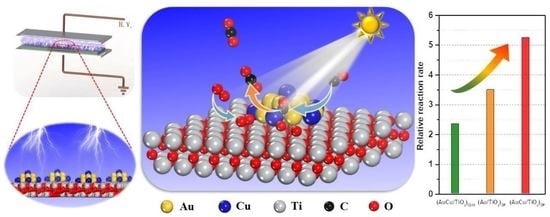
Boosting the Photocatalysis of Plasmonic Au-Cu Nanocatalyst by AuCu-TiO2 Interface Derived from O2 Plasma Treatment
Abstract
:1. Introduction
2. Results
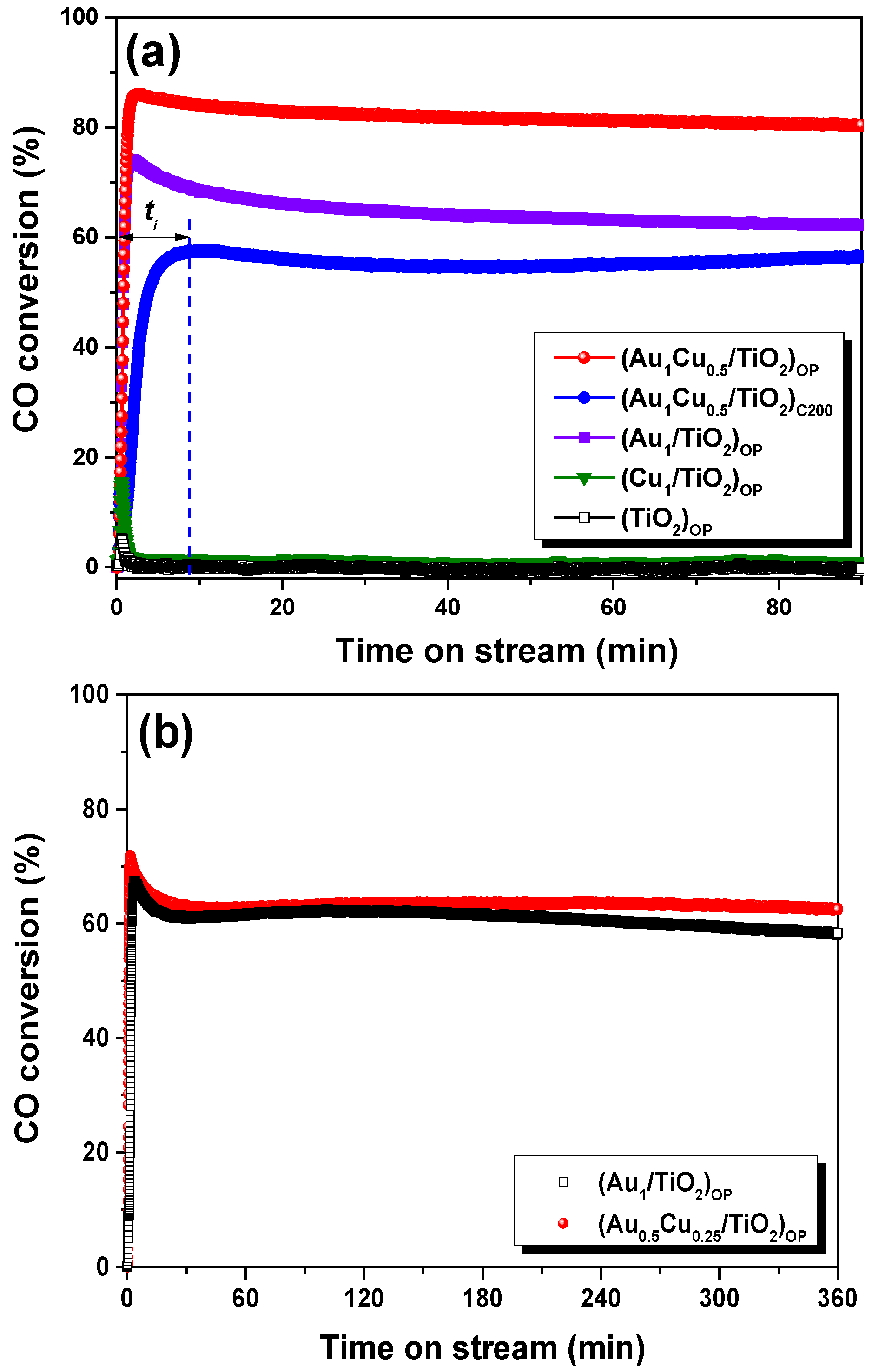
2.1. Studies of VL PCO of CO
2.2. Electronic State of the Plasmonic Nanocatalysts
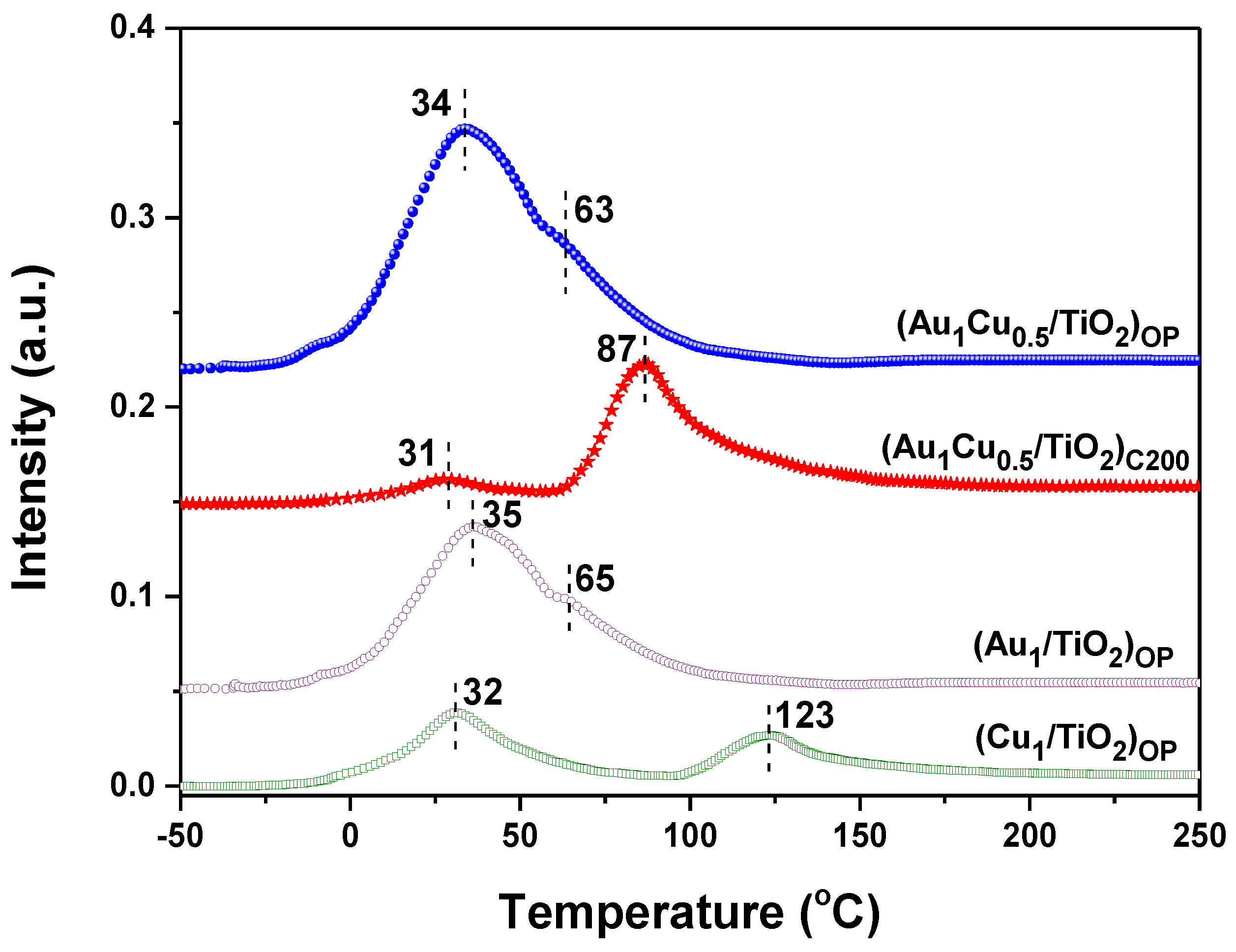
2.3. Structural States of the Plasmonic Nanocatalysts
2.4. Photo-Response Properties of the Plasmonic Nanocatalysts
3. Discussion
3.1. AuCu-TiO2 Interfacial Sites Construction
3.2. The Boost of VL Photocatalysis on AuCu-TiO2 Interfacial Sites
4. Materials and Methods
4.1. Catalysts Preparation
4.2. Catalysts Characterization
4.3. DFT Calculation
4.4. Photocatalytic Reaction Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linic, S.; Chavez, S.; Elias, R. Flow and extraction of energy and charge carriers in hybrid plasmonic nanostructures. Nat. Mater. 2021, 20, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Kim, Y.H.; Lee, S.Y.; Song, H.D.; Yi, J. Hot-electron-transfer enhancement for the efficient energy conversion of visible light. Angew. Chem. Int. Ed. 2014, 53, 11203–11207. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, D.; Shiraishi, Y.; Sugano, Y.; Ichikawa, S.; Tanaka, S.; Hirai, T. Gold nanoparticles located at the interface of anatase/rutile TiO2 particles as active plasmonic photocatalysts for aerobic oxidation. J. Am. Chem. Soc. 2012, 134, 6309–6315. [Google Scholar] [CrossRef] [PubMed]
- Dao, D.V.; Nguyen, T.T.D.; Uthirakumar, P.; Cho, Y.-H.; Kim, G.-C.; Yang, J.-K.; Tran, D.-T.; Le, T.D.; Choi, H.; Kim, H.Y.; et al. Insightful understanding of hot-carrier generation and transfer in plasmonic Au@CeO2 core-shell photocatalysts for light-driven hydrogen evolution improvement. Appl. Catal. B 2021, 286, 119947. [Google Scholar] [CrossRef]
- Naya, S.-I.; Tada, H. Dependence of the plasmonic activity of Au/TiO2 for the decomposition of 2-naphthol on the crystal form of TiO2 and Au particle size. J. Catal. 2018, 364, 328–333. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, B.; Li, C. Effects of Au nanoparticle size and metal-support interaction on plasmon-induced photocatalytic water oxidation. Chin. J. Catal. 2018, 39, 1219–1227. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, W.; Zhu, L.; Li, G.; Yang, K.; Jin, R. Integrating plasmonic Au nanorods with dendritic like α-Bi2O3/Bi2O2CO3 heterostructures for superior visible-light-driven photocatalysis. Appl. Catal. B 2016, 184, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Zhang, L.-Y.; Liu, J.-L.; Zhang, X.-M.; Li, X.-S.; Zhu, A.-M. TiO2-supported Au-Ag plasmonic nanocatalysts achieved by plasma restructuring and activation. J. Hazar. Mater. 2021, 402, 123508. [Google Scholar] [CrossRef]
- Neaţu, S.; Macia-Agullo, J.A.; Concepción, P.; Garcia, H. Gold-copper nanoalloys supported on TiO2 as photocatalysts for CO2 reduction by water. J. Am. Chem. Soc. 2014, 136, 15969–15976. [Google Scholar] [CrossRef]
- Tan, T.H.; Scott, J.A.; Ng, Y.H.; Taylor, R.A.; Aguey-Zinsou, K.-F.; Amal, R. Plasmon enhanced selective electronic pathways in TiO2 supported atomically ordered bimetallic Au-Cu alloys. J. Catal. 2017, 352, 638–648. [Google Scholar] [CrossRef]
- Dmitry, B.S.; Vishal, J.; Valery, A.N.; Johanna, T.; Maria, E.M.; Jakob, B.W.; Olof, P.; Rainer, T.; Anders, M.; Ivan, M.; et al. Strong Schottky barrier reduction at Au-catalyst/GaAs-nanowire interfaces by electric dipole formation and Fermi-level unpinning. Nat. Commun. 2014, 5, 3221. [Google Scholar]
- Zhu, B.; Li, X.; Deng, X.-Q.; Wang, Y.-Q.; Lu, L.-L. Activation of Au-Ag Plasmonic Bimetallic Nanocatalysts with Cold Plasma: The Role of Loading Sequence of Plasmonic Metals and Discharge Atmosphere. Plasma Chem. Plasma Process. 2022, 42, 671–687. [Google Scholar] [CrossRef]
- Sugano, Y.; Shiraishi, Y.; Tsukamoto, D.; Ichikawa, S.; Tanaka, S.; Hirai, T. Supported Au-Cu Bimetallic Alloy Nanoparticles: An Aerobic Oxidation Catalyst with Regenerable Activity by Visible-Light Irradiation. Angew. Chem. Int. Ed. 2013, 125, 5403–5407. [Google Scholar] [CrossRef]
- Deng, X.-Q.; Zhu, B.; Li, X.-S.; Liu, J.-L.; Zhu, X.; Zhu, A.-M. Visible-light photocatalytic oxidation of CO over plasmonic Au/TiO2: Unusual features of oxygen plasma activation. Appl. Catal. B 2016, 188, 48–55. [Google Scholar] [CrossRef]
- Liu, J.-H.; Wang, A.-Q.; Chi, Y.-S.; Lin, H.-P.; Mou, C.-Y. Synergistic Effect in an Au-Ag Alloy Nanocatalyst: CO Oxidation. J. Phys. Chem. B 2005, 109, 40–43. [Google Scholar] [CrossRef]
- Liu, X.; Wang, A.; Li, L.; Zhang, T.; Mou, C.-Y.; Lee, J.-F. Structural changes of Au-Cu bimetallic catalysts in CO oxidation: In situ XRD, EPR, XANES, and FT-IR characterizations. J. Catal. 2011, 278, 288–296. [Google Scholar] [CrossRef]
- Du, X.R.; Huang, Y.K.; Pan, X.L.; Han, B.; Su, Y.; Jiang, Q.K.; Li, M.R.; Tang, H.L.; Li, G.; Qiao, B.T. Size-dependent strong metal-support interaction in TiO2 supported Au nanocatalysts. Nat. Commun. 2020, 11, 5811. [Google Scholar] [CrossRef]
- Jin, L.; Liu, B.; Louis, M.E.; Li, G.H.; He, J. Highly Crystalline Mesoporous Titania Loaded with Monodispersed Gold Nanoparticles: Controllable Metal-Support Interaction in Porous Materials. ACS Appl. Mater. Interfaces 2020, 12, 9617–9627. [Google Scholar] [CrossRef]
- Wang, Y.C.; Widmann, D.; Behm, R.J. Influence of TiO2 Bulk Defects on CO Adsorption and CO Oxidation on Au/TiO2: Electronic Metal-Support Interactions (EMSIs) in Supported Au Catalysts. ACS Catal. 2017, 7, 2339–2345. [Google Scholar] [CrossRef]
- Zhu, B.; Li, X.-S.; Liu, J.-L.; Liu, J.-B.; Zhu, X.; Zhu, A.-M. In-situ regeneration of Au nanocatalysts by atmospheric-pressure air plasma: Significant contribution of water vapor. Appl. Catal. B 2015, 179, 69–77. [Google Scholar] [CrossRef]
- Hu, S.L.; Li, W.-X. Sabatier principle of metal-support interaction for design of ultrastable metal nanocatalysts. Science 2021, 374, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Wang, H.P.; Liu, C.J.; Zeng, Z.Y.; Zhang, H.; Zhou, C.M.; Jia, X.L.; Yang, Y.H. Formation of monometallic Au and Pd and bimetallic Au-Pd nanoparticles confined in mesopores via Ar glow-discharge plasma reduction and their catalytic applications in aerobic oxidation of benzyl alcohol. J. Catal. 2012, 289, 105–117. [Google Scholar] [CrossRef]
- Kim, H.H.; Tsubota, S.; Daté, M.; Ogata, A.; Futamura, S. Catalyst regeneration and activity enhancement of Au/TiO2 by atmospheric pressure nonthermal plasma. Appl. Catal. A 2007, 329, 93–98. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Neyts, E.C.; Cao, X.X.; Zhang, X.S.; Jang, B.W.-L.; Liu, C.J. Catalyst Preparation with Plasmas: How Does It Work? ACS Catal. 2018, 8, 2093–2110. [Google Scholar] [CrossRef]
- Liang, X.; Wang, Z.J.; Liu, C.J. Size-Controlled Synthesis of Colloidal Gold Nanoparticles at Room Temperature Under the Influence of Glow Discharge. Nanoscale Res. Lett. 2010, 5, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.H.; Kim, J.H.; Ogata, A. Microscopic observation of discharge plasma on the surface of zeolites supported metallic nanoparticles. J. Phys. D Appl. Phys. 2009, 42, 135210. [Google Scholar] [CrossRef]
- Mei, D.H.; Zhu, X.B.; He, Y.L.; Yan, J.D.; Tu, X. Plasma-assisted conversion of CO2 in a dielectric barrier discharge reactor: Understanding the effect of packing materials. Plasma Sources Sci. Technol. 2014, 24, 015011. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Yan, Y.; Li, M.; Li, X.-S.; Liu, J.-L.; Zhu, Y.-M. Low temperature removal of toluene over Ag/CeO2/Al2O3 nanocatalyst in an atmospheric plasma catalytic system. Plasma Process Polym. 2018, 15, 1700215. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, X.S.; Zhu, B.; Zhu, X.; Lian, H.Y.; Zhu, A.M. A facile approach to direct preparation of Pt nanocatalysts from oxidative dechloridation of supported H2PtCl6 by oxygen plasma. J. Catal. 2022, 414, 16–24. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Chen, Z.P.; Qu, C.L.; Chen, L.X. Highly Sensitive Visual Detection of Copper Ions Based on the Shape-Dependent LSPR Spectroscopy of Gold Nanorods. Langmuir 2014, 30, 3625–3630. [Google Scholar] [CrossRef]
- Sun, Z.-G.; Li, X.-S.; Liu, J.-L.; Li, Y.-C.; Zhu, B.; Zhu, A.-M. A promising visible-light photocatalyst: H2 plasma-activated amorphous-TiO2-supported Au nanoparticles. J. Catal. 2019, 375, 380–388. [Google Scholar] [CrossRef]
- Fu, Q.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Active Nonmetallic Au and Pt Species on Ceria-Based Water-Gas Shift Catalysts. Science 2003, 301, 935–938. [Google Scholar] [CrossRef]
- Bollinger, M.A.; Vannice, M.A. A kinetic and DRIFTS study of low-temperature carbon monoxide oxidation over Au-TiO2 catalysts. Appl. Catal. B 1996, 8, 417–443. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Chiorino, A. FTIR Study of CO Oxidation on Au/TiO2 at 90 K and Room Temperature. An Insight into the Nature of the Reaction Centers. J. Phys. Chem. B 2000, 104, 5414–5416. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Z.; Li, Z.; Huang, W. Understanding morphology-dependent CuOx-CeO2 interactions from the very beginning. Chin. J. Catal. 2020, 41, 1006–1016. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Tsoncheva, T.; Dimitrov, M.; Minchev, C.; Knözinger, H. Characterization of Cu/MCM-41 and Cu/MCM-48 mesoporous catalysts by FTIR spectroscopy of adsorbed CO. Appl. Catal. A 2003, 241, 331–340. [Google Scholar] [CrossRef]
- Li, Y.-C.; Li, X.-S.; Zhu, B.; Zhu, A.-M. Boosting low-temperature water gas shift reaction over Au/TiO2 nanocatalyst activated by oxygen plasma. Chem. Eng. J. 2022, 430, 133013. [Google Scholar] [CrossRef]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef] [Green Version]
- Ferry, V.E.; Munday, J.N.; Atwater, H.A. Design Considerations for Plasmonic Photovoltaics. Adv. Mater. 2010, 22, 4794–4808. [Google Scholar] [CrossRef]
- Patra, K.K.; Gopinath, C.S. Bimetallic and Plasmonic Ag-Au on TiO2 for Solar Water Splitting: An Active Nanocomposite for Entire Visible-Light-Region Absorption. Chemcatchem 2016, 8, 3294–3311. [Google Scholar] [CrossRef]
- Kreibig, U. Interface-induced dephasing of Mie plasmon polaritons. Appl. Phys. B 2008, 93, 79–89. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, H.; Renzas, J.R.; Zhang, Y.; Somorjai, G.A. Probing Hot Electron Flow Generated on Pt Nanoparticles with Au/TiO2 Schottky Diodes during Catalytic CO Oxidation. Nano Lett. 2008, 8, 2388–2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, G.-Y.; Zhao, Z.-Y.; Liu, Q.-L.; Dong, X.-D.; Zhao, Q.-M. Theoretical calculations for localized surface plasmon resonance effects of Cu/TiO2 nanosphere: Generation, modulation, and application in photocatalysis. Sol. Energ. Mat. Sol. C 2020, 208, 110385. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, L.-Y.; Li, M.; Yan, Y.; Zhang, X.-M.; Zhu, Y.-M. High-performance of plasma-catalysis hybrid system for toluene removal in air using supported Au nanocatalysts. Chem. Eng. J. 2020, 381, 122599. [Google Scholar] [CrossRef]
- Avanesian, T.; Dai, S.; Kale, J.M.; Graham, W.G.; Pan, X.; Christopher, P. Quantitative and Atomic-Scale View of CO-Induced Pt Nanoparticle Surface Reconstruction at Saturation Coverage via DFT Calculations Coupled with in Situ TEM and IR. J. Am. Chem. Soc. 2017, 139, 4551–4558. [Google Scholar] [CrossRef]
- Barmparis, G.D.; Remediakis, I.N. Dependence on CO adsorption of the shapes of multifaceted gold nanoparticles: A density functional theory. Rev. B Condens. Matter Mater. Phys. 2012, 86, 085457. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.H.; Le, V.K.; Minh, C.L.; Nguyen, N.H. A theoretical study of carbon dioxide adsorption and activation on metal-doped (Fe, Co, Ni) carbon nanotube. Comput. Theor. Chem. 2017, 1100, 46–51. [Google Scholar] [CrossRef]
- Liu, C.; Lourenco, M.P.; Hedstrom, S.; Cavalca, F.; Diaz-Morales, O.; Duarte, H.A.; Nilsson, A.; Pettersson, L.G.M. Stability and Effects of Subsurface Oxygen in Oxide-Derived Cu Catalyst for CO2 Reduction. J. Phys. Chem. C 2017, 121, 25010–25017. [Google Scholar] [CrossRef]
- Liu, L.M.; McAllister, B.; Ye, H.Q.; Hu, P. Identifying an O2 Supply Pathway in CO Oxidation on Au/TiO2 (110): A Density Functional Theory Study on the Intrinsic Role of Water. J. Am. Chem. Soc. 2006, 128, 4017–4022. [Google Scholar] [CrossRef]
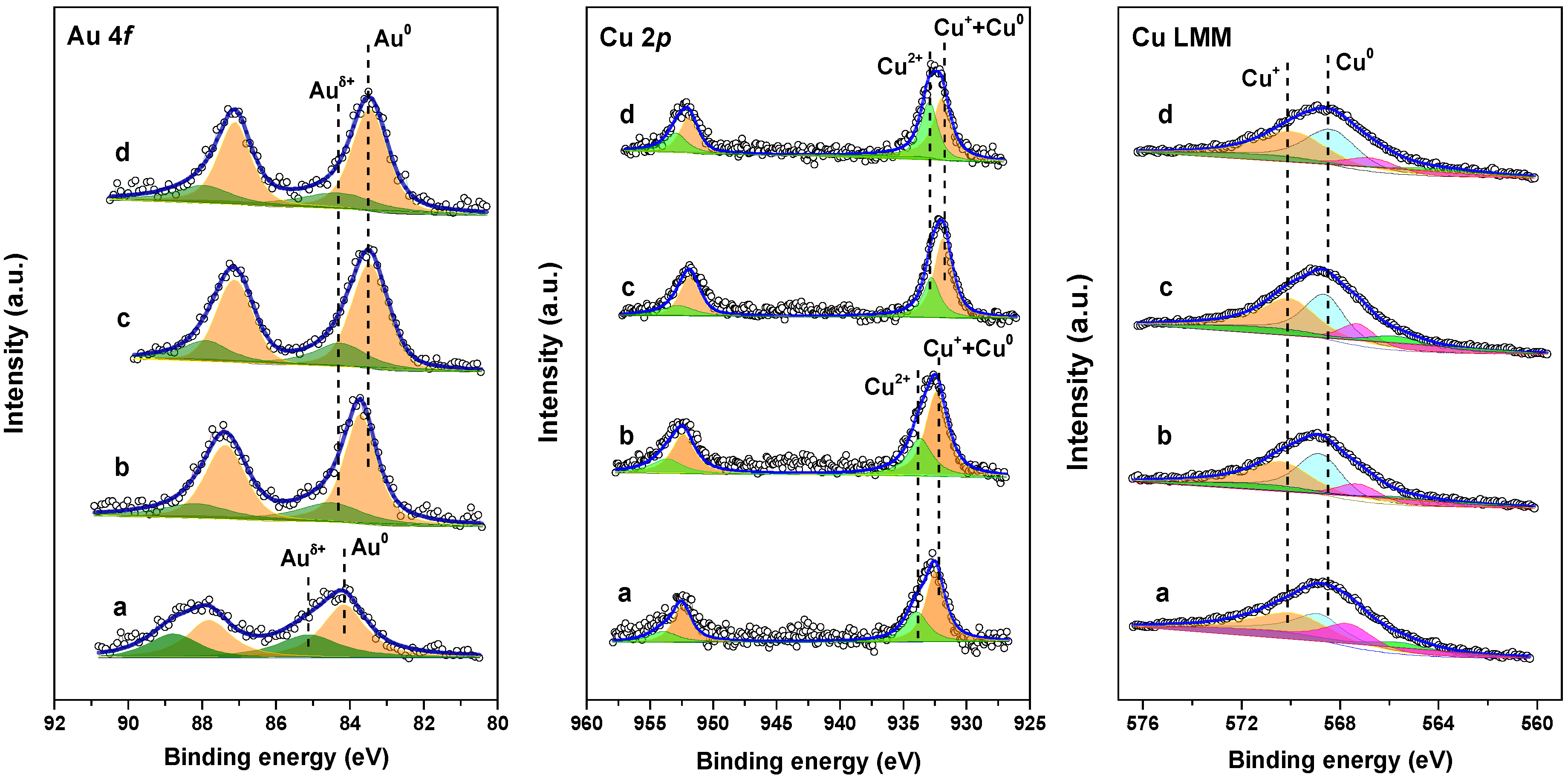
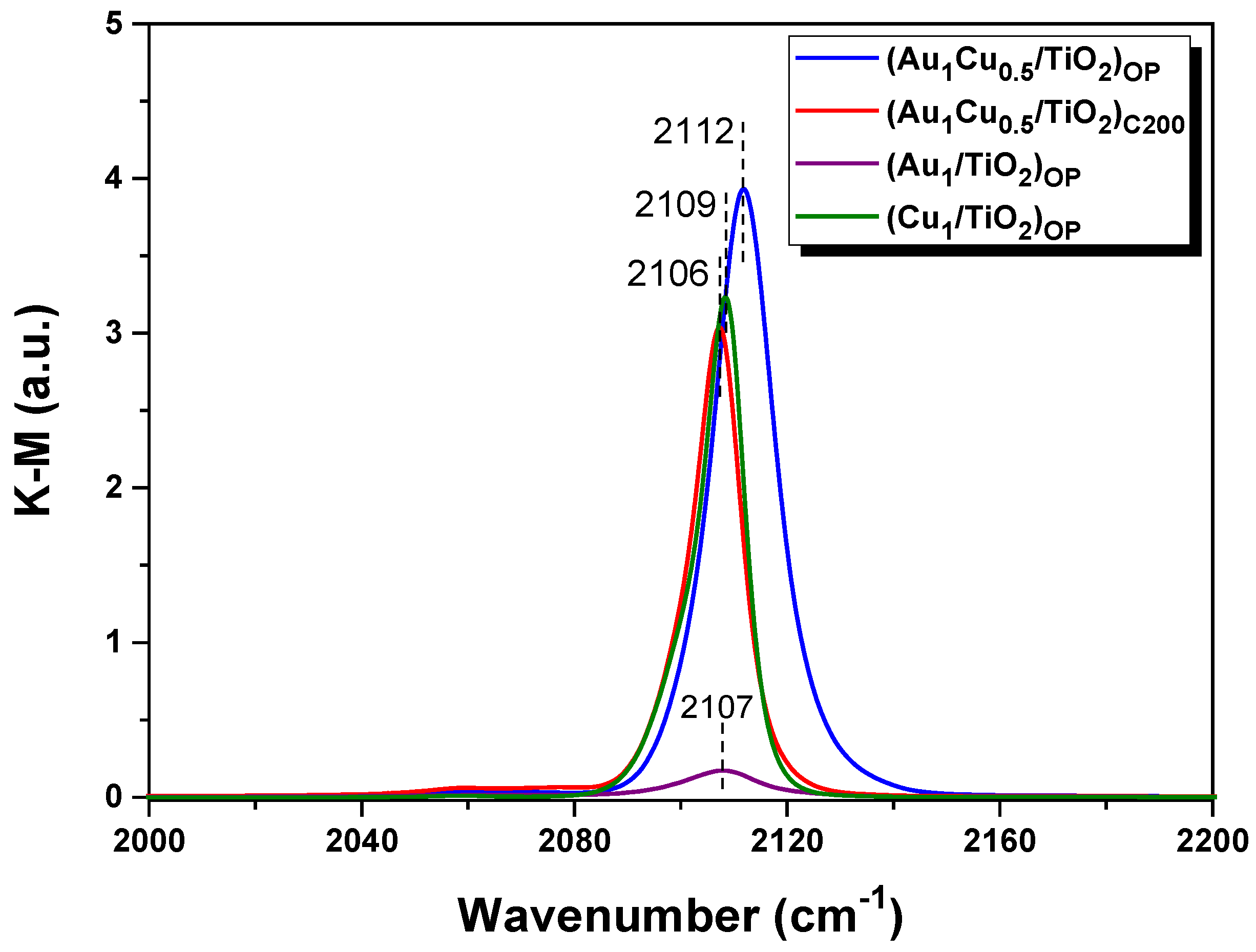
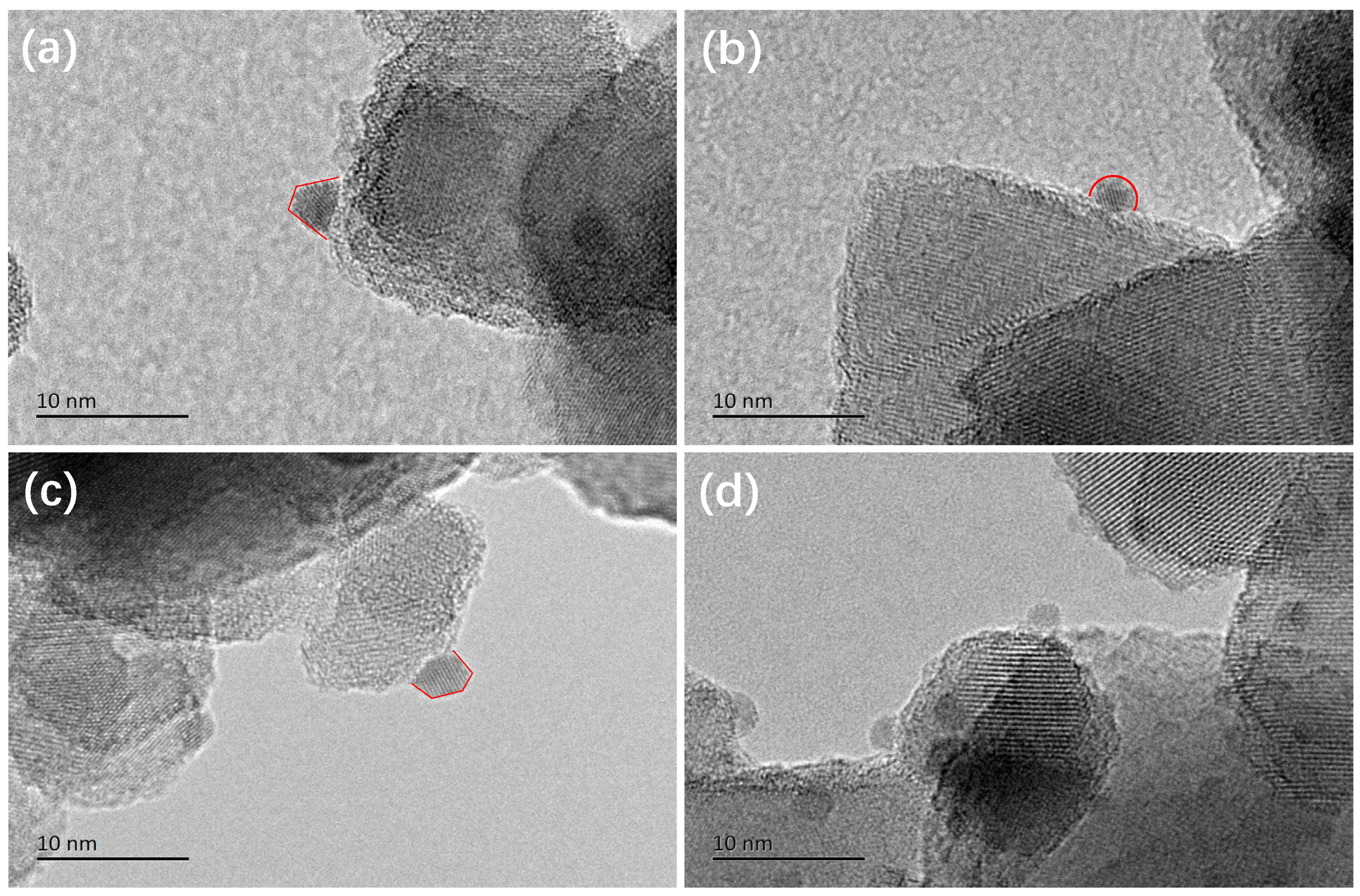

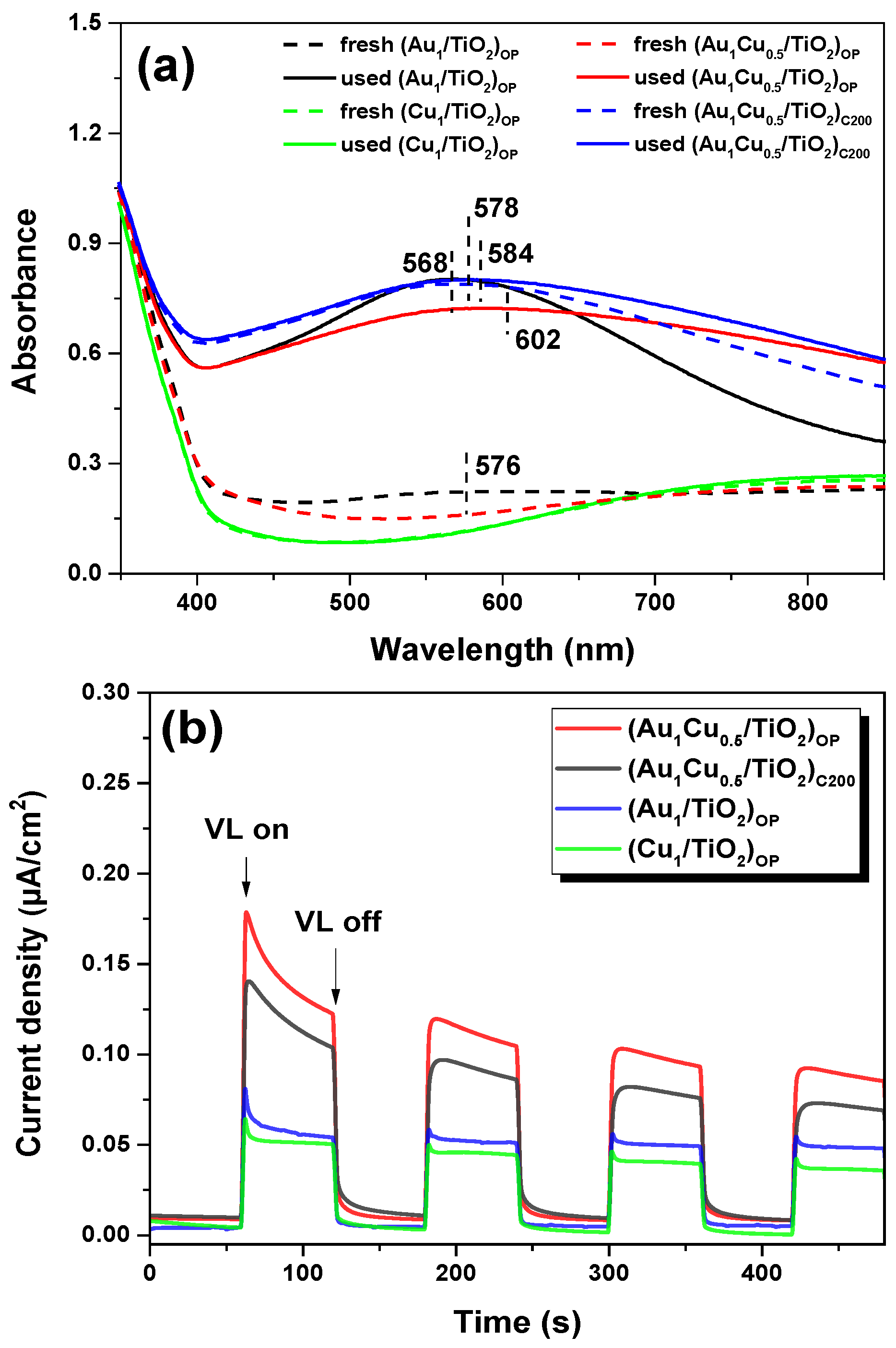

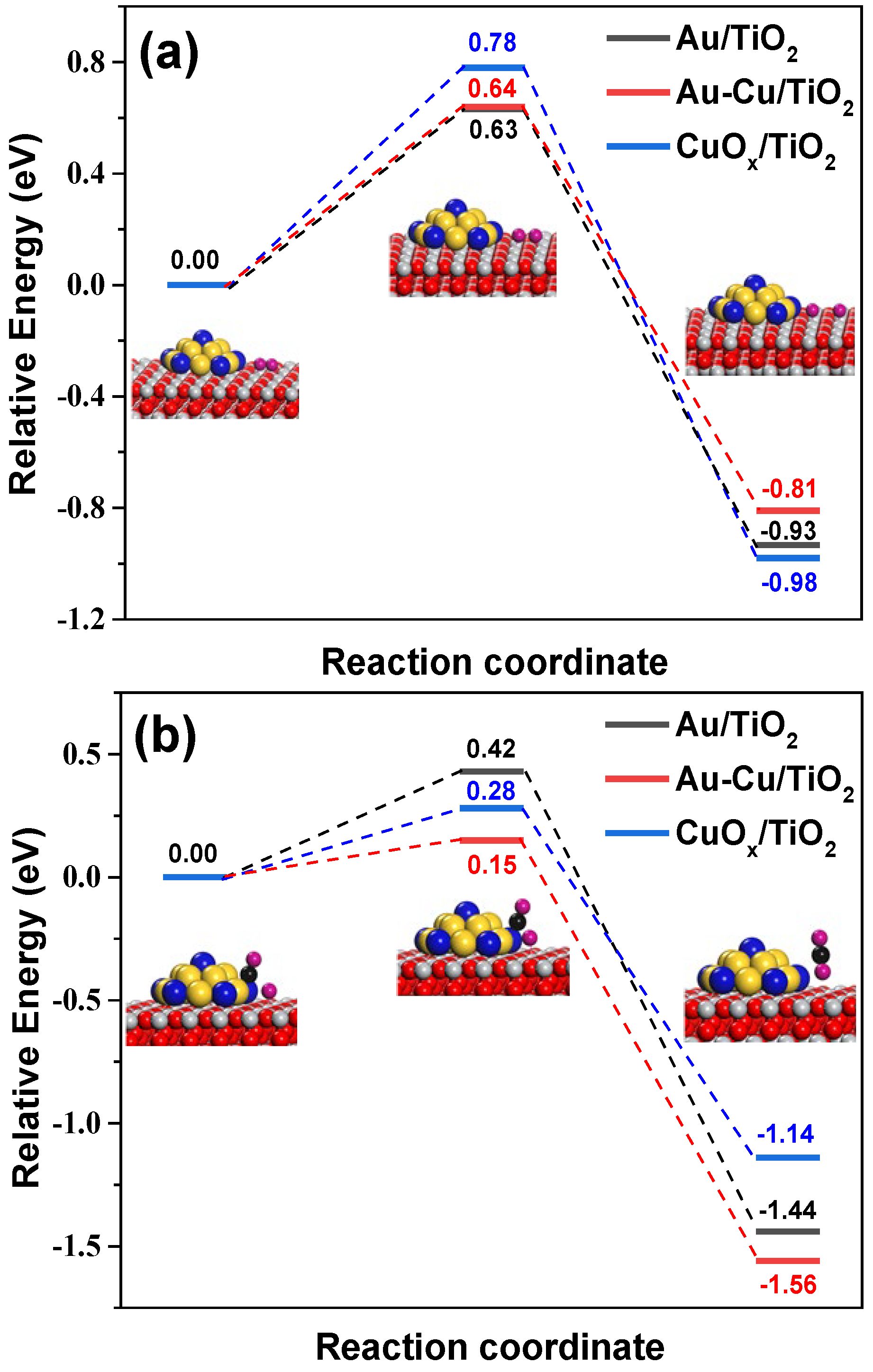
| Sample | Au 4f7/2 (eV) | Cu 2p3/2 (eV) | Cu LMM (eV) | Auδ+/Au (at.%) | Cu2+/Cu (at.%) | Cu0/Cu+ | |||
|---|---|---|---|---|---|---|---|---|---|
| Auδ+ | Au0 | Cu2+ | Cu0 + Cu+ | Cu0 | Cu+ | ||||
| a | 85.1 | 84.2 | 934.0 | 932.5 | 568.7 | 570.1 | 43 | 35 | 0.88 |
| b | 84.5 | 83.7 | 933.8 | 932.4 | 568.8 | 570.2 | 29 | 32 | 0.97 |
| c | 84.2 | 83.4 | 932.8 | 931.9 | 568.7 | 570.3 | 38 | 40 | 0.83 |
| d | 84.3 | 83.4 | 933.0 | 932.0 | 568.5 | 570.0 | 24 | 36 | 0.91 |
| Samples | Proportion of Perimeter Atoms (%) | ||
|---|---|---|---|
| (Au1Cu0.5/TiO2)OP | 16 | 267 | 6.0 |
| (Au1Cu0.5/TiO2)C200 | 28 | 601 | 4.7 |
| (Au1/TiO2)OP | 30 | 564 | 5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.; Li, X.; Li, Y.; Liu, J.; Zhang, X. Boosting the Photocatalysis of Plasmonic Au-Cu Nanocatalyst by AuCu-TiO2 Interface Derived from O2 Plasma Treatment. Int. J. Mol. Sci. 2023, 24, 10487. https://doi.org/10.3390/ijms241310487
Zhu B, Li X, Li Y, Liu J, Zhang X. Boosting the Photocatalysis of Plasmonic Au-Cu Nanocatalyst by AuCu-TiO2 Interface Derived from O2 Plasma Treatment. International Journal of Molecular Sciences. 2023; 24(13):10487. https://doi.org/10.3390/ijms241310487
Chicago/Turabian StyleZhu, Bin, Xue Li, Yecheng Li, Jinglin Liu, and Xiaomin Zhang. 2023. "Boosting the Photocatalysis of Plasmonic Au-Cu Nanocatalyst by AuCu-TiO2 Interface Derived from O2 Plasma Treatment" International Journal of Molecular Sciences 24, no. 13: 10487. https://doi.org/10.3390/ijms241310487
APA StyleZhu, B., Li, X., Li, Y., Liu, J., & Zhang, X. (2023). Boosting the Photocatalysis of Plasmonic Au-Cu Nanocatalyst by AuCu-TiO2 Interface Derived from O2 Plasma Treatment. International Journal of Molecular Sciences, 24(13), 10487. https://doi.org/10.3390/ijms241310487