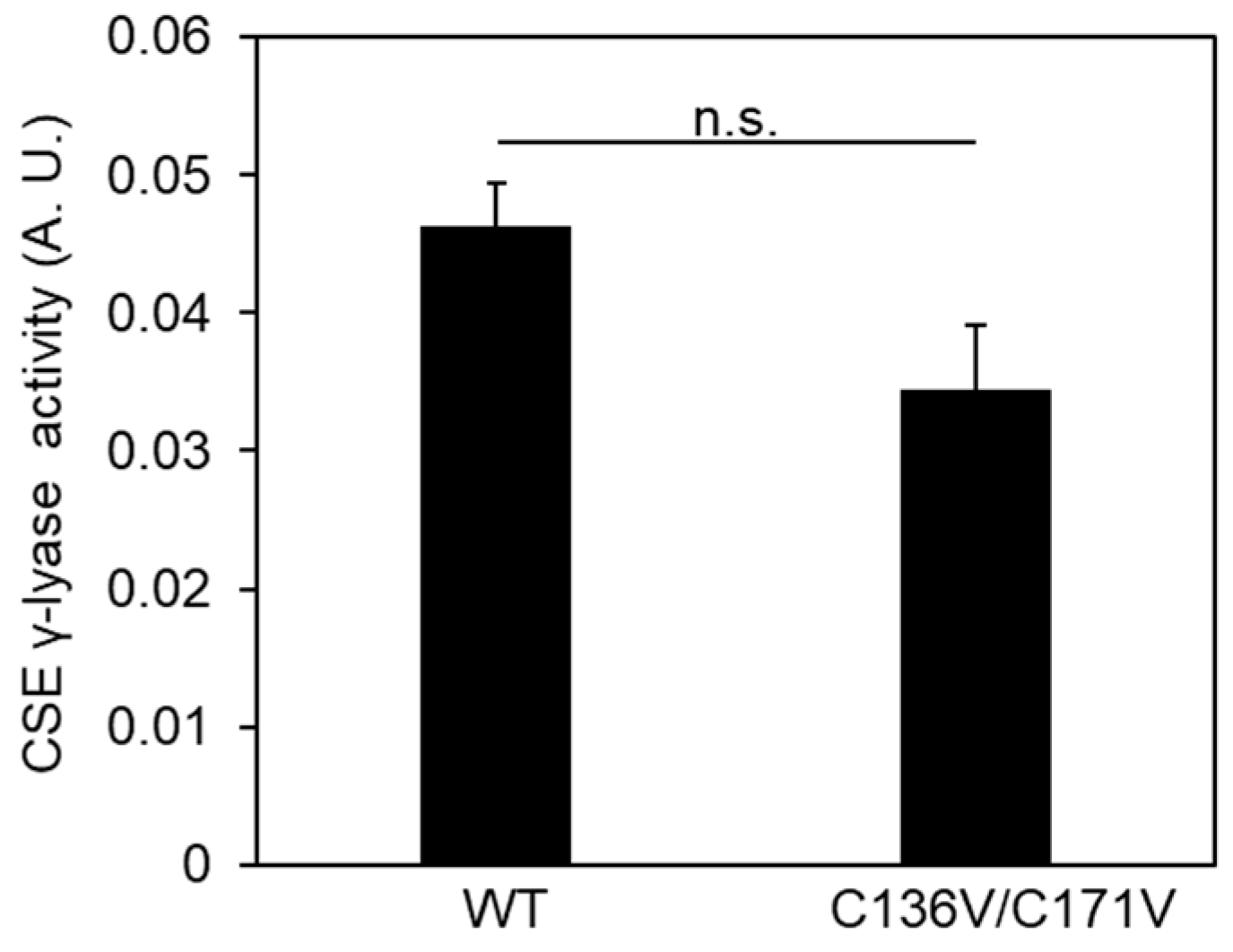
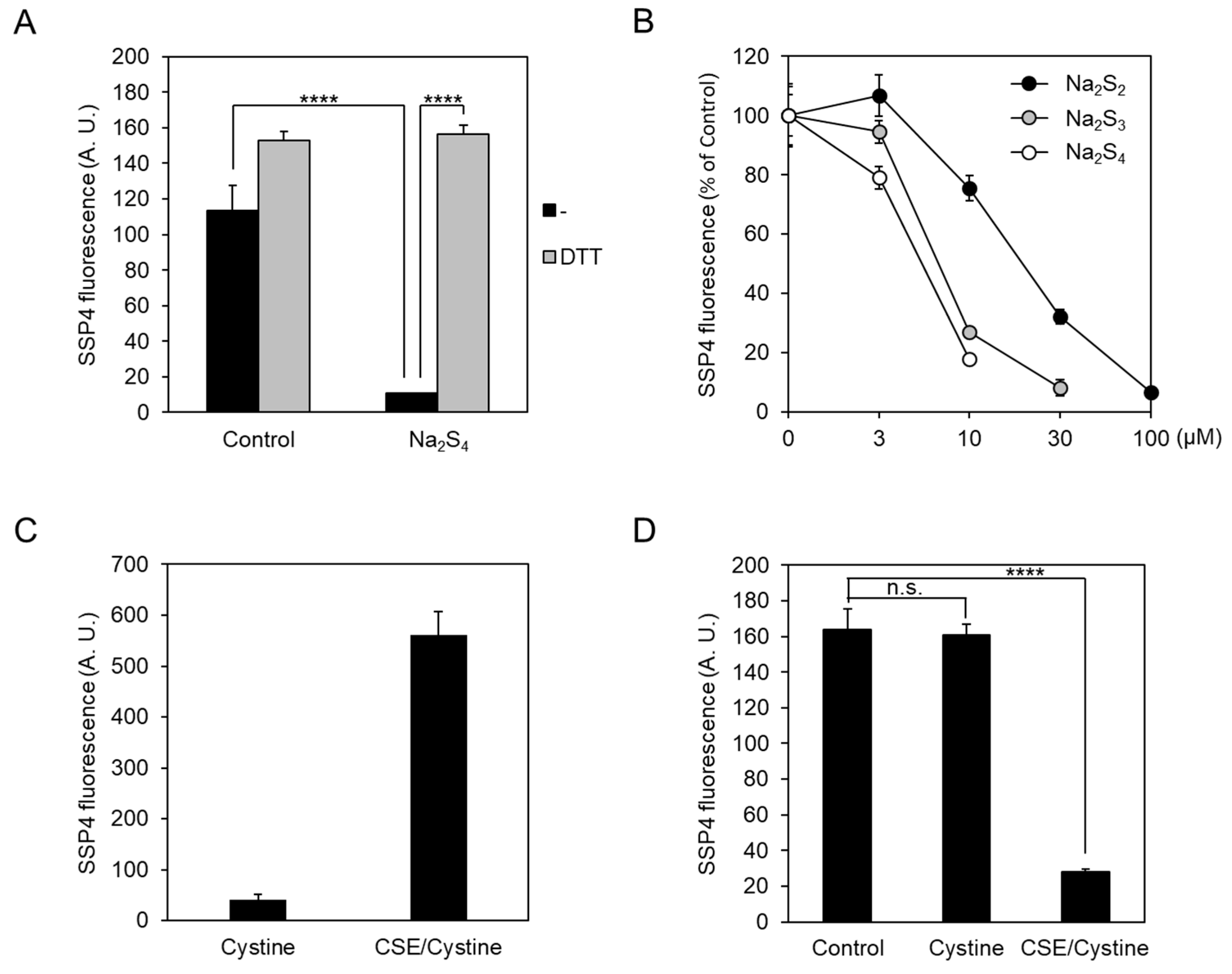
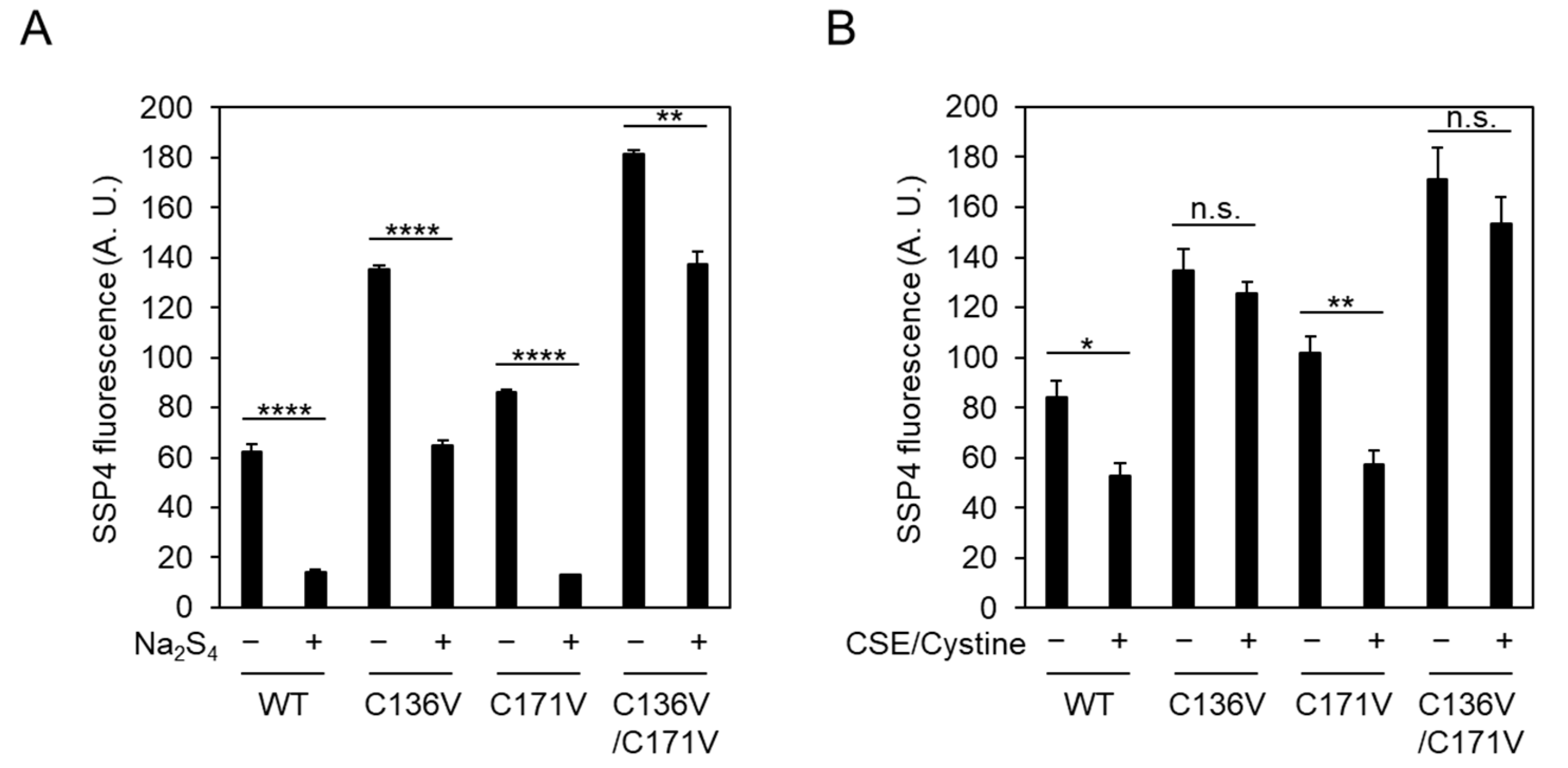
| CSE | WT | C136V | C171V | C136V/C171V |
|---|---|---|---|---|
| Km (mM) | 7.57 ± 2.23 | 11.77 ± 2.58 | 6.13 ± 0.43 | 8.40 ± 0.64 |
| Vmax (µmol/min/mg CSE) | 29.93 ± 12.06 | 28.30 ± 6.67 | 22.00 ± 4.39 | 29.12 ± 3.52 |
| Vmax/Km | 3.95 | 2.40 | 3.58 | 3.46 |
| CSE | WT | C136V | C171V | C136V/C171V |
|---|---|---|---|---|
| Km (mM) | 0.271 ± 0.026 | 0.554 ± 0.046 | 0.308 ± 0.029 | 0.32 ± 0.024 |
| Vmax (Fluorescence Intensity) | 57.79 ± 3.61 | 193.92 ± 14.31 | 78.10 ± 5.96 | 180.64 ± 7.94 |
| Vmax/Km | 213.35 | 349.91 | 253.78 | 563.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araki, S.; Takata, T.; Ono, K.; Sawa, T.; Kasamatsu, S.; Ihara, H.; Kumagai, Y.; Akaike, T.; Watanabe, Y.; Tsuchiya, Y. Cystathionine γ-Lyase Self-Inactivates by Polysulfidation during Cystine Metabolism. Int. J. Mol. Sci. 2023, 24, 9982. https://doi.org/10.3390/ijms24129982
Araki S, Takata T, Ono K, Sawa T, Kasamatsu S, Ihara H, Kumagai Y, Akaike T, Watanabe Y, Tsuchiya Y. Cystathionine γ-Lyase Self-Inactivates by Polysulfidation during Cystine Metabolism. International Journal of Molecular Sciences. 2023; 24(12):9982. https://doi.org/10.3390/ijms24129982
Chicago/Turabian StyleAraki, Shoma, Tsuyoshi Takata, Katsuhiko Ono, Tomohiro Sawa, Shingo Kasamatsu, Hideshi Ihara, Yoshito Kumagai, Takaaki Akaike, Yasuo Watanabe, and Yukihiro Tsuchiya. 2023. "Cystathionine γ-Lyase Self-Inactivates by Polysulfidation during Cystine Metabolism" International Journal of Molecular Sciences 24, no. 12: 9982. https://doi.org/10.3390/ijms24129982
APA StyleAraki, S., Takata, T., Ono, K., Sawa, T., Kasamatsu, S., Ihara, H., Kumagai, Y., Akaike, T., Watanabe, Y., & Tsuchiya, Y. (2023). Cystathionine γ-Lyase Self-Inactivates by Polysulfidation during Cystine Metabolism. International Journal of Molecular Sciences, 24(12), 9982. https://doi.org/10.3390/ijms24129982