Mitochondria Deregulations in Cancer Offer Several Potential Targets of Therapeutic Interventions
Abstract
1. Introduction
2. Molecules Targeting Mitochondrial Metabolic Pathways
2.1. Drugs Targeting the Krebs Cycle
2.2. Drugs Targeting Fatty Acid Oxidation
2.3. Drugs Targeting Glutamine Metabolism
2.4. Drugs Targeting the Oxidative Phosphorylation (OXPHOS) System
2.5. Drugs Targeting Heme Biosynthesis
3. Molecules Targeting Mitochondrial Homeostasis
3.1. Drugs Targeting Mitochondrial Biogenesis
3.2. Drugs Targeting Apoptosis by Bcl-2 Family Proteins
3.3. Drugs Targeting Mitochondrial Dynamics
3.4. Drugs Targeting Mitophagy
3.5. Drugs Targeting Mitochondrial Proteostasis
3.5.1. Drugs Targeting Mitochondrial Protein Synthesis
3.5.2. Drugs Targeting Mitochondrial Proteases
3.6. Drugs Targeting the Cellular Redox Balance
4. Photodynamic, Photothermal and Chemodynamic Therapies
5. How to Target Mitochondrial Drugs
6. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Patti, G.J. The Warburg effect: A signature of mitochondrial overload. Trends Cell Biol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 10, 669–680. [Google Scholar] [CrossRef]
- Jia, D.; Park, J.H.; Jung, K.H.; Levine, H.; Kaipparettu, B.A. Elucidating the Metabolic Plasticity of Cancer: Mitochondrial Reprogramming and Hybrid Metabolic States. Cells 2018, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Duong, Q.V.; Levitsky, Y.; Dessinger, M.J.; Strubbe-Rivera, J.O.; Bazil, J.N. Identifying Site-Specific Superoxide and Hydrogen Peroxide Production Rates from the Mitochondrial Electron Transport System Using a Computational Strategy. Function 2021, 2, zqab050. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Margreiter, R.; Ausserlechner, M.J.; Hagenbuchner, J. The Complex Interplay between Mitochondria, ROS and Entire Cellular Metabolism. Antioxidants 2022, 11, 1995. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Mahmood, M.; Reznik, E.; Gammage, P.A. Mitochondrial DNA is a major source of driver mutations in cancer. Trends Cancer 2022, 12, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
- Iommarini, L.; Ghelli, A.; Gasparre, G.; Porcelli, A.M. Mitochondrial metabolism and energy sensing in tumor progression. Biochim. Biophys. Acta Bioenerg. 2017, 8, 582–590. [Google Scholar] [CrossRef]
- Palorini, R.; De Rasmo, D.; Gaviraghi, M.; Sala Danna, L.; Signorile, A.; Cirulli, C.; Chiaradonna, F.; Alberghina, L.; Papa, S. Oncogenic K-ras expression is associated with derangement of the cAMP/PKA pathway and forskolin-reversible alterations of mitochondrial dynamics and respiration. Oncogene 2013, 32, 352–362. [Google Scholar] [CrossRef]
- Signorile, A.; De Rasmo, D.; Cormio, A.; Musicco, C.; Rossi, R.; Fortarezza, F.; Palese, L.L.; Loizzi, V.; Resta, L.; Scillitani, G.; et al. Human Ovarian Cancer Tissue Exhibits Increase of Mitochondrial Biogenesis and Cristae Remodeling. Cancers 2019, 11, 1350. [Google Scholar] [CrossRef]
- Eniafe, J.; Jiang, S. The functional roles of TCA cycle metabolites in cancer. Oncogene 2021, 40, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Sciacovelli, M.; Frezza, C. Fumarate hydratase in cancer: A multifaceted tumour suppressor Semin. Cell Dev. Biol. 2020, 98, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Roboz, G.J.; DiNardo, C.D.; Stein, E.M.; de Botton, S.; Mims, A.S.; Prince, G.T.; Altman, J.K.; Arellano, M.L.; Donnellan, W.; Erba, H.P.; et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood 2020, 135, 463–471. [Google Scholar] [CrossRef]
- Mellinghoff, I.K.; van den Bent, M.J.; Blumenthal, D.T.; Touat, M.; Peters, K.B.; Clarke, J.; Mendez, J.; Yust-Katz, S.; Welsh, L.; Mason, W.P.; et al. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N. Engl. J. Med. 2023. [Google Scholar] [CrossRef]
- Corbet, C.; Pinto, A.; Martherus, R.; Santiago de Jesus, J.P.; Polet, F.; Feron, O. Acidosis Drives the Reprogramming of Fatty Acid Metabolism in Cancer Cells through Changes in Mitochondrial and Histone Acetylation. Cell Metab. 2016, 24, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Giannoudis, A.; Sharma, V. The role of CPT1A as a biomarker of breast cancer progression: A bioinformatic approach. Sci. Rep. 2022, 12, 16441. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, B.T.; Qamar, L.; Yamamoto, T.M.; McMellen, A.; Watson, Z.L.; Richer, J.K.; Behbakht, K.; Schlaepfer, I.R.; Bitler, B.G. Targeting fatty acid oxidation to promote anoikis and inhibit ovarian cancer progression. Mol. Cancer Res. 2020, 18, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, I.R.; Joshi, M. CPT1A-mediated Fat Oxidation, Mechanisms, and Therapeutic Potential. Endocrinology 2020, 161, bqz046. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-K.; Choi, S.; Yoon, S.-J.; Choi, R.J.; Park, J.; Lee, E.H.; Cho, H.J.; Lee, S.; Teo, W.-Y.; Moon, J.H.; et al. Etomoxir, a carnitine palmitoyltransferase 1 inhibitor, combined with temozolomide reduces stemness and invasiveness in patient-derived glioblastoma tumorspheres. Cancer Cell Int. 2022, 22, 309. [Google Scholar] [CrossRef]
- Lin, H.; Patel, S.; Affleck, V.S.; Wilson, I.; Turnbull, D.M.; Joshi, A.R.; Maxwell, R.; Stoll, E.A. Fatty acid oxidation is required for the respiration and proliferation of malignant glioma cells. Neuro Oncol. 2017, 19, 43–54. [Google Scholar] [CrossRef]
- Ricciardi, M.R.; Mirabilii, S.; Allegretti, M.; Licchetta, R.; Calarco, A.; Torrisi, M.R.; Foà, R.; Nicolai, R.; Peluso, G.; Tafuri, A. Targeting the leukemia cell metabolism by the CPT1a inhibition: Functional preclinical effects in leukemias. Blood 2015, 126, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.; Tomita, Y.; Drew, P.; Price, T.; Maddern, G.; Smith, E.; Fenix, K. Perhexiline: Old Drug, New Tricks? A Summary of Its Anti-Cancer Effects. Molecules 2023, 28, 3624. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.; Li, C.M.Y.; Li, R.; Yeo, K.; Wright, J.A.; Gieniec, K.A.; Vrbanac, L.; Sammour, T.; Lawrence, M.; Thomas, M.; et al. The Antianginal Drug Perhexiline Displays Cytotoxicity against Colorectal Cancer Cells In Vitro: A Potential for Drug Repurposing. Cancers 2022, 14, 1043. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.L.; Fu, A.; Zhao, P.; Li, J.; Geng, L.; Smith, S.T.; Kondo, J.; Coffey, R.J.; Johnson, M.O.; Rathmell, J.C.; et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat. Med. 2018, 24, 194–202. [Google Scholar] [CrossRef]
- Lampa, M.; Arlt, H.; He, T.; Ospina, B.; Reeves, J.; Zhang, B.; Murtie, J.; Deng, G.; Barberis, C.; Hoffmann, D.; et al. Glutaminase is essential for the growth of triple-negative breast cancer cells with a deregulated glutamine metabolism pathway and its suppression synergizes with mTOR inhibition. PLoS ONE 2017, 12, e0185092. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhang, H.S.; Liu, M.Y.; Li, H.M.; Wang, X.Y.; Wang, M. GLS1 depletion inhibited colorectal cancer proliferation and migration via redox/Nrf2/autophagy-dependent pathway. Arch. Biochem. Biophys. 2021, 708, 108964. [Google Scholar] [CrossRef]
- Hou, W.; Lu, S.; Zhao, H.; Yu, Y.; Xu, H.; Yu, B.; Su, L.; Lin, C.; Ruan, B.H. Propylselen inhibits cancer cell growth by targeting glutamate dehydrogenase at the NADP+ binding site. Biochem. Biophys. Res. Commun. 2019, 509, 262–267. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, Z.; Chen, Z.; Liu, J.; Ding, H.; Luo, C.; Wang, M.; Du, D. The discovery of a non-competitive GOT1 inhibitor, hydralazine hydrochloride, via a coupling reaction-based high-throughput screening assay. Bioorg. Med. Chem. Lett. 2022, 73, 128883. [Google Scholar] [CrossRef]
- Sun, W.; Luan, S.; Qi, C.; Tong, Q.; Yan, S.; Li, H.; Zhang, Y. Aspulvinone O, a natural inhibitor of GOT1 suppresses pancreatic ductal adenocarcinoma cells growth by interfering glutamine metabolism. Cell Commun. Signal 2019, 17, 111. [Google Scholar] [CrossRef]
- Kim, M.; Gwak, J.; Hwang, S.; Yang, S.; Jeong, S.M. Mitochondrial GPT2 plays a pivotal role in metabolic adaptation to the perturbation of mitochondrial glutamine metabolism. Oncogene 2019, 38, 4729–4738. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, J.M.; Nelson, K.K.; Clem, B.F.; Lane, A.N.; Arumugam, S.; Simmons, A.; Eaton, J.W.; Telang, S.; Chesney, J. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res. 2008, 10, R84. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2014. [Google Scholar]
- Di Gregorio, J.; Petricca, S.; Iorio, R.; Toniato, E.; Flati, V. Mitochondrial and metabolic alterations in cancer cells. Eur. J. Cell Biol. 2022, 101, 151225. [Google Scholar] [CrossRef]
- Zhou, Y.; Zou, J.; Xu, J.; Zhou, Y.; Cen, X.; Zhao, Y. Recent advances of mitochondrial complex I inhibitors for cancer therapy: Current status and future perspectives. Eur. Med. Chem. 2023, 251, 115219. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Jiang, Y.; Liang, G.; Feng, Y.; Qu, F. Metformin: A Promising Antidiabetic Medication for Cancer Treatment. Curr. Drug Targets 2023, 24, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.; Chun, K.H.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kim, Y.S.; Woo, J.T.; Nam, M.S.; Baik, S.H.; et al. Metformin reduces the risk of cancer in patients with type 2 diabetes: An analysis based on the Korean National Diabetes Program Cohort. Medicine 2018, 97, e0036. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.R.; Harris, A.L. Is it still worth pursuing the repurposing of metformin as a cancer therapeutic? Br. J. Cancer 2023, 128, 958–966. [Google Scholar] [CrossRef]
- Wheaton, W.W.; Weinberg, S.E.; Hamanaka, R.B.; Soberanes, S.; Sullivan, L.B.; Anso, E.; Glasauer, A.; Dufour, E.; Mutlu, G.M.; Budigner, G.S.; et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 2014, 3, e02242. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 2023, 1–17. [Google Scholar] [CrossRef]
- Al-Wahab, Z.; Mert, I.; Tebbe, C.; Chhina, J.; Hijaz, M.; Morris, R.T.; Ali-Fehmi, R.; Giri, S.; Munkarah, A.R.; Rattan, R. Metformin prevents aggressive ovarian cancer growth driven by high-energy diet: Similarity with calorie restriction. Oncotarget 2015, 6, 10908–19023. [Google Scholar] [CrossRef]
- Beebe-Dimmer, J.L.; Cooney, K.A. Mitochondrial alterations may underlie race-specific differences in cancer risk and outcome. J. Clin. Investig. 2019, 129, 2187–2188. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.K.; Padhukasahasram, B.; Ahmedani, B.K.; Peterson, E.L.; Wells, K.E.; Burchard, E.G.; Lanfear, D.E. Differing effects of metformin on glycemic control by race-ethnicity. J. Clin. Endocrinol. Metab. 2014, 99, 3160–3168. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, F.; Chadderton, N.; Farrar, G.J.; Zisterer, D.M.; Porter, R.K. Direct effects of phenformin on metabolism/bioenergetics and viability of SH-SY5Y neuroblastoma cells. Oncol. Lett. 2017, 14, 6298–6306. [Google Scholar] [CrossRef] [PubMed]
- Masoud, R.; Reyes-Castellanos, G.; Lac, S.; Garcia, J.; Dou, S.; Shintu, L.; Abdel Hadi, N.; Gicquel, T.; El Kaoutari, A.; Diémé, B.; et al. Targeting Mitochondrial Complex I Overcomes Chemoresistance in High OXPHOS Pancreatic Cancer. Cell Rep. Med. 2020, 1, 100143. [Google Scholar] [CrossRef]
- Basit, F.; Van Oppen, L.M.P.E.; Schöckel, L.; Bossenbroek, H.M.; Van Emst-de Vries, S.E.; Hermeling, J.C.W.; Grefte, S.; Kopitz, C.; Heroult, M.; Willems, P.H.; et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017, 8, e2716. [Google Scholar] [CrossRef]
- Wilk, A.; Wyczechowska, D.; Zapata, A.; Dean, M.; Mullinax, J.; Marrero, L.; Parsons, C.; Peruzzi, F.; Culicchia, F.; Ochoa, A.; et al. Molecular mechanisms of fenofibrate-induced metabolic catastrophe and glioblastoma cell death. Mol. Cell. Biol. 2015, 35, 182–198. [Google Scholar] [CrossRef]
- Chen, L.; Peng, J.; Wang, Y.; Jiang, H.; Wang, W.; Dai, J.; Tang, M.; Wei, Y.; Kuang, H.; Xu, G.; et al. Fenofibrate-induced mitochondrial dysfunction and metabolic reprogramming reversal: The antitumor effects in gastric carcinoma cells mediated by the PPAR pathway. Am. J. Transl. Res. 2020, 12, 428–446. [Google Scholar]
- Wang, J.; Xu, Y.; Wan, H.; Hu, J. Antibiotic ivermectin selectively induces apoptosis in chronic myeloid leukemia through inducing mitochondrial dysfunction and oxidative stress. Biochem. Biophys. Res. Commun. 2018, 497, 241–247. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Y.; Zhou, Z. Antibiotic ivermectin preferentially targets renal cancer through inducing mitochondrial dysfunction and oxidative damage. Biochem. Biophys. Res. Commun. 2017, 492, 373–378. [Google Scholar] [CrossRef]
- Molina, J.R.; Sun, Y.; Protopopova, M.; Gera, S.; Bandi, M.; Bristow, C.; McAfoos, T.; Morlacchi, P.; Ackroyd, J.; Agip, A.A.N.; et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat. Med. 2018, 24, 1036–1046. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, G.; Pan, J.; Zielonka, J.; Xiong, D.; Myers, C.R.; Feng, L.; Shin, S.S.; Kim, Y.H.; Bui, D.; et al. Magnolia extract is effective for the chemoprevention of oral cancer through its ability to inhibit mitochondrial respiration at complex I. Cell Commun. Signal 2020, 18, 58. [Google Scholar] [CrossRef] [PubMed]
- Min, H.Y.; Pei, H.; Hyun, S.Y.; Boo, H.J.; Jang, H.J.; Cho, J.; Kim, J.H.; Sonm, J.; Lee, H.Y. Potent anticancer effect of the natural steroidal saponin gracillin is produced by inhibiting glycolysis and oxidative phosphorylation-mediated bioenergetics. Cancers 2020, 12, 913. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shestov, A.A.; Worth, A.J.; Nath, K.; Nelson, D.S.; Leeper, D.B.; Glickson, J.D.; Blair, I.A. Inhibition of mitochondrial complex II by the anticancer agent lonidamine. J. Biol. Chem. 2016, 291, 42–57. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, Q.; Pan, J.; Lee, Y.; Ouari, O.; Hardy, M.; Zielonka, M.; Myers, C.R.; Zielonka, J.; Weh, K.; et al. Targeting lonidamine to mitochondria mitigates lung tumorigenesis and brain metastasis. Nat. Commun. 2019, 10, 2205. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Hardy, M.; Topchyan, P.; Zander, R.; Volberding, P.; Cui, W.; Kalyanaraman, B. Potent inhibition of tumour cell proliferation and immunoregulatory function by mitochondria-targeted atovaquone. Sci. Rep. 2020, 10, 17872. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.C.; Board, P.G.; Blackburn, A.C. Targeting metabolism with arsenic trioxide and dichloroacetate in breast cancer cells. Mol. Cancer 2011, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Mun, Y.C.; Ahn, J.Y.; Yoo, E.S.; Lee, K.E.; Nam, E.M.; Huh, J.; Woo, H.A.; Rhee, S.G.; Seong, C.M. Peroxiredoxin 3 Has Important Roles on Arsenic Trioxide Induced Apoptosis in Human Acute Promyelocytic Leukemia Cell Line via Hyperoxidation of Mitochondrial Specific Reactive Oxygen Species. Mol. Cells 2020, 43, 813–820. [Google Scholar]
- Fiorillo, M.; Scatena, C.; Naccarato, A.G.; Sotgia, F.; Lisanti, M.P. Bedaquiline, an FDA-approved drug, inhibits mitochondrial ATP production and metastasis in vivo, by targeting the gamma subunit (ATP5F1C) of the ATP synthase. Cell Death Differ. 2021, 28, 2797–2817. [Google Scholar] [CrossRef]
- Wu, R.; Yang, X.; Zhou, Q.; Yu, W.; Li, M.; Wo, J.; Shan, W.; Zhao, H.; Chen, Y.; Zhan, Z. Aurovertin B exerts potent antitumor activity against triple-negative breast cancer in vivo and in vitro via regulating ATP synthase activity and DUSP1 expression. Pharmazie 2020, 75, 261–265. [Google Scholar]
- Shi, Y.; Lim, S.K.; Liang, Q.; Iyer, S.V.; Wang, H.Y.; Wang, Z.; Xie, X.; Sun, D.; Chen, Y.J.; Tabar, V.; et al. Gboxin is an oxidative phosphorylation inhibitor that targets glioblastoma. Nature 2019, 567, 341–346. [Google Scholar] [CrossRef]
- Voltarelli, V.A.; Alves de Souza, R.W.; Miyauchi, K.; Hauser, C.J.; Otterbein, L.E. Heme: The Lord of the Iron Ring. Antioxidants 2023, 12, 1074. [Google Scholar] [CrossRef] [PubMed]
- Sohoni, S.; Ghosh, P.; Wang, T.; Kalainayakan, S.P.; Vidal, C.; Dey, S.; Konduri, P.C.; Zhang, L. Elevated Heme Synthesis and Uptake Underpin Intensified Oxidative Metabolism and Tumorigenic Functions in Non–Small Cell Lung Cancer Cells. Cancer Res. 2019, 79, 2511–2525. [Google Scholar] [CrossRef] [PubMed]
- Kiening, M.; Lange, N.A. Recap of Heme Metabolism towards Understanding Protoporphyrin IX Selectivity in Cancer Cells. Int. J. Mol. Sci. 2022, 23, 7974. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Wang, Y.; Lian, S.; Lynch, J.; Nagai, S.; Fanshawe, B.; Kandilci, A.; Janke, L.J.; Neale, G.; Fan, Y.; et al. Upregulated heme biosynthesis, an exploitable vulnerability in MYCN-driven leukemogenesis. JCI Insight 2017, 2, e92409. [Google Scholar] [CrossRef]
- Weinbach, E.C.; Ebert, P.S. Effects of succinylacetone on growth and respiration of L1210 leukemia cells. Cancer Lett. 1985, 26, 253–259. [Google Scholar] [CrossRef]
- Lee, P.J.; Woo, S.J.; Yoo, H.M.; Cho, N.; Kim, H.P. Differential Mechanism of ATP Production Occurs in Response to Succinylacetone in Colon Cancer Cells. Molecules 2019, 24, 3575. [Google Scholar] [CrossRef]
- Kalainayakan, S.P.; Ghosh, P.; Dey, S.; Fitzgerald, K.E.; Sohoni, S.; Konduri, P.C.; Garrossian, M.; Liu, L.; Zhang, L. Cyclopamine tartrate, a modulator of hedgehog signaling and mitochondrial respiration, effectively arrests lung tumor growth and progression. Sci. Rep. 2019, 9, 1405. [Google Scholar] [CrossRef]
- Sasaki, R.; Suzuki, Y.; Yonezawa, Y.; Ota, Y.; Okamoto, Y.; Demizu, Y.; Huang, P.; Yoshida, H.; Sugimura, K.; Mizushina, Y. DNA polymerase gamma inhibition by vitamin K3 induces mitochondria-mediated cytotoxicity in human cancer cells. Cancer Sci. 2008, 99, 1040–1048. [Google Scholar] [CrossRef]
- Bonekamp, N.A.; Peter, B.; Hillen, H.S.; Felser, A.; Bergbrede, T.; Choidas, A.; Horn, M.; Unger, A.; Di Lucrezia, R.; Atanassov, I.; et al. Small-molecule inhibitors of human mitochondrial DNA transcription. Nature 2020, 588, 712–716. [Google Scholar] [CrossRef]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef]
- Bost, F.; Kaminski, L. The metabolic modulator PGC-1α in cancer. Am. J. Cancer Res. 2019, 9, 198–211. [Google Scholar] [PubMed]
- Cormio, A.; Guerra, F.; Cormio, G.; Pesce, V.; Fracasso, F.; Loizzi, V.; Cantatore, P.; Selvaggi, L.; Gadaleta, M.N. The PGC-1alpha-dependent pathway of mitochondrial biogenesis is upregulated in type I endometrial cancer. Biochem. Biophys. Res. Commun. 2009, 390, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Chen, X.F.; Li, Q.; Zhang, S.T.; Si, L.N. PGC-1α activates SIRT3 to modulate cell proliferation and glycolytic metabolism in breast cancer. Neoplasma 2021, 68, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Li, X.; Ma, Y. miR-23b-3p suppressing PGC1α promotes proliferation through reprogramming metabolism in osteosarcoma. Cell Death Dis. 2019, 10, 381. [Google Scholar] [CrossRef]
- Sun, X.; Liu, X.; Yu, K.; Xu, S.; Qiu, P.; Lv, Z.; Zhang, X.; Xu, Y. Targeting PGC1α to wrestle cancer: A compelling therapeutic opportunity. J. Cancer Res. Clin. Oncol. 2022, 148, 767–774. [Google Scholar] [CrossRef]
- Seo, W.; Yoo, S.; Zhong, Y.; Lee, S.H.; Woo, S.Y.; Choi, H.S.; Won, M.; Roh, T.; Jeon, S.M.; Kim, K.T.; et al. Targeting ERRα promotes cytotoxic effects against acute myeloid leukemia through suppressing mitochondrial oxidative phosphorylation. J. Hematol. Oncol. 2022, 15, 156. [Google Scholar] [CrossRef]
- Liu, S.L.; Liang, H.B.; Yang, Z.Y.; Cai, C.; Wu, Z.Y.; Wu, X.S.; Dong, P.; Li, M.L.; Zheng, L.; Gong, W. Gemcitabine and XCT790, an ERRα inverse agonist, display a synergistic anticancer effect in pancreatic cancer. Int. J. Med. Sci. 2022, 19, 286–298. [Google Scholar] [CrossRef]
- Schoepke, E.; Billon, C.; Haynes, K.M.; Avdagic, A.; Sitaula, S.; Sanders, R.; Adeyemi, C.M.; Walker, J.K.; Burris, T.P. A Selective ERRα/γ Inverse Agonist, SLU-PP-1072, Inhibits the Warburg Effect and Induces Apoptosis in Prostate Cancer Cells. ACS Chem. Biol. 2020, 15, 2338–2345. [Google Scholar] [CrossRef]
- Morita, M.; Gravel, S.P.; Hulea, L.; Larsson, O.; Pollak, M.; St-Pierre, J.; Topisirovic, I. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle 2015, 14, 473–480. [Google Scholar] [CrossRef]
- Ali, E.S.; Mitra, K.; Akter, S.; Ramproshad, S.; Mondal, B.; Khan, I.N.; Islam, M.T.; Sharifi-Rad, J.; Calina, D.; Cho, W.C. Recent advances and limitations of mTOR inhibitors in the treatment of cancer. Cancer Cell Int. 2022, 22, 284. [Google Scholar] [CrossRef]
- Malizzia, L.J.; Hsu, A. Temsirolimus, an mTOR inhibitor for treatment of patients with advanced renal cell carcinoma. Clin. J. Oncol. Nurs. 2008, 12, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Witzig, T.E.; Geyer, S.M.; Ghobrial, I.; Inwards, D.J.; Fonseca, R.; Kurtin, P.; Ansell, S.M.; Luyun, R.; Flynn, P.J.; Morton, R.F. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J. Clin. Oncol. 2005, 23, 5347–5356. [Google Scholar] [CrossRef]
- Jhanwar-Uniyal, M.; Dominguez, J.F.; Mohan, A.L.; Tobias, M.E.; Gandhi, C.D. Disentangling the signaling pathways of mTOR complexes, mTORC1 and mTORC2, as a therapeutic target in glioblastoma. Adv. Biol. Regul. 2022, 83, 100854. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Sarosiek, K.A.; Wood, K.C. Endogenous and imposed determinants of apoptotic vulnerabilities in cancer. Trends Cancer 2023, 9, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Liang, J.; Li, Y.; He, J. Drugs and Clinical Approaches Targeting the Antiapoptotic Protein: A Review. Biomed. Res. Int. 2019, 2019, 1212369. [Google Scholar] [CrossRef]
- Melo, G.; Silva, C.A.B.; Hague, A.; Parkinson, E.K.; Rivero, E.R.C. Anticancer effects of putative and validated BH3-mimetic drugs in head and neck squamous cell carcinomas: An overview of current knowledge. Oral Oncol. 2022, 132, 105979. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- El-Cheikh, J.; Bidaoui, G.; Saleh, M.; Moukalled, N.; Abou Dalle, I.; Bazarbachi, A. Venetoclax: A New Partner in the Novel Treatment Era for Acute Myeloid Leukemia and Myelodysplastic Syndrome. Clin. Hematol. Int. 2023, 5, 143–154. [Google Scholar] [CrossRef]
- Quintana-Cabrera, R.; Scorrano, L. Determinants and outcomes of mitochondrial dynamics. Mol. Cell. 2023, 83, 857–876. [Google Scholar] [CrossRef]
- Trotta, A.P.; Chipuk, J.E. Mitochondrial Dynamics as Regulators of Cancer Biology. Cell. Mol. Life Sci. 2017, 74, 1999–2017. [Google Scholar] [CrossRef]
- Cormio, A.; Musicco, C.; Gasparre, G.; Cormio, G.; Pesce, V.; Sardanelli, A.M.; Gadaleta, M.N. Increase in proteins involved in mitochondrial fission, mitophagy, proteolysis and antioxidant response in type I endometrial cancer as an adaptive response to respiratory complex I deficiency. Biochem. Biophys. Res. Commun. 2017, 491, 85–90. [Google Scholar] [CrossRef]
- Courtois, S.; de Luxán-Delgado, B.; Penin-Peyta, L.; Royo-García, A.; Parejo-Alonso, B.; Jagust, P.; Alcalá, S.; Rubiolo, J.A.; Sánchez, L.; Sainz, B., Jr.; et al. Inhibition of Mitochondrial Dynamics Preferentially Targets Pancreatic Cancer Cells with Enhanced Tumorigenic and Invasive Potential. Cancers 2021, 13, 698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, L.; Wang, L.; Wang, J.; Wang, D.; Jiang, J.; Zhang, J.; Zhou, Q. Mitochondrial division inhibitor (mdivi-1) inhibits proliferation and epithelial-mesenchymal transition via the NF-κB pathway in thyroid cancer cells. Toxicol. Vitr. 2023, 88, 105552. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Dasgupta, A.; Chen, K.H.; Neuber-Hess, M.; Patel, J.; Hurst, T.E.; Mewburn, J.D.; Lima, P.D.A.; Alizadeh, E.; Martin, A.; et al. Identification of novel dynamin-related protein 1 (Drp1) GTPase inhibitors: Therapeutic potential of Drpitor1 and Drpitor1a in cancer and cardiac ischemia-reperfusion injury. FASEB J. 2020, 34, 1447–1464. [Google Scholar] [CrossRef]
- Fu, Y.; Dong, W.; Xu, Y.; Li, L.; Yu, X.; Pang, Y.; Chan, L.; Deng, Y.; Qian, C. Targeting mitochondrial dynamics by AZD5363 in triple-negative breast cancer MDA-MB-231 cell-derived spheres. Naunyn. Schmiedebergs Arch. Pharmacol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, Z.; Zhang, S.; Zhang, T.; Liu, Y.; Zhang, L. Cellular mitophagy: Mechanism, roles in diseases and small molecule pharmacological regulation. Theranostics 2023, 13, 736–766. [Google Scholar] [CrossRef]
- Song, C.; Pan, S.; Zhang, J.; Li, N.; Geng, Q. Mitophagy: A novel perspective for insighting into cancer and cancer treatment. Cell Prolif. 2022, 55, e13327. [Google Scholar] [CrossRef] [PubMed]
- Daido, S.; Kanzawa, T.; Yamamoto, A.; Takeuchi, H.; Kondo, Y.; Kondo, S. Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res. 2004, 64, 4286–4293. [Google Scholar] [CrossRef]
- Chang, S.H.; Lee, A.Y.; Yu, K.N.; Park, J.; Kim, K.P.; Cho, M.H. Dihydroergotamine tartrate induces lung cancer cell death through apoptosis and mitophagy. Chemotherapy 2016, 61, 304–312. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Li, Y.; Gao, J. Melatonin increases human cervical cancer HeLa cells apoptosis induced by cisplatin via inhibition of JNK/Parkin/mitophagy axis. Vitr. Cell Dev. Biol. Anim. 2018, 54, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Abbas, M.N.; Kausar, S.; Yang, J.; Li, L.; Tan, L.; Cui, H. Biological Functions and Molecular Mechanisms of Antibiotic Tigecycline in the Treatment of Cancers. Int. J. Mol. Sci. 2019, 20, 3577. [Google Scholar] [CrossRef]
- Gao, Y.; Shang, Q.; Li, W.; Guo, W.; Stojadinovic, A.; Mannion, C.; Man, Y.G.; Chen, T. Antibiotics for cancer treatment: A double-edged sword. J. Cancer. 2020, 11, 5135–5149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiao, W.; Luo, Y. Mitochondrial quality control proteases and their modulation for cancer therapy. Med. Res. Rev. 2023, 43, 399–436. [Google Scholar] [CrossRef] [PubMed]
- Nouri, K.; Feng, Y.; Schimmer, A.D. Mitochondrial ClpP serine protease-biological function and emerging target for cancer therapy. Cell Death Dis. 2020, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, J.A.; Guarné, A.; Ortega, J. ClpP: A structurally dynamic protease regulated by AAA+ proteins. J. Struct. Biol. 2012, 179, 202–210. [Google Scholar] [CrossRef]
- Cormio, A.; Sanguedolce, F.; Pesce, V.; Musicco, C. Mitochondrial Caseinolytic Protease P: A Possible Novel Prognostic Marker and Therapeutic Target in Cancer. Int. J. Mol. Sci. 2021, 22, 6228. [Google Scholar] [CrossRef]
- Cole, A.; Wang, Z.; Coyaud, E.; Voisin, V.; Gronda, M.; Jitkova, Y.; Mattson, R.; Hurren, R.; Babovic, S.; MacLean, N.; et al. Inhibition of the mitochondrial protease ClpP as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell 2015, 27, 864–876. [Google Scholar] [CrossRef]
- Gronauer, T.F.; Mandl, M.M.; Lakemeyer, M.; Hackl, M.W.; Meßner, M.; Korotkov, V.S.; Pachmayr, J.; Sieber, S.A. Design and synthesis of tailored human caseinolytic protease P inhibitors. Chem. Commun. 2018, 54, 9833–9836. [Google Scholar] [CrossRef]
- Wong, K.S.; Mabanglo, M.F.; Seraphim, T.V.; Mollica, A.; Mao, Y.Q.; Rizzolo, K.; Leung, E.; Moutaoufik, M.T.; Hoell, L.; Phanse, S.; et al. Acyldepsipeptide Analogs Dysregulate Human Mitochondrial ClpP Protease Activity and Cause Apoptotic Cell Death. Cell Chem. Biol. 2018, 25, 1017–1030.e9. [Google Scholar] [CrossRef]
- Graves, P.R.; Aponte-Collazo, L.J.; Fennell, E.M.J.; Graves, A.C.; Hale, A.E.; Dicheva, N.; Herring, L.E.; Gilbert, T.S.K.; East, M.P.; McDonald, I.M.; et al. Mitochondrial protease ClpP is a target for the anticancer compounds ONC201 and related analogues. ACS Chem. Biol. 2019, 14, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, J.; Zarabi, S.F.; Davis, R.E.; Halgas, O.; Nii, T.; Jitkova, Y.; Zhao, R.; St-Germain, J.; Heese, L.E.; Egan, G.; et al. Mitochondrial ClpP-Mediated Proteolysis Induces Selective Cancer Cell Lethality. Cancer Cell 2019, 35, 721–737.e9. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, L.; De Gaetano, A.; Mandrioli, M.; Van Tongeren, E.; Bortolotti, C.A.; Cossarizza, A.; Pinti, M. The biology of Lonp1: More than a mitochondrial protease. Int. Rev. Cell Mol. Biol. 2020, 354, 1–61. [Google Scholar] [PubMed]
- Cheng, C.W.; Kuo, C.Y.; Fan, C.C.; Fang, W.C.; Jiang, S.S.; Lo, Y.K.; Wang, T.Y.; Kao, M.C.; Lee, A.Y. Overexpression of Lon contributes to survival and aggressive phenotype of cancer cells through mitochondrial complex I-mediated generation of reactive oxygen species. Cell Death Dis. 2013, 4, e681. [Google Scholar] [CrossRef]
- Zanini, G.; Selleri, V.; De Gaetano, A.; Gibellini, L.; Malerba, M.; Mattioli, A.V.; Nasi, M.; Apostolova, N.; Pinti, M. Differential Expression of Lonp1 Isoforms in Cancer Cells. Cells 2022, 11, 3940. [Google Scholar] [CrossRef]
- Gibellini, L.; Pinti, M.; Bartolomeo, R.; De Biasi, S.; Cormio, A.; Musicco, C.; Carnevale, G.; Pecorini, S.; Nasi, M.; De Pol, A.; et al. Inhibition of Lon protease by triterpenoids alters mitochondria and is associated to cell death in human cancer cells. Oncotarget 2015, 6, 25466–25483. [Google Scholar] [CrossRef]
- Wang, H.M.; Cheng, K.C.; Lin, C.J.; Hsu, S.W.; Fang, W.C.; Hsu, T.F.; Chiu, C.C.; Chang, H.W.; Hsu, C.H.; Lee, A.Y. Obtusilactone A and (-)-sesamin induce apoptosis in human lung cancer cells by inhibiting mitochondrial Lon protease and activating DNA damage checkpoints. Cancer Sci. 2010, 101, 2612–2620. [Google Scholar] [CrossRef]
- Chakraborty, A.; Bose, R.; Bose, K. Unraveling the Dichotomy of Enigmatic Serine Protease HtrA2. Front. Mol. Biosci. 2022, 9, 824846. [Google Scholar] [CrossRef]
- Wu, L.; Li, X.; Li, Z.; Cheng, Y.; Wu, F.; Lv, C.; Zhang, W.; Tang, W. HtrA serine proteases in cancers: A target of interest for cancer therapy. Biomed. Pharmacother. 2021, 39, 111603. [Google Scholar] [CrossRef]
- Bohovych, I.; Fernandez, M.R.; Rahn, J.J.; Stackley, K.D.; Bestman, J.E.; Anandhan, A.; Franco, R.; Claypool, S.M.; Lewis, R.E.; Chan, S.S.; et al. Metalloprotease OMA1 Fine-tunes Mitochondrial Bioenergetic Function and Respiratory Supercomplex Stability. Sci. Rep. 2015, 5, 13989. [Google Scholar] [CrossRef]
- Noh, S.; Phorl, S.; Naskar, R.; Oeum, K.; Seo, Y.; Kim, E.; Kweon, H.S.; Lee, J.Y. p32/C1QBP regulates OMA1-dependent proteolytic processing of OPA1 to maintain mitochondrial connectivity related to mitochondrial dysfunction and apoptosis. Sci. Rep. 2020, 10, 10618. [Google Scholar] [CrossRef] [PubMed]
- Daverey, A.; Levytskyy, R.M.; Stanke, K.M.; Viana, M.P.; Swenson, S.; Hayward, S.L.; Narasimhan, M.; Khalimonchuk, O.; Kidambi, S. Depletion of mitochondrial protease OMA1 alters proliferative properties and promotes metastatic growth of breast cancer cells. Sci. Rep. 2019, 9, 14746. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Zinovkin, R.A.; Lyamzaev, K.G.; Chernyak, B.V. Current perspectives of mitochondria-targeted antioxidants in cancer prevention and treatment. Front. Cell Dev. Biol. 2023, 11, 1048177. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.S.; Kim, D.H.; Surh, Y.J. Role of Reductive versus Oxidative Stress in Tumor Progression and Anticancer Drug Resistance. Cells 2021, 10, 758. [Google Scholar] [CrossRef]
- Hanczko, R.; Fernandez, D.R.; Doherty, E.; Qian, Y.; Vas, G.; Niland, B.; Telarico, T.; Garba, A.; Banerjee, S.; Middleton, F.A.; et al. Prevention of hepatocarcinogenesis and increased susceptibility to acetaminophen-induced liver failure in transaldolase-deficient mice by N-acetylcysteine. J. Clin. Investig. 2009, 119, 1546–1557. [Google Scholar] [CrossRef]
- Oaks, Z.; Patel, A.; Huang, N.; Choudhary, G.; Winans, T.; Faludi, T.; Krakko, D.; Duarte, M.; Lewis, J.; Beckford, M.; et al. Cytosolic aldose metabolism contributes to progression from cirrhosis to hepatocarcinogenesis. Nat. Metab. 2023, 5, 41–60. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Wang, Z.; Li, B.; Zhu, H. Modulation of redox homeostasis: A strategy to overcome cancer drug resistance. Front. Pharmacol. 2023, 14, 1156538. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef]
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef]
- Hong, Y.H.; Uddin, M.H.; Jo, U.; Kim, B.; Song, J.; Suh, D.H.; Kim, H.S.; Song, Y.S. ROS Accumulation by PEITC Selectively Kills Ovarian Cancer Cells via UPR-Mediated Apoptosis. Front. Oncol. 2015, 5, 167. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhen, C.; Liu, J.; Shang, P. β-Phenethyl Isothiocyanate Induces Cell Death in Human Osteosarcoma through Altering Iron Metabolism, Disturbing the Redox Balance, and Activating the MAPK Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 5021983. [Google Scholar] [CrossRef] [PubMed]
- Tusskorn, O.; Prawan, A.; Senggunprai, L.; Kukongviriyapan, U.; Kukongviriyapan, V. Phenethyl isothiocyanate induces apoptosis of cholangiocarcinoma cells through interruption of glutathione and mitochondrial pathway. Naunyn. Schmiedebergs Arch. Pharmacol. 2013, 386, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, K.; Wang, J.; Jiang, H.; Zhang, M.; Liu, Y.; Shan, C.; Hu, F.; Fu, W.; Zhang, C.; et al. A rational foundation for micheliolide-based combination strategy by targeting redox and metabolic circuit in cancer cells. Biochem. Pharmacol. 2022, 200, 115037. [Google Scholar] [CrossRef] [PubMed]
- Dorr, R.T.; Raymond, M.A.; Landowski, T.H.; Roman, N.O.; Fukushima, S. Induction of apoptosis and cell cycle arrest by imexon in human pancreatic cancer cell lines. Int. J. Gastrointest. Cancer 2005, 36, 15–28. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A. Understanding the Prooxidant Action of Plant Polyphenols in the Cellular Microenvironment of Malignant Cells: Role of Copper and Therapeutic Implications. Front. Pharmacol. 2022, 13, 929853. [Google Scholar] [CrossRef]
- Benot-Dominguez, R.; Tupone, M.G.; Castelli, V.; d’Angelo, M.; Benedetti, E.; Quintiliani, M.; Cinque, B.; Forte, I.M.; Cifone, M.G.; Ippoliti, R.; et al. Olive leaf extract impairs mitochondria by pro-oxidant activity in MDA-MB-231 and OVCAR-3 cancer cells. Biomed. Pharmacother. 2021, 134, 111139. [Google Scholar] [CrossRef]
- Khamphio, M.; Barusrux, S.; Weerapreeyakul, N. Sesamol induces mitochondrial apoptosis pathway in HCT116 human colon cancer cells via pro-oxidant effect. Life Sci. 2016, 158, 46–56. [Google Scholar] [CrossRef]
- Liu, Z.H.; Yang, C.X.; Zhang, L.; Yang, C.Y.; Xu, X.Q. Baicalein, as a Prooxidant, Triggers Mitochondrial Apoptosis in MCF-7 Human Breast Cancer Cells Through Mobilization of Intracellular Copper and Reactive Oxygen Species Generation. Oncotargets Ther. 2019, 12, 10749–10761. [Google Scholar] [CrossRef]
- Lin, X.; Wang, L.; Zhao, L.; Zhu, Z.; Chen, T.; Chen, S.; Tao, Y.; Zeng, T.; Zhong, Y.; Sun, H.; et al. Curcumin micelles suppress gastric tumor cell growth by upregulating ROS generation, disrupting redox equilibrium and affecting mitochondrial bioenergetics. Food Funct. 2020, 11, 4146–4159. [Google Scholar] [CrossRef]
- Shi, L.; Gao, L.L.; Cai, S.Z.; Xiong, Q.W.; Ma, Z.R. A novel selective mitochondrial-targeted curcumin analog with remarkable cytotoxicity in glioma cells. Eur. J. Med. Chem. 2021, 221, 113528. [Google Scholar] [CrossRef] [PubMed]
- Kadkhoda, J.; Tarighatnia, A.; Nader, N.D.; Aghanejad, A. Targeting mitochondria in cancer therapy: Insight into photodynamic and photothermal therapies. Life Sci. 2022, 307, 120898. [Google Scholar] [CrossRef]
- Guo, X.; Yang, N.; Ji, W.; Zhang, H.; Dong, X.; Zhou, Z.; Li, L.; Shen, H.M.; Yao, S.Q.; Huang, W. Mito-Bomb: Targeting Mitochondria for Cancer Therapy. Adv. Mater. 2021, 33, e2007778. [Google Scholar] [CrossRef]
- Allison, R.R.; Ferguson, J.S. Photodynamic therapy to a primary cancer of the peripheral lung: Case report. Photodiagnosis Photodyn. Ther. 2022, 39, 103001. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Osuchowski, M.; Adamczyk, M.; Stopa, J.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Advancements in photodynamic therapy of esophageal cancer. Front. Oncol. 2022, 12, 1024576. [Google Scholar] [CrossRef] [PubMed]
- Sauraj Kang, J.H.; Lee, O.; Ko, Y.T. Novel aggregation-induced emission-photosensitizers with built-in capability of mitochondria targeting and glutathione depletion for efficient photodynamic therapy. Nanoscale 2023, 15, 4882–4892. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, N.; Wang, X.; Yang, Y.; Xiang, Y.; Long, X.; Zhang, J.; Huang, J.; Chen, L.; Huang, Q. Mitochondria-Targeting Chemodynamic Therapy Nanodrugs for Cancer Treatment. Front. Pharmacol. 2022, 13, 847048. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Qin, Y.T.; Feng, Y.S.; He, X.W.; Li, W.Y.; Zhang, Y.K. Phosphate-Degradable Nanoparticles Based on Metal-Organic Frameworks for Chemo-Starvation-Chemodynamic Synergistic Antitumor Therapy. ACS Appl. Mater. Interfaces 2021, 13, 37713–37723. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, S.; Zhang, L.; Deng, Q.; Ren, J.; Qu, X. A Metabolic Multistage Glutathione Depletion Used for Tumor-Specific Chemodynamic Therapy. ACS Nano 2022, 16, 4228–4238. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Waseem, R.; Zehra, Z.; Aiman, A.; Bhardwaj, P.; Ansari, J.; Hassan, M.I.; Islam, A. Mitochondrial Dysfunction: Pathophysiology and Mitochondria-Targeted Drug Delivery Approaches. Pharmaceutics 2022, 14, 2657. [Google Scholar] [CrossRef]
- Lu, Z.; Li, J.; Chen, B.; Feng, J.; Hu, Q.; Jin, Y.; Fu, Z. Mitochondria Targeted Nanoparticles Potentiate Tumor Chemo-Phototherapy by Toxic Oxidative Stress Mediated Oxeiptosis. Macromol. Biosci. 2023, e2300151. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.J.; Smith, R.A.; Murphy, M.P. Synthesis and characterization of thiobutyltriphenylphosphonium bromide, a novel thiol reagent targeted to the mitochondrial matrix. Arch. Biochem. Biophys. 1995, 322, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Kafle, U.; Agrawal, S.; Dash, A.K. Injectable Nano Drug Delivery Systems for the Treatment of Breast Cancer. Pharmaceutics 2022, 14, 2783. [Google Scholar] [CrossRef] [PubMed]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability ParametersWith Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zeng, Y.; Chen, M.; Liu, G. Application of Nanomicelles in Enhancing Bioavailability and Biological Efficacy of Bioactive Nutrients. Polymer 2022, 14, 3278. [Google Scholar] [CrossRef]
- Lyu, Z.; Ding, L.; Tintaru, A.; Peng, L. Self-Assembling Supramolecular Dendrimers for Biomedical Applications: Lessons Learned from Poly(amidoamine) Dendrimers. Acc. Chem. Res. 2020, 53, 2936–2949. [Google Scholar] [CrossRef]
- Ganji, C.; Muppala, V.; Khan, M.; Purnachandra Nagaraju, G.; Farran, B. Mitochondrial-targeted nanoparticles: Delivery and therapeutic agents in cancer. Drug Discov. Today 2023, 28, 103469. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Giri, P.M.; Banerjee, A.; Layek, B. A Recent Review on Cancer Nanomedicine. Cancers 2023, 15, 2256. [Google Scholar] [CrossRef]
- Herst, P.M.; Rowe, M.R.; Carson, G.M.; Berridge, M.V. Functional Mitochondria in Health and Disease. Front. Endocrinol. 2017, 8, 296. [Google Scholar] [CrossRef]
- Zhang, J.; Simpson, C.M.; Berner, J.; Chong, H.B.; Fang, J.; Ordulu, Z.; Weiss-Sadan, T.; Possemato, A.P.; Harry, S.; Takahashi, M.; et al. Systematic identification of anticancer drug targets reveals a nucleus-to-mitochondria ROS-sensing pathway. Cell 2023, 186, 2361–2379.e25. [Google Scholar] [CrossRef] [PubMed]


| Drugs | Targets | Effects | Ref. |
|---|---|---|---|
| Krebs cycle | |||
| Ivosidenib, Vorasidenib | Mutant IDH | Increase remission and survival in patients with leukemia and glioma | [13,14] |
| Fatty acid oxidation | |||
| Etomoxir, ST1326, Perhexiline | CPT-1 | Prevent the proliferation of glioblastoma, glioma, leukemia and colorectal cancer cells | [19,20,21,22,23] |
| Glutamine metabolism | |||
| V-9302 | Glutamine transporter (ASCT2) | Reduces the proliferation of lung, breast and colorectal cancer cells | [25] |
| CB-839, BPTES, UPGL00004 | GLS | Decrease the proliferation of triple-negative breast and colorectal cancer cells | [26,27] |
| Propylselen | GDH | Decreases the proliferation of liver and lung cancer cells | [28] |
| Hydralazine hydrochloride, Aspulvinone O | GOT1 | Inhibit the proliferation of colorectal, breast and pancreatic cancer cells | [29,30] |
| Aminooxyacetate, Oxamate | GPT2 | Suppress the proliferation of breast, lung, colon and pancreatic cancer cells | [31,32] |
| OXPHOS | |||
| Metformin, Phenformin, BAY 87-2243, Fenofibrate, Ivermectin, IACS-010759, Magnolia extracts | Complex I | Antitumor activity in osteosarcoma, neuroblastoma, melanoma, glioblastoma, myeloid leukemia, gastric, breast, ovarian, endometrial, pancreatic, liver, lung, renal, colorectal, brain and oral cancers | [37,38] [44,45,46,47,48,49,50,51,52] |
| Gracillin, Lonidamine | Complex II | Inhibit the proliferation of melanoma, brain and lung cancer cells | [53,54,55] |
| Atovaquone | Complex III | Reduces tumor proliferation in pancreatic, breast and lung cancer cells | [56] |
| Arsenic trioxide | Complex IV | Reduces tumor proliferation in breast and promyelocytic leukemia cancer cells | [57,58] |
| Bedaquiline, Aurovertin B, Gboxin | Complex V | Induce apoptosis in breast and glioblastoma cancer cells | [59,60,61] |
| Heme biosynthesis | |||
| Succinylacetone | ALA-D | Reduces growth in leukemic and colon cancer cells | [65,66,67] |
| Cyclopamine tartrate | ALAS1 | Suppresses growth in non-small cell lung cancer (NSCLC) | [68] |
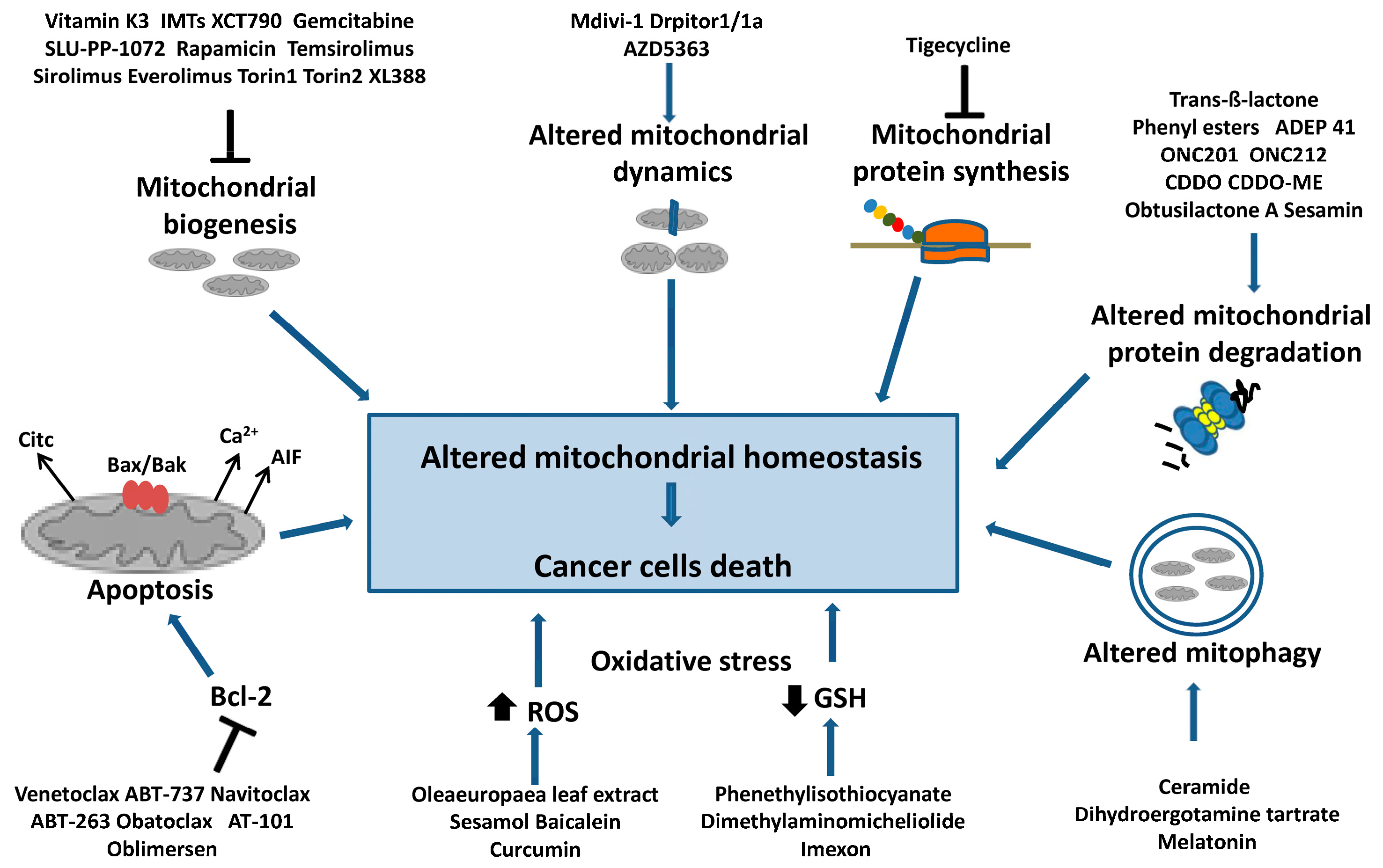
| Drugs | Targets | Effects | Ref. | |
|---|---|---|---|---|
| Biogenesis | ||||
| Vitamin K3, | mtDNA polymerase γ | Induces apoptosis in colon, lung, breast, pancreatic, prostate, leukemic cancer cells | [69] | |
| IMTs | mtRNA polymerase | Induce an antitumor response in xenografts of human cancer cells | [70] | |
| XCT790, Gemcitabine, SLU-PP-1072 | ERRα | Anticancer effects in acute myeloid leukemia, pancreatic and prostate cancer | [77,78,79] | |
| Rapamycin, Temsirolimus, Sirolimus, Everolimus Torin1, Torin2, XL388 | mTOR | Anticancer effects in renal cancer, glioblastoma, glioma, breast and endometrial cancer, lymphomas, multiple myeloma | [81,82,83,84] | |
| Apoptosis | ||||
| Venetoclax, ABT-737, Navitoclax, ABT-263, Obatoclax, AT-101, Oblimersen | Bcl-2 | Tumor regression in chronic lymphocytic leukemia, acute myeloid leukemia, breast cancer, gastric cancers, small-cell lung cancer, head and neck squamous cancer | [87,88,89,90] | |
| Dynamics | ||||
| Mdivi-1, Drpitor1/1a | Drp1 | Reduce proliferation in lung, breast, pancreatic, thyroid cancer cells | [94,96] | |
| AZD536 | Mfn1 | Reduces proliferation in breast cancer cell lines | [97] | |
| Mitophagy | ||||
| Ceramide | BNIP3 | Reduces proliferation in glioma cell line | [100] | |
| Dihydroergotamine tartrate Melatonin | PINK1/Parkin | Induce lung and cervical cancer cell death | [101,102] | |
| Proteostasis | ||||
| Protein synthesis | Tigecycline | 28S subunit | Slows proliferation in acute myeloid leukemia, gastric, glioma cancer cells | [103] |
| Protein degradation | Trans-ß-lactone, Phenyl esters | ClpP (inhibition) | Induce apoptosis in AML, osteosarcoma, and liver cell lines | [108,109,110] |
| ADEP 41, ONC201, ONC212 | ClpP (activation) | Induce apoptosis in kidney and cervical cancers and in osteosarcoma, neuroblastoma, leukemia, and lymphoma cell lines | [108,111,112,113] | |
| CDDO, CDDO-ME, Obtusilactone A, Sesamin | Lonp1 | Induce apoptosis in colon, liver, breast, and lung cancer cells | [117,118] | |
| Redox balance | ||||
| Phenethylisothiocyanate, Dimethylaminomicheliolide, Buthionine sulfoximine, Imexon | GSH | Induce apoptosis in ovarian cancer, osteosarcoma, cholangiocarcinoma, leukemia, glioblastoma, and pancreatic cancer cells | [132,133,134,135,136] | |
| Olea europaea leaf extract, Sesamol, Baicalein, Curcumin | ROS | Show antiproliferative and pro-apoptotic activity in ovarian, colon, pancreatic, breast, gastric, and glioma cancer cells | [138,139,140,141,142] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musicco, C.; Signorile, A.; Pesce, V.; Loguercio Polosa, P.; Cormio, A. Mitochondria Deregulations in Cancer Offer Several Potential Targets of Therapeutic Interventions. Int. J. Mol. Sci. 2023, 24, 10420. https://doi.org/10.3390/ijms241310420
Musicco C, Signorile A, Pesce V, Loguercio Polosa P, Cormio A. Mitochondria Deregulations in Cancer Offer Several Potential Targets of Therapeutic Interventions. International Journal of Molecular Sciences. 2023; 24(13):10420. https://doi.org/10.3390/ijms241310420
Chicago/Turabian StyleMusicco, Clara, Anna Signorile, Vito Pesce, Paola Loguercio Polosa, and Antonella Cormio. 2023. "Mitochondria Deregulations in Cancer Offer Several Potential Targets of Therapeutic Interventions" International Journal of Molecular Sciences 24, no. 13: 10420. https://doi.org/10.3390/ijms241310420
APA StyleMusicco, C., Signorile, A., Pesce, V., Loguercio Polosa, P., & Cormio, A. (2023). Mitochondria Deregulations in Cancer Offer Several Potential Targets of Therapeutic Interventions. International Journal of Molecular Sciences, 24(13), 10420. https://doi.org/10.3390/ijms241310420







