Environmental Air Pollutants Affecting Skin Functions with Systemic Implications
Abstract
:1. Introduction
2. Molecular Mechanisms of Pollutant-Affected Skin Physiology
2.1. Skin-A Dynamic Barrier with the Capacity to Maintain Homeostasis
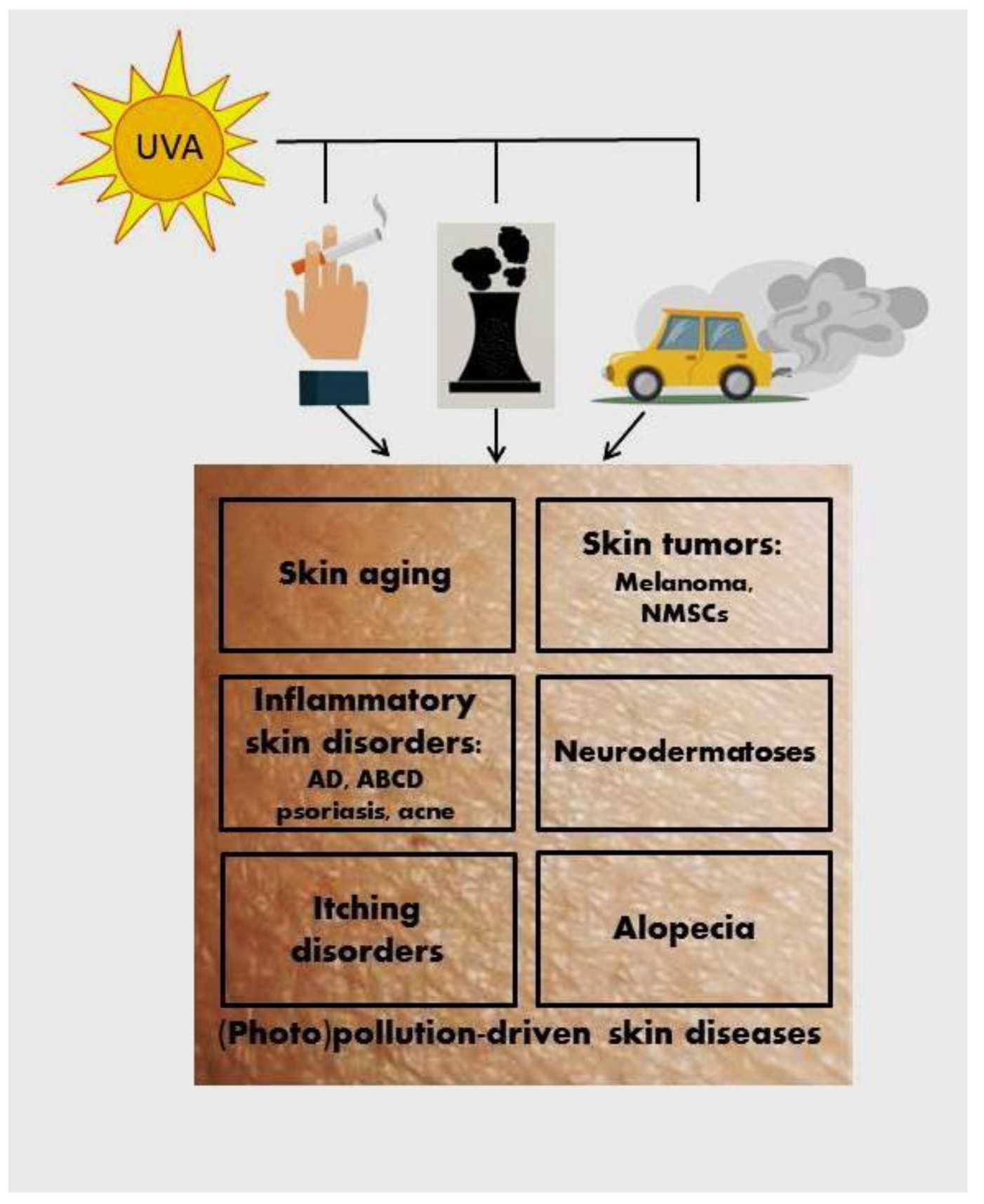
2.2. Cutaneous Response to Air Pollutants, Photopollution, and Cigarette Smoke
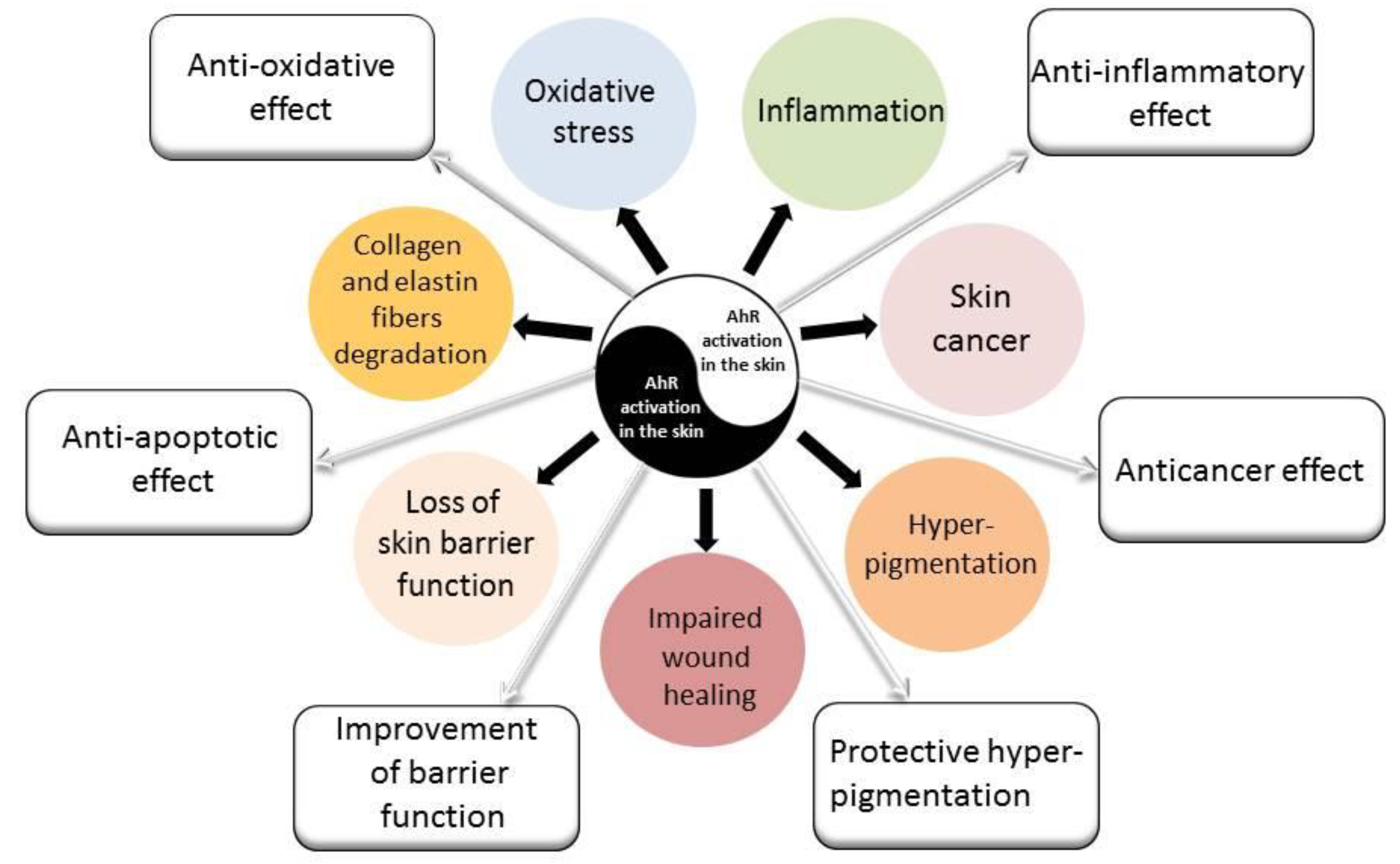
2.3. AhR Signaling Pathway–A Target of Air Pollutants
2.4. Pollution Affects Vitamin D Production in the Skin
3. Pollution-Induced Skin Pathologies
3.1. Premature Skin Aging
3.2. Skin Cancers
3.3. Atopic Dermatitis
3.4. Psoriasis
3.5. Acne
3.6. Melasma and Other Hyperpigmentations
3.7. Hair Loss
4. Preventive and Treatment Options for Air Pollutant-Damaged Skin
4.1. Anti-Pollution Skin Care Strategies
4.2. Modulation of AhR in Air Pollution-Induced Skin Diseases
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef] [Green Version]
- de Bont, J.; Jaganathan, S.; Dahlquist, M.; Persson, Å.; Stafoggia, M.; Ljungman, P. Ambient air pollution and cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. J. Intern. Med. 2022, 291, 779–800. [Google Scholar] [CrossRef] [PubMed]
- Damevska, K.; Nikolovska, S.; Kazandjieva, J.; Kotevska, B.; Bocheva, G. Skin and pollution. In Advances in Integrative Dermatology; França, K., Lotti, T., Eds.; Wiley, J. & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 379–392. [Google Scholar]
- Araviiskaia, E.; Berardesca, E.; Bieber, T.; Gontijo, G.; Sanchez Viera, M.; Marrot, L.; Chuberre, B.; Dreno, B. The impact of airborne pollution on skin. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, W.H.; Kim, Y.Y.; Park, H.Y. Air Pollution and Central Nervous System Disease: A Review of the Impact of Fine Particulate Matter on Neurological Disorders. Front. Public Health 2020, 8, 575330. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.C.; Coburn, J.; Garrick, J.M. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol. Ther. 2020, 210, 107523. [Google Scholar] [CrossRef]
- Lee, H.W.; Kang, S.C.; Kim, S.Y.; Cho, Y.J.; Hwang, S. Long-term Exposure to PM10 Increases Lung Cancer Risks: A Cohort Analysis. Cancer Res. Treat. 2022, 54, 1030–1037. [Google Scholar] [CrossRef]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Real, Á.D.; Santurtún, A.; Teresa Zarrabeitia, M. Epigenetic related changes on air quality. Environ. Res. 2021, 197, 111155. [Google Scholar] [CrossRef]
- Grippo, A.; Zhang, J.; Chu, L.; Guo, Y.; Qiao, L.; Zhang, J.; Myneni, A.A.; Mu, L. Air pollution exposure during pregnancy and spontaneous abortion and stillbirth. Rev. Environ Health 2018, 33, 247–264. [Google Scholar] [CrossRef]
- Koohgoli, R.; Hudson, L.; Naidoo, K.; Wilkinson, S.; Chavan, B.; Birch-Machin, M.A. Bad air gets under your skin. Exp. Dermatol. 2017, 26, 384–387. [Google Scholar] [CrossRef] [Green Version]
- Mathew, B.B.; Singh, H.; Biju, V.G.; Krishnamurthy, N.B. Classification, Source, and Effect of Environmental Pollutants and Their Biodegradation. J. Environ. Pathol. Toxicol. Oncol. 2017, 36, 55–71. [Google Scholar] [CrossRef]
- Pan, T.L.; Wang, P.W.; Aljuffali, I.A.; Huang, C.T.; Lee, C.W.; Fang, J.Y. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J. Dermatol. Sci. 2015, 78, 51–60. [Google Scholar] [CrossRef]
- Larese Filon, F.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. International Agency for Research on Cancer Monograph Working Group IARC. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013, 14, 1262–1263. [Google Scholar] [CrossRef]
- Donaldson, K.; Tran, L.; Jimenez, L.A.; Duffin, R.; Newby, D.E.; Mills, N.; MacNee, W.; Stone, V. Combustion-derived nanoparticles: A review of their toxicology following inhalation exposure. Part. Fibre Toxicol. 2005, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.S.; Ryu, M.H.; Carlsten, C. Ultrafine particles: Unique physicochemical properties relevant to health and disease. Exp. Mol. Med. 2020, 52, 318–328. [Google Scholar] [CrossRef]
- Toriba, A.; Kuramae, Y.; Chetiyanukornkul, T.; Kizu, R.; Makino, T.; Nakazawa, H.; Hayakawa, K. Quantification of polycyclic aromatic hydrocarbons (PAHs) in human hair by HPLC with fluorescence detection: A biological monitoring method to evaluate the exposure to PAHs. Biomed. Chromatogr. 2003, 17, 126–132. [Google Scholar] [CrossRef]
- Hearn, B.A.; Ding, Y.S.; Watson, C.H.; Johnson, T.L.; Zewdie, G.; Jeong-Im, J.H.; Walters, M.J.; Holman, M.R.; Rochester, C.G. Multi-year Study of PAHs in Mainstream Cigarette Smoke. Tob. Regul. Sci. 2018, 4, 96–106. [Google Scholar] [CrossRef]
- Appenzeller, B.M.; Mathon, C.; Schummer, C.; Alkerwi, A.; Lair, M.L. Simultaneous determination of nicotine and PAH metabolites in human hair specimen: A potential methodology to assess tobacco smoke contribution in PAH exposure. Toxicol. Lett. 2012, 210, 211–219. [Google Scholar] [CrossRef]
- Dimitrov, A.; Zanini, M.; Zucchi, H.; Boudah, S.; Lima, J.; Soeur, J.; Marrot, L. Vitamin C prevents epidermal damage induced by PM-associated pollutants and UVA1 combined exposure. Exp. Dermatol. 2021, 30, 1693–1698. [Google Scholar] [CrossRef]
- Guerreiro, C.B.B.; Horálek, J.; de Leeuw, F.; Couvidat, F. Benzo(a)pyrene in Europe: Ambient air concentrations, population exposure and health effects. Environ. Pollut. 2016, 214, 657–667. [Google Scholar] [CrossRef] [PubMed]
- von Koschembahr, A.; Youssef, A.; Béal, D.; Gudimard, L.; Giot, J.P.; Douki, T. Toxicity and DNA repair in normal human keratinocytes co-exposed to benzo[a]pyrene and sunlight. Toxicol. In Vitro 2020, 63, 104744. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J.; Traber, M.G.; Tsang, K.; Cross, C.E.; Packer, L. In vivo exposure to ozone depletes vitamins C and E and induces lipid peroxidation in epidermal layers of murine skin. Free Radic. Biol. Med. 1997, 23, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Pecorelli, A.; Belmonte, G.; Pambianchi, E.; Cervellati, F.; Lynch, S.; Krol, Y.; Oresajo, C. Protective Effects of Topical Vitamin C Compound Mixtures against Ozone-Induced Damage in Human Skin. J. Investig. Dermatol. 2017, 137, 1373–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef] [Green Version]
- Bernerd, F.; Passeron, T.; Castiel, I.; Marionnet, C. The Damaging Effects of Long UVA (UVA1) Rays: A Major Challenge to Preserve Skin Health and Integrity. Int. J. Mol. Sci. 2022, 23, 8243. [Google Scholar] [CrossRef]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef]
- Marionnet, C.; Tricaud, C.; Bernerd, F. Exposure to non-extreme solar UV daylight: Spectral characterization, effects on skin and photoprotection. Int. J. Mol. Sci. 2014, 16, 68–90. [Google Scholar] [CrossRef]
- Burns, E.M.; Ahmed, H.; Isedeh, P.N.; Kohli, I.; Van Der Pol, W.; Shaheen, A.; Muza_ar, A.F.; Al-Sadek, C.; Foy, T.M.; Abdelgawwad, M.S.; et al. Ultraviolet radiation, both UVA and UVB, influences the composition of the skin microbiome. Exp. Dermatol. 2019, 28, 136–141. [Google Scholar] [CrossRef]
- Soeur, J.; Belaïdi, J.P.; Chollet, C.; Denat, L.; Dimitrov, A.; Jones, C.; Perez, P.; Zanini, M.; Zobiri, O.; Mezzache, S.; et al. Photo-pollution stress in skin: Traces of pollutants (PAH and particulate matter) impair redox homeostasis in keratinocytes exposed to UVA1. J. Dermatol Sci. 2017, 86, 162–169. [Google Scholar] [CrossRef]
- Nakashima, Y.; Ohta, S.; Wolf, A.M. Blue light-induced oxidative stress in live skin. Free Radic. Biol. Med. 2017, 108, 300–310. [Google Scholar] [CrossRef]
- Dong, K.; Goyarts, E.C.; Pelle, E.; Trivero, J.; Pernodet, N. Blue light disrupts the circadian rhythm and create damage in skin cells. Int. J. Cosmet. Sci. 2019, 41, 558–562. [Google Scholar] [CrossRef]
- Ouyang, X.; Yang, J.; Hong, Z.; Wu, Y.; Xie, Y.; Wang, G. Mechanisms of blue light-induced eye hazard and protective measures: A review. Biomed. Pharmacother. 2020, 130, 110577. [Google Scholar] [CrossRef]
- Li, X.; Zhu, S.; Qi, F. Blue light pollution causes retinal damage and degeneration by inducing ferroptosis. J. Photochem. Photobiol. B 2023, 238, 112617. [Google Scholar] [CrossRef]
- Campiche, R.; Curpen, S.J.; Lutchmanen-Kolanthan, V.; Gougeon, S.; Cherel, M.; Laurent, G.; Gempeler, M.; Schuetz, R. Pigmentation effects of blue light irradiation on skin and how to protect against them. Int. J. Cosmet. Sci. 2020, 42, 399–406. [Google Scholar] [CrossRef]
- Mancebo, S.E.; Wang, S.Q. Recognizing the impact of ambient air pollution on skin health. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2326–2332. [Google Scholar] [CrossRef] [Green Version]
- Abolhasani, R.; Araghi, F.; Tabary, M.; Aryannejad, A.; Mashinchi, B.; Robati, R.M. The impact of air pollution on skin and related disorders: A comprehensive review. Dermatol. Ther. 2021, 34, e14840. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012, 212, 1–115. [Google Scholar]
- Zouboulis, C.C. Human skin: An independent peripheral endocrine organ. Horm. Res. 2000, 54, 230–242. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Tobin, D.J. The cutaneous serotoninergic/melatoninergic system: Securing a place under the sun. FASEB J. 2005, 19, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Kleszczyński, K.; Semak, I.; Janjetovic, Z.; Sweatman, T.; Skobowiat, C.; Steketee, J.D.; Lin, Z.; Postlethwaite, A.; et al. Characterization of serotonin and N-acetylserotonin systems in the human epidermis and skin cells. J. Pineal Res. 2020, 68, e12626. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zbytek, B.; Nikolakis, G.; Manna, P.R.; Skobowiat, C.; Zmijewski, M.; Li, W.; Janjetovic, Z.; Postlethwaite, A.; Zouboulis, C.C.; et al. Steroidogenesis in the skin: Implications for local immune functions. J. Steroid Biochem. Mol. Biol. 2013, 137, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Slominski, R.M.; Raman, C.; Chen, J.Y.; Athar, M.; Elmets, C. Neuroendocrine signaling in the skin with a special focus on the epidermal neuropeptides. Am. J. Physiol. Cell Physiol. 2022, 323, C1757–C1776. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Kohn, L.; Ain, K.B.; Venkataraman, G.M.; Pisarchik, A.; Chung, J.H.; Giuliani, C.; Thornton, M.; Slugocki, G.; et al. Expression of hypothalamic-pituitary-thyroid axis related genes in the human skin. J. Investig. Dermatol. 2002, 119, 1449–1455. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Wortsman, J.; Pisarchik, A.; Zbytek, B.; Linton, E.A.; Mazurkiewicz, J.E.; Wei, E.T. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001, 15, 1678–1693. [Google Scholar] [CrossRef]
- Holick, M.F. Ultraviolet B Radiation: The Vitamin D Connection. Adv. Exp. Med. Biol. 2017, 996, 137–154. [Google Scholar]
- Slominski, A.; Kim, T.K.; Zmijewski, M.A.; Janjetovic, Z.; Li, W.; Chen, J.; Kusniatsova, E.I.; Semak, I.; Postlethwaite, A.; Miller, D.D.; et al. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinology 2013, 5, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Kim, T.K.; Li, W.; Yi, A.K.; Postlethwaite, A.; Tuckey, R.C. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J. Steroid Biochem. Mol. Biol. 2014, 144, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Roosterman, D.; Goerge, T.; Schneider, S.W.; Bunnett, N.W.; Steinhoff, M. Neuronal control of skin function: The skin as a neuroimmunoendocrine organ. Physiol. Rev. 2006, 86, 1309–1379. [Google Scholar] [CrossRef] [PubMed]
- Luger, T.A.; Paus, R.; Slominski, A.; Lipton, J. The proopiomelanocortin system in cutaneous neuroimmunomodulation. An introductory overview. Ann. N. Y. Acad. Sci. 1999, 885, xi–xiv. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, R.L.; Lipper, G.; Lerner, E.A. The neuro-immuno-cutaneous-endocrine network: Relationship of mind and skin. Arch. Dermatol. 1998, 134, 1431–1435. [Google Scholar] [CrossRef]
- Slominski, A.T.; Manna, P.R.; Tuckey, R.C. Cutaneous glucocorticosteroidogenesis: Securing local homeostasis and the skin integrity. Exp. Dermatol. 2014, 23, 369–374. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Zmijewski, M.A.; Zbytek, B.; Tobin, D.J.; Theoharides, T.C.; Rivier, J. Key role of CRF in the skin stress response system. Endocr. Rev. 2013, 34, 827–884. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kleszczyński, K.; Semak, I.; Janjetovic, Z.; Zmijewski, M.A.; Kim, T.K.; Slominski, R.M.; Reiter, R.J.; Fischer, T.W. Local melatoninergic system as the protector of skin integrity. Int. J. Mol. Sci. 2014, 15, 17705–17732. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Wortsman, J.; Paus, R.; Elias, P.M.; Tobin, D.J.; Feingold, K.R. Skin as an endocrine organ: Implications for its function. Drug Discov. Today Dis. Mech. 2008, 5, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Tončić, R.J.; Kezić, S.; Hadžavdić, S.L.; Marinović, B. Skin barrier and dry skin in the mature patient. Clin. Dermatol. 2017, 36, 109–115. [Google Scholar] [CrossRef]
- Marrot, L. Pollution and Sun Exposure: A Deleterious Synergy. Mechanisms and Opportunities for Skin Protection. Curr. Med. Chem. 2018, 25, 5469–5486. [Google Scholar] [CrossRef]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Ryu, Y.S.; Hyun, Y.J.; Shilnikova, K.; Zhen, A.X.; Jeong, J.W.; Choi, Y.H.; Kang, H.K.; et al. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch. Toxicol. 2018, 92, 2077–2091. [Google Scholar] [CrossRef] [Green Version]
- Wortzman, M.; Nelson, D.B. A comprehensive topical antioxidant inhibits oxidative stress induced by blue light exposure and cigarette smoke in human skin tissue. J. Cosmet. Dermatol. 2021, 20, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Mokrzyński, K.; Krzysztyńska-Kuleta, O.; Zawrotniak, M.; Sarna, M.; Sarna, T. Fine Particulate Matter-Induced Oxidative Stress Mediated by UVA-Visible Light Leads to Keratinocyte Damage. Int. J. Mol. Sci. 2021, 22, 10645. [Google Scholar] [CrossRef]
- Romani, A.; Cervellati, C.; Muresan, X.M.; Belmonte, G.; Pecorelli, A.; Cervellati, F.; Benedusi, M.; Evelson, P.; Valacchi, G. Keratinocytes oxidative damage mechanisms related to airbone particle matter exposure. Mech. Ageing Dev. 2018, 172, 86–95. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, K.A.; Zhen, A.X.; Fernando, P.D.S.M.; Ahn, M.J.; Koh, Y.S.; Kang, H.K.; Yi, J.M.; Choi, Y.H.; Hyun, J.W. Particulate matter 2.5 mediates cutaneous cellular injury by inducing mitochondria-associated endoplasmic reticulum stress: Protective effects of ginsenoside Rb1. Antioxidants 2019, 8, 383. [Google Scholar] [CrossRef] [Green Version]
- Grevendonk, L.; Janssen, B.G.; Vanpoucke, C.; Lefebvre, W.; Hoxha, M.; Bollati, V.; Nawrot, T.S. Mitochondrial oxidative DNA damage and exposure to particulate air pollution in mother-newborn pairs. Environ. Health 2016, 15, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, D.; Ripley, S.; Weichenthal, S.; Godri Pollitt, K.J. Ambient particulate matter oxidative potential: Chemical determinants, associated health effects, and strategies for risk management. Free Radic. Biol. Med. 2020, 151, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Woodby, B.; Prieux, R.; Valacchi, G. Involvement of 4-hydroxy-2-nonenal in pollution-induced skin damage. Biofactors 2019, 45, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Verdin, A.; Cazier, F.; Fitoussi, R.; Blanchet, N.; Vié, K.; Courcot, D.; Momas, I.; Seta, N.; Achard, S. An in vitro model to evaluate the impact of environmental fine particles (PM0.3–2.5) on skin damage. Toxicol. Lett. 2019, 305, 94–102. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [Green Version]
- Valacchi, G.; Fortino, V.; Bocci, V. The dual action of ozone on the skin. Br. J. Dermatol. 2005, 153, 1096–1100. [Google Scholar] [CrossRef]
- Weber, S.U.; Jothi, S.; Thiele, J.J. High-pressure liquid chromatography analysis of ozone-induced depletion of hydrophilic and lipophilic antioxidants in murine skin. Methods Enzymol. 2000, 319, 536–546. [Google Scholar]
- Weber, S.U.; Thiele, J.J.; Cross, C.E.; Packer, L. Vitamin C, uric acid, and glutathione gradients in murine stratum corneum and their susceptibility to ozone exposure. J. Investig. Dermatol. 1999, 113, 1128–1132. [Google Scholar] [CrossRef] [Green Version]
- De Luca, C.; Valacchi, G. Surface lipids as multifunctional mediators of skin responses to environmental stimuli. Mediat. Inflamm. 2010, 2010, 321494. [Google Scholar] [CrossRef]
- Thiele, J.J.; Weber, S.U.; Packer, L. Sebaceous gland secretion is a major physiologic route of vitamin E delivery to skin. J. Investig. Dermatol. 1999, 113, 1006–1010. [Google Scholar]
- Ekanayake Mudiyanselage, S.; Hamburger, M.; Elsner, P.; Thiele, J.J. Ultraviolet a induces generation of squalene monohydroperoxide isomers in human sebum and skin surface lipids in vitro and in vivo. J. Investig. Dermatol. 2003, 120, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Pham, D.M.; Boussouira, B.; Moyal, D.; Nguyen, Q.L. Oxidization of squalene, a human skin lipid: A new and reliable marker of environmental pollution studies. Int. J. Cosmet. Sci. 2015, 37, 357–365. [Google Scholar] [CrossRef]
- Lefebvre, M.A.; Pham, D.M.; Boussouira, B.; Bernard, D.; Camus, C.; Nguyen, Q.L. Evaluation of the impact of urban pollution on the quality of skin: A multicentre study in Mexico. Int. J. Cosmet. Sci. 2015, 37, 329–338. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, S.; Huang, G.; Liu, H. Cigarettes, a skin killer! Cigarette smoke may cause ferroptosis in female skin. J. Cosmet. Dermatol. 2022, 21, 3085–3094. [Google Scholar] [CrossRef]
- Esser, C.; Bargen, I.; Weighardt, H.; Haarmann-Stemmann, T.; Krutmann, J. Functions of the aryl hydrocarbon receptor in the skin. Semin. Immunopathol. 2013, 35, 677–691. [Google Scholar] [CrossRef]
- Furue, M.; Takahara, M.; Nakahara, T.; Uchi, H. Role of AhR/ARNT system in skin homeostasis. Arch. Dermatol. Res. 2014, 306, 769–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szelest, M.; Walczak, K.; Plech, T. A New Insight into the Potential Role of Tryptophan-Derived AhR Ligands in Skin Physiological and Pathological Processes. Int. J. Mol. Sci. 2021, 22, 1104. [Google Scholar] [CrossRef] [PubMed]
- Jux, B.; Kadow, S.; Luecke, S.; Rannug, A.; Krutmann, J.; Esser, C. The aryl hydrocarbon receptor mediates UVB radiation-induced skin tanning. J. Investig. Dermatol. 2011, 131, 203–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, Y.; Lee, E.J.; Luo, E.; Choi, J.; Kim, J.Y.; Kim, S.; Kim, S.-H.; Bae, Y.J.; Park, S.; Lee, J.; et al. Particulate Matter Promotes Melanin Production through Endoplasmic Reticulum Stress-Mediated IRE1α Signaling. J. Investig. Dermatol. 2022, 142, 1425–1434.e6. [Google Scholar] [CrossRef]
- Esser, C.; Lawrence, B.P.; Sherr, D.H.; Perdew, G.H.; Puga, A.; Barouki, R.; Coumoul, X. Old Receptor, New Tricks-The Ever-Expanding Universe of Aryl Hydrocarbon Receptor Functions. Report from the 4th AHR Meeting, 29–31 August 2018 in Paris, France. Int. J. Mol. Sci. 2018, 19, 3603. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.; Chen, R.; Chen, X.; Huang, B.; Lin, R.; Xie, X.; Chen, J.; Jiang, J.; Deng, Y.; Wen, J. AhR regulates the expression of human cytochrome P450 1A1 (CYP1A1) by recruiting Sp1. FEBS J. 2019, 286, 4215–4231. [Google Scholar] [CrossRef]
- Mimura, J.; Ema, M.; Sogawa, K.; Fujii-Kuriyama, Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999, 13, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.Q.; Wang, Y.N.; Feng, H.Y.; Guo, Z.Y.; Li, X.; Nie, X.L.; Zhao, Y.Y. Host/microbiota interactions-derived tryptophan metabolites modulate oxidative stress and inflammation via aryl hydrocarbon receptor signaling. Free Radic. Biol. Med. 2022, 184, 30–41. [Google Scholar] [CrossRef]
- Uberoi, A.; Bartow-McKenney, C.; Zheng, Q.; Flowers, L.; Campbell, A.; Knight, S.A.B.; Chan, N.; Wei, M.; Lovins, V.; Bugayev, J.; et al. Commensal microbiota regulates skin barrier function and repair via signaling through the aryl hydrocarbon receptor. Cell Host Microbe 2021, 29, 1235–1248.e8. [Google Scholar] [CrossRef]
- Tsuji, G.; Takahara, M.; Uchi, H.; Matsuda, T.; Chiba, T.; Takeuchi, S.; Yasukawa, F.; Moroi, Y.; Furue, M. Identification of Ketoconazole as an AhR-Nrf2 Activator in Cultured Human Keratinocytes: The Basis of Its Anti-Inflammatory Effect. J. Investig. Dermatol. 2012, 132, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Contador-Troca, M.; Alvarez-Barrientos, A.; Barrasa, E.; Rico-Leo, E.M.; Catalina-Fernández, I.; Menacho-Márquez, M.; Bustelo, X.R.; García-Borrón, J.C.; Gómez-Durán, A.; Sáenz-Santamaría, J.; et al. The dioxin receptor has tumor suppressor activity in melanoma growth and metastasis. Carcinogenesis 2013, 34, 2683–2693. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Kim, T.K.; Janjetovic, Z.; Brożyna, A.A.; Żmijewski, M.A.; Xu, H.; Sutter, T.R.; Tuckey, R.C.; Jetten, A.M.; Crossman, D.K. Differential and Overlapping Effects of 20,23(OH)2D3 and 1,25(OH)2D3 on Gene Expression in Human Epidermal Keratinocytes: Identification of AhR as an Alternative Receptor for 20,23(OH)2D3. Int. J. Mol. Sci. 2018, 19, 3072. [Google Scholar] [CrossRef] [Green Version]
- Brzeminski, P.; Fabisiak, A.; Slominski, R.M.; Kim, T.K.; Janjetovic, Z.; Podgorska, E.; Song, Y.; Saleem, M.; Reddy, S.B.; Qayyum, S.; et al. Chemical synthesis, biological activities and action on nuclear receptors of 20S(OH)D3, 20S,25(OH)2D3, 20S,23S(OH)2D3 and 20S,23R(OH)2D3. Bioorg. Chem. 2022, 121, 105660. [Google Scholar] [CrossRef]
- Song, Y.; Slominski, R.M.; Qayyum, S.; Kim, T.K.; Janjetovic, Z.; Raman, C.; Tuckey, R.C.; Song, Y.; Slominski, A.T. Molecular and structural basis of interactions of vitamin D3 hydroxyderivatives with aryl hydrocarbon receptor (AhR): An integrated experimental and computational study. Int. J. Biol. Macromol. 2022, 209 Pt A, 1111–1123. [Google Scholar] [CrossRef]
- Grishanova, A.Y.; Perepechaeva, M.L. Aryl Hydrocarbon Receptor in Oxidative Stress as a Double Agent and Its Biological and Therapeutic Significance. Int. J. Mol. Sci. 2022, 23, 6719. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.H.; Jeong, G.J.; Park, K.Y.; Lee, M.K.; Seo, S.J. Particulate matter induces pro-inflammatory cytokines via phosphorylation of p38 MAPK possibly leading to dermal inflammaging. Exp. Dermatol. 2019, 28, 809–815. [Google Scholar] [CrossRef]
- Magnani, N.D.; Muresan, X.M.; Belmonte, G.; Cervellati, F.; Sticozzi, C.; Pecorelli, A.; Miracco, C.; Marchini, T.; Evelson, P.; Valacchi, G. Skin Damage Mechanisms Related to Airborne Particulate Matter Exposure. Toxicol. Sci. 2016, 149, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.S.; Lim, H.K.; Hong, K.K.; Shin, M.K.; Lee, J.W.; Lee, S.W.; Kim, N.I. Cigarette smoke-induced interleukin-1 alpha may be involved in the pathogenesis of adult acne. Ann. Dermatol. 2014, 26, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, G.; Takahara, M.; Uchi, H.; Takeuchi, S.; Mitoma, C.; Moroi, Y.; Furue, M. An environmental contaminant, benzo(a)pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J. Dermatol Sci. 2011, 62, 42–49. [Google Scholar] [CrossRef]
- Suvanprakorn, P.; Tongyen, T.; Prakhongcheep, O.; Laoratthaphong, P.; Chanvorachote, P. Establishment of an Anti-acne Vulgaris Evaluation Method Based on TLR2 and TLR4-mediated Interleukin-8 Production. In Vivo 2019, 33, 1929–1934. [Google Scholar] [CrossRef] [Green Version]
- Skov, L.; Beurskens, F.J.; Zachariae, C.O.; Reitamo, S.; Teeling, J.; Satijn, D.; Knudsen, K.M.; Boot, E.P.; Hudson, D.; Baadsgaard, O.; et al. IL-8 as antibody therapeutic target in inflammatory diseases: Reduction of clinical activity in palmoplantar pustulosis. J. Immunol. 2008, 181, 669–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valacchi, G.; Sticozzi, C.; Belmonte, G.; Cervellati, F.; Demaude, J.; Chen, N.; Krol, Y.; Oresajo, C. Vitamin C Compound Mixtures Prevent Ozone-Induced Oxidative Damage in Human Keratinocytes as Initial Assessment of Pollution Protection. PLoS ONE 2015, 10, e0131097. [Google Scholar] [CrossRef] [PubMed]
- Haarmann-Stemmann, T.; Esser, C.; Krutmann, J. The Janus-Faced Role of Aryl Hydrocarbon Receptor Signaling in the Skin: Consequences for Prevention and Treatment of Skin Disorders. J. Investig. Dermatol. 2015, 135, 2572–2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.W.; Lin, Z.C.; Hu, S.C.; Chiang, Y.C.; Hsu, L.F.; Lin, Y.C.; Lee, I.T.; Tsai, M.H.; Fang, J.Y. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci. Rep. 2016, 6, 27995. [Google Scholar] [CrossRef] [Green Version]
- Ono, Y.; Torii, K.; Fritsche, E.; Shintani, Y.; Nishida, E.; Nakamura, M.; Shirakata, Y.; Haarmann-Stemmann, T.; Abel, J.; Krutmann, J.; et al. Role of the aryl hydrocarbon receptor in tobacco smoke extract-induced matrix metalloproteinase-1 expression. Exp. Dermatol. 2013, 22, 349–353. [Google Scholar] [CrossRef]
- Lecas, S.; Boursier, E.; Fitoussi, R.; Vié, K.; Momas, I.; Seta, N.; Achard, S. In vitro model adapted to the study of skin ageing induced by air pollution. Toxicol. Lett. 2016, 259, 60–68. [Google Scholar] [CrossRef]
- Birkedal-Hansen, H. Catabolism and turnover of collagens: Collagenases. Methods Enzymol. 1987, 144, 140–171. [Google Scholar]
- Sárdy, M. Role of matrix metalloproteinases in skin ageing. Connect. Tissue Res. 2009, 50, 132–138. [Google Scholar] [CrossRef]
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne particle exposure and extrinsic skin aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Li, Q.; Du, H.Y.; Wang, Q.W.; Huang, Y.; Liu, W. Airborne polycyclic aromatic hydrocarbons trigger human skin cells aging through aryl hydrocarbon receptor. Biochem. Biophys. Res. Commun. 2017, 488, 445–452. [Google Scholar] [CrossRef]
- Sutter, C.H.; Yin, H.; Li, Y.; Mammen, J.S.; Bodreddigari, S.; Stevens, G.; Cole, J.A.; Sutter, T.R. EGF receptor signaling blocks aryl hydrocarbon receptor-mediated transcription and cell differentiation in human epidermal keratinocytes. Proc. Natl. Acad. Sci. USA 2009, 106, 4266–4271. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, L.H.; Sutter, C.H.; Leon Carrion, S.; Tran, Q.T.; Bodreddigari, S.; Kensicki, E.; Mohney, R.P.; Sutter, T.R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated production of reactive oxygen species is an essential step in the mechanism of action to accelerate human keratinocyte differentiation. Toxicol. Sci. 2013, 132, 235–249. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Morita, A.; Tsuji, T. Tobacco smoke extract induces age-related changes due to modulation of TGF-beta. Exp. Dermatol. 2003, 12, 51–56. [Google Scholar] [CrossRef]
- Huang, N.; Mi, T.; Xu, S.; Dadd, T.; Ye, X.; Chen, G.; Du, Y.; Santhanam, U. Traffic-derived air pollution compromises skin barrier function and stratum corneum redox status: A population study. J. Cosmet. Dermatol. 2019, 19, 1751–1759. [Google Scholar] [CrossRef]
- Hieda, D.S.; Anastacio da Costa Carvalho, L.; Vaz de Mello, B.; Oliveira, E.A.; Romano de Assis, S.; Wu, J.; Du-Thumm, L.; Viana da Silva, C.L.; Roubicek, D.A.; Maria-Engler, S.S.; et al. Air Particulate Matter Induces Skin Barrier Dysfunction and Water Transport Alteration on a Reconstructed Human Epidermis Model. J. Investig. Dermatol. 2020, 140, 2343–2352.e3. [Google Scholar] [CrossRef]
- Haas, K.; Weighardt, H.; Deenen, R.; Köhrer, K.; Clausen, B.; Zahner, S.; Boukamp, P.; Bloch, W.; Krutmann, J.; Esser, C. Aryl Hydrocarbon Receptor in Keratinocytes Is Essential for Murine Skin Barrier Integrity. J. Investig. Dermatol. 2016, 136, 2260–2269. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.E.; Amini, H.; Heydarpour, P.; Amini Chermahini, F.; Godderis, L. Air pollution, environmental chemicals, and smoking may trigger vitamin D deficiency: Evidence and potential mechanisms. Environ. Int. 2019, 122, 67–90. [Google Scholar] [CrossRef]
- Johns, L.E.; Ferguson, K.K.; Meeker, J.D. Relationships Between Urinary Phthalate Metabolite and Bisphenol A Concentrations and Vitamin D Levels in U.S. Adults: National Health and Nutrition Examination Survey (NHANES), 2005–2010. J. Clin. Endocrinol. Metab. 2016, 101, 4062–4069. [Google Scholar] [CrossRef] [Green Version]
- Shinkov, A.; Borissova, A.M.; Dakovska, L.; Vlahov, J.; Kassabova, L.; Svinarov, D. Winter 25-hydroxyvitamin D levels in young urban adults are affected by smoking, body mass index and educational level. Eur. J. Clin. Nutr. 2015, 69, 355–360. [Google Scholar] [CrossRef]
- Kmieć, P.; Żmijewski, M.; Waszak, P.; Sworczak, K.; Lizakowska-Kmieć, M. Vitamin D deficiency during winter months among an adult, predominantly urban, population in Northern Poland. Endokrynol. Pol. 2014, 65, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.T.; Kim, T.K.; Shehabi, H.Z.; Semak, I.; Tang, E.K.; Nguyen, M.N.; Benson, H.A.; Korik, E.; Janjetovic, Z.; Chen, J.; et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012, 26, 3901–3915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuckey, R.C.; Cheng, C.Y.S.; Slominski, A.T. The serum vitamin D metabolome: What we know and what is still to discover. J. Steroid Biochem. Mol. Biol. 2019, 186, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Li, W.; Tuckey, R.C. Classical and non-classical metabolic transformation of vitamin D in dermal fibroblasts. Exp. Dermatol. 2016, 25, 231–232. [Google Scholar] [CrossRef] [Green Version]
- Baïz, N.; Dargent-Molina, P.; Wark, J.D.; Souberbielle, J.C.; Slama, R.; Annesi-Maesano, I.; EDEN Mother-Child Cohort Study Group. Gestational exposure to urban air pollution related to a decrease in cord blood vitamin d levels. J. Clin. Endocrinol. Metab. 2012, 97, 4087–4095. [Google Scholar] [CrossRef] [Green Version]
- Hoseinzadeh, E.; Taha, P.; Wei, C.; Godini, H.; Ashraf, G.M.; Taghavi, M.; Miri, M. The impact of air pollutants, UV exposure and geographic location on vitamin D deficiency. Food Chem. Toxicol. 2018, 113, 241–254. [Google Scholar] [CrossRef]
- Oda, Y.; Hu, L.; Nguyen, T.; Fong, C.; Zhang, J.; Guo, P.; Bikle, D.D. Vitamin D Receptor Is Required for Proliferation, Migration, and Differentiation of Epidermal Stem Cells and Progeny during Cutaneous Wound Repair. J. Investig. Dermatol. 2018, 138, 2423–2431. [Google Scholar] [CrossRef] [Green Version]
- Reichrath, J.; Saternus, R.; Vogt, T. Challenge and perspective: The relevance of ultraviolet (UV) radiation and the vitamin D endocrine system (VDES) for psoriasis and other inflammatory skin diseases. Photochem. Photobiol. Sci. 2017, 16, 433–444. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. The impact of vitamin D on skin aging. Int. J. Mol. Sci. 2021, 22, 9097. [Google Scholar] [CrossRef]
- Reichrath, J.; Zouboulis, C.C.; Vogt, T.; Holick, M.F. Targeting the vitamin D endocrine system (VDES) for the management of inflammatory and malignant skin diseases: An historical view and outlook. Rev. Endocr. Metab. Disord. 2016, 17, 405–417. [Google Scholar] [CrossRef]
- Slominski, A.T.; Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Stefan, J.; Slominski, R.M.; Hanumanthu, V.S.; Raman, C.; Qayyum, S.; Song, Y.; et al. Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives. Cell Biochem. Biophys. 2020, 78, 165–180. [Google Scholar] [CrossRef]
- Scott, J.F.; Das, L.M.; Ahsanuddin, S.; Qiu, Y.; Binko, A.M.; Traylor, Z.P.; Debanne, S.M.; Cooper, K.D.; Boxer, R.; Lu, K.Q. Oral Vitamin D Rapidly Attenuates Inflammation from Sunburn: An Interventional Study. J. Investig. Dermatol. 2017, 137, 2078–2086. [Google Scholar] [CrossRef] [Green Version]
- Tongkao-On, W.; Carter, S.; Reeve, V.E.; Dixon, K.M.; Gordon-Thomson, C.; Halliday, G.M.; Tuckey, R.C.; Mason, R.S. CYP11A1 in skin: An alternative route to photoprotection by vitamin D compounds. J. Steroid Biochem. Mol. Biol. 2015, 148, 72–78. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Tuckey, R.C.; Li, W.; Raman, C.; Panich, U.; Slominski, A.T. CYP11A1-derived vitamin D3 products protect against UVB-induced inflammation and promote keratinocytes differentiation. Free Radic. Biol. Med. 2020, 155, 87–98. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Jarrett, S.G.; D’Orazio, J.A.; Holick, M.F.; Tang, E.K.Y.; Tuckey, R.C.; Panich, U.; Li, W.; et al. Protective effects of novel derivatives of vitamin D3 and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms. Redox Biol. 2019, 24, 101206. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Schwartz, C.J.; Tuckey, R.C.; Tang, E.K.Y.; Raman, C.; Panich, U.; Slominski, A.T. Hydroxylumisterols, Photoproducts of Pre-Vitamin D3, Protect Human Keratinocytes against UVB-Induced Damage. Int. J. Mol. Sci. 2020, 21, 9374. [Google Scholar] [CrossRef]
- De Silva, W.G.M.; Han, J.Z.R.; Yang, C.; Tongkao-On, W.; McCarthy, B.Y.; Ince, F.A.; Holland, A.J.A.; Tuckey, R.C.; Slominski, A.T.; Abboud, M.; et al. Evidence for Involvement of Nonclassical Pathways in the Protection From UV-Induced DNA Damage by Vitamin D-Related Compounds. JBMR Plus 2021, 5, e10555. [Google Scholar] [CrossRef]
- Slominski, A.T.; Brożyna, A.A.; Skobowiat, C.; Zmijewski, M.A.; Kim, T.K.; Janjetovic, Z.; Oak, A.S.; Jozwicki, W.; Jetten, A.M.; Mason, R.S.; et al. On the role of classical and novel forms of vitamin D in melanoma progression and management. J. Steroid Biochem. Mol. Biol. 2018, 177, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Brożyna, A.A.; Hoffman, R.M.; Slominski, A.T. Relevance of Vitamin D in Melanoma Development, Progression and Therapy. Anticancer Res. 2020, 40, 473–489. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Janjetovic, Z.; Kim, T.K.; Wright, A.C.; Grese, L.N.; Riney, S.J.; Nguyen, M.N.; Tuckey, R.C. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012, 32, 3733–3742. [Google Scholar] [PubMed]
- Slominski, A.T.; Brożyna, A.A.; Zmijewski, M.A.; Janjetovic, Z.; Kim, T.K.; Slominski, R.M.; Tuckey, R.C.; Mason, R.S.; Jetten, A.M.; Guroji, P.; et al. The Role of Classical and Novel Forms of Vitamin D in the Pathogenesis and Progression of Nonmelanoma Skin Cancers. Adv. Exp. Med. Biol. 2020, 1268, 257–283. [Google Scholar] [PubMed]
- Slominski, A.T.; Brożyna, A.A.; Kim, T.K.; Elsayed, M.M.; Janjetovic, Z.; Qayyum, S.; Slominski, R.M.; Oak, A.S.W.; Li, C.; Podgorska, E.; et al. CYP11A1-derived vitamin D hydroxyderivatives as candidates for therapy of basal and squamous cell carcinomas. Int. J. Oncol. 2022, 61, 96. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.M.; Norman, A.W.; Sequeira, V.B.; Mohan, R.; Rybchyn, M.S.; Reeve, V.E.; Halliday, G.M.; Mason, R.S. 1α,25(OH)2-vitamin D and a nongenomic vitamin D analogue inhibit ultraviolet radiation-induced skin carcinogenesis. Cancer Prev. Res. 2011, 4, 1485–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. Neuroendocrine Aspects of Skin Aging. Int. J. Mol. Sci. 2019, 20, 2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Passeron, T.; Krutmann, J.; Andersen, M.L.; Katta, R.; Zouboulis, C.C. Clinical and biological impact of the exposome on the skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34 (Suppl. 4), 4–25. [Google Scholar] [CrossRef]
- Green, A.C.; Wallingford, S.C.; McBride, P. Childhood exposure to ultraviolet radiation and harmful skin effects: Epidemiological evidence. Prog. Biophys. Mol. Biol. 2011, 107, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Vierkötter, A.; Krutmann, J. Environmental influences on skin aging and ethnic-specific manifestations. Dermatoendocrinology 2012, 4, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Martic, I.; Jansen-Dürr, P.; Cavinato, M. Effects of Air Pollution on Cellular Senescence and Skin Aging. Cells 2022, 11, 2220. [Google Scholar] [CrossRef]
- Kennedy, C.; Bastiaens, M.T.; Bajdik, C.D.; Willemze, R.; Westendorp, R.G.; Bouwes Bavinck, J.N. Leiden Skin Cancer Study. Effect of smoking and sun on the aging skin. J. Investig. Dermatol. 2003, 120, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Wondrak, G.T.; Jacobson, M.K.; Jacobson, E.L. Endogenous UVA-photosensitizers: Mediators of skin photodamage and novel targets for skin photoprotection. Photochem Photobiol Sci 2006, 5, 215–237. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Ye, K.; Picard, M.; Gu, Z. Independent impacts of aging on mitochondrial DNA quantity and quality in humans. BMC Genom. 2017, 18, 890. [Google Scholar] [CrossRef] [Green Version]
- Papaccio, F.; D’Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef]
- Fuks, K.B.; Hüls, A.; Sugiri, D.; Altug, H.; Vierkötter, A.; Abramson, M.J.; Goebel, J.; Wagner, G.G.; Demuth, I.; Krutmann, J.; et al. Tropospheric ozone and skin aging: Results from two German cohort studies. Environ. Int. 2019, 124, 139–144. [Google Scholar] [CrossRef]
- Hüls, A.; Sugiri, D.; Fuks, K.; Krutmann, J.; Schikowski, T. Lentigine formation in caucasian women-interaction between particulate matter and solar UVR. J. Investig. Dermatol. 2018, 139, 974–976. [Google Scholar] [CrossRef] [Green Version]
- Peng, F.; Xue, C.H.; Hwang, S.K.; Li, W.H.; Chen, Z.; Zhang, J.Z. Exposure to fine particulate matter associated with senile lentigo in Chinese women: A cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Bernhard, D.; Moser, C.; Backovic, A.; Wick, G. Cigarette smoke—an aging accelerator? Exp. Gerontol. 2007, 42, 160–165. [Google Scholar] [CrossRef]
- Morita, A. Tobacco smoke causes premature skin aging. J. Dermatol. Sci. 2007, 48, 169–175. [Google Scholar] [CrossRef]
- Chaichalotornkul, S.; Nararatwanchai, T.; Narkpinit, S.; Dararat, P.; Kikuchi, K.; Maruyama, I.; Tancharoen, S. Secondhand smoke exposure-induced nucleocytoplasmic shuttling of HMGB1 in a rat premature skin aging model. Biochem. Biophys. Res. Commun. 2015, 456, 92–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, A.; Torii, K.; Maeda, A.; Yamaguchi, Y. Molecular basis of tobacco smoke-induced premature skin aging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, W.J.; Hanson, P.S.; Critchley, A.; Griffiths, B.; Chavan, B.; Birch-Machin, M.A. Exposing human primary dermal fibroblasts to particulate matter induces changes associated with skin aging. FASEB J. 2020, 34, 14725–14735. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Byun, E.J.; Lee, J.D.; Kim, S.; Kim, H.S. Air pollution, autophagy, and skin aging impact of particulate matter (pm10) on human dermal fibroblasts. Int. J. Mol. Sci. 2018, 19, E2727. [Google Scholar] [CrossRef] [Green Version]
- Moan, J.; Grigalavicius, M.; Baturaite, Z.; Dahlback, A.; Juzeniene, A. The relationship between UV exposure and incidence of skin cancer. Photodermatol. Photoimmunol. Photomed. 2015, 31, 26–35. [Google Scholar] [CrossRef]
- Lin, M.J.; Torbeck, R.L.; Dubin, D.P.; Lin, C.E.; Khorasani, H. Climate change and skin cancer. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e324–e325. [Google Scholar] [CrossRef]
- Umar, S.A.; Tasduq, S.A. Ozone Layer Depletion and Emerging Public Health Concerns—An Update on Epidemiological Perspective of the Ambivalent Effects of Ultraviolet Radiation Exposure. Front. Oncol. 2022, 12, 866733. [Google Scholar] [CrossRef]
- Balato, N.; Megna, M.; Ayala, F.; Balato, A.; Napolitano, M.; Patruno, C. Effects of climate changes on skin diseases. Expert Rev. Anti-Infect. Ther. 2014, 12, 171–181. [Google Scholar] [CrossRef]
- Green, A.C.; Olsen, C.M. Cutaneous squamous cell carcinoma: An epidemiological review. Br. J. Dermatol. 2017, 177, 373–381. [Google Scholar] [CrossRef]
- Datzmann, T.; Markevych, I.; Trautmann, F.; Heinrich, J.; Schmitt, J.; Tesch, F. Outdoor air pollution, green space, and cancer incidence in Saxony: A semi-individual cohort study. BMC Public Health 2018, 18, 715. [Google Scholar] [CrossRef]
- Siddens, L.K.; Larkin, A.; Krueger, S.K.; Bradfield, C.A.; Waters, K.M.; Tilton, S.C.; Pereira, C.B.; Löhr, C.V.; Arlt, V.M.; Phillips, D.H.; et al. Polycyclic aromatic hydrocarbons as skin carcinogens: Comparison of benzo[a]pyrene, dibenzo[def,p]chrysene and three environmental mixtures in the FVB/N mouse. Toxicol. Appl. Pharmacol. 2012, 264, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Hopf, N.B.; Spring, P.; Hirt-Burri, N.; Jimenez, S.; Sutter, B.; Vernez, D.; Berthet, A. Polycyclic aromatic hydrocarbons (PAHs) skin permeation rates change with simultaneous exposures to solar ultraviolet radiation (UV-S). Toxicol. Lett. 2018, 287, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Dusingize, J.C.; Olsen, C.M.; Pandeya, N.P.; Subramaniam, P.; Thompson, B.S.; Neale, R.E.; Green, A.C.; Whiteman, D.C. QSkin Study. Cigarette Smoking and the Risks of Basal Cell Carcinoma and Squamous Cell Carcinoma. J. Investig. Dermatol. 2017, 137, 1700–1708. [Google Scholar] [CrossRef]
- Bukowska, B.; Mokra, K.; Michałowicz, J. Benzo[a]pyrene-Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. Int. J. Mol. Sci. 2022, 23, 6348. [Google Scholar] [CrossRef]
- McNeill, J.M.; Wills, E.D. The formation of mutagenic derivatives of benzo[a]pyrene by peroxidising fatty acids. Chem. Biol. Interact. 1985, 53, 197–207. [Google Scholar] [CrossRef]
- Bukowska, B.; Duchnowicz, P. Molecular Mechanisms of Action of Selected Substances Involved in the Reduction of Benzo[a]pyrene-Induced Oxidative Stress. Molecules 2022, 27, 1379. [Google Scholar] [CrossRef]
- Vogeley, C.; Rolfes, K.M.; Krutmann, J.; Haarmann-Stemmann, T. The Aryl Hydrocarbon Receptor in the Pathogenesis of Environmentally-Induced Squamous Cell Carcinomas of the Skin. Front. Oncol. 2022, 12, 841721. [Google Scholar] [CrossRef]
- Andrysík, Z.; Vondráček, J.; Marvanová, S.; Ciganek, M.; Neča, J.; Pěnčíková, K.; Mahadevan, B.; Topinka, J.; Baird, W.M.; Kozubík, A.; et al. Activation of the aryl hydrocarbon receptor is the major toxic mode of action of an organic extract of a reference urban dust particulate matter mixture: The role of polycyclic aromatic hydrocarbons. Mutat. Res. 2011, 714, 53–62. [Google Scholar] [CrossRef]
- Costa, C.; Catania, S.; De Pasquale, R.; Stancanelli, R.; Scribano, G.M.; Melchini, A. Exposure of human skin to benzo[a]pyrene: Role of CYP1A1 and aryl hydrocarbon receptor in oxidative stress generation. Toxicology 2010, 271, 83–86. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Ide, F.; Kishi, R.; Akutagawa, T.; Sakai, S.; Nakamura, M.; Ishikawa, T.; Fujii-Kuriyama, Y.; Nakatsuru, Y. Aryl hydrocarbon receptor plays a significant role in mediating airborne particulate-induced carcinogenesis in mice. Environ. Sci. Technol. 2007, 41, 3775–3780. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Nakatsuru, Y.; Ichinose, M.; Takahashi, Y.; Kume, H.; Mimura, J.; Fujii-Kuriyama, Y.; Ishikawa, T. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 779–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modi, B.G.; Neustadter, J.; Binda, E.; Lewis, J.; Filler, R.B.; Roberts, S.J.; Kwong, B.Y.; Reddy, S.; Overton, J.D.; Galan, A.; et al. Langerhans cells facilitate epithelial DNA damage and squamous cell carcinoma. Science 2012, 335, 104–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuka, A.; Nomura, T.; Rerknimitr, P.; Seidel, J.A.; Honda, T.; Kabashima, K. The interplay between genetic and environmental factors in the pathogenesis of atopic dermatitis. Immunol. Rev. 2017, 278, 246–262. [Google Scholar] [CrossRef]
- Bocheva, G.S.; Slominski, R.M.; Slominski, A.T. Immunological Aspects of Skin Aging in Atopic Dermatitis. Int. J. Mol. Sci. 2021, 22, 5729. [Google Scholar] [CrossRef]
- Ständer, S. Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1136–1143. [Google Scholar] [CrossRef]
- Luger, T.; Amagai, M.; Dreno, B.; Dagnelie, M.A.; Liao, W.; Kabashima, K.; Schikowski, T.; Proksch, E.; Elias, P.M.; Simon, M.; et al. Atopic dermatitis: Role of the skin barrier, environment, microbiome, and therapeutic agents. J. Dermatol. Sci. 2021, 102, 142–157. [Google Scholar] [CrossRef]
- Kim, J.; Kim, E.H.; Oh, I.; Jung, K.; Han, Y.; Cheong, H.K.; Ahn, K. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J. Allergy Clin. Immunol. 2013, 132, 495–498.e1. [Google Scholar] [CrossRef]
- Fadadu, R.P.; Grimes, B.; Jewell, N.P.; Vargo, J.; Young, A.T.; Abuabara, K.; Balmes, J.R.; Wei, M.L. Association of Wildfire Air Pollution and Health Care Use for Atopic Dermatitis and Itch. JAMA Dermatol. 2021, 157, 658–666. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, J.S.; Seo, H.M. Exposure to air pollution and incidence of atopic dermatitis in the general population: A national population-based retrospective cohort study. J. Am. Acad. Dermatol. 2022, 87, 1321–1327. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lamichhane, D.K.; Lee, M.; Ye, S.; Kwon, J.H.; Park, M.S.; Kim, H.C.; Leem, J.H.; Hong, Y.C.; Kim, Y.; et al. Preventive Effect of Residential Green Space on Infantile Atopic Dermatitis Associated with Prenatal Air Pollution Exposure. Int. J. Environ. Res. Public Health 2018, 15, 102. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.; Huang, D.; Wang, W.; Yan, H.; Li, S.; Xiang, H. Associations of gestational and the first year of life exposure to ambient air pollution with childhood eczema in Hubei, China. Environ. Sci. Pollut Res Int. 2019, 26, 23842–23849. [Google Scholar] [CrossRef]
- Yao, T.C.; Huang, H.Y.; Pan, W.C.; Wu, C.Y.; Tsai, S.Y.; Hung, C.Y.; Lu, K.L.; Chang-Chien, J.; Tseng, C.L.; Wu, C.D.; et al. Association of prenatal exposure to fine particulate matter pollution with childhood eczema. Allergy 2021, 76, 2241–2245. [Google Scholar] [CrossRef]
- Kim, S.Y.; Sim, S.; Choi, H.G. Atopic dermatitis is associated with active and passive cigarette smoking in adolescents. PLoS ONE 2017, 12, e0187453. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Chuang, H.Y.; Hong, C.H.; Huang, S.K.; Chang, Y.C.; Ko, Y.C.; Yu, H.S. Lifetime exposure to cigarette smoking and the development of adult-onset atopic dermatitis. Br. J. Dermatol. 2011, 164, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Kantor, R.; Kim, A.; Thyssen, J.P.; Silverberg, J.I. Association of atopic dermatitis with smoking: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2016, 75, 1119–1125.e1. [Google Scholar] [CrossRef] [Green Version]
- Green, M.; Kashetsky, N.; Feschuk, A.; Maibach, H.I. Transepidermal water loss (TEWL): Environment and pollution-A systematic review. Skin Health Dis. 2022, 2, e104. [Google Scholar] [CrossRef]
- Shamsipour, M.; Nasrollahi, S.A.; Hassanvand, M.S.; Yazdanparast, T.; Samadi, A.; Yunesian, M.; Mahdavi, M.; Kassir, M.; Firooz, A. Short-term effects of exposure to air pollution on biophysical parameters of skin in a panel of healthy adults. Dermatol. Ther. 2020, 33, e14536. [Google Scholar] [CrossRef]
- Leung, M.H.Y.; Tong, X.; Shen, Z.; Du, S.; Bastien, P.; Appenzeller, B.M.R.; Betts, R.J.; Mezzache, S.; Bourokba, N.; Cavusoglu, N.; et al. Skin microbiome differentiates into distinct cutotypes with unique metabolic functions upon exposure to polycyclic aromatic hydrocarbons. Microbiome 2023, 11, 124. [Google Scholar] [CrossRef]
- Lopez, D.J.; Lodge, C.J.; Bui, D.S.; Waidyatillake, N.T.; Su, J.C.; Perret, J.L.; Knibbs, L.D.; Erbas, B.; Thomas, P.S.; Hamilton, G.S.; et al. Association between ambient air pollution and development and persistence of atopic and non-atopic eczema in a cohort of adults. Allergy 2021, 76, 2524–2534. [Google Scholar] [CrossRef]
- Celebi Sozener, Z.; Özbey Yücel, Ü.; Altiner, S.; Ozdel Oztürk, B.; Cerci, P.; Türk, M.; Gorgülü Akin, B.; Akdis, M.; Yilmaz, I.; Ozdemir, C.; et al. The External Exposome and Allergies: From the Perspective of the Epithelial Barrier Hypothesis. Front. Allergy 2022, 3, 887672. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.P.; Li, Z.; Choi, E.K.; Lee, S.; Kim, Y.K.; Seo, E.Y.; Chung, J.H.; Cho, S. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J. Dermatol. Sci. 2018, 91, 175–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Xu, Z.; Jiang, F.; Li, S.; Liu, S.; Wu, M.; Yan, C.; Tan, J.; Yu, G.; Hu, Y.; et al. Relative impact of meteorological factors and air pollutants on childhood allergic diseases in Shanghai, China. Sci. Total. Environ. 2020, 706, 135975. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Xiong, X.; Liang, F.; Tian, L.; Liu, W.; Wang, Z.; Pan, X. The interactive effects between air pollution and meteorological factors on the hospital outpatient visits for atopic dermatitis in Beijing, China: A time-series analysis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2362–2370. [Google Scholar] [CrossRef] [PubMed]
- Isler, M.F.; Coates, S.J.; Boos, M.D. Climate change, the cutaneous microbiome and skin disease: Implications for a warming world. Int. J. Dermatol. 2023, 62, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Iida, K.; Imaji, M.; Nakahara, T. Microbiome analysis of forehead skin in patients with atopic dermatitis and healthy subjects: Implication of Staphylococcus and Corynebacterium. J. Dermatol. 2018, 45, 876–877. [Google Scholar] [CrossRef]
- Fadadu, R.P.; Abuabara, K.; Balmes, J.R.; Hanifin, J.M.; Wei, M.L. Air Pollution and Atopic Dermatitis, from Molecular Mechanisms to Population-Level Evidence: A Review. Int. J. Environ. Res. Public Health 2023, 20, 2526. [Google Scholar] [CrossRef]
- Pan, Z.; Dai, Y.; Akar-Ghibril, N.; Simpson, J.; Ren, H.; Zhang, L.; Hou, Y.; Wen, X.; Chang, C.; Tang, R.; et al. Impact of Air Pollution on Atopic Dermatitis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2023. [Google Scholar] [CrossRef]
- Woo, Y.R.; Park, S.Y.; Choi, K.; Hong, E.S.; Kim, S.; Kim, H.S. Air Pollution and Atopic Dermatitis (AD): The Impact of Particulate Matter (PM10) on an AD Mouse-Model. Int. J. Mol. Sci. 2020, 21, 6079. [Google Scholar] [CrossRef]
- Kim, H.O.; Kim, J.H.; Chung, B.Y.; Choi, M.G.; Park, C.W. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp. Dermatol. 2014, 23, 278–281. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Liu, P.; Mu, Z.L.; Zhang, J.Z. Aryl hydrocarbon receptor expression in serum, peripheral blood mononuclear cells, and skin lesions of patients with atopic dermatitis and its correlation with disease severity. Chin. Med. J. 2020, 133, 148–153. [Google Scholar] [CrossRef]
- Hong, C.H.; Lee, C.H.; Yu, H.S.; Huang, S.K. Benzopyrene, a major polyaromatic hydrocarbon in smoke fume, mobilizes Langerhans cells and polarizes Th2/17 responses in epicutaneous protein sensitization through the aryl hydrocarbon receptor. Int. Immunopharmacol. 2016, 36, 111–117. [Google Scholar] [CrossRef]
- Hidaka, T.; Ogawa, E.; Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Fujimura, T.; Aiba, S.; Nakayama, K.; Okuyama, R.; et al. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat. Immunol. 2017, 18, 64–73. [Google Scholar] [CrossRef]
- Grän, F.; Kerstan, A.; Serfling, E.; Goebeler, M.; Muhammad, K. Current Developments in the Immunology of Psoriasis. Yale J. Biol. Med. 2020, 93, 97–110. [Google Scholar]
- Bellinato, F.; Adami, G.; Vaienti, S.; Benini, C.; Gatti, D.; Idolazzi, L.; Fassio, A.; Rossini, M.; Girolomoni, G.; Gisondi, P. Association Between Short-term Exposure to Environmental Air Pollution and Psoriasis Flare. JAMA Dermatol. 2022, 158, 375–381. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Yang, R.; Yu, H.; Shang, S.; Hu, Y. Short-term exposure to ambient fine particulate matter and psoriasis: A time-series analysis in Beijing, China. Front. Public Health 2022, 10, 1015197. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Harskamp, C.T.; Dhillon, J.S.; Armstrong, E.J. Psoriasis and smoking: A systematic review and meta-analysis. Br. J. Dermatol. 2014, 170, 304–314. [Google Scholar] [CrossRef]
- van Voorhis, M.; Knopp, S.; Julliard, W.; Fechner, J.H.; Zhang, X.; Schauer, J.J.; Mezrich, J.D. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PLoS ONE 2013, 8, e82545. [Google Scholar] [CrossRef] [Green Version]
- Afaq, F.; Zaid, M.A.; Pelle, E.; Khan, N.; Syed, D.N.; Matsui, M.S.; Maes, D.; Mukhtar, H. Aryl hydrocarbon receptor is an ozone sensor in human skin. J. Investig. Dermatol. 2009, 129, 2396–2403. [Google Scholar] [CrossRef] [Green Version]
- Guillon, C.; Meziani, M.; Abdelli, S.; Sigaudo Roussel, D.; Bonod, C.; Nosbaum, A. The aryl hydrocarbon receptor pathway plays a central role in the cutaneous response to pollutants. Eur. J. Dermatol. 2022, 32, 305–311. [Google Scholar]
- Kim, H.R.; Kang, S.Y.; Kim, H.O.; Park, C.W.; Chung, B.Y. Role of Aryl Hydrocarbon Receptor Activation and Autophagy in Psoriasis-Related Inflammation. Int. J. Mol. Sci. 2020, 21, 2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dréno, B.; Bettoli, V.; Araviiskaia, E.; Sanchez Viera, M.; Bouloc, A. The influence of exposome on acne. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 812–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolkenstein, P.; Machovcová, A.; Szepietowski, J.C.; Tennstedt, D.; Veraldi, S.; Delarue, A. Acne prevalence and associations with lifestyle: A cross-sectional online survey of adolescents/young adults in 7 European countries. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, N. Acne vulgaris: New evidence in pathogenesis and future modalities of treatment. J. Dermatolog. Treat. 2021, 32, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Moyal, D.; Liu, W.; Kandahari, S.; Lee, G.S.; Nopadon, N.; Xiang, L.F.; Seité, S. Pollution and acne: Is there a link? Clin. Cosmet. Investig. Dermatol. 2017, 10, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Pan, X.; Vierkötter, A.; Guo, Q.; Wang, X.; Wang, Q.; Seité, S.; Moyal, D.; Schikowski, T.; Krutmann, J. A Time-Series Study of the Effect of Air Pollution on Outpatient Visits for Acne Vulgaris in Beijing. Skin Pharmacol. Physiol. 2018, 31, 107–113. [Google Scholar] [CrossRef]
- Li, X.; Cao, Y.; An, S.J.; Xiang, Y.; Huang, H.X.; Xu, B.; Zhang, Y.; Li, Y.F.; Lu, Y.G.; Cai, T.J. The association between short-term ambient air pollution and acne vulgaris outpatient visits: A hospital-based time-series analysis in Xi’an. Environ. Sci. Pollut. Res. Int. 2022, 29, 14624–14633. [Google Scholar] [CrossRef]
- Mourelatos, K.; Eady, E.A.; Cunliffe, W.J.; Clark, S.M.; Cove, J.H. Temporal changes in sebum excretion and propionibacterial colonization in preadolescent children with and without acne. Br. J. Dermatol. 2007, 156, 22–31. [Google Scholar] [CrossRef]
- Capitanio, B.; Lora, V.; Ludovici, M.; Sinagra, J.L.; Ottaviani, M.; Mastrofrancesco, A.; Ardigò, M.; Camera, E. Modulation of sebum oxidation and interleukin-1α levels associates with clinical improvement of mild comedonal acne. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1792–1797. [Google Scholar] [CrossRef]
- Zouboulis, C.C. Acne and sebaceous gland function. Clin. Dermatol. 2004, 22, 360–366. [Google Scholar] [CrossRef]
- Lefebvre, M.A.; Pham, D.M.; Boussouira, B.; Qiu, H.; Ye, C.; Long, X.; Chen, R.; Gu, W.; Laurent, A.; Nguyen, Q.L. Consequences of urban pollution upon skin status. A controlled study in Shanghai area. Int. J. Cosmet. Sci. 2016, 38, 217–223. [Google Scholar] [CrossRef]
- El Haddad, C.; Gerbaka, N.E.; Hallit, S.; Tabet, C. Association between exposure to ambient air pollution and occurrence of inflammatory acne in the adult population. BMC Public Health 2021, 21, 1664. [Google Scholar] [CrossRef]
- Abd El All, H.S.; Shoukry, N.S.; El Maged, R.A.; Ayada, M.M. Immunohistochemical expression of interleukin 8 in skin biopsies from patients with inflammatory acne vulgaris. Diagn. Pathol. 2007, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, J.; Watterson, S.; Layton, A.M.; Bjourson, A.J.; Barnard, E.; McDowell, A. Propionibacterium acnes and Acne Vulgaris: New Insights from the Integration of Population Genetic, Multi-Omic, Biochemical and Host-Microbe Studies. Microorganisms 2019, 7, 128. [Google Scholar] [CrossRef] [Green Version]
- Sorg, O.; Zennegg, M.; Schmid, P.; Fedosyuk, R.; Valikhnovskyi, R.; Gaide, O.; Kniazevych, V.; Saurat, J.H. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) poisoning in Victor Yushchenko: Identification and measurement of TCDD metabolites. Lancet 2009, 374, 1179–1185. [Google Scholar] [CrossRef]
- Furue, M.; Tsuji, G. Chloracne and Hyperpigmentation Caused by Exposure to Hazardous Aryl Hydrocarbon Receptor Ligands. Int. J. Environ. Res. Public Health 2019, 16, 4864. [Google Scholar] [CrossRef] [Green Version]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef]
- Eller, M.S.; Gilchrest, B.A. Tanning as part of the eukaryotic SOS response. Pigment Cell Res. 2000, 13 (Suppl. S8), 94–97. [Google Scholar] [CrossRef]
- Roberts, W.E. Pollution as a risk factor for the development of melasma and other skin disorders of facial hyperpigmentation—Is there a case to be made? J. Drugs Dermatol. 2015, 14, 337–341. [Google Scholar]
- Shi, Y.; Zeng, Z.; Liu, J.; Pi, Z.; Zou, P.; Deng, Q.; Ma, X.; Qiao, F.; Xiong, W.; Zhou, C.; et al. Particulate matter promotes hyperpigmentation via AhR/MAPK signaling activation and by increasing α-MSH paracrine levels in keratinocytes. Environ. Pollut. 2021, 278, 116850. [Google Scholar] [CrossRef]
- Rajput, R. Understanding Hair Loss due to Air Pollution and the Approach to Management. Hair Transpl. 2015, 5, 133. [Google Scholar]
- Jun, M.S.; Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Particulate Matters Induce Apoptosis in Human Hair Follicular Keratinocytes. Ann. Dermatol. 2020, 32, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.S.; Ibrahim, H.S.; Abdelaziz, H.H.; Elsaie, M.L. Implications of cigarette smoking on early-onset androgenetic alopecia: A cross-sectional Study. J. Cosmet. Dermatol. 2021, 20, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- D’Agostini, F.; Balansky, R.; Pesce, C.; Fiallo, P.; Lubet, R.A.; Kelloff, G.J.; De Flora, S. Induction of alopecia in mice exposed to cigarette smoke. Toxicol. Lett. 2000, 114, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Mistry, N. Guidelines for Formulating Anti-Pollution Products. Cosmetics 2017, 4, 57. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Oh, S.J.; Park, S.; Park, J.H.; Lee, J.H. Anti-pollution skincare: Research on effective ways to protect skin from particulate matter. Dermatol. Ther. 2021, 34, e14960. [Google Scholar] [CrossRef]
- Peres, D.D.; Sarruf, F.D.; de Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic acid photoprotective properties in association with UV filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B 2018, 185, 46–49. [Google Scholar] [CrossRef]
- Krutmann, J.; Schalka, S.; Watson, R.E.B.; Wei, L.; Morita, A. Daily photoprotection to prevent photoaging. Photodermatol. Photoimmunol. Photomed. 2021, 37, 482–489. [Google Scholar] [CrossRef]
- Farris, P.K.; Valacchi, G. Ultraviolet Light Protection: Is It Really Enough? Antioxidants 2022, 11, 1484. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Krutmann, J. Effective photoprotection of human skin against infrared A radiation by topically applied antioxidants: Results from a vehicle controlled, double-blind, randomized study. Photochem. Photobiol. 2015, 91, 248–250. [Google Scholar] [CrossRef]
- Diao, P.; He, H.; Tang, J.; Xiong, L.; Li, L. Natural compounds protect the skin from airborne particulate matter by attenuating oxidative stress. Biomed. Pharmacother. 2021, 138, 111534. [Google Scholar] [CrossRef]
- Tran, H.M.; Yang, C.Y.; Wu, T.H.; Yen, F.L. Liposomes Encapsulating Morin: Investigation of Physicochemical Properties, Dermal Absorption Improvement and Anti-Aging Activity in PM-Induced Keratinocytes. Antioxidants 2022, 11, 1183. [Google Scholar] [CrossRef]
- McDaniel, D.H.; Waugh, J.M.; Jiang, L.I.; Stephens, T.J.; Yaroshinsky, A.; Mazur, C.; Wortzman, M.; Nelson, D.B. Evaluation of the Antioxidant Capacity and Protective Effects of a Comprehensive Topical Antioxidant Containing Water-soluble, Enzymatic, and Lipid-soluble Antioxidants. J. Clin. Aesthet. Dermatol. 2019, 12, 46–53. [Google Scholar]
- Pecorelli, A.; McDaniel, D.H.; Wortzman, M.; Nelson, D.B. Protective effects of a comprehensive topical antioxidant against ozone-induced damage in a reconstructed human skin model. Arch. Dermatol. Res. 2021, 313, 139–146. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.K.; Böhm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczyński, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef]
- Kleszczyński, K.; Bilska, B.; Stegemann, A.; Flis, D.J.; Ziolkowski, W.; Pyza, E.; Luger, T.A.; Reiter, R.J.; Böhm, M.; Slominski, A.T. Melatonin and its metabolites ameliorate UVR-induced mitochondrial oxidative stress in human MNT-1 melanoma cells. Int. J. Mol. Sci. 2018, 19, 3786. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Pisarchik, A.; Semak, I.; Sweatman, T.; Wortsman, J.; Szczesniewski, A.; Slugocki, G.; McNulty, J.; Kauser, S.; Tobin, D.J.; et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002, 16, 896–898. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Semak, I.; Fischer, T.W.; Kim, T.K.; Kleszczyński, K.; Hardeland, R.; Reiter, R.J. Metabolism of melatonin in the skin: Why is it important? Exp. Dermatol. 2017, 26, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal. Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin, Its Metabolites and Their Interference with Reactive Nitrogen Compounds. Molecules 2021, 26, 4105. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.W.; Slominski, A.; Zmijewski, M.A.; Reiter, R.J.; Paus, R. Melatonin as a major skin protectant: From free radical scavenging to DNA damage repair. Exp. Dermatol. 2008, 17, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Kilańczyk, E.; Bryszewska, M. The effect of melatonin on antioxidant enzymes in human diabetic skin fibroblasts. Cell. Mol. Biol. Lett. 2003, 8, 333–336. [Google Scholar]
- Fischer, T.W.; Kleszczyński, K.; Hardkop, L.H.; Kruse, N.; Zillikens, D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J. Pineal Res. 2013, 54, 303–312. [Google Scholar] [CrossRef]
- Bielach-Bazyluk, A.; Zbroch, E.; Mysliwiec, H.; Rydzewska-Rosolowska, A.; Kakareko, K.; Flisiak, I.; Hryszko, T. Sirtuin 1 and skin: Implications in intrinsic and extrinsic aging-a systematic review. Cells 2021, 10, 813. [Google Scholar] [CrossRef]
- Granger, C.; Brown, A.; Aladren, S.; Narda, M. Night Cream Containing Melatonin, Carnosine and Helichrysum italicum Extract Helps Reduce Skin Reactivity and Signs of Photodamage: Ex Vivo and Clinical Studies. Dermatol. Ther. 2020, 10, 1315–1329. [Google Scholar] [CrossRef]
- Sagan, D.; Stepniak, J.; Gesing, A.; Lewinski, A.; Karbownik-Lewinska, M. Melatonin reverses the enhanced oxidative damage to membrane lipids and improves skin biophysical characteristics in former-smokers—A study in postmenopausal women. Ann. Agric. Environ. Med. 2017, 24, 659–666. [Google Scholar] [CrossRef]
- Furue, M.; Uchi, H.; Mitoma, C.; Hashimoto-Hachiya, A.; Chiba, T.; Ito, T.; Nakahara, T.; Tsuji, G. Antioxidants for Healthy Skin: The Emerging Role of Aryl Hydrocarbon Receptors and Nuclear Factor-Erythroid 2-Related Factor-2. Nutrients 2017, 9, 223. [Google Scholar] [CrossRef]
- Uchi, H.; Yasumatsu, M.; Morino-Koga, S.; Mitoma, C.; Furue, M. Inhibition of aryl hydrocarbon receptor signaling and induction of NRF2-mediated antioxidant activity by cinnamaldehyde in human keratinocytes. J. Dermatol. Sci. 2017, 85, 36–43. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Nrf2—A regulator of keratinocyte redox signaling. Free Radic. Biol. Med. 2015, 88, 243–252. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Arena, A.; Romeo, M.A.; Benedetti, R.; Gilardini Montani, M.S.; Santarelli, R.; Gonnella, R.; D’Orazi, G.; Cirone, M. NRF2 and STAT3: Friends or foes in carcinogenesis? Discov. Oncol. 2023, 14, 37. [Google Scholar] [CrossRef]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef]
- Kim, H.B.; Um, J.Y.; Chung, B.Y.; Kim, J.C.; Kang, S.Y.; Park, C.W.; Kim, H.O. Aryl Hydrocarbon Receptors: Evidence of Therapeutic Targets in Chronic Inflammatory Skin Diseases. Biomedicines 2022, 10, 1087. [Google Scholar] [CrossRef]
- Hwang, J.; Newton, E.M.; Hsiao, J.; Shi, V.Y. Aryl hydrocarbon receptor/nuclear factor E2-related factor 2 (AHR/NRF2) signalling: A novel therapeutic target for atopic dermatitis. Exp. Dermatol. 2022, 31, 485–497. [Google Scholar] [CrossRef]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Aryl Hydrocarbon Receptor in Atopic Dermatitis and Psoriasis. Int. J. Mol. Sci. 2019, 20, 5424. [Google Scholar] [CrossRef] [Green Version]
- Paller, A.S.; Stein Gold, L.; Soung, J.; Tallman, A.M.; Rubenstein, D.S.; Gooderham, M. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J. Am. Acad. Dermatol. 2021, 84, 632–638. [Google Scholar] [CrossRef]
- Peppers, J.; Paller, A.S.; Maeda-Chubachi, T.; Wu, S.; Robbins, K.; Gallagher, K.; Kraus, J.E. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2019, 80, 89–98. [Google Scholar] [CrossRef]
- Bissonnette, R.; Stein Gold, L.; Rubenstein, D.S.; Tallman, A.M.; Armstrong, A. Tapinarof in the treatment of psoriasis: A review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J. Am. Acad. Dermatol. 2021, 84, 1059–1067. [Google Scholar] [CrossRef]
- Smith, S.H.; Jayawickreme, C.; Rickard, D.J.; Nicodeme, E.; Bui, T.; Simmons, C.; Coquery, C.M.; Neil, J.; Pryor, W.M.; Mayhew, D.; et al. Tapinarof Is a Natural AhR Agonist that Resolves Skin Inflammation in Mice and Humans. J. Investig. Dermatol. 2017, 137, 2110–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furue, M.; Nakahara, T. Revival of AHR Agonist for the Treatment of Atopic Dermatitis: Tapinarof. Curr. Treat. Options Allergy 2020, 7, 414–421. [Google Scholar] [CrossRef]
- Vu, Y.H.; Hashimoto-Hachiya, A.; Takemura, M.; Yumine, A.; Mitamura, Y.; Nakahara, T.; Furue, M.; Tsuji, G. IL-24 Negatively Regulates Keratinocyte Differentiation Induced by Tapinarof, an Aryl Hydrocarbon Receptor Modulator: Implication in the Treatment of Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 9412. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.H.; Choi, D.; Chun, Y.J.; Noh, M. Keratinocyte-derived IL-24 plays a role in the positive feedback regulation of epidermal inflammation in response to environmental and endogenous toxic stressors. Toxicol. Appl. Pharmacol. 2014, 280, 199–206. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocheva, G.; Slominski, R.M.; Slominski, A.T. Environmental Air Pollutants Affecting Skin Functions with Systemic Implications. Int. J. Mol. Sci. 2023, 24, 10502. https://doi.org/10.3390/ijms241310502
Bocheva G, Slominski RM, Slominski AT. Environmental Air Pollutants Affecting Skin Functions with Systemic Implications. International Journal of Molecular Sciences. 2023; 24(13):10502. https://doi.org/10.3390/ijms241310502
Chicago/Turabian StyleBocheva, Georgeta, Radomir M. Slominski, and Andrzej T. Slominski. 2023. "Environmental Air Pollutants Affecting Skin Functions with Systemic Implications" International Journal of Molecular Sciences 24, no. 13: 10502. https://doi.org/10.3390/ijms241310502
APA StyleBocheva, G., Slominski, R. M., & Slominski, A. T. (2023). Environmental Air Pollutants Affecting Skin Functions with Systemic Implications. International Journal of Molecular Sciences, 24(13), 10502. https://doi.org/10.3390/ijms241310502





