Tat-CIAPIN1 Prevents Pancreatic β-Cell Death in hIAPP-Induced RINm5F Cells and T2DM Animal Model
Abstract
:1. Introduction
2. Results
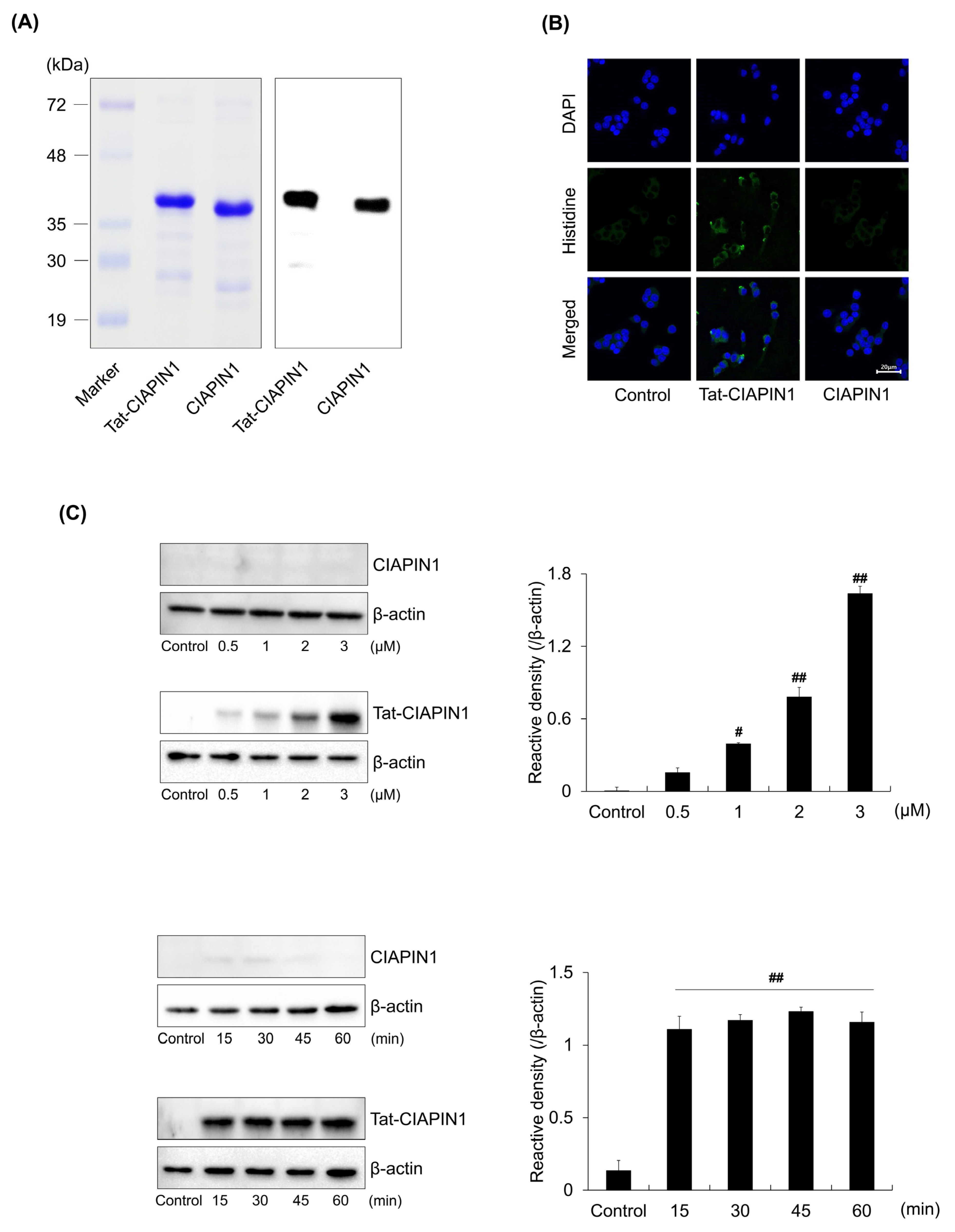
2.1. Delivery of Tat-CIAPIN1 Protein into RINm5F Cells
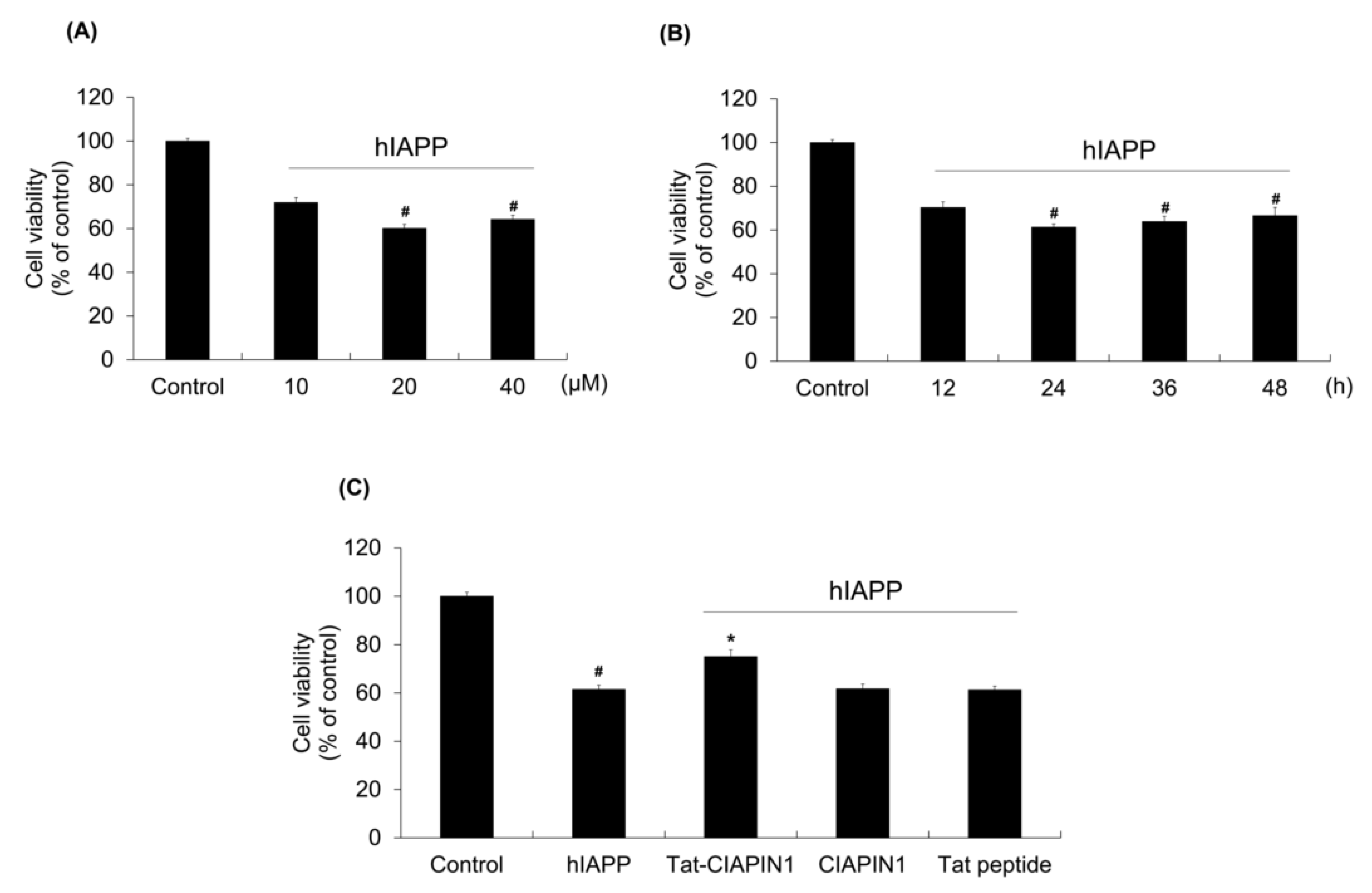
2.2. Effects of Tat-CIAPIN1 Protein on hIAPP-Induced RINm5F Cell Viability
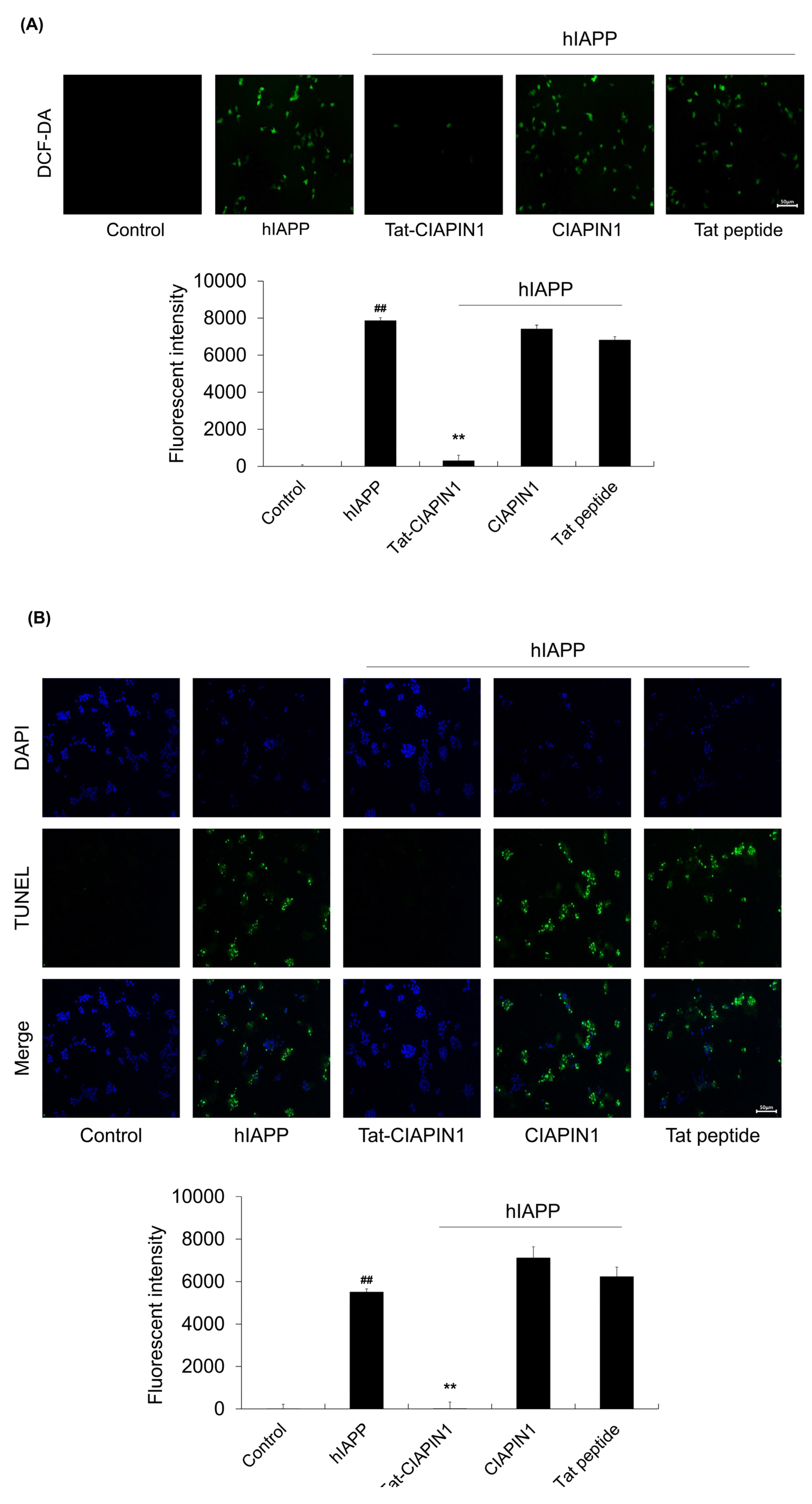
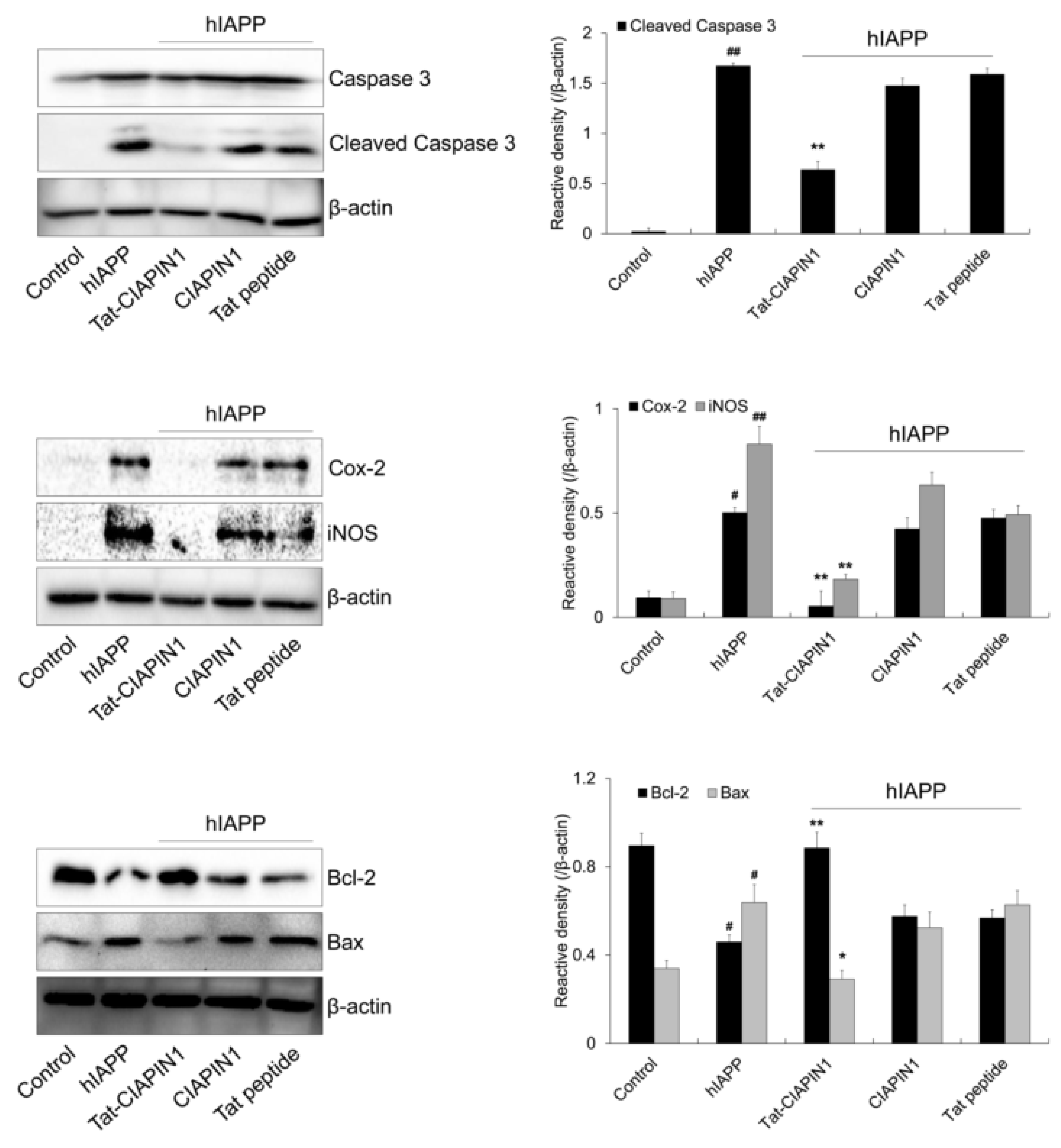
2.3. Effects of Tat-CIAPIN1 Protein on hIAPP-Induced MAPK and Apoptotic Protein Expression in RINm5F Cells
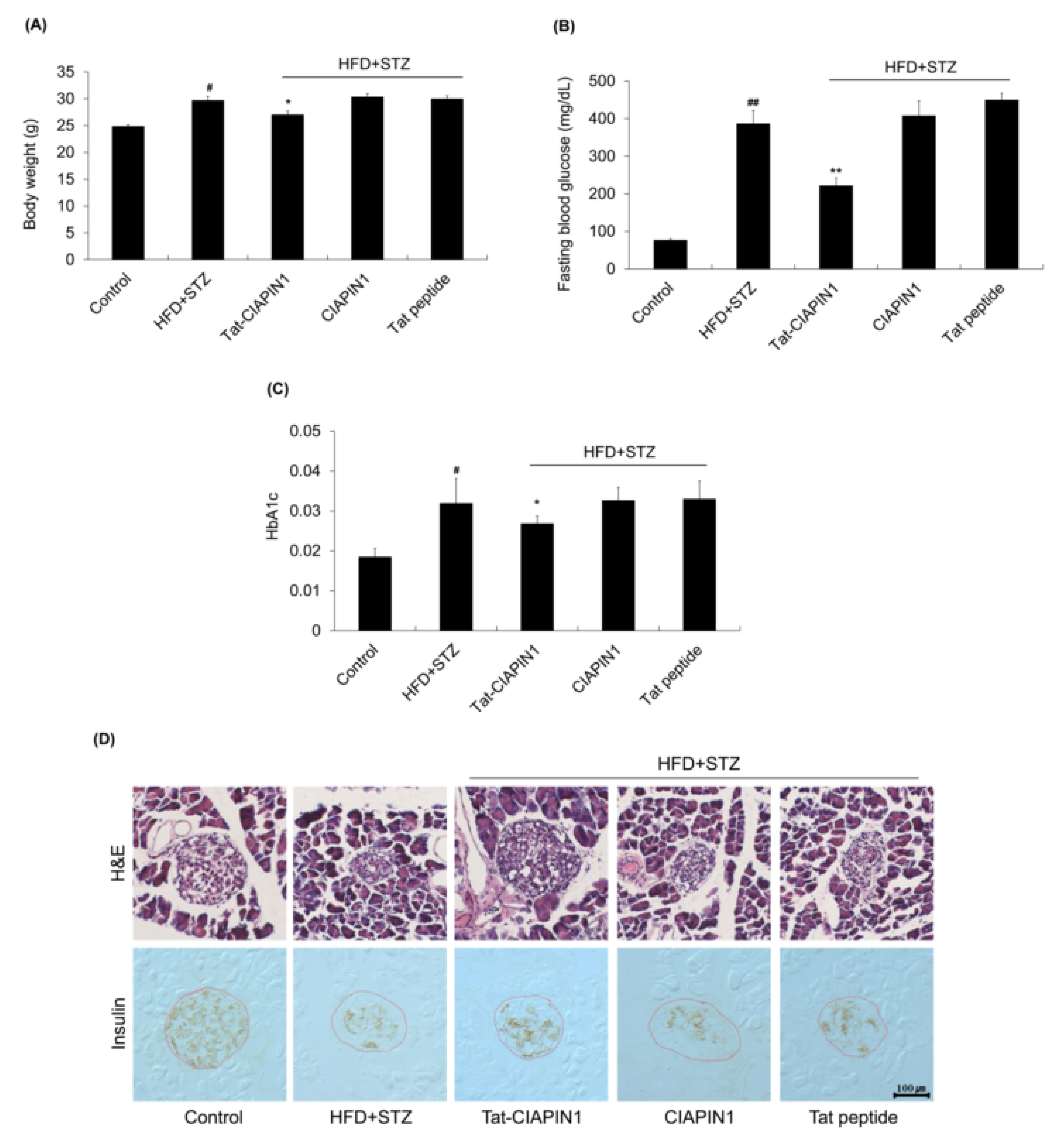
2.4. Effects of Tat-CIAPIN1 in T2DM Mice Model
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Purification of Tat-CIAPIN1 Protein
4.3. Cell Culture and Delivery of Tat-CIAPIN1 Protein into RINm5F Cells
4.4. Cell Viability Assay
4.5. Western Blot Analysis
4.6. Measurement of ROS Levels
4.7. TUNEL Assay
4.8. Animal Model and Treatments
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clark, A.; Nilsson, M.R. Islet amyloid: A complication of islet dysfunction or an aetiological factor in Type 2 diabetes? Diabetologia 2004, 47, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Hoppener, J.W.; Lips, C.J. Role of islet amyloid in type 2 diabetes mellitus. Int. J. Biochem. Cell Biol. 2006, 38, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Haataja, L.; Gurlo, T.; Huang, C.J.; Butler, P.C. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr. Rev. 2008, 29, 303–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronsky, J.; Chada, M.; Kotaska, K.; Prusa, R. Amylin-its physiological role in humans. Cesk Fysiol. 2002, 51, 176–180. [Google Scholar]
- Ritzel, R.A.; Meier, J.J.; Lin, C.Y.; Veldhuis, J.D.; Butler, P.C. Human islet amyloid polypeptide oligomers disrupt cell coupling, induce apoptosis, and impair insulin secretion in isolated human islets. Diabetes 2007, 56, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Clementi, G.; Caruso, A.; Cutuli, V.M.; de Bernardis, E.; Prato, A.; Amico-Roxas, M. Amylin given by central or peripheral routes decreases gastric emptying and intestinal transit in the rat. Experientia 1996, 52, 677–679. [Google Scholar] [CrossRef]
- Hayden, M.R. Islet amyloid, metabolic syndrome, and the natural progressive history of type 2 diabetes mellitus. J. Pancreas 2002, 3, 86–108. [Google Scholar]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiological and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef] [Green Version]
- Ge, Q.; Xie, X.X.; Xiao, X.; Li, X.; Saisho, Y. Exosome-like vesicles as new mediators and therapeutic targets for treating insulin resistance and β-cell mass failure in type 2 diabetes mellitus. J. Diabetes Res. 2019, 2019, 3256060. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Luo, Y.; Jang, H.; Yu, X.; Wei, G.; Nussinov, R.; Zheng, J. Probing ion channel activity of human islet amyloid polypeptide (amylin). Biochim. Biophys. Acta 2012, 1818, 3121–3130. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.Y.; Gurlo, T.; Kayed, R.; Butler, A.E.; Haataja, L.; Glabe, C.G.; Butler, P.C. Toxic human islet amyloid polypeptide (h-IAPP) oligomers are intracellular, and vaccination to induce anti-toxic oligomer antibodies does not prevent h-IAPP-induced beta-cell apoptosis in h-IAPP transgenic mice. Diabetes 2007, 56, 1324–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevilacqua, E.; Gomes, S.Z.; Lorenzon, A.R.; Hoshida, M.S.; Amarante-Paffaro, A.M. NADPH oxidase as an important source of reactive oxygen species at the mouse maternal-fetal interface: Putative biological roles. Reprod. Biomed. Online 2012, 25, 31–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burtenshaw, D.; Kitching, M.; Redmond, E.M.; Megson, I.L.; Cahill, P.A. Reactive Oxygen Species (ROS), intimal thickening, and subclinical atherosclerotic disease. Front. Cardiovasc. Med. 2019, 6, 89. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Shibayama, H.; Takai, E.; Matsumura, I.; Kouno, M.; Morii, E.; Kitamura, Y.; Takeda, J.; Kanakura, Y. Identification of a cytokine-induced antiapoptotic molecule anamorsin essential for definitive hematopoiesis. J. Exp. Med. 2004, 199, 581–592. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Su, G.F.; Hu, W.J.; Bi, X.X.; Zhang, L.; Wan, G. The study on expression of CIAPIN1 interfering hepatocellular carcinoma cell proliferation and its mechanisms. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3054–3060. [Google Scholar]
- Hao, Z.; Li, X.; Qiao, T.; Du, R.; Hong, L.; Fan, D. CIAPIN1 confers multidrug resistance by upregulating the expression of MDR-1 and MRP-1 in gastric cancer cells. Cancer Biol. Ther. 2006, 5, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, K.; Fan, D. CIAPIN1 as a therapeutic target in cancer. Expert. Opin. Ther. Targets 2010, 14, 603–610. [Google Scholar] [CrossRef]
- Park, K.A.; Yun, N.; Shin, D.I.; Choi, S.Y.; Kim, H.; Kim, W.K.; Kanakura, Y.; Shibayama, H.; Oh, Y. Nuclear translocation of anamorsin during drug-induced dopaminergic neurodegeneration in culture and in rat brain. J. Neural Transm. 2011, 118, 433–444. [Google Scholar] [CrossRef]
- Yeo, H.J.; Shin, M.J.; Yeo, E.J.; Choi, Y.J.; Kim, D.W.; Kim, D.S.; Eum, W.S.; Choi, S.Y. Tat-CIAPIN1 inhibits hippocampal neuronal cell damage through the MAPK and apoptotic signaling pathways. Free Radic. Biol. Med. 2019, 135, 68–78. [Google Scholar] [CrossRef]
- Gump, J.M.; Dowdy, S.F. TAT transduction: The molecular mechanism and therapeutic prospects. Trends Mol. Med. 2007, 13, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Kardani, K.; Milani, A.; Shabani, S.H.; Bolhassani, A. Cell penetrating peptides: The potent multi-cargo intracellular carriers. Expert Opin. Drug Deliv. 2019, 16, 1227–1258. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.L.; Dowdy, S.F. Cell penetrating peptides in drug delivery. Pharm. Res. 2004, 21, 389–393. [Google Scholar] [CrossRef]
- Lindsay, M.A. Peptide-mediated cell delivery: Application in protein target validation. Curr. Opin. Pharmacol. 2002, 2, 587–594. [Google Scholar] [CrossRef]
- Dietz, G.P. Cell-penetrating peptide technology to deliver chaperones and associated factors in diseases and basic research. Curr. Pharm. Biotechnol. 2010, 11, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, L.; Wu, Q.; Yang, E.; Zhang, G.; Sun, H.; Wang, F. A recombinant trans-membrane protein hMnSOD-R9 inhibits the proliferation of cervical cancer cells in vitro. Mol. Cell. Biochem. 2014, 385, 79–86. [Google Scholar] [CrossRef]
- Liu, L.; Yu, R.; Shi, Y.; Dai, Y.; Zeng, Z.; Guo, X.; Ji, Q.; Wang, G.; Zhong, J. Transduced protein transduction domain linked HSP27 protected LECs against UVB radiation-induced damage. Exp. Eye Res. 2014, 120, 36–42. [Google Scholar] [CrossRef]
- Jia, X.; Tian, H.; Tang, L.; Zheng, L.; Zheng, L.; Yang, T.; Yu, B.; Wang, Z.; Lin, P.; Li, X.; et al. High-efficiency expression of TAT-bFGF fusion protein in Escherichia coli and effect on hypertrophic scar tissue. PLoS ONE 2015, 10, e0117448. [Google Scholar] [CrossRef]
- Zhu, Y.; Bu, Q.; Liu, X.; Hu, W.; Wang, Y. Neuroprotective effect of TAT-14–3-3ε fusion protein against cerebral ischemia/reperfusion injury in rats. PLoS ONE 2014, 9, e93334. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, M.; Kim, D.W.; Shin, M.J.; Son, O.; Jo, H.S.; Yeo, H.J.; Cho, S.B.; Park, J.H.; Lee, C.H.; et al. Transduced PEP-1-PON1 protein regulate microglial activation and dopaminergic neuronal death in a Parkinson’s model. Biomaterials 2015, 64, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, D.W.; Park, J.H.; Kim, S.J.; Lee, C.H.; Yong, J.I.; Ryu, E.J.; Cho, S.B.; Yeo, H.J.; Hyeon, J.; et al. PEP-1-SIRT2 inhibits inflammatory response and oxidative stress-induced cell death via expression of antioxidant enzymes in murine macrophages. Free Radic. Biol. Med. 2013, 63, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.S.; Kim, D.W.; Shin, M.J.; Cho, S.B.; Park, J.H.; Lee, C.H.; Yeo, E.J.; Choi, Y.J.; Yeo, H.J.; Sohn, E.J.; et al. Tat-HSP22 inhibits oxidative stress-induced hippocampal neuronal cell death by regulation of the mitochondrial pathway. Mol. Brain 2017, 10, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Shin, M.J.; Kim, D.W.; Yeo, H.J.; Yeo, E.J.; Choi, Y.J.; Sohn, E.J.; Han, K.H.; Park, J.; Lee, K.W.; et al. Tat-biliverdin reductase A exerts a protective role in oxidative stress-induced hippocampal neuronal cell damage by regulating the apoptosis and MAPK signaling. Int. J. Mol. Sci. 2020, 21, 2672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, W.; Ruangkittisakul, A.; MacTavish, D.; Shi, J.Y.; Ballanyi, K.; Jhamandas, J.H. Amyloid β (Aβ) peptide directly activates amylin-3 receptor subtype by triggering multiple intracellular signaling pathways. J. Biol. Chem. 2012, 287, 18820–18830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signgh, S.; Bhowmick, D.C.; Pany, S.; Joe, M.; Zaghlula, N.; Jeremic, A.M. Apoptosis signal regulating kinase-1 and NADPH oxidase mediate human amylin evoked redox stress and apoptosis in pancreatic beta-cells. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1721–1733. [Google Scholar] [CrossRef]
- Subramanian, S.L.; Hull, R.L.; Zraika, S.; Aston-Mourney, K.; Udayasankar, J.; Kahn, S.E. cJUN Nterminal kinase (JNK) activation mediates islet amyloid-induced beta cell apoptosis in cultured human islet amyloid polypeptide transgenic mouse islets. Diabetologia 2012, 55, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Rumora, L.; Hadzija, M.; Barisic, K.; Maysinger, D.; Grubiic, T.Z. Amylin-induced cytotoxicity is associated with activation of caspase-3 and MAP kinases. Biol. Chem. 2002, 383, 1751–1758. [Google Scholar] [CrossRef]
- Kubo, E.; Fatma, N.; Akagi, Y.; Beier, D.R.; Singh, S.P.; Singh, D.P. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am. J. Physiol. Cell Physiol. 2008, 294, C842–C855. [Google Scholar] [CrossRef] [Green Version]
- Nagel, F.; Falkenburger, B.H.; Tonges, L.; Kowsky, S.; Poppelmeyer, C.; Schulz, J.B.; Bahr, M.; Dietz, G.P.H. Tat-Hsp70 protects dopaminergic neurons in midbrain cultures and in the substantia nigra in models of Parkinson’s disease. J. Neurochem. 2008, 105, 853–864. [Google Scholar] [CrossRef]
- Yeo, H.J.; Shin, M.J.; Kim, D.W.; Kwon, H.Y.; Eum, W.S.; Choi, S.Y. Tat-CIAPIN1 protein prevents cytokine-induced cytotoxicity in pancreatic RINm5F β-cells. BMB Rep. 2021, 54, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Zraika, S.; Hull, R.L.; Udayasankar, J.; Aston-Mournet, K.; Subramanian, S.L.; Kisilevsky, R.; Szarek, W.A.; Kahn, S.E. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia 2009, 52, 626–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.; Gorgun, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [Green Version]
- Padgett, L.E.; Broniowska, K.A.; Hansen, P.A.; Corbett, J.A.; Tse, H.M. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann. N.Y. Acad. Sci. 2013, 1281, 16–35. [Google Scholar] [CrossRef] [Green Version]
- Miraee-Nedjad, S.; Sims, P.F.G.; Schwartz, J.M.; Doig, A.J. Effect of IAPP on the proteome of cultured Rin-5F cells. BMC Biochem. 2018, 19, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Almazan, S.; Filisola-Villasenor, J.G.; Aleman-Gonzalez-Duhart, D.; Tamay-Cach, F.; Mendieta-Wejebe, J.E. Current molecular aspects in the development and treatment of diabetes. J. Physiol. Biochem. 2020, 76, 13–35. [Google Scholar] [CrossRef]
- Abbasihormozi, S.H.; Babapour, V.; Kouhkan, A.; Naslji, A.N.; Afraz, K.; Zolfaghary, Z.; Shahverdi, A.H. Stress hormone and oxidative stress biomarkers link obesity and diabetes with reduced fertility potential. Cell J. 2019, 21, 307–313. [Google Scholar]
- Hsieh, C.C.; Papaconstantinou, J. Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. FASEB J. 2006, 20, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Yun, N.; Lee, Y.M.; Kim, C.; Shibayama, H.; Tanimura, A.; Hamanaka, Y.; Kanakura, Y.; Park, I.S.; Jo, A.; Shin, J.H.; et al. Anamorsin, a novel caspase-3 substrate in neurodegeneration. J. Biol. Chem. 2014, 289, 22183–22195. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, Q.; Wang, C.; Xiong, Q.; Lin, Y.; Sun, Q.; Jin, H.; Yang, F.; Ren, X.; Pang, T. Knock-down of CIAPIN1 sensitizes K562 chronic myeloid leukemia cells to Imatinib by regulation of cell cycle and apoptosis-associated members via NF-κB and ERK5 signaling pathways. Biochem. Pharmacol. 2016, 99, 132–145. [Google Scholar] [CrossRef]
- Luo, Z.; Li, T.; Gao, Q.; Chen, Y.; Su, G.; Zhao, Y. Impact of licochalcone A on the progression of diabetic nephropathy in type 2 diabetes mellitus of C57BL/6 mice. Food Funct. 2021, 12, 10676–10689. [Google Scholar] [CrossRef]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, H.; Yang, F.; Ma, X.; Wang, Y.; Chen, Q.; Yuan, L. Long-term liraglutide administration induces pancreas neogenesis in adult T2DM mice. Cell Transplant. 2020, 29, 963689720927392. [Google Scholar] [CrossRef]
- Li, F.; Liu, G.; Xue, P.; Ren, Z.; Dai, P.; Niu, W.; Xin, M. YiQi YangYin Decoction attenuates nonalcoholic fatty liver disease in type 2 diabetes rats. Evid. Based Complement Alternat. Med. 2021, 2021, 5511019. [Google Scholar] [CrossRef]
- Son, Y.; Lee, H.; Son, S.Y.; Lee, C.H.; Kim, S.Y.; Lim, Y. Ameliorative effect of Annona muricata (Graviola) extract on hyperglycemia induced hepatic damage in type 2 diabeteic mice. Antioxidants 2021, 10, 1546. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, K.; Sun, P.; Zhao, R.; Liu, B.; Lu, P. Exercise improves bone formation by upregulating the Wnt3a/β-catenin signalling pathway in type 2 diabetic mice. Diabetol. Metab. Syndr. 2021, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Purnamasari, D.; Tetrasiwi, E.N.; Kartiko, G.J.; Astrella, C.; Husam, K.; Laksmi, P.W. Sarcopenia and chronic complications of type 2 diabetes mellitus. Rev. Diabet. Stud. 2022, 18, 157–165. [Google Scholar] [CrossRef]
- Goyal, R.; Faizy, A.F.; Siddiqui, S.S.; Singhai, M. Evaluation of TNF-alpha and IL-6 levels in obese and non-obese diabetics: Pre- and postinsulin effects. N. Am. J. Med. Sci. 2012, 4, 180–184. [Google Scholar]
- King, D.E.; Mainous, A.G.; Buchanan, T.A.; Pearson, W.S. C-reactive protein and glycemic control in adults with diabetes. Diabetes Care. 2003, 26, 1535–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vozarova, B.; Weyer, C.; Hanson, K.; Tataranni, P.A.; Bogardus, C.; Pratley, R.E. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes. Res. 2001, 9, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.J.; Shin, M.J.; You, J.H.; Kim, J.S.; Kim, M.Y.; Kim, D.W.; Kim, D.S.; Eum, W.S.; Choi, S.Y. Transduced Tat-CIAPIN1 reduces the inflammatory response on LPS- and TPA-induced damages. BMB Rep. 2019, 52, 695–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Kaneto, H.; Nakatani, Y.; Miyatsuka, T.; Kawamori, D.; Matsuoka, T.A.; Matsuhisa, M.; Kajimoto, Y.; Ichijo, H.; Yamasaki, Y.; Hori, M. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat. Med. 2004, 10, 1128–1132. [Google Scholar] [CrossRef]
- Kamata, H.; Honda, S.I.; Maeda, S.; Chang, L.; Hirata, H.; Karin, M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005, 120, 649–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Shin, M.J.; Kim, D.W.; Jo, H.S.; Cho, S.B.; Park, J.H.; Lee, C.H.; Yeo, E.J.; Choi, Y.J.; Kim, J.A.; Hwang, J.S.; et al. Tat-PRAS40 prevents hippocampal HT-22 cell death and oxidative stress induced animal brain ischemic insults. Free Radic. Biol. Med. 2016, 97, 250–262. [Google Scholar] [CrossRef]
- Lee, B.S.; Kang, S.U.; Huang, M.; Kim, Y.S.; Lee, Y.S.; Park, J.Y.; Kim, C.H. OTUB1 knockdown promotes apoptosis in melanoma cells by upregulating TRAIL expression. BMB Rep. 2021, 54, 608–613. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, W.; Wang, Q.; Zhao, J.; Yang, D.; Yang, Y. Inhibition of VRK1 suppresses proliferation and migration of vascular smooth muscle cells and intima hyperplasia after injury via mTORC1/β-catenin axis. BMB Rep. 2022, 55, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.H.; Kim, B.; Kim, S.H.; Jung, B.C.; Lee, Y.; Kim, Y.S. Pulsed electromagnetic field potentiates etoposide-induced MCF-7 cell death. BMB Rep. 2022, 55, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Deng, X.; Jia, J.; Zhao, L.; Wang, C.; Cai, Z.; Guo, C.; Yang, L.; Wand, D.; Ma, S.; et al. Angiopoietin-like protein 8 (betatrophin) inhibits hepatic gluconeogenesis through PI3K/Akt signaling pathway in diabetic mice. Metab. Clin. Exp. 2022, 126, 154921. [Google Scholar] [CrossRef]
- Yeo, H.J.; Yeo, E.J.; Shin, M.J.; Choi, E.J.; Lee, C.H.; Kwon, H.Y.; Kim, D.W.; Eum, W.S.; Choi, S.Y. Protective effects of Tat-DJ-1 protein against streptozotocin-induced diabetes in a mice model. BMB Rep. 2018, 51, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Ahn, E.H.; Kim, D.W.; Shin, M.J.; Ryu, E.J.; Yong, J.I.; Chung, S.Y.; Cha, H.J.; Kim, S.J.; Choi, Y.J.; Kim, D.S.; et al. Tat-Atox1 inhibits streptozotocin-induced cell death in pancreatic RINm5F cells and attenuates diabetes in a mouse model. Int. J. Mol. Med. 2016, 38, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, H.J.; Shin, M.J.; Yoo, K.-Y.; Jung, B.H.; Eum, W.S.; Choi, S.Y. Tat-CIAPIN1 Prevents Pancreatic β-Cell Death in hIAPP-Induced RINm5F Cells and T2DM Animal Model. Int. J. Mol. Sci. 2023, 24, 10478. https://doi.org/10.3390/ijms241310478
Yeo HJ, Shin MJ, Yoo K-Y, Jung BH, Eum WS, Choi SY. Tat-CIAPIN1 Prevents Pancreatic β-Cell Death in hIAPP-Induced RINm5F Cells and T2DM Animal Model. International Journal of Molecular Sciences. 2023; 24(13):10478. https://doi.org/10.3390/ijms241310478
Chicago/Turabian StyleYeo, Hyeon Ji, Min Jea Shin, Ki-Yeon Yoo, Bo Hyun Jung, Won Sik Eum, and Soo Young Choi. 2023. "Tat-CIAPIN1 Prevents Pancreatic β-Cell Death in hIAPP-Induced RINm5F Cells and T2DM Animal Model" International Journal of Molecular Sciences 24, no. 13: 10478. https://doi.org/10.3390/ijms241310478



