Antimicrobial Activity of Biogenic Metal Oxide Nanoparticles and Their Synergistic Effect on Clinical Pathogens
Abstract
:1. Introduction
2. Results
2.1. Synthesis of CuO, ZnO, and WO3 Nanoparticles
2.2. Characterization of the Nanoparticles
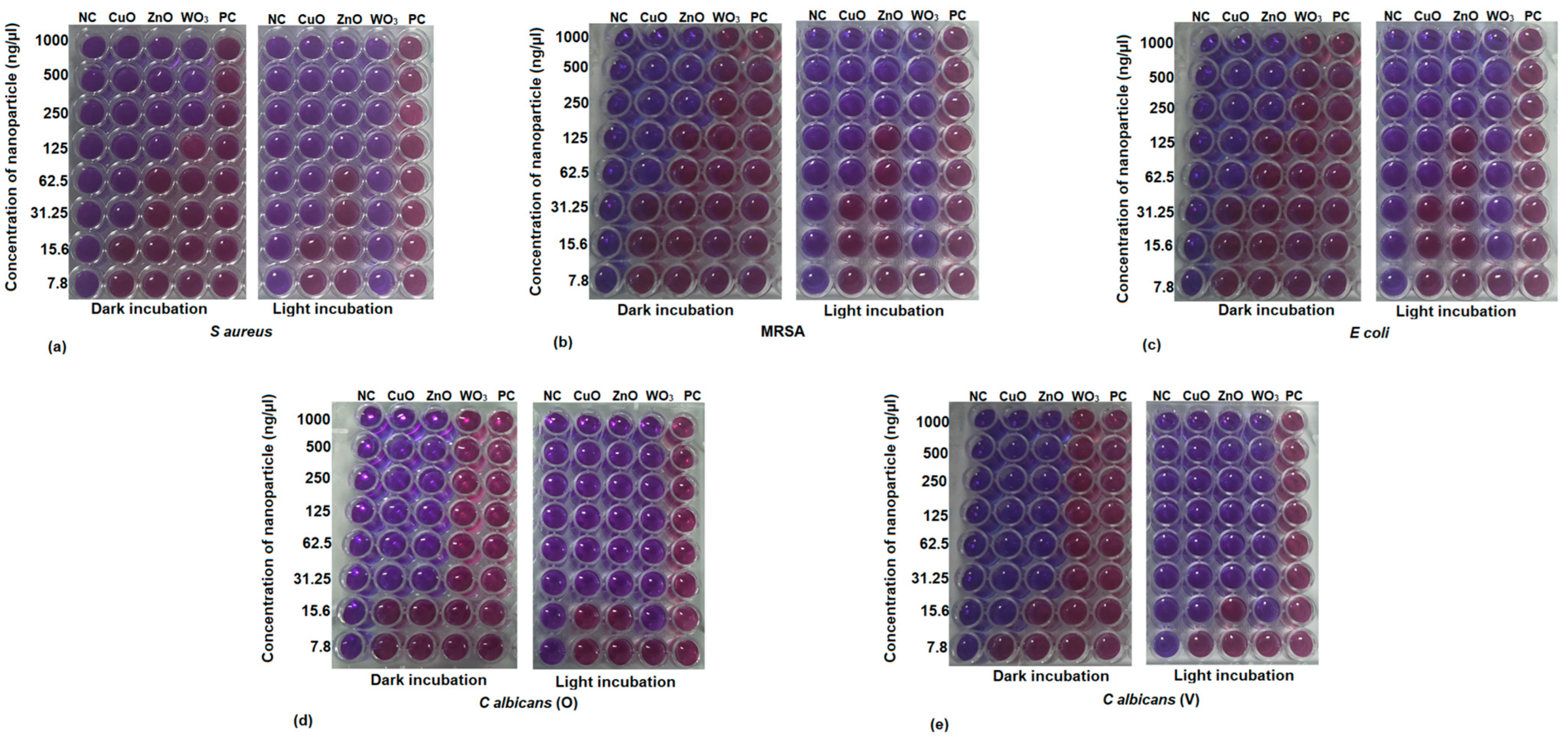
2.3. Minimum Inhibitory Concentration (MIC) by Resazurin Assay
2.4. Minimum Microbicidal Concentration (MMC)
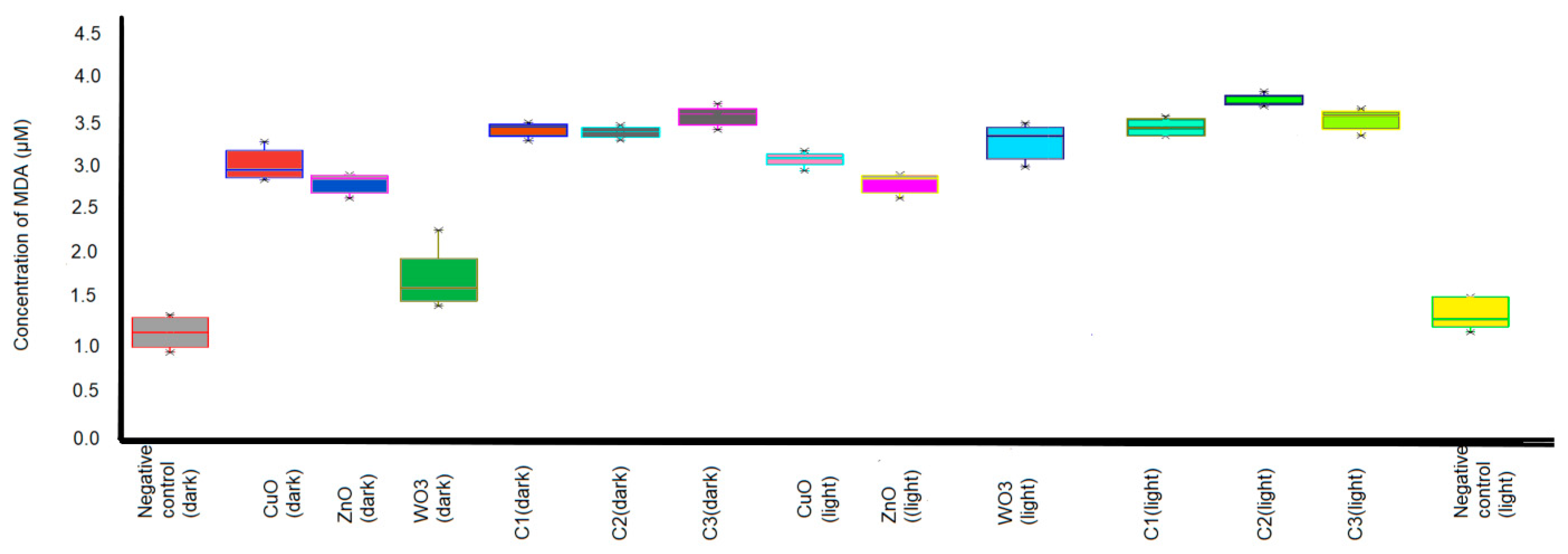
2.5. Determination of Malondialdehyde (MDA)
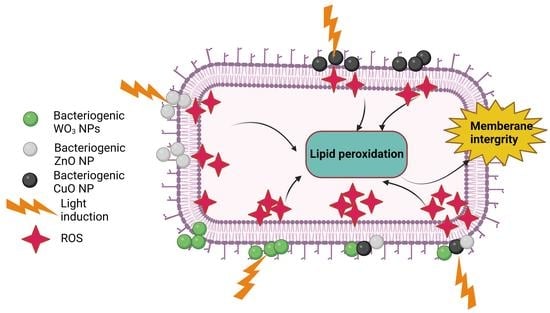
2.6. Flow Cytometry Analysis of Dead/Live Microbial Cells and Membrane Integrity Assay by Fluorescence Microscopy Imaging
3. Discussion
4. Materials and Methods
4.1. Synthesis of CuO, ZnO, and WO3 Nanoparticle
4.2. Collection of Clinical Isolates
4.3. Minimum Inhibitory Concentration by Resazurin Assay
4.4. Minimal Microbicidal Activity
4.5. Combination of Nanoparticle Formulation
4.6. Determination of Malondialdehyde (MDA)
4.7. Flow Cytometry Analysis of Dead/Live Microbial Cells and Membrane Integrity Assay by Fluoresce Microscopy Imaging
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bentivegna, E.; Luciani, M.; Arcari, L.; Santino, I.; Simmaco, M.; Martelletti, P. Reduction of Multidrug-Resistant (MDR) Bacterial Infections during the COVID-19 Pandemic: A Retrospective Study. Int. J. Environ. Res. Public Health 2021, 18, 1003. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial Approaches for Textiles: From Research to Market. Materials 2016, 9, 498. [Google Scholar] [CrossRef] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Bankier, C.; Matharu, R.K.; Cheong, Y.K.; Ren, G.G.; Cloutman-Green, E.; Ciric, L. Synergistic Antibacterial Effects of Metallic Nanoparticle Combinations. Sci. Rep. 2019, 9, 16074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, F.; Tchetchik, A.; Furxhi, I. Reduction of Health Care-Associated Infections (HAIs) with Antimicrobial Inorganic Nanoparticles Incorporated in Medical Textiles: An Economic Assessment. Nanomaterials 2020, 10, 999. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Das, S.S.; Khatoon, A.; Ansari, M.T.; Afzal, M.; Hasnain, M.S.; Nayak, A.K. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Ghaffar, N.; Javad, S.; Farrukh, M.A.; Shah, A.A.; Gatasheh, M.K.; Al-Munqedhi, B.M.; Chaudhry, O. Metal nanoparticles assisted revival of Streptomycin against MDRS Staphylococcus aureus. PLoS ONE 2022, 17, e0264588. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Shi, Y.; Song, H.; Yu, C. Antibiotic-Free Antibacterial Strategies Enabled by Nanomaterials: Progress and Perspectives. Adv. Mater. 2020, 32, e1904106. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh-Narbat, M.; Cheng, H.; Chabok, R.; Alvarez, M.M.; De La Fuente-Nunez, C.; Phillips, K.S.; Khademhosseini, A. Strategies for antimicrobial peptide coatings on medical devices: A review and regulatory science perspective. Crit. Rev. Biotechnol. 2021, 41, 94–120. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Metzler, D.M.; Cha, D.K.; Huang, C.P. The short-term toxic effects of TiO2 nanoparticles toward bacteria through viability, cellular respiration, and lipid peroxidation. Environ. Sci. Pollut. Res. 2015, 22, 17917–17924. [Google Scholar] [CrossRef] [PubMed]
- Domon, H.; Hiyoshi, T.; Maekawa, T.; Yonezawa, D.; Tamura, H.; Kawabata, S.; Yanagihara, K.; Kimura, O.; Kunitomo, E.; Terao, Y. Antibacterial activity of hinokitiol against both antibiotic-resistant and -susceptible pathogenic bacteria that predominate in the oral cavity and upper airways. Microbiol. Immunol. 2019, 63, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, P.R.; Chen, M.L.; Sun, C.C.; Chen, W.H.; Pan, H.J.; Yang, L.S.; Chang, S.C.; Ho, S.W.; Lee, C.Y.; Hsieh, W.C.; et al. Antimicrobial Drug Resistance in Pathogens Causing Nosocomial Infections at a University Hospital in Taiwan, 1981–1999. Emerg. Infect. Dis. 2002, 8, 63–68. [Google Scholar] [CrossRef]
- Li, Y.; Cao, X.; Ge, H.; Jiang, Y.; Zhou, H.; Zheng, W. Targeted surveillance of nosocomial infection in intensive care units of 176 hospitals in Jiangsu province, China. J. Hosp. Infect. 2017, 99, 36–41. [Google Scholar] [CrossRef]
- Francis, D.V.; Thaliyakattil, S.; Cherian, L.; Sood, N.; Gokhale, T. Metallic Nanoparticle Integrated Ternary Polymer Blend of PVA/Starch/Glycerol: A Promising Antimicrobial Food Packaging Material. Polymers 2022, 14, 1379. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.V.; Aiswarya, T.; Gokhale, T. Optimization of the incubation parameters for biogenic synthesis of WO3 nanoparticles using Taguchi method. Heliyon 2022, 8, e10640. [Google Scholar] [CrossRef]
- Francis, D.V.; Sood, N.; Gokhale, T. Biogenic CuO and ZnO Nanoparticles as Nanofertilizers for Sustainable Growth of Amaranthus hybridus. Plants 2022, 11, 2776. [Google Scholar] [CrossRef]
- He, Y.; Ingudam, S.; Reed, S.; Gehring, A.; Strobaugh, T.P.; Irwin, P. Study on the mechanism of antibacterial action of magnesium oxide nanoparticles against foodborne pathogens. J. Nanobiotechnol. 2016, 14, 54. [Google Scholar] [CrossRef] [Green Version]
- Matalkeh, M.; Nasrallah, G.K.; Shurrab, F.M.; Al-Absi, E.S.; Mohammed, W.; Elzatahry, A.; Saoud, K.M. Visible Light Photocatalytic Activity of Ag/WO3 Nanoparticles and its Antibacterial Activity Under Ambient Light and in The Dark. Results Eng. 2022, 13, 100313. [Google Scholar] [CrossRef]
- Hoiczyk, E.; Hansel, A. Cyanobacterial Cell Walls: News from an Unusual Prokaryotic Envelope. J. Bacteriol. 2000, 182, 1191–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurunathan, S.; Han, J.W.; Kwon, D.-N.; Kim, J.-H. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res. Lett. 2014, 9, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadipour, S.; Field, R.A.; Miller, G.J. Prospects for anti-Candida therapy through targeting the cell wall: A mini-review. Cell Surf. 2021, 7, 100063. [Google Scholar] [CrossRef]
- Sumanth, B.; Lakshmeesha, T.R.; Ansari, M.A.; Alzohairy, M.A.; Udayashankar, A.C.; Shobha, B.; Niranjana, S.R.; Srinivas, C.; Almatroudi, A. Mycogenic Synthesis of Extracellular Zinc Oxide Nanoparticles from Xylaria acuta and Its Nanoantibiotic Potential. Int. J. Nanomed. 2020, 15, 8519–8536. [Google Scholar] [CrossRef]
- Lallo da Silva, B.; Abuçafy, M.P.; Berbel Manaia, E.; Oshiro Junior, J.A.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: An overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef] [Green Version]
- Gangadoo, S.; Xu, C.; Cozzolino, D.; Latham, K.; Della Gaspera, E.; Chapman, J.; Truong, V.K. Probing Nanoscale Interactions of Antimicrobial Zinc Oxide Quantum Dots on Bacterial and Fungal Cell Surfaces. Adv. Mater. Interfaces 2022, 9, 2101484. [Google Scholar] [CrossRef]
- Dayem, A.A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [Green Version]
- Alekish, M.; Ismail, Z.B.; Albiss, B.; Nawasrah, S. In vitro antibacterial effects of zinc oxide nanoparticles on multiple drug-resistant strains of Staphylococcus aureus and Escherichia coli: An alternative approach for antibacterial therapy of mastitis in sheep. Veter World 2018, 11, 1428–1432. [Google Scholar] [CrossRef] [Green Version]
- Nabila, M.I.; Kannabiran, K. Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatal. Agric. Biotechnol. 2018, 15, 56–62. [Google Scholar] [CrossRef]
- Muthuvel, A.; Jothibas, M.; Manoharan, C. Synthesis of copper oxide nanoparticles by chemical and biogenic methods: Photocatalytic degradation and in vitro antioxidant activity. Nanotechnol. Environ. Eng. 2020, 5, 14. [Google Scholar] [CrossRef]
- Subramani, T.; Thimmarayan, G.; Balraj, B.; Chandrasekar, N.; Palanisamy, M.; Nagarajan, S.K.; Amirtharajan, S.; Kumar, M.; Sivakumar, C. Surfactants assisted synthesis of WO3 nanoparticles with improved photocatalytic and antibacterial activity: A strong impact of morphology. Inorg. Chem. Commun. 2022, 142, 109709. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Ghosh, R.; Adhikari, T.; Mukhopadhyay, S.; Chattopadhyay, A.; Pal, S.K. Development of Nanomedicine from Copper Mine Tailing Waste: A Pavement towards Circular Economy with Advanced Redox Nanotechnology. Catalysts 2023, 13, 369. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Newton, K.A.; Osterloh, F.E. Size and Morphology of Suspended WO3 Particles Control Photochemical Charge Carrier Extraction and Photocatalytic Water Oxidation Activity. Top. Catal. 2016, 59, 750–756. [Google Scholar] [CrossRef]
- Copper(II) Oxide Nanopowder, Particle Size 50 nm TEM 1317-38-0. Available online: https://www.sigmaaldrich.com/IN/en/product/aldrich/544868?gclid=CjwKCAjw1YCkBhAOEiwA5aN4AXZ35hypTdt2vpsnAQFFqov6HrQBlKL4-tLGDDqlTSe_gZLirgWWJhoCFpkQAvD_BwE&gclsrc=aw.ds (accessed on 6 June 2023).
- Zinc Oxide Nanopowder, Particle Size 97 1314-13-2. Available online: https://www.sigmaaldrich.com/IN/en/product/aldrich/677450?gclid=CjwKCAjw1YCkBhAOEiwA5aN4AQB7goh8wzNvlKSVYt_G7F1nBw4f_Uyr9lSJG0YZsXCyOePlNavSGhoCkuYQAvD_BwE&gclsrc=aw.ds (accessed on 6 June 2023).
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Memic, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamwenda, G.R.; Arakawa, H. The visible light induced photocatalytic activity of tungsten trioxide powders. Appl. Catal. A Gen. 2001, 210, 181–191. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Alaya, L.; Saeedi, A.M.; Alsaigh, A.A.; Almalki, M.H.K.; Alonizan, N.H.; Hjiri, M. ZnO:V Nanoparticles with Enhanced Antimicrobial Activities. J. Compos. Sci. 2023, 7, 190. [Google Scholar] [CrossRef]
- Zhou, X.; Taghizadeh, K.; Dedon, P.C. Chemical and Biological Evidence for Base Propenals as the Major Sourceof the Endogenous M1dG Adduct in CellularDNA. J. Biol. Chem. 2005, 280, 25377–25382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belenky, P.; Ye, J.D.; Porter, C.B.M.; Cohen, N.R.; Lobritz, M.A.; Ferrante, T.; Jain, S.; Korry, B.J.; Schwarz, E.G.; Walker, G.C.; et al. Bactericidal Antibiotics Induce Toxic Metabolic Perturbations that Lead to Cellular Damage. Cell Rep. 2015, 13, 968–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosaka, Y.; Nosaka, A.Y. Identification and Roles of the Active Species Generated on Various Photocatalysts. In Photocatalysis and Water Purification: From Fundamentals to Recent Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 3–24. [Google Scholar] [CrossRef]
- Pawar, M.; Sendoğdular, S.T.; Gouma, P. A Brief Overview of TiO2 Photocatalyst for Organic Dye Remediation: Case Study of Reaction Mechanisms Involved in Ce-TiO2 Photocatalysts System. J. Nanomater. 2018, 2018, 5953609. [Google Scholar] [CrossRef] [Green Version]
- Matharu, R.K.; Ciric, L.; Ren, G.; Edirisinghe, M. Comparative Study of the Antimicrobial Effects of Tungsten Nanoparticles and Tungsten Nanocomposite Fibres on Hospital Acquired Bacterial and Viral Pathogens. Nanomaterials 2020, 10, 1017. [Google Scholar] [CrossRef]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [Green Version]
- Yadav, H.M.; Otari, S.V.; Bohara, R.A.; Mali, S.S.; Pawar, S.H.; Delekar, S.D. Synthesis and visible light photocatalytic antibacterial activity of nickel-doped TiO2 nanoparticles against Gram-positive and Gram-negative bacteria. J. Photochem. Photobiol. A Chem. 2014, 294, 130–136. [Google Scholar] [CrossRef]
- Hirota, K.; Sugimoto, M.; Kato, M.; Tsukagoshi, K.; Tanigawa, T.; Sugimoto, H. Preparation of zinc oxide ceramics with a sustainable antibacterial activity under dark conditions. Ceram. Int. 2010, 36, 497–506. [Google Scholar] [CrossRef]
- Xu, X.; Chen, D.; Yi, Z.; Jiang, M.; Wang, L.; Zhou, Z.; Fan, X.; Wang, Y.; Hui, D. Antimicrobial Mechanism Based on H2O2 Generation at Oxygen Vacancies in ZnO Crystals. Langmuir 2013, 29, 5573–5580. [Google Scholar] [CrossRef]
- Prasanna, V.L.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef]
- Khurana, C.; Sharma, P.; Pandey, O.; Chudasama, B. Synergistic Effect of Metal Nanoparticles on the Antimicrobial Activities of Antibiotics against Biorecycling Microbes. J. Mater. Sci. Technol. 2016, 32, 524–532. [Google Scholar] [CrossRef]
- Jin, S.E.; Jin, H.E. Antimicrobial Activity of Zinc Oxide Nano/Microparticles and Their Combinations against Pathogenic Microorganisms for Biomedical Applications: From Physicochemical Character-istics to Pharmacological Aspects. Nanomaterials 2021, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, P. Estimation of the Size and Internal Structure of Colloidal Particles by Means of Röntgen; Mathematical and physical class; News from the Society of Sciences: Goettingen, Germany, 1918; pp. 98–100. [Google Scholar]
- Teh, C.H.; Nazni, W.A.; Nurulhusna, A.H.; Norazah, A.; Lee, H.L. Determination of antibacterial activity and minimum inhibitory concentration of larval extract of fly via resazurin-based turbidometric assay. BMC Microbiol. 2017, 17, 36. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, R.T.; Galvão, C.N.; Betancourt, Y.P.; Mathiazzi, B.I.; Carmona-Ribeiro, A.M. Microbicidal Dispersions and Coatings from Hybrid Nanoparticles of Poly (Methyl Methacrylate), Poly (Diallyl Dimethyl Ammonium) Chloride, Lipids, and Surfactants. Int. J. Mol. Sci. 2019, 20, 6150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taha, M.A.; Badawy, M.E.; Abdel-Razik, R.K.; Younis, H.M.; Abo-El-Saad, M.M. Mitochondrial dysfunction and oxidative stress in liver of male albino rats after exposing to sub-chronic intoxication of chlorpyrifos, cypermethrin, and imidacloprid. Pestic. Biochem. Physiol. 2021, 178, 104938. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Azevedo, N.F.; Ivask, A. Propidium iodide staining underestimates viability of adherent bacterial cells. Sci. Rep. 2019, 9, 6483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiefel, P.; Schmidt-Emrich, S.; Maniura-Weber, K.; Ren, Q. Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol. 2015, 15, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, M.H.; Beck-Nielsen, H.; Andersen, M.N.; Handberg, A. A flow cytometric method for characterization of circulating cell-derived microparticles in plasma. J. Extracell. Vesicles 2014, 3, 20795. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Zahediasl, S. Normality Tests for Statistical Analysis: A Guide for Non-Statisticians. Int. J. Endocrinol. 2012, 10, 486. [Google Scholar] [CrossRef] [Green Version]
- Sawyer, S.F. Analysis of Variance: The Fundamental Concepts. J. Man. Manip. Ther. 2009, 17, 27E–38E. [Google Scholar] [CrossRef]
- Gerald, B. A brief review of independent, dependent and one sample t-test. Int. J. Appl. Math. 2018, 14, 50–54. [Google Scholar] [CrossRef] [Green Version]
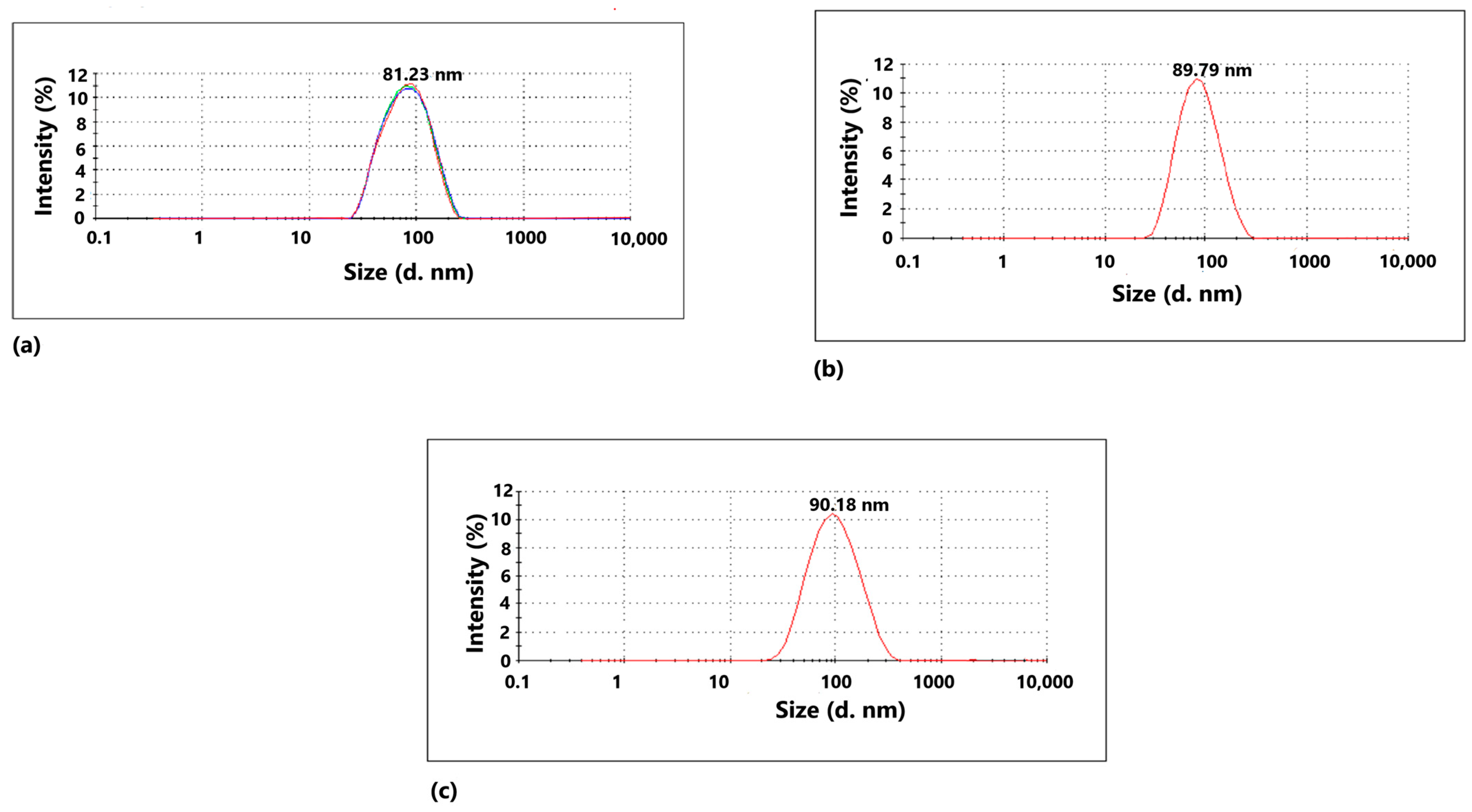




| Peak Position | FWHM | Crystallite Size (nm) | Average Grain Size (nm) | |
|---|---|---|---|---|
| CuO | 20.90853 | 0.22918 | 35.24957 | 38.96 (±5) |
| 35.37912 | 0.27172 | 30.68807 | ||
| 38.61757 | 0.1803 | 46.68871 | ||
| 48.62369 | 0.27196 | 32.05383 | ||
| 61.41574 | 0.25925 | 35.64097 | ||
| 66.0172 | 0.17732 | 53.42576 | ||
| WO3 | 23.19039 | 0.18668 | 43.44222 | 53.03 (±5) |
| 23.67589 | 0.20875 | 38.88346 | ||
| 24.41808 | 0.19304 | 42.10592 | ||
| 31.77072 | 0.1311 | 63.00318 | ||
| 45.52266 | 0.18972 | 45.41009 | ||
| 66.26026 | 0.09757 | 97.22758 | ||
| 56.53233 | 0.21911 | 41.16581 | ||
| ZnO | 23.28048 | 0.26135 | 31.03541 | 42.64 (±3) |
| 31.8997 | 0.24101 | 34.28227 | ||
| 33.47986 | 0.21567 | 38.46548 | ||
| 34.76525 | 0.17377 | 47.90504 | ||
| 50.91306 | 0.14305 | 61.50663 |
| Clinical Isolate | Incubation in Dark | Incubation in Light | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CuO | ZnO | WO3 | CuO | ZnO | WO3 | |||||||
| Concentration of Nanoparticles (ng/µL) | Concentration of Nanoparticles (ng/µL) | |||||||||||
| MIC | MMC | MIC | MMC | MIC | MMC | MIC | MMC | MIC | MMC | MIC | MMC | |
| S. aureus | 31.25 | 62.5 | 125 | 250 | 250 | - | 31.25 | 62.5 | 125 | 125 | <7.8 | 7.8 |
| MRSA | 62.5 | 125 | 250 | 250 | - | - | 62.5 | 125 | 250 | 250 | 15.6 | 15.6 |
| E. coli | 62.5 | 62.5 | 250 | 250 | - | - | 62.5 | 62.5 | 250 | 31.25 | 15.6 | 7.8 |
| C. albicans (O) | 31.25 | 125 | 31.25 | 62.5 | - | - | 31.25 | 125 | 31.25 | 62.5 | 15.6 | 15.6 |
| C. albicans (V) | 15.6 | 31.25 | 31.25 | 62.5 | - | - | 15.6 | 31.25 | 31.25 | 62.5 | 15.6 | 15.6 |
| Nanoparticle | Test Organism | Minimal Inhibitory Concentration in Literature | Minimal Inhibitory Concentration of S. maltophilia Synthesized Nanoparticles |
|---|---|---|---|
| ZnO (chemically synthesized) | S. aureus | 3.9 µg/mL [39] | 31.25 ng/mL |
| E. coli | 7.81 µg/mL [39] | 62.5 ng/mL | |
| CuO (biogenic) | S. aureus | 11.6 µg/mL [40] | 31.25 ng/mL |
| CuO (chemically synthesized) | E. coli | 100 µg/mL [30] | 62.5 ng/mL |
| WO3 (chemically synthesized) | S. aureus | 100 µg/mL [41] | 7.8 ng/mL |
| Nanoparticle Combinations | Concentration (ng/µL) | ||
|---|---|---|---|
| CuO | WO3 | ZnO | |
| Combination 1 (C1) | 15.6 | 1.95 | 15.6 |
| Combination 2 (C2) | 7.8 | 7.8 | 7.8 |
| Combination 3 (C3) | 3.9 | 3.9 | 31.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francis, D.V.; Jayakumar, M.N.; Ahmad, H.; Gokhale, T. Antimicrobial Activity of Biogenic Metal Oxide Nanoparticles and Their Synergistic Effect on Clinical Pathogens. Int. J. Mol. Sci. 2023, 24, 9998. https://doi.org/10.3390/ijms24129998
Francis DV, Jayakumar MN, Ahmad H, Gokhale T. Antimicrobial Activity of Biogenic Metal Oxide Nanoparticles and Their Synergistic Effect on Clinical Pathogens. International Journal of Molecular Sciences. 2023; 24(12):9998. https://doi.org/10.3390/ijms24129998
Chicago/Turabian StyleFrancis, Dali Vilma, Manju Nidagodu Jayakumar, Hafiz Ahmad, and Trupti Gokhale. 2023. "Antimicrobial Activity of Biogenic Metal Oxide Nanoparticles and Their Synergistic Effect on Clinical Pathogens" International Journal of Molecular Sciences 24, no. 12: 9998. https://doi.org/10.3390/ijms24129998