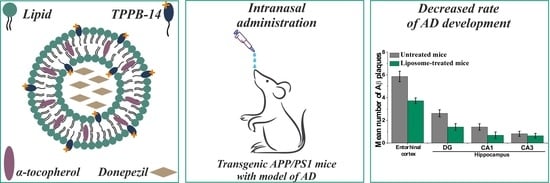
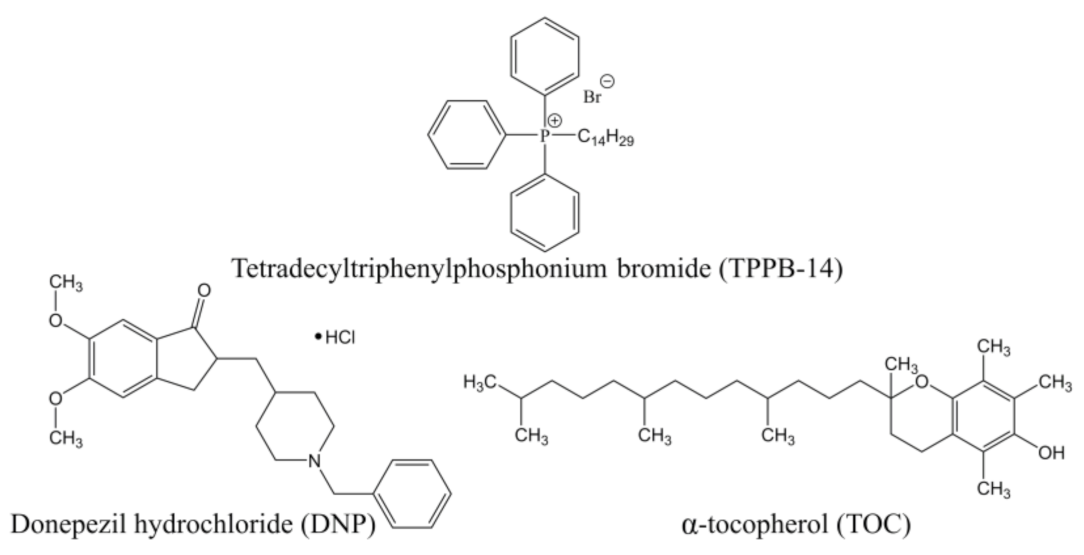
Mitochondria-Targeted Delivery Strategy of Dual-Loaded Liposomes for Alzheimer’s Disease Therapy
Abstract
1. Introduction
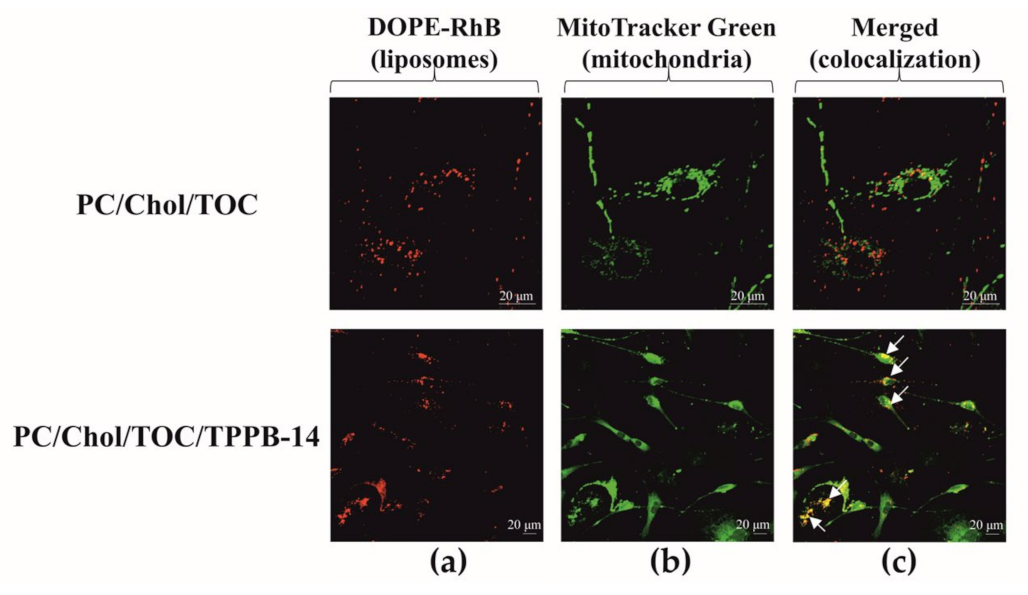
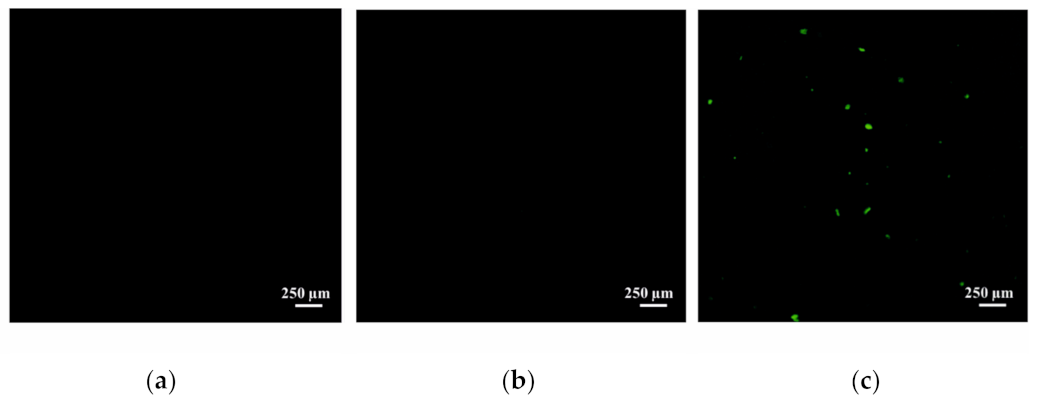
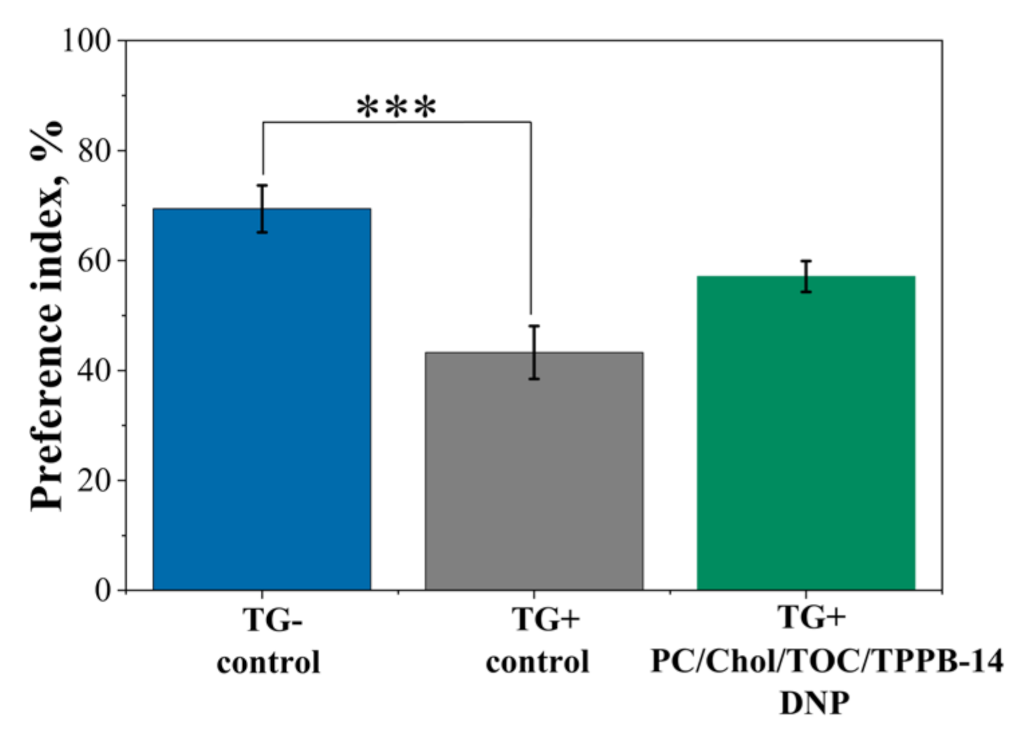
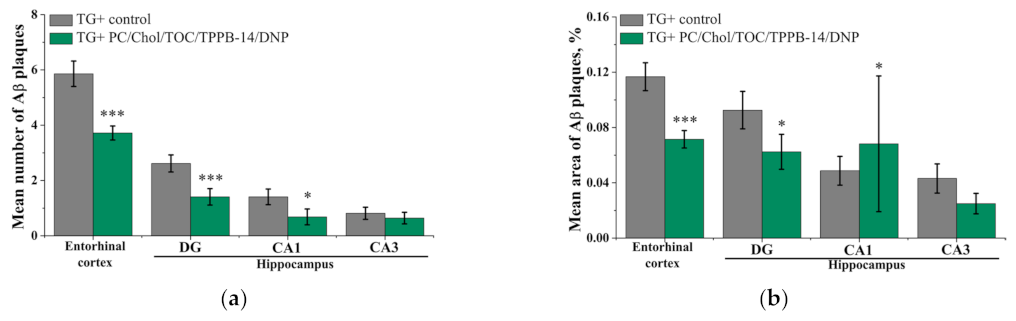
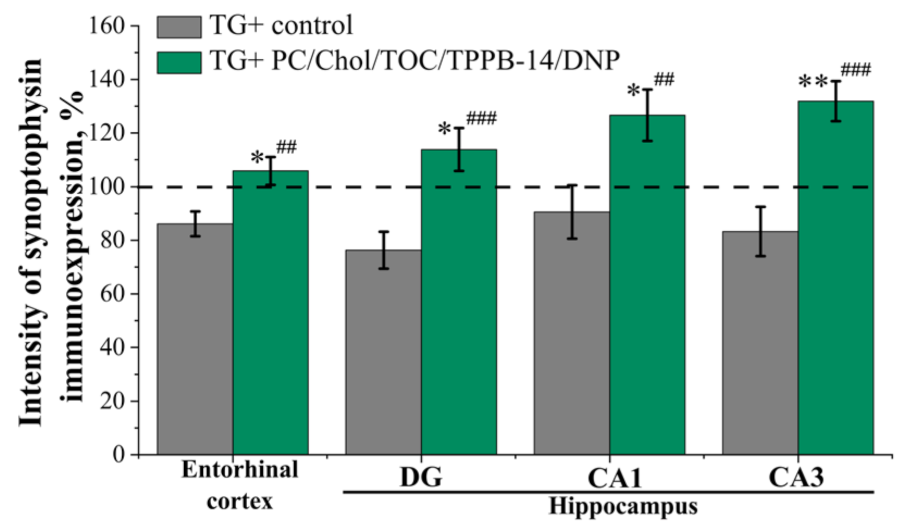
2. Results
3. Discussion
4. Materials and Methods
4.1. Objects
4.2. Liposome Preparation Protocol
4.3. Determination of the Size, Zeta Potential, and Morphology of Liposomes
4.4. Quantification of Encapsulation Efficiency (EE%)
4.5. Quantification of DNP Release Rate In Vitro and Release Kinetic Model Fitting
4.6. Colocalization
4.7. Antioxidant Activity In Vitro
4.8. Animals
4.9. Visualization of Liposomes into the Rat Brain
4.10. Novel Object Recognition Test
4.11. Thioflavin S Staining and Immunohistochemistry
4.12. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, M.M.; El-Salamouni, N.S.; El-Refaie, W.M.; Hazzah, H.A.; Ali, M.M.; Tosi, G.; Farid, R.M.; Blanco-Prieto, M.J.; Billa, N.; Hanafy, A.S. Nanotechnology-based drug delivery systems for Alzheimer’s disease management: Technical, industrial, and clinical challenges. J. Control. Release 2017, 245, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, L.; Gaynanova, G.; Vasilieva, E.; Vasileva, L.; Pavlov, R.; Kashapov, R.; Petrov, K.; Sinyashin, O. Recent nanoscale carriers for therapy of Alzheimer’s disease: Current strategies and perspectives. Curr. Med. Chem. 2023, 30, 3743–3774. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; Van Der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Murray, M.E.; Graff-Radford, N.R.; Ross, O.A.; Petersen, R.C.; Duara, R.; Dickson, D.W. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: A retrospective study. Lancet Neurol. 2011, 10, 785–796. [Google Scholar] [CrossRef]
- Davidson, Y.S.; Raby, S.; Foulds, P.G.; Robinson, A.; Thompson, J.C.; Sikkink, S.; Yusuf, I.; Amin, H.; DuPlessis, D.; Troakes, C.; et al. TDP-43 Pathological changes in early onset familial and sporadic Alzheimer’s disease, late onset Alzheimer’s disease and down’s syndrome: Association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol. 2011, 122, 703–713. [Google Scholar] [CrossRef]
- Alonso Vilatela, M.E.; López-López, M.; Yescas-Gómez, P. Genetics of Alzheimer’s disease. Arch. Med. Res. 2012, 43, 622–631. [Google Scholar] [CrossRef]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef]
- Craig, L.A.; Hong, N.S.; McDonald, R.J. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2011, 35, 1397–1409. [Google Scholar] [CrossRef]
- Hardy, J. The amyloid hypothesis for Alzheimer’s disease: A critical reappraisal. J. Neurochem. 2009, 110, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.-L.; Yardin, C.; Terro, F. Tau protein kinases: Involvement in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.; Selman, A.; Sehar, U.; Rawat, P.; Reddy, A.P.; Reddy, P.H. Therapeutics of Alzheimer’s disease: Recent developments. Antioxidants 2022, 11, 2402. [Google Scholar] [CrossRef] [PubMed]
- Arnst, N.; Redolfi, N.; Lia, A.; Bedetta, M.; Greotti, E.; Pizzo, P. Mitochondrial Ca2+ signaling and bioenergetics in Alzheimer’s disease. Biomedicines 2022, 10, 3025. [Google Scholar] [CrossRef]
- Li, Y.; Xia, X.; Wang, Y.; Zheng, J.C. Mitochondrial dysfunction in microglia: A novel perspective for pathogenesis of Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 248. [Google Scholar] [CrossRef]
- Atlante, A.; Amadoro, G.; Latina, V.; Valenti, D. Therapeutic potential of targeting mitochondria for Alzheimer’s disease treatment. J. Clin. Med. 2022, 11, 6742. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Sehar, N.; Dar, N.J.; Khan, A.; Arafah, A.; Rashid, S.; Rashid, S.M.; Ganaie, M.A. Mitochondrial dysfunctions, oxidative stress and neuroinflammation as therapeutic targets for neurodegenerative diseases: An update on current advances and impediments. Neurosci. Biobehav. Rev. 2023, 144, 104961. [Google Scholar] [CrossRef]
- Castellani, R.; Hirai, K.; Aliev, G.; Drew, K.L.; Nunomura, A.; Takeda, A.; Cash, A.D.; Obrenovich, M.E.; Perry, G.; Smith, M.A. Role of mitochondrial dysfunction in Alzheimer’s disease. J. Neurosci. Res. 2002, 70, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Friedland-Leuner, K.; Stockburger, C.; Denzer, I.; Eckert, G.P.; Müller, W.E. Mitochondrial dysfunction: Cause and consequence of Alzheimer’s disease. In Progress in Molecular Biology and Translational Science; Heinz, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 127, pp. 183–210. [Google Scholar] [CrossRef]
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s disease: Current therapeutic significance and future prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in Alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, W.-J.; Chen, W.-W. Microwaves and Alzheimer’s disease. Exp. Ther. Med. 2016, 12, 1969–1972. [Google Scholar] [CrossRef]
- Mumtaz, S.; Rana, J.N.; Choi, E.H.; Han, I. Microwave radiation and the brain: Mechanisms, current status, and future prospects. Int. J. Mol. Sci. 2022, 23, 9288. [Google Scholar] [CrossRef] [PubMed]
- Carreiras, M.; Mendes, E.; Perry, M.; Francisco, A.; Marco-Contelles, J. The multifactorial nature of Alzheimer’s disease for developing potential therapeutics. Curr. Top. Med. Chem. 2013, 13, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, P.R.; Bates, K.A.; Porter, T.; Teimouri, E.; Perry, G.; Steele, J.W.; Gandy, S.; Groth, D.; Martins, R.N.; Verdile, G. Latrepirdine: Molecular mechanisms underlying potential therapeutic roles in Alzheimer’s and other neurodegenerative diseases. Transl. Psychiatry 2013, 3, e332. [Google Scholar] [CrossRef]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment combinations for Alzheimer’s disease: Current and future pharmacotherapy options. J. Alzheimers Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef]
- Farlow, M.P. Utilizing combination therapy in the treatment of Alzheimer’s disease. Expert Rev. Neurother. 2004, 4, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, M.; Guo, J.; Cao, D.; Luo, J.; Wan, Y.; Fang, Y.; Jin, Y.; Xie, S.-S.; Liu, J. Dual functional antioxidant and butyrylcholinesterase inhibitors for the treatment of Alzheimer’s disease: Design, synthesis and evaluation of novel melatonin-alkylbenzylamine hybrids. Bioorg. Med. Chem. 2023, 78, 117146. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liang, X.; Xie, G.; Chen, L.; Liu, W.; Luo, G.; Zhang, P.; Yu, L.; Zheng, X.; Ji, H.; et al. Synthesis and evaluation of novel ligustrazine derivatives as multi-targeted inhibitors for the treatment of Alzheimer’s disease. Molecules 2018, 23, 2540. [Google Scholar] [CrossRef] [PubMed]
- Canto, R.F.S.; Barbosa, F.A.R.; Nascimento, V.; De Oliveira, A.S.; Brighente, I.M.C.; Braga, A.L. Design, Synthesis and evaluation of seleno-dihydropyrimidinones as potential multi-targeted therapeutics for Alzheimer’s disease. Org. Biomol. Chem. 2014, 12, 3470–3477. [Google Scholar] [CrossRef]
- Keri, R.S.; Quintanova, C.; Marques, S.M.; Esteves, A.R.; Cardoso, S.M.; Santos, M.A. Design, synthesis and neuroprotective evaluation of novel tacrine–benzothiazole hybrids as multi-targeted compounds against Alzheimer’s disease. Bioorg. Med. Chem. 2013, 21, 4559–4569. [Google Scholar] [CrossRef]
- Séguy, L.; Groo, A.-C.; Malzert-Fréon, A. How nano-engineered delivery systems can help marketed and repurposed drugs in Alzheimer’s disease treatment? Drug Discov. Today 2022, 27, 1575–1589. [Google Scholar] [CrossRef]
- Delbreil, P.; Rabanel, J.-M.; Banquy, X.; Brambilla, D. Therapeutic nanotechnologies for Alzheimer’s disease: A critical analysis of recent trends and findings. Adv. Drug Deliv. Rev. 2022, 187, 114397. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Zafar, A.; Noorulla, K.M.; Tura, A.J.; Sara, U.V.S.; Panjwani, D.; Khalid, M.; Haji, M.J.; Gobena, W.G.; Gebissa, T.; et al. Nose to brain delivery of donepezil through surface modified NLCs: Formulation development, optimization, and brain targeting study. J. Drug Deliv. Sci. Technol. 2022, 75, 103631. [Google Scholar] [CrossRef]
- Eloy, J.O.; Claro De Souza, M.; Petrilli, R.; Barcellos, J.P.A.; Lee, R.J.; Marchetti, J.M. Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery. Colloids Surf. B 2014, 123, 345–363. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Li, Z.; Loh, X.J. Small molecule therapeutic-loaded liposomes as therapeutic carriers: From development to clinical applications. RSC Adv. 2016, 6, 70592–70615. [Google Scholar] [CrossRef]
- Vasileva, L.; Gaynanova, G.; Zueva, I.; Lyubina, A.; Amerhanova, S.; Buzyurova, D.; Babaev, V.; Voloshina, A.; Petrov, K.; Zakharova, L. Transdermal delivery of 2-PAM as a tool to increase the effectiveness of traditional treatment of organophosphate poisoning. Int. J. Mol. Sci. 2022, 23, 14992. [Google Scholar] [CrossRef] [PubMed]
- Kashapov, R.; Gaynanova, G.; Gabdrakhmanov, D.; Kuznetsov, D.; Pavlov, R.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-assembly of amphiphilic compounds as a versatile tool for construction of nanoscale drug carriers. Int. J. Mol. Sci. 2020, 21, 6961. [Google Scholar] [CrossRef]
- Kashapov, R.; Ibragimova, A.; Pavlov, R.; Gabdrakhmanov, D.; Kashapova, N.; Burilova, E.; Zakharova, L.; Sinyashin, O. Nanocarriers for biomedicine: From lipid formulations to inorganic and hybrid nanoparticles. Int. J. Mol. Sci. 2021, 22, 7055. [Google Scholar] [CrossRef]
- Pavlov, R.; Romanova, E.; Kuznetsov, D.; Lyubina, A.; Amerhanova, S.; Voloshina, A.; Buzyurova, D.; Babaev, V.; Zueva, I.; Petrov, K.; et al. The formation of morphologically stable lipid nanocarriers for glioma therapy. Int. J. Mol. Sci. 2023, 24, 3632. [Google Scholar] [CrossRef]
- Gaynanova, G.; Vasileva, L.; Kashapov, R.; Kuznetsova, D.; Kushnazarova, R.; Tyryshkina, A.; Vasilieva, E.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-assembling drug formulations with tunable permeability and biodegradability. Molecules 2021, 26, 6786. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Hou, J.; Xiong, W.; Kim, H.; Chen, J.; Zheng, C.; Jiang, X.; Yoon, J.; Shen, J. Tumor selective metabolic reprogramming as a prospective PD-L1 depression strategy to reactivate immunotherapy. Adv. Mater. 2022, 34, 2206121. [Google Scholar] [CrossRef]
- Jebastin, K.; Narayanasamy, D. Rationale utilization of phospholipid excipients: A distinctive tool for progressing state of the art in research of emerging drug carriers. J. Liposome Res. 2023, 33, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Sharma, A.; Narang, R.K.; Rawal, R.K. Recent nanocarrier approaches for targeted drug delivery in cancer therapy. Curr. Mol. Pharmacol. 2021, 14, 350–366. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A review of liposomes as a drug delivery system: Current status of approved products, regulatory environments, and future perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Pingale, P.; Dhapte-Pawar, V. Nasal delivery of neurotherapeutics via nanocarriers: Facets, aspects, and prospects. Front. Pharmacol. 2022, 13, 979682. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, R.; Musumeci, T.; Carbone, C.; Pignatello, R. Nanotechnologies for intranasal drug delivery: An update of literature. Pharm. Dev. Technol. 2021, 26, 824–845. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Recent Advances in intranasal liposomes for drug, gene, and vaccine delivery. Pharmaceutics 2023, 15, 207. [Google Scholar] [CrossRef]
- Vasilieva, E.A.; Kuznetsova, D.A.; Valeeva, F.G.; Kuznetsov, D.M.; Zakharov, A.V.; Amerhanova, S.K.; Voloshina, A.D.; Zueva, I.V.; Petrov, K.A.; Zakharova, L.Y. Therapy of organophosphate poisoning via intranasal administration of 2-PAM-loaded chitosomes. Pharmaceutics 2022, 14, 2846. [Google Scholar] [CrossRef]
- Lissi, E. Evaluation of total antioxidant potential (TRAP) and total antioxidant reactivity from luminol-enhanced chemiluminescence measurements. Free Radic. Biol. Med. 1995, 18, 153–158. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gaynanova, G.A.; Vasileva, L.A.; Sibgatullina, G.V.; Samigullin, D.V.; Sapunova, A.S.; Voloshina, A.D.; Galkina, I.V.; Petrov, K.A.; Zakharova, L.Y. Mitochondria-targeted cationic liposomes modified with alkyltriphenylphosphonium bromides loaded with hydrophilic drugs: Preparation, cytotoxicity and colocalization assay. J. Mater. Chem. B 2019, 7, 7351–7362. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Jiang, X.; Zheng, C.; Luo, W.; Xiang, X.; Qi, X.; Shen, J. Metformin modified chitosan as a multi-functional adjuvant to enhance cisplatin-based tumor chemotherapy efficacy. Int. J. Biol. Macromol. 2023, 224, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, H.; Huang, X.; Lu, Z.; Zhang, L.; Hu, J.; Tian, D.; Fu, J.; Zhang, G.; Meng, Y.; et al. Formulation and evaluation of a two-stage targeted liposome coated with hyaluronic acid for improving lung cancer chemotherapy and overcoming multidrug resistance. J. Biomater. Sci. Polym. Ed. 2023, 1–24. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, J.; Liu, D.; Chen, J.; Liu, S.; Peng, Q.; Tian, Y.; Du, M.; Zhang, J.; Xiao, W.; et al. Development of artesunate intelligent prodrug liposomes based on mitochondrial targeting strategy. J. Nanobiotechnol. 2022, 20, 376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ling, L.; Lu, J.; Jiang, F.; Sun, J.; Zhang, Z.; Huang, Y.; Liu, X.; Zhu, Y.; Fu, X.; et al. Reactive oxygen species-responsive mitochondria-targeted liposomal quercetin attenuates retinal ischemia–reperfusion injury via regulating SIRT1/FOXO3A and P38 MAPK signaling pathways. Bioeng. Transl. Med. 2023, 8, e10460. [Google Scholar] [CrossRef]
- Forini, F.; Canale, P.; Nicolini, G.; Iervasi, G. Mitochondria-targeted drug delivery in cardiovascular disease: A long road to nano-cardio medicine. Pharmaceutics 2020, 12, 1122. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-L.; Wang, W.; Han, N.; Sun, H.-L.; Dong, F.-M.; Song, Y.-X.; Feng, R.-F.; Wang, J.-H. The mitochondria-targeted small molecule SS31 delays progression of behavioral deficits by attenuating β-amyloid plaque formation and mitochondrial/synaptic deterioration in APP/PS1 mice. Biochem. Biophys. Res. Commun. 2023, 658, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Liu, H.; Zhang, H.; Han, Y.; Yuan, J.; Wang, T.; Gao, Y.; Li, Z. Ameliorating mitochondrial dysfunction of neurons by biomimetic targeting nanoparticles mediated mitochondrial biogenesis to boost the therapy of Parkinson’s disease. Adv. Sci. 2023, 2300758. [Google Scholar] [CrossRef]
- Chavarria, D.; Da Silva, O.; Benfeito, S.; Barreiro, S.; Garrido, J.; Cagide, F.; Soares, P.; Remião, F.; Brazzolotto, X.; Nachon, F.; et al. Fine-tuning the biological profile of multitarget mitochondriotropic antioxidants for neurodegenerative diseases. Antioxidants 2021, 10, 329. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Liu, J.C. Mitochondrial calcium and reactive oxygen species in cardiovascular disease. Cardiovasc. Res. 2023, 119, 1105–1116. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kuk, M.U.; So, M.K.; Song, E.S.; Lee, H.; Ahn, S.K.; Kwon, H.W.; Park, J.T.; Park, S.C. Targeting mitochondrial oxidative stress as a strategy to treat aging and age-related diseases. Antioxidants 2023, 12, 934. [Google Scholar] [CrossRef]
- Pires, M.; Rego, A.C. Apoe4 and Alzheimer’s disease pathogenesis—Mitochondrial deregulation and targeted therapeutic strategies. Int. J. Mol. Sci. 2023, 24, 778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, J.; Zhu, Z.; He, Y.; Fang, R. Mitochondrion: A bridge linking aging and degenerative diseases. Life Sci. 2023, 322, 121666. [Google Scholar] [CrossRef]
- Trigo, D.; Vitória, J.; Da Cruz, E.; Silva Odete, A.B. Novel therapeutic strategies targeting mitochondria as a gateway in neurodegeneration. Neural. Regen. Res. 2023, 18, 991. [Google Scholar] [CrossRef]
- Sharma, C.; Kim, S. Oxidative stress: Culprit or consequence in Alzheimer’s amyloidopathy. Neural. Regen. Res. 2023, 18, 1948–1949. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, E.; Weaver, E.; Meziane, A.; Lamprou, D.A. Microfluidic paclitaxel-loaded lipid nanoparticle formulations for chemotherapy. Int. J. Pharm. 2022, 628, 122320. [Google Scholar] [CrossRef] [PubMed]
- Cauzzo, J.; Nystad, M.; Holsæter, A.M.; Basnet, P.; Škalko-Basnet, N. Following the fate of dye-containing liposomes in vitro. Int. J. Mol. Sci. 2020, 21, 4847. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-based nanoparticles for drug delivery systems. In Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–76. [Google Scholar] [CrossRef]
- Gaikwad, V.L.; Choudhari, P.B.; Bhatia, N.M.; Bhatia, M.S. Characterization of pharmaceutical nanocarriers: In vitro and in vivo studies. In Nanomaterials for Drug Delivery and Therapy; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–58. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Van Der Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Lipid-based antimicrobial delivery-systems for the treatment of bacterial infections. Front. Chem. 2020, 7, 872. [Google Scholar] [CrossRef]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef]
- Piwowarczyk, L.; Mlynarczyk, D.T.; Krajka-Kuźniak, V.; Majchrzak-Celińska, A.; Budzianowska, A.; Tomczak, S.; Budzianowski, J.; Woźniak-Braszak, A.; Pietrzyk, R.; Baranowski, M.; et al. Natural compounds in liposomal nanoformulations of potential clinical application in glioblastoma. Cancers 2022, 14, 6222. [Google Scholar] [CrossRef]
- Long, P.; Zhu, L.; Lai, H.; Xu, S.; Dong, X.; Shao, Y.; Wang, L.; Cheng, S.; Liu, G.; He, J.; et al. Monascus red pigment liposomes: Microstructural characteristics, stability, and anticancer activity. Foods 2023, 12, 447. [Google Scholar] [CrossRef]
- Gainanova, G.A.; Vagapova, G.I.; Syakaev, V.V.; Ibragimova, A.R.; Valeeva, F.G.; Tudriy, E.V.; Galkina, I.V.; Kataeva, O.N.; Zakharova, L.Y.; Latypov, S.K.; et al. Self-assembling systems based on amphiphilic alkyltriphenylphosphonium bromides: Elucidation of the role of head group. J. Colloid Interface Sci. 2012, 367, 327–336. [Google Scholar] [CrossRef]
- Ren, J.; Jiang, F.; Wang, S.; Hu, H.; Zhang, B.; Zhao, Y.P.; Chen, L.; Lv, Z.; Dai, F. Hydrophilic Hindering and Hydrophobic Growing: A vesicle glycometabolism multi-drug combination therapeutic against Alzheimer’s disease. Biomater. Sci. 2021, 9, 6444–6460. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Xu, L.; Zou, Y.; Wang, S. Romidepsin and metformin nanomaterials delivery on streptozocin for the treatment of Alzheimer’s disease in animal model. Biomed. Pharmacother. 2021, 141, 111864. [Google Scholar] [CrossRef] [PubMed]
- Sarathlal, K.C.; Kakoty, V.; Krishna, K.V.; Dubey, S.K.; Chitkara, D.; Taliyan, R. Neuroprotective efficacy of co-encapsulated rosiglitazone and vorinostat nanoparticle on streptozotocin induced mice model of Alzheimer disease. ACS Chem. Neurosci. 2021, 12, 1528–1541. [Google Scholar] [CrossRef]
- Yang, X.; Yang, W.; Xia, X.; Lei, T.; Yang, Z.; Jia, W.; Zhou, Y.; Cheng, G.; Gao, H. Intranasal delivery of BACE1 SiRNA and rapamycin by dual targets modified nanoparticles for Alzheimer’s disease therapy. Small 2022, 18, 2203182. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, W.J.; Grossberg, G.T. A Fixed-dose combination of memantine extended-release and donepezil in the treatment of moderate-to-severe Alzheimer’s disease. Drug Des. Devel. Ther. 2016, 10, 3267–3279. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.S.; Ramos, I.I.; Fernandes, S.R.; Barreiros, L.; Lima, S.A.C.; Reis, S.; Domingues, M.R.M.; Segundo, M.A. Insights on ultrafiltration-based separation for the purification and quantification of methotrexate in nanocarriers. Molecules 2020, 25, 1879. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gaynanova, G.A.; Vasilieva, E.A.; Pavlov, R.V.; Zueva, I.V.; Babaev, V.M.; Kuznetsov, D.M.; Voloshina, A.D.; Petrov, K.A.; Zakharova, L.Y.; et al. Oxime Therapy for brain AChE reactivation and neuroprotection after organophosphate poisoning. Pharmaceutics 2022, 14, 1950. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, D.A.; Vasilieva, E.A.; Kuznetsov, D.M.; Lenina, O.A.; Filippov, S.K.; Petrov, K.A.; Zakharova, L.Y.; Sinyashin, O.G. Enhancement of the transdermal delivery of nonsteroidal anti-inflammatory drugs using liposomes containing cationic surfactants. ACS Omega 2022, 7, 25741–25750. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Zhukova, N.A.; Lukashenko, S.S.; Valeeva, F.G.; Burilova, E.A.; Sapunova, A.S.; Voloshina, A.D.; Mirgorodskaya, A.B.; Zakharova, L.Y.; Sinyashin, O.G.; et al. Multi-targeted approach by 2-benzimidazolylquinoxalines-loaded cationic arginine liposomes against cervical cancer cells in vitro. Colloids Surf. B 2019, 178, 317–328. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kuznetsova, D.A.; Kushnazarova, R.A.; Gabdrakhmanov, D.R.; Zhukova, N.A.; Lukashenko, S.S.; Sapunova, A.S.; Voloshina, A.D.; Sinyashin, O.G.; Mamedov, V.A.; et al. Soft nanocarriers for new poorly soluble conjugate of pteridine and benzimidazole: Synthesis and cytotoxic activity against tumor cells. J. Mol. Liq. 2020, 317, 114007. [Google Scholar] [CrossRef]
- Rajput, A.; Butani, S. Donepezil HCl liposomes: Development, characterization, cytotoxicity, and pharmacokinetic study. AAPS PharmSciTech 2022, 23, 74. [Google Scholar] [CrossRef] [PubMed]
- Al Harthi, S.; Alavi, S.E.; Radwan, M.A.; El Khatib, M.M.; AlSarra, I.A. Nasal delivery of donepezil HCl-loaded hydrogels for the treatment of Alzheimer’s disease. Sci. Rep. 2019, 9, 9563. [Google Scholar] [CrossRef]
- Ullah, Z.; Al-Asmari, A.; Tariq, M.; Fatani, A. Preparation, characterization, and in vivo evaluation of intranasally administered liposomal formulation of donepezil. Drug Des. Devel. Ther. 2016, 10, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Alruwaili, N.K.; Zafar, A.; Alsaidan, O.A.; Yasir, M.; Mostafa, E.M.; Alnomasy, S.F.; Rawaf, A.; Alquraini, A.; Alomar, F.A. Development of surface modified bilosomes for the oral delivery of quercetin: Optimization, characterization in-vitro antioxidant, antimicrobial, and cytotoxicity study. Drug Deliv. 2022, 29, 3035–3050. [Google Scholar] [CrossRef] [PubMed]
- Sumathi, R. Formulation and characterization of soya lecithin-based liposomes for encapsulating a weakly soluble naringenin. J. Med. Pharm. Allied Sci. 2021, 10, 2018–4023. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Keramati, M.; Azadi, A.; Afzali, E.; Shahbazi, R.; Chiani, M.; Norouzian, D.; Bakhshandeh, H. Optimization, physicochemical characterization, and antimicrobial activity of a novel simvastatin nano-niosomal gel against E. Coli and S. Aureus. Chem. Phys. Lipids 2021, 234, 105019. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, D.A.; Vasileva, L.A.; Gaynanova, G.A.; Pavlov, R.V.; Sapunova, A.S.; Voloshina, A.D.; Sibgatullina, G.V.; Samigullin, D.V.; Petrov, K.A.; Zakharova, L.Y.; et al. Comparative study of cationic liposomes modified with triphenylphosphonium and imidazolium surfactants for mitochondrial delivery. J. Mol. Liq. 2021, 330, 115703. [Google Scholar] [CrossRef]
- Jayachandran, P.; Ilango, S.; Suseela, V.; Nirmaladevi, R.; Shaik, M.R.; Khan, M.; Khan, M.; Shaik, B. Green synthesized silver nanoparticle-loaded liposome-based nanoarchitectonics for cancer management: In vitro drug release analysis. Biomedicines 2023, 11, 217. [Google Scholar] [CrossRef]
- Llesuy, S.; Evelson, P.; Campos, A.M.; Lissi, E. Methodologies for evaluation of total antioxidant activities in complex mixtures. A critical review. Biol. Res. 2001, 34, 51–73. [Google Scholar] [CrossRef]
- Vladimirov, G.; Sergunova, E.; Izmailov, D.; Vladimirov, Y. Chemiluminescent determination of total antioxidant capacity in medicinal plant material. Bull. RSMU 2016, 2, 62–68. [Google Scholar] [CrossRef]
- Alamoudi, A.A.; Méndez, P.A.; Workman, D.; Schätzlein, A.G.; Uchegbu, I.F. Brain gene silencing with cationic amino-capped poly(ethylene glycol) polyplexes. Biomedicines 2022, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Porfiryeva, N.N.; Semina, I.I.; Moustafine, R.I.; Khutoryanskiy, V.V. Intranasal administration as a route to deliver drugs to the brain (Review). Drug Dev. Regist. 2021, 10, 117–127. [Google Scholar] [CrossRef]
- Burilova, E.A.; Pashirova, T.N.; Zueva, I.V.; Gibadullina, E.M.; Lushchekina, S.V.; Sapunova, A.S.; Kayumova, R.M.; Rogov, A.M.; Evtjugin, V.G.; Sudakov, I.A.; et al. Bi-functional sterically hindered phenol lipid-based delivery systems as potential multi-target agents against Alzheimer’s disease via an intranasal route. Nanoscale 2020, 12, 13757–13770. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, E.; Feng, Y.; Qi, J.; Fan, W.; Ma, Y.; He, H.; Xia, F.; Dong, X.; Zhao, W.; Lu, Y.; et al. Evidence of nose-to-brain delivery of nanoemulsions: Cargoes but not vehicles. Nanoscale 2017, 9, 1174–1183. [Google Scholar] [CrossRef]
- Sutthapitaksakul, L.; Dass, C.R.; Sriamornsak, P. Donepezil—An updated review of challenges in dosage form design. J. Drug Deliv. Sci. Technol. 2021, 63, 102549. [Google Scholar] [CrossRef]
- Bhavna, M.; Ali, S.; Ali, M.; Bhatnagar, A.; Baboota, S.; Ali, J. Donepezil nanosuspension intended for nose to brain targeting: In Vitro and in vivo safety evaluation. Int. J. Biol. Macromol. 2014, 67, 418–425. [Google Scholar] [CrossRef]
- Khunt, D.; Shrivas, M.; Polaka, S.; Gondaliya, P.; Misra, M. Role of omega-3 fatty acids and butter oil in targeting delivery of donepezil hydrochloride microemulsion to brain via the intranasal route: A comparative study. AAPS PharmSciTech 2020, 21, 45. [Google Scholar] [CrossRef]
- Yasir, M.; Sara, U.V.S.; Chauhan, I.; Gaur, P.K.; Singh, A.P.; Puri, D.; Ameeduzzafar. Solid lipid nanoparticles for nose to brain delivery of donepezil: Formulation, optimization by Box–Behnken design, in vitro and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1838–1851. [Google Scholar] [CrossRef]
- Espinoza, L.C.; Silva-Abreu, M.; Clares, B.; Rodríguez-Lagunas, M.J.; Halbaut, L.; Cañas, M.-A.; Calpena, A.C. Formulation strategies to improve nose-to-brain delivery of donepezil. Pharmaceutics 2019, 11, 64. [Google Scholar] [CrossRef]
- Patil, R.P.; Pawara, D.D.; Gudewar, C.S.; Tekade, A.R. Nanostructured cubosomes in an in situ nasal gel system: An alternative approach for the controlled delivery of donepezil HCl to brain. J. Liposome Res. 2019, 29, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Yamamoto, H.; Ohtaki, T.; Ishikawa, Y.; Noda, S.; Maeda, K.; Fujitani, K.; Miyazaki, H.; Takabayashi, F.; Sasaki, T.; et al. Active component in green tea catechins and effective intake period for prevention of age-related brain dysfunction. Anti-Aging Med. 2011, 8, 75–81. [Google Scholar] [CrossRef]
- Pedziwiatr-Werbicka, E.; Horodecka, K.; Shcharbin, D.; Bryszewska, M. Nanoparticles in combating cancer: Opportunities and limitations. A brief review. Curr Med. Chem. 2020, 28, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Taléns-Visconti, R.; De Julián-Ortiz, J.V.; Vila-Busó, O.; Diez-Sales, O.; Nácher, A. Intranasal drug administration in Alzheimer-type dementia: Towards clinical applications. Pharmaceutics 2023, 15, 1399. [Google Scholar] [CrossRef] [PubMed]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s disease: Treatment strategies and their limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef]
- Zakharova, L.; Pashirova, T.; Doktorovova, S.; Fernandes, A.R.; Sanchez-Lopez, E.; Silva, A.M.; Souto, S.B.; Souto, E.B. Cationic surfactants: Self-assembly, structure-activity correlation and their biological applications. Int. J. Mol. Sci. 2019, 20, 5534. [Google Scholar] [CrossRef]
- Sibgatullina, G.V.; Malomouzh, A.I. GABA in developing rat skeletal muscle and motor neurons. Protoplasma 2020, 257, 1009–1015. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]
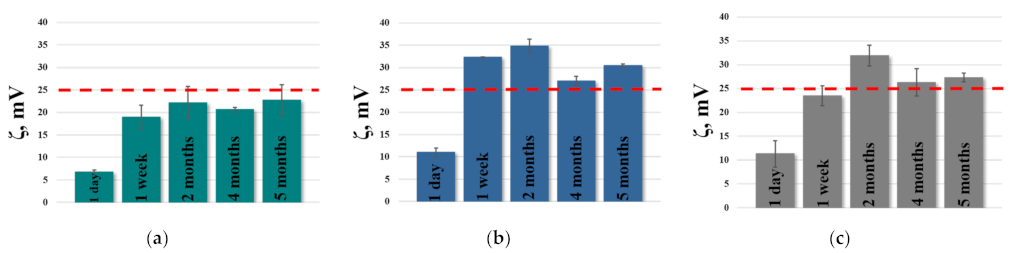

| System | Dh, nm | PdI | ζ, mV | Dh, nm | PdI | ζ, mV |
|---|---|---|---|---|---|---|
| 1st Day | 5th Month | |||||
| 15 mM | ||||||
| PC/Chol | 107 ± 2 | 0.071 ± 0.027 | −9.2 ± 1.2 | 116 ± 4 | 0.116 ± 0.005 # | −10.6 ± 1.7 |
| PC/Chol/TPPB-14 | 105 ± 2 | 0.109 ± 0.010 | 8.2 ± 1.3 | 115 ± 3 | 0.114 ± 0.014 | 24.3 ± 1.9 |
| PC/Chol/TOC | 105 ± 2 | 0.067 ± 0.012 | −1.8 ± 0.8 *** | 114 ± 3 | 0.115 ± 0.004 ## | −17.0 ± 1.4 ** |
| PC/Chol/TOC/TPPB-14 | 110 ± 3 | 0.133 ± 0.011 | 6.8 ± 0.4 | 121 ± 2 | 0.101 ± 0.010 # | 22.7 ± 3.4 |
| 20 mM | ||||||
| PC/Chol | 108 ± 2 | 0.078 ± 0.006 | −7.6 ± 0.5 | 116 ± 2 | 0.085 ± 0.008 | −1.6 ± 0.5 |
| PC/Chol/TPPB-14 | 110 ± 2 | 0.193 ± 0.010 | 14.3 ± 2.2 | 115 ± 1 | 0.114 ± 0.013 ## | 36.6 ± 0.5 |
| PC/Chol/TOC | 104 ± 3 | 0.071 ± 0.01 | −2.9 ± 1.4 ** | 116 ± 2 | 0.101 ± 0.005 ## | −7.9 ± 1.4 ** |
| PC/Chol/TOC/TPPB-14 | 113 ± 2 | 0.231 ± 0.015 | 11.0 ± 1.0 | 122 ± 1 *** | 0.105 ± 0.008 ### | 30.5 ± 0.3 *** |
| 30 mM | ||||||
| PC/Chol | 115 ± 2 | 0.067 ± 0.015 | −4.9 ± 0.5 | 121 ± 1 | 0.083 ± 0.012 | −5.0 ± 0.9 |
| PC/Chol/TPPB-14 | 115 ± 2 | 0.120 ± 0.01 | 23.9 ± 3.4 | 126 ± 1 | 0.142 ± 0.029 | 35.1 ± 1.4 |
| PC/Chol/TOC | 103 ± 2 ** | 0.077 ± 0.005 | −3.6 ± 0.7 * | 158 ± 1 *** | 0.147 ± 0.016 ## | −4.0 ± 0.2 |
| PC/Chol/TOC/TPPB-14 | 110 ± 1 * | 0.148 ± 0.017 | 11.3 ± 2.8 ** | 120 ± 1 ** | 0.103 ± 0.021 # | 27.3 ± 0.9 ** |
| System | Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Korsmeyer–Peppas | First-Order | Higuchi | Zero-Order | ||||||
| n | kKP, %/minn | R2 | k1, 1/min | R2 | kH, %/min1/2 | R2 | k0, %/min | R2 | |
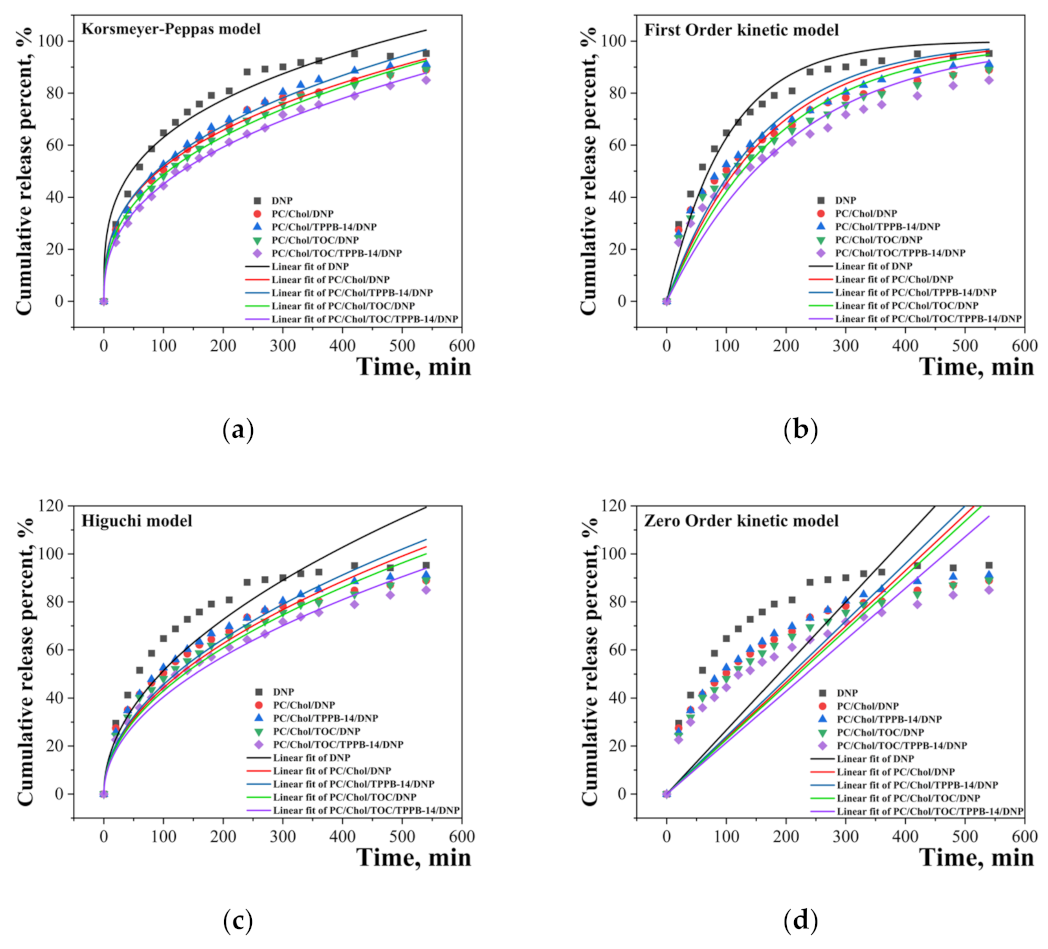
| DNP | 0.299 | 15.878 | 0.9680 | 0.00987 | 0.9591 | 5.139 | 0.8077 | 0.267 | - |
| PC/Chol * | 0.352 | 10.210 | 0.9926 | 0.00602 | 0.8953 | 4.431 | 0.9198 | 0.233 | 0.0729 |
| PC/Chol/TPPB-14 * | 0.364 | 9.777 | 0.9923 | 0.00641 | 0.9291 | 4.562 | 0.9342 | 0.240 | 0.1441 |
| PC/Chol/TOC * | 0.381 | 8.385 | 0.9961 | 0.00547 | 0.9120 | 4.305 | 0.9542 | 0.227 | 0.2376 |
| PC/Chol/TOC/TPPB-14 * | 0.396 | 7.269 | 0.9972 | 0.0047 | 0.8967 | 4.047 | 0.9665 | 0.214 | 0.3071 |
| System | Values Received from Chemiluminescence Curves | |
|---|---|---|
| TAR, % | TRAP, s | |
| TOC | 36.4 ± 2.9 | 221 ± 25 |
| PC/Chol/TOC/TPPB-14 * | 98.2 ± 0.1 | Over 30,000 |
| System | PC, mM | Chol, mM | TOC, mM | TPPB-14, mM |
|---|---|---|---|---|
| 15 mM | ||||
| PC/Chol | 12 | 3 | - | - |
| PC/Chol/TPPB-14 | 12 | 3 | - | 0.24 |
| PC/Chol/TOC | 12 | 1.5 | 1.5 | - |
| PC/Chol/TOC/TPPB-14 | 12 | 1.5 | 1.5 | 0.24 |
| 20 mM | ||||
| PC/Chol | 16 | 4 | - | - |
| PC/Chol/TPPB-14 | 16 | 4 | - | 0.32 |
| PC/Chol/TOC | 16 | 2 | 2 | - |
| PC/Chol/TOC/TPPB-14 | 16 | 2 | 2 | 0.32 |
| 30 mM | ||||
| PC/Chol | 24 | 6 | - | - |
| PC/Chol/TPPB-14 | 24 | 6 | - | 0.48 |
| PC/Chol/TOC | 24 | 3 | 3 | - |
| PC/Chol/TOC/TPPB-14 | 24 | 3 | 3 | 0.48 |
| Kinetic Model | Equation |
|---|---|
| Korsmeyer-Peppas | Qt = kKP ∙ tn |
| Higuchi | Qt = kH ∙ t1/2 |
| First-Order | Qt = Q∞ ∙ (1 − e−k1t) |
| Zero-Order | Qt = k0 ∙ t |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileva, L.; Gaynanova, G.; Valeeva, F.; Belyaev, G.; Zueva, I.; Bushmeleva, K.; Sibgatullina, G.; Samigullin, D.; Vyshtakalyuk, A.; Petrov, K.; et al. Mitochondria-Targeted Delivery Strategy of Dual-Loaded Liposomes for Alzheimer’s Disease Therapy. Int. J. Mol. Sci. 2023, 24, 10494. https://doi.org/10.3390/ijms241310494
Vasileva L, Gaynanova G, Valeeva F, Belyaev G, Zueva I, Bushmeleva K, Sibgatullina G, Samigullin D, Vyshtakalyuk A, Petrov K, et al. Mitochondria-Targeted Delivery Strategy of Dual-Loaded Liposomes for Alzheimer’s Disease Therapy. International Journal of Molecular Sciences. 2023; 24(13):10494. https://doi.org/10.3390/ijms241310494
Chicago/Turabian StyleVasileva, Leysan, Gulnara Gaynanova, Farida Valeeva, Grigory Belyaev, Irina Zueva, Kseniya Bushmeleva, Guzel Sibgatullina, Dmitry Samigullin, Alexandra Vyshtakalyuk, Konstantin Petrov, and et al. 2023. "Mitochondria-Targeted Delivery Strategy of Dual-Loaded Liposomes for Alzheimer’s Disease Therapy" International Journal of Molecular Sciences 24, no. 13: 10494. https://doi.org/10.3390/ijms241310494
APA StyleVasileva, L., Gaynanova, G., Valeeva, F., Belyaev, G., Zueva, I., Bushmeleva, K., Sibgatullina, G., Samigullin, D., Vyshtakalyuk, A., Petrov, K., Zakharova, L., & Sinyashin, O. (2023). Mitochondria-Targeted Delivery Strategy of Dual-Loaded Liposomes for Alzheimer’s Disease Therapy. International Journal of Molecular Sciences, 24(13), 10494. https://doi.org/10.3390/ijms241310494