ABCG2 Gene and ABCG2 Protein Expression in Colorectal Cancer—In Silico and Wet Analysis
Abstract
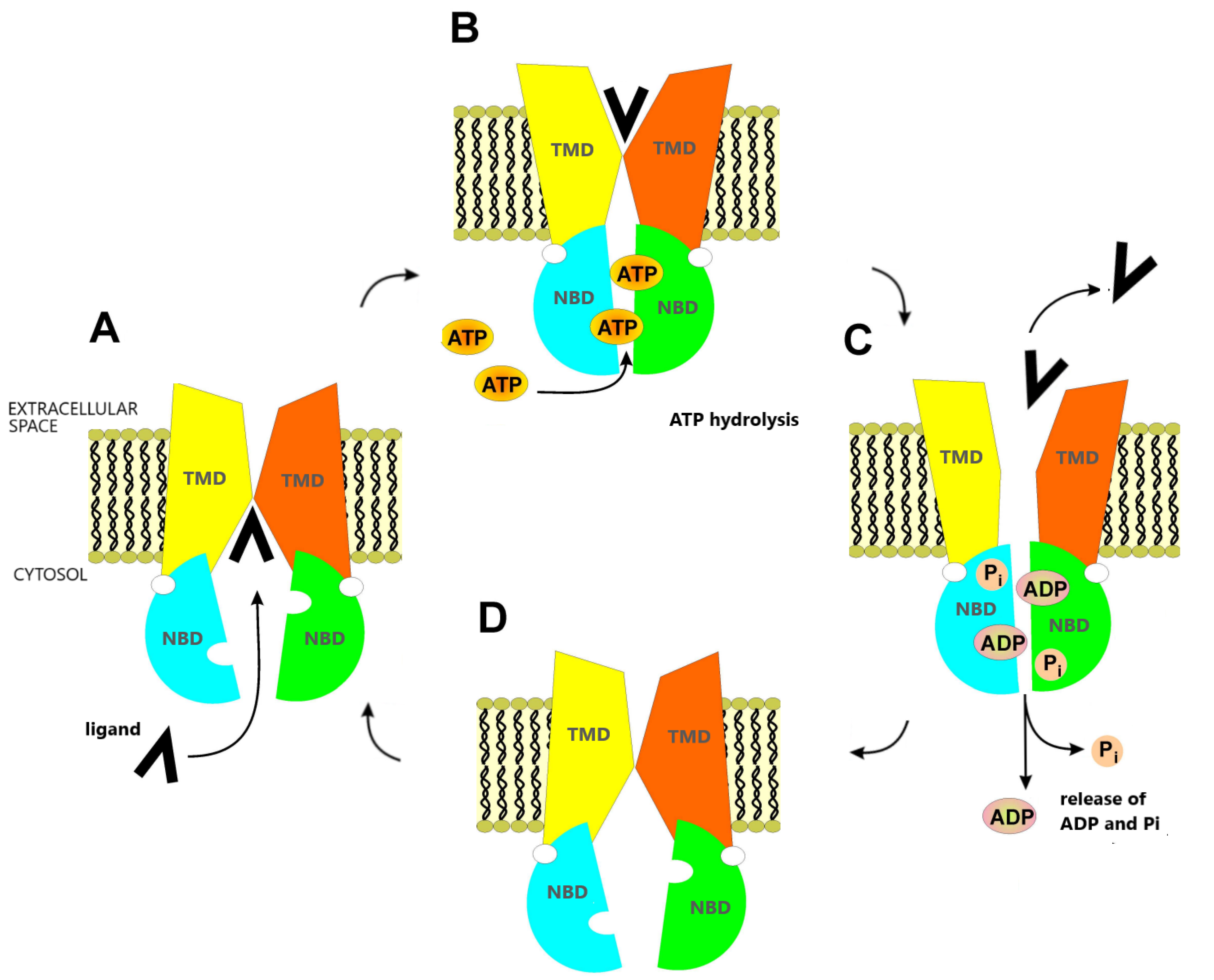
1. Introduction
2. Results
2.1. In Silico Analysis of OMICS Data Regarding ABCG2 Gene and ABCG2 Protein Expression in Colorectal Cancer
2.1.1. A Decrease in ABCG2 mRNA Expression Level Is Common in Multiple Carcinomas
2.1.2. ABCG2 Is Underexpressed in Colorectal Cancer in Comparison to Both Adjacent and Unpaired Normal Colorectal Tissue
2.1.3. ABCG2 Expression Is Higher in Metastatic Tissues Than in Primary Tumours of Colon Cancer
2.1.4. ABCG2 Protein Could Be Detected in Colon and Rectum Normal Tissue but Not in Colorectal Cancer
2.1.5. ABCG2 Gene Expression Level in Colorectal Cancer Could Be Connected with Methylation Status but Not with DNA Alterations of the ABCG2 Gene
2.1.6. ABCG2 Gene Expression Level in Colorectal Cancer Could Be Connected with Some Clinical Features
2.1.7. Neither ABCG2 Gene nor ABCG2 Protein Expression Level Is Related to a Prognosis in Colorectal Cancer
2.1.8. ABCG2 Protein Interacts with Proteins Involved in, e.g., Leukotriene Transport, Endodermal Cell Fate Specification, and Histon Methylation and Ubiquitination
2.2. ABCG2 Gene and ABCG2 Protein Expression in Colorectal Cancer Samples—Wet Validation of the In Silico Analysis Results
2.2.1. ABCG2 Protein Could Be Detected in the Cytoplasm and Membrane of Colorectal Cancer Cells
2.2.2. ABCG2 Gene Is Underexpressed in Nearly Two-Thirds of Colorectal Cancer Cases
2.2.3. No Association Was Found between ABCG2 Gene Expression and ABCG2 Protein Levels in Colorectal Cancer Samples
2.2.4. Neither ABCG2 Gene Expression Level nor ABCG2 Protein Level Is Connected with Selected Clinicopathological Factors in Colorectal Cancer Samples
2.2.5. Neither ABCG2 Gene Expression Level nor ABCG2 Protein Level Is Connected with the Overall Survival Probability of Colorectal Cancer Samples
3. Discussion
4. Materials and Methods
4.1. In Silico Analysis
4.1.1. ABCG2 Gene Expression and ABCG2 Protein Level Analysis
Oncomine
TIMER2.0
TMNplot
Human Protein Atlas
4.1.2. Analysis of DNA Alteration and Methylation of the ABCG2 Gene
cBioPortal
UALCAN
MEXPRESS
4.1.3. Analysis of Connection of ABCG2 and Clinicopathological Features
MEXPRESS
UALCAN
4.1.4. Prognosis and Survival Analysis
GEPIA2
Human Protein Atlas
PrognoScan
4.1.5. Protein–Protein Interaction Analysis
STRING
4.2. ABCG2 Gene Expression and ABCG2 Protein Analysis in Colorectal Cancer Samples
4.2.1. Patients and Tissue Samples
4.2.2. ABCG2 Protein Level Analysis by Immunohistochemistry
4.2.3. RNA Isolation and cDNA Synthesis
4.2.4. Real-Time PCR Reaction
4.2.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Global Cancer Observatory; International Agency for Research on Cancer; World Health Organization. Colorectal Cancer-Fact Sheet. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (accessed on 1 May 2023).
- The Global Cancer Observatory; International Agency for Research on Cancer; World Health Organization. Cancer Tomorrow. 2020. Available online: https://gco.iarc.fr/tomorrow/en (accessed on 1 May 2023).
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [PubMed]
- Nielsen, D.L.; Palshof, J.A.; Brünner, N.; Stenvang, J.; Viuff, B.M. Implications of ABCG2 Expression on Irinotecan Treatment of Colorectal Cancer Patients: A Review. Int. J. Mol. Sci. 2017, 18, 1926. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.J.; Hollenstein, K.; Locher, K.P. Uptake or extrusion: Crystal structures of full ABC transporters suggest a common mechanism. Mol. Microbiol. 2007, 65, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.F.; Linton, K.J. The ATP switch model for ABC transporters. Nat. Struct. Mol. Biol. 2004, 11, 918–926. [Google Scholar]
- Linton, K.J. Structure and function of ABC transporters. Physiology 2007, 22, 122–130. [Google Scholar]
- Hollenstein, K.; Dawson, R.J.; Locher, K.P. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 2007, 17, 412–418. [Google Scholar] [CrossRef]
- Dietrich, C.G.; Vehr, A.K.; Martin, I.V.; Gassler, N.; Rath, T.; Roeb, E.; Schmitt, J.; Trautwein, C.; Geier, A. Downregulation of breast cancer resistance protein in colon adenomas reduces cellular xenobiotic resistance and leads to accumulation of a food-derived carcinogen. Int. J. Cancer 2011, 129, 546–552. [Google Scholar]
- Alvarez, A.I.; Real, R.; Pérez, M.; Mendoza, G.; Prieto, J.G.; Merino, G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J. Pharm. Sci. 2010, 99, 598–617. [Google Scholar]
- Uno, S.; Uraki, M.; Ito, A.; Shinozaki, Y.; Yamada, A.; Kawase, A.; Iwaki, M. Changes in mRNA expression of ABC and SLC transporters in liver and intestines of the adjuvant-induced arthritis rat. Biopharm. Drug Dispos. 2009, 30, 49–54. [Google Scholar]
- Englund, G.; Jacobson, A.; Rorsman, F.; Artursson, P.; Kindmark, A.; Rönnblom, A. Efflux transporters in ulcerative colitis: Decreased expression of BCRP (ABCG2) and Pgp (ABCB1). Inflamm. Bowel Dis. 2007, 13, 291–297. [Google Scholar]
- Mosaffa, F.; Kalalinia, F.; Lage, H.; Afshari, J.T.; Behravan, J. Pro-inflammatory cytokines interleukin-1 beta, interleukin 6, and tumor necrosis factor-alpha alter the expression and function of ABCG2 in cervix and gastric cancer cells. Mol. Cell. Biochem. 2012, 363, 385–393. [Google Scholar] [CrossRef]
- Deuring, J.J.; de Haar, C.; Koelewijn, C.L.; Kuipers, E.J.; Peppelenbosch, M.P.; van der Woude, C.J. Absence of ABCG2-mediated mucosal detoxification in patients with active inflammatory bowel disease is due to impeded protein folding. Biochem. J. 2012, 441, 87–93. [Google Scholar]
- Albermann, N.; Schmitz-Winnenthal, F.H.; Z’graggen, K.; Volk, C.; Hoffmann, M.M.; Haefeli, W.E.; Weiss, J. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem. Pharmacol. 2005, 70, 949–958. [Google Scholar]
- Sarkadi, B.; Homolya, L.; Hegedűs, T. The ABCG2/BCRP transporter and its variants-from structure to pathology. FEBS Lett. 2020, 594, 4012–4034. [Google Scholar]
- To, K.K.; Zhan, Z.; Litman, T.; Bates, S.E. Regulation of ABCG2 expression at the 3′ untranslated region of its mRNA through modulation of transcript stability and protein translation by a putative microRNA in the S1 colon cancer cell line. Mol. Cell. Biol. 2008, 28, 5147–5161. [Google Scholar] [CrossRef]
- Campa, D.; Pardini, B.; Naccarati, A.; Vodickova, L.; Novotny, J.; Försti, A.; Hemminki, K.; Barale, R.; Vodicka, P.; Canzian, F. A gene-wide investigation on polymorphisms in the ABCG2/BRCP transporter and susceptibility to colorectal cancer. Mutat. Res. 2008, 645, 56–60. [Google Scholar]
- Kopp, T.I.; Andersen, V.; Tjonneland, A.; Vogel, U. Polymorphisms in ATP-binding cassette transporter genes and interaction with diet and life style factors in relation to colorectal cancer in a Danish prospective case-cohort study. Scand. J. Gastroenterol. 2015, 50, 1469–1481. [Google Scholar] [CrossRef]
- Andersen, V.; Ostergaard, M.; Christensen, J.; Overvad, K.; Tjonneland, A. Polymorphisms in the xenobiotic transporter Multidrug Resistance 1 (MDR1) gene and interaction with meat intake in relation to risk of colorectal cancer in a Danish prospective case-cohort study. BMC Cancer 2009, 9, 407. [Google Scholar]
- Haraguchi, N.; Utsunomiya, T.; Inoue, H.; Tanaka, F.; Mimori, K.; Barnard, G.F.; Mori, M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells 2006, 24, 506–513. [Google Scholar]
- Xie, Z.Y.; Lv, K.; Xiong, Y.; Guo, W.H. ABCG2-meditated multidrug resistance and tumor-initiating capacity of side population cells from colon cancer. Oncol. Res. Treat. 2014, 37, 666–668, 670–672. [Google Scholar]
- Ma, L.; Li, T.; Jin, Y.; Wei, J.; Yang, Y.; Zhang, H. ABCG2 is required for self-renewal and chemoresistance of CD133-positive human colorectal cancer cells. Tumour. Biol. 2016, 37, 12889–12896. [Google Scholar] [PubMed]
- Hu, J.; Li, J.; Yue, X.; Wang, J.; Liu, J.; Sun, L.; Kong, D. Expression of the cancer stem cell markers ABCG2 and OCT-4 in right-sided colon cancer predicts recurrence and poor outcomes. Oncotarget 2017, 8, 28463–28470. [Google Scholar] [PubMed]
- Krishnamurthy, P.; Ross, D.D.; Nakanishi, T.; Bailey-Dell, K.; Zhou, S.; Mercer, K.E.; Sarkadi, B.; Sorrentino, B.P.; Schuetz, J.D. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J. Biol. Chem. 2004, 279, 24218–24225. [Google Scholar] [PubMed]
- Gupta, N.; Martin, P.M.; Miyauchi, S.; Ananth, S.; Herdman, A.V.; Martindale, R.G.; Podolsky, R.; Ganapathy, V. Down-regulation of BCRP/ABCG2 in colorectal and cervical cancer. Biochem. Biophys. Res. Commun. 2006, 343, 571–577. [Google Scholar]
- Chen, J.S.; Pardo, F.S.; Wang-Rodriguez, J.; Chu, T.S.; Lopez, J.P.; Aguilera, J.; Altuna, X.; Weisman, R.A.; Ongkeko, W.M. EGFR regulates the side population in head and neck squamous cell carcinoma. Laryngoscope 2006, 116, 401–406. [Google Scholar]
- Bhatia, P.; Bernier, M.; Sanghvi, M.; Moaddel, R.; Schwarting, R.; Ramamoorthy, A.; Wainer, I.W. Breast cancer resistance protein (BCRP/ABCG2) localises to the nucleus in glioblastoma multiforme cells. Xenobiotica 2012, 42, 748–755. [Google Scholar] [CrossRef]
- Liang, S.C.; Yang, C.Y.; Tseng, J.Y.; Wang, H.L.; Tung, C.Y.; Liu, H.W.; Chen, C.Y.; Yeh, Y.C.; Chou, T.Y.; Yang, M.H.; et al. ABCG2 localizes to the nucleus and modulates CDH1 expression in lung cancer cells. Neoplasia 2015, 17, 265–278. [Google Scholar]
- Wang, X.; Xia, B.; Liang, Y.; Peng, L.; Wang, Z.; Zhuo, J.; Wang, W.; Jiang, B. Membranous ABCG2 expression in colorectal cancer independently correlates with shortened patient survival. Cancer Biomark. 2013, 13, 81–88. [Google Scholar] [CrossRef]
- Benderra, Z.; Faussat, A.M.; Sayada, L.; Perrot, J.Y.; Chaoui, D.; Marie, J.P.; Legrand, O. Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin. Cancer Res. 2004, 10, 7896–7902. [Google Scholar]
- Uggla, B.; Ståhl, E.; Wågsäter, D.; Paul, C.; Karlsson, M.G.; Sirsjö, A.; Tidefel, U. BCRP mRNA expression v. clinical outcome in 40 adult AML patients. Leuk. Res. 2005, 29, 141–146. [Google Scholar] [CrossRef]
- Yoh, K.; Ishii, G.; Yokose, T.; Minegishi, Y.; Tsuta, K.; Goto, K.; Nishiwaki, Y.; Kodama, T.; Suga, M.; Ochiai, A. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin. Cancer Res. 2004, 10, 1691–1697. [Google Scholar]
- Kim, Y.H.; Ishii, G.; Goto, K.; Ota, S.; Kubota, K.; Murata, Y.; Mishima, M.; Saijo, N.; Nishiwaki, Y.; Ochiai, A. Expression of breast cancer resistance protein is associated with a poor clinical outcome in patients with small-cell lung cancer. Lung Cancer 2009, 65, 105–111. [Google Scholar] [CrossRef]
- Hang, D.; Dong, H.C.; Ning, T.; Dong, B.; Hou, D.L.; Xu, W.G. Prognostic value of the stem cell markers CD133 and ABCG2 expression in esophageal squamous cell carcinoma. Dis. Esophagus 2012, 25, 638–644. [Google Scholar]
- Huang, L.; Lu, Q.; Han, Y.; Li, Z.; Zhang, Z.; Li, X. ABCG2/V-ATPase was associated with the drug resistance and tumor metastasis of esophageal squamous cancer cells. Diagn. Pathol. 2012, 7, 180. [Google Scholar]
- Tsunoda, S.; Okumura, T.; Ito, T.; Kondo, K.; Ortiz, C.; Tanaka, E.; Watanabe, G.; Itami, A.; Sakai, Y.; Shimada, Y. ABCG2 expression is an independent unfavorable prognostic factor in esophageal squamous cell carcinoma. Oncology 2006, 71, 251–258. [Google Scholar]
- Lee, S.H.; Kim, H.; Hwang, J.H.; Lee, H.S.; Cho, J.Y.; Yoon, Y.S.; Han, H.S. Breast cancer resistance protein expression is associated with early recurrence and decreased survival in resectable pancreatic cancer patients. Pathol. Int. 2012, 62, 167–175. [Google Scholar]
- Yuan, Y.; Yang, Z.; Miao, X.; Li, D.; Liu, Z.; Zou, Q. The clinical significance of FRAT1 and ABCG2 expression in pancreatic ductal adenocarcinoma. Tumour. Biol. 2015, 36, 9961–9968. [Google Scholar]
- Shen, B.; Dong, P.; Li, D.; Gao, S. Expression and function of ABCG2 in head and neck squamous cell carcinoma and cell lines. Exp. Ther. Med. 2011, 2, 1151–1157. [Google Scholar]
- Yamada, A.; Ishikawa, T.; Ota, I.; Kimura, M.; Shimizu, D.; Tanabe, M.; Chishima, T.; Sasaki, T.; Ichikawa, Y.; Morita, S.; et al. High expression of ATP-binding cassette transporter ABCC11 in breast tumors is associated with aggressive subtypes and low disease-free survival. Breast Cancer Res. Treat. 2013, 137, 773–782. [Google Scholar]
- Xiang, L.; Su, P.; Xia, S.; Liu, Z.; Wang, Y.; Gao, P.; Zhou, G. ABCG2 is associated with HER-2 expression, lymph node metastasis and clinical stage in breast invasive ductal carcinoma. Diagn. Pathol. 2011, 6, 90. [Google Scholar]
- Larbcharoensub, N.; Sornmayura, P.; Sirachainan, E.; Wilasrusmee, C.; Wanmoung, H.; Janvilisri, T. Prognostic value of ABCG2 in moderately and poorly differentiated intrahepatic cholangiocarcinoma. Histopathology 2011, 59, 235–246. [Google Scholar] [PubMed]
- Glasgow, S.C.; Yu, J.; Carvalho, L.P.; Shannon, W.D.; Fleshman, J.W.; McLeod, H.L. Unfavourable expression of pharmacologic markers in mucinous colorectal cancer. Br. J. Cancer 2005, 92, 259–264. [Google Scholar] [PubMed]
- Giampieri, R.; Scartozzi, M.; Loretelli, C.; Piva, F.; Mandolesi, A.; Lezoche, G.; Del Prete, M.; Bittoni, A.; Faloppi, L.; Bianconi, M.; et al. Cancer stem cell gene profile as predictor of relapse in high risk stage II and stage III, radically resected colon cancer patients. PLoS ONE 2013, 8, e72843. [Google Scholar]
- Liu, H.G.; Pan, Y.F.; You, J.; Wang, O.C.; Huang, K.T.; Zhang, X.H. Expression of ABCG2 and its significance in colorectal cancer. Asian Pac. J. Cancer Prev. 2010, 11, 845–848. [Google Scholar] [PubMed]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef]
- Andersen, V.; Vogel, L.K.; Kopp, T.I.; Sæbø, M.; Nonboe, A.W.; Hamfjord, J.; Kure, E.H.; Vogel, U. High ABCC2 and low ABCG2 gene expression are early events in the colorectal adenoma-carcinoma sequence. PLoS ONE 2015, 10, e0119255. [Google Scholar]
- Hlavata, I.; Mohelnikova-Duchonova, B.; Vaclavikova, R.; Liska, V.; Pitule, P.; Novak, P.; Bruha, J.; Vycital, O.; Holubec, L.; Treska, V.; et al. The role of ABC transporters in progression and clinical outcome of colorectal cancer. Mutagenesis 2012, 27, 187–196. [Google Scholar]
- Candeil, L.; Gourdier, I.; Peyron, D.; Vezzio, N.; Copois, V.; Bibeau, F.; Orsetti, B.; Scheffer, G.L.; Ychou, M.; Khan, Q.A.; et al. ABCG2 overexpression in colon cancer cells resistant to SN38 and in irinotecan-treated metastases. Int. J. Cancer 2004, 109, 848–854. [Google Scholar]
- To, K.K.; Zhan, Z.; Bates, S.E. Aberrant promoter methylation of the ABCG2 gene in renal carcinoma. Mol. Cell. Bio. 2006, 26, 8572–8585. [Google Scholar] [CrossRef]
- Turner, J.G.; Gump, J.L.; Zhang, C.; Cook, J.M.; Marchion, D.; Hazlehurst, L.; Munster, P.; Schell, M.J.; Dalton, W.S.; Sullivan, D.M. ABCG2 expression, function, and promoter methylation in human multiple myeloma. Blood 2006, 108, 3881–3889. [Google Scholar]
- Peng, Y.; Xu, B.; Tang, J.; Wan, Z.; Sun, H.; Wang, G.; Zhu, Y.S. Analysis of ABCG2 methylation in stool samples of Chinese healthy males by pyrosequencing. Pharmazie 2016, 71, 447–454. [Google Scholar]
- Maliepaard, M.; Scheffer, G.L.; Faneyte, I.F.; van Gastelen, M.A.; Pijnenborg, A.C.; Schinkel, A.H.; van De Vijver, M.J.; Scheper, R.J.; Schellens, J.H. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001, 61, 3458–3464. [Google Scholar]
- Lin, P.C.; Lin, H.H.; Lin, J.K.; Lin, C.C.; Yang, S.H.; Li, A.F.; Chen, W.S.; Chang, S.C. Expression of ABCG2 associated with tumor response in metastatic colorectal cancer patients receiving first-line FOLFOX therapy—Preliminary evidence. Int. J. Biol. Markers 2013, 28, 182–186. [Google Scholar]
- Han, S.H.; Kim, J.W.; Kim, M.; Kim, J.H.; Lee, K.W.; Kim, B.H.; Oh, H.K.; Kim, D.W.; Kang, S.B.; Kim, H.; et al. Prognostic implication of ABC transporters and cancer stem cell markers in patients with stage III colon cancer receiving adjuvant FOLFOX-4 chemotherapy. Oncol. Lett. 2019, 17, 5572–5580. [Google Scholar]
- Kang, D.; Park, J.M.; Jung, C.K.; Lee, B.I.; Oh, S.T.; Choi, M.G. Prognostic impact of membranous ATP-binding cassette Sub-family G member 2 expression in patients with colorectal carcinoma after surgical resection. Cancer Biol. Ther. 2015, 16, 1438–1444. [Google Scholar]
- Palshof, J.A.; Cederbye, C.N.; Høgdall, E.V.S.; Poulsen, T.S.; Linnemann, D.; Nygaard, S.B.; Stenvang, J.; Christensen, I.J.; Jensen, B.V.; Pfeiffer, P.; et al. ABCG2 Protein Levels and Association to Response to First-Line Irinotecan-Based Therapy for Patients with Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 5027. [Google Scholar]
- Mogi, M.; Yang, J.; Lambert, J.F.; Colvin, G.A.; Shiojima, I.; Skurk, C.; Summer, R.; Fine, A.; Quesenberry, P.J.; Walsh, K. Akt signaling regulates side population cell phenotype via Bcrp1 translocation. J. Biol. Chem. 2003, 278, 39068–39075. [Google Scholar]
- Meyer zu Schwabedissen, H.E.; Grube, M.; Dreisbach, A.; Jedlitschky, G.; Meissner, K.; Linnemann, K.; Fusch, C.; Ritter, C.A.; Völker, U.; Kroemer, H.K. Epidermal growth factor-mediated activation of the map kinase cascade results in altered expression and function of ABCG2 (BCRP). Drug Metab. Dispos. 2006, 34, 524–533. [Google Scholar]
- Silvestris, N.; Simone, G.; Partipilo, G.; Scarpi, E.; Lorusso, V.; Brunetti, A.E.; Maiello, E.; Paradiso, A.; Mangia, A. CES2, ABCG2, TS and Topo-I primary and synchronous metastasis expression and clinical outcome in metastatic colorectal cancer patients treated with first-line FOLFIRI regimen. Int. J. Mol. Sci. 2014, 15, 15767–15777. [Google Scholar] [CrossRef]
- Kim, B.H.; Oh, H.K.; Kim, D.W.; Kang, S.B.; Choi, Y.; Shin, E. Clinical Implications of Cancer Stem Cell Markers and ABC Transporters as a Predictor of Prognosis in Colorectal Cancer Patients. Anticancer. Res. 2020, 40, 4481–4489. [Google Scholar] [CrossRef]
- Rosenthal, S.B.; Bush, K.T.; Nigam, S.K. A Network of SLC and ABC Transporter and DME Genes Involved in Remote Sensing and Signaling in the Gut-Liver-Kidney Axis. Sci. Rep. 2019, 9, 11879. [Google Scholar] [PubMed]
- De Mattia, E.; Toffoli, G.; Polesel, J.; D’Andrea, M.; Corona, G.; Zagonel, V.; Buonadonna, A.; Dreussi, E.; Cecchin, E. Pharmacogenetics of ABC and SLC transporters in metastatic colorectal cancer patients receiving first-line FOLFIRI treatment. Pharm. Genom. 2013, 23, 549–557. [Google Scholar]
- Ghatak, S.; Misra, S.; Toole, B.P. Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells. J. Biol. Chem. 2005, 280, 8875–8883. [Google Scholar] [PubMed]
- Misra, S.; Toole, B.P.; Ghatak, S. Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J. Biol. Chem. 2006, 281, 34936–34941. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.J.; Jiang, J.Y.; Wang, M.; Li, M.Y.; Zheng, L.M.; Feng, Z.X.; Liu, L. Cetuximab enhances the efficiency of irinotecan through simultaneously inhibiting the MAPK signaling and ABCG2 in colorectal cancer cells. Pathol. Res. Pract. 2020, 216, 152798. [Google Scholar]
- Pick, A.; Wiese, M. Tyrosine kinase inhibitors influence ABCG2 expression in EGFR-positive MDCK BCRP cells via the PI3K/Akt signaling pathway. ChemMedChem 2012, 7, 650–662. [Google Scholar]
- Ma, H.; Yao, Q.; Zhang, A.M.; Lin, S.; Wang, X.X.; Wu, L.; Sun, J.G.; Chen, Z.T. The effects of artesunate on the expression of EGFR and ABCG2 in A549 human lung cancer cells and a xenograft model. Molecules 2011, 16, 10556–10569. [Google Scholar]
- Bleau, A.M.; Hambardzumyan, D.; Ozawa, T.; Fomchenko, E.I.; Huse, J.T.; Brennan, C.W.; Holland, E.C. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell 2009, 4, 226–235. [Google Scholar]
- Guha, D.; Saha, T.; Bose, S.; Chakraborty, S.; Dhar, S.; Khan, P.; Adhikary, A.; Das, T.; Sa, G. Integrin-EGFR interaction regulates anoikis resistance in colon cancer cells. Apoptosis 2019, 24, 958–971. [Google Scholar]
- Sogawa, C.; Eguchi, T.; Namba, Y.; Okusha, Y.; Aoyama, E.; Ohyama, K.; Okamoto, K. Gel-Free 3D Tumoroids with Stem Cell Properties Modeling Drug Resistance to Cisplatin and Imatinib in Metastatic Colorectal Cancer. Cells 2021, 10, 344. [Google Scholar]
- Zhao, W.; Li, Y.; Zhang, X. Stemness-Related Markers in Cancer. Cancer Transl. Med. 2017, 3, 87–95. [Google Scholar]
- Xiong, B.; Ma, L.; Hu, X.; Zhang, C.; Cheng, Y. Characterization of side population cells isolated from the colon cancer cell line SW480. Int. J. Oncol. 2014, 45, 1175–1183. [Google Scholar]
- Hervieu, C.; Christou, N.; Battu, S.; Mathonnet, M. The Role of Cancer Stem Cells in Colorectal Cancer: From the Basics to Novel Clinical Trials. Cancers 2021, 13, 1092. [Google Scholar]
- Gheytanchi, E.; Naseri, M.; Karimi-Busheri, F.; Atyabi, F.; Mirsharif, E.S.; Bozorgmehr, M.; Ghods, R.; Madjd, Z. Morphological and molecular characteristics of spheroid formation in HT-29 and Caco-2 colorectal cancer cell lines. Cancer Cell Int. 2021, 21, 204. [Google Scholar]
- Sándor, S.; Jordanidisz, T.; Schamberger, A.; Várady, G.; Erdei, Z.; Apáti, Á.; Sarkadi, B.; Orbán, T.I. Functional characterization of the ABCG2 5’ non-coding exon variants: Stem cell specificity, translation efficiency and the influence of drug selection. Biochim. Biophys. Acta 2016, 1859, 943–951. [Google Scholar]
- Susanto, J.; Lin, Y.H.; Chen, Y.N.; Shen, C.R.; Yan, Y.T.; Tsai, S.T.; Chen, C.H.; Shen, C.N. Porphyrin homeostasis maintained by ABCG2 regulates self-renewal of embryonic stem cells. PLoS ONE 2008, 3, e4023. [Google Scholar]
- Alowaidi, F.; Hashimi, S.M.; Alqurashi, N.; Alhulais, R.; Ivanovski, S.; Bellette, B.; Meedenyia, A.; Lam, A.; Wood, S. Assessing stemness and proliferation properties of the newly established colon cancer ‘stem’ cell line, CSC480 and novel approaches to identify dormant cancer cells. Oncol. Rep. 2018, 39, 2881–2891. [Google Scholar] [CrossRef]
- Olszewski, U.; Liedauer, R.; Ausch, C.; Thalhammer, T.; Hamilton, G. Overexpression of CYP3A4 in a COLO 205 Colon Cancer Stem Cell Model in vitro. Cancers 2011, 3, 1467–1479. [Google Scholar]
- Tomita, H.; Tanaka, K.; Tanaka, T.; Hara, A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget 2016, 7, 11018–11032. [Google Scholar]
- Rhodes, D.R.; Yu, J.; Shanker, K.; Deshpandez, N.; Varambally, R.; Ghosh, D.; Barrette, T.; Pandeyb, A.; Chinnaiyan, A.M. ONCOMINE: A cancer microarray database and integrated data-mining platform. Neoplasia 2004, 6, 1–6. [Google Scholar]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [PubMed]
- Bartha, Á.; Győrffy, B. TNMplot.com: A web tool for the comparison of gene expression in normal, tumor and metastatic tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [PubMed]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar]
- Koch, A.; De Meyer, T.; Jeschke, J.; Van Criekinge, W. MEXPRESS: Visualizing expression, DNA methylation and clinical TCGA data. BMC Genom. 2015, 16, 636. [Google Scholar]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar]
- Mizuno, H.; Kitada, K.; Nakai, K.; Sarai, A. PrognoScan: A new database for meta-analysis of the prognostic value of genes. BMC Med. Genom. 2009, 2, 18. [Google Scholar]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional character-ization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar]
- Diestra, J.E.; Scheffer, G.L.; Català, I.; Maliepaard, M.; Schellens, J.H.; Scheper, R.J.; Germà-Lluch, J.R.; Izquierdo, M.A. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffinembedded material. J. Pathol. 2002, 198, 213–219. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar]



















| Dataset | End-Point * | Probe ID | N | Cut-Point | Cox p-Value | HR [95% CI] |
|---|---|---|---|---|---|---|
| GSE12945 | DFS | 209735_at | 51 | 0.75 | 0.3906 | 0.53 [0.13–2.24] |
| GSE12945 | OS | 209735_at | 62 | 0.29 | 0.7889 | 1.07 [0.66–1.72] |
| GSE17536 | OS | 209735_at | 177 | 0.87 | 0.0126 | 1.45 [1.08–1.94] |
| GSE17536 | DFS | 209735_at | 145 | 0.90 | 0.1210 | 1.44 [0.91–2.28] |
| GSE17536 | DSS | 209735_at | 177 | 0.87 | 0.0034 | 1.58 [1.16–2.14] |
| GSE14333 | DFS | 209735_at | 226 | 0.82 | 0.1959 | 1.23 [0.90–1.69] |
| GSE17537 | DFS | 209735_at | 55 | 0.15 | 0.1856 | 1.32 [0.87–2.00] |
| GSE17537 | DSS | 209735_at | 49 | 0.82 | 0.6398 | 1.17 [0.61–2.21] |
| GSE17537 | OS | 209735_at | 55 | 0.76 | 0.3611 | 1.22 [0.80–1.86] |
| Feature | ABCG2 Protein Level | |||||
|---|---|---|---|---|---|---|
| Absent | Present | p-Value | Low | High | p-Value | |
| Age | ||||||
| up to 60 y | 11 | 34 | 0.906 * | 16 | 18 | 0.844 * |
| over 60 y | 13 | 38 | 17 | 21 | ||
| Sex | ||||||
| female | 13 | 37 | 0.814 * | 17 | 20 | 0.984 * |
| male | 11 | 35 | 16 | 19 | ||
| Tumour localization | ||||||
| rectum | 8 | 27 | 0.939 & | 13 | 14 | 0.825 * |
| colon | 16 | 44 | 20 | 24 | ||
| Histological type | ||||||
| adenocarcinoma tubulare | 19 | 64 | 0.389 # | 31 | 33 | 0.380 # |
| adenocarcinoma mucinosum | 5 | 8 | 2 | 6 | ||
| Histological grade | ||||||
| G1 or G2 | 15 | 52 | 0.898 & | 22 | 30 | 0.336 & |
| G3 | 9 | 20 | 11 | 19 | ||
| Depth of tumour invasion | ||||||
| pT1 or pT2 | 5 | 24 | 0.898 & | 9 | 15 | 0.316 * |
| pT3 or pT4 | 19 | 48 | 24 | 24 | ||
| Lymph nodes metastasis | ||||||
| pN0 | 12 | 43 | 0.811 & | 20 | 23 | 0.702 * |
| pN1 and pN2 | 12 | 24 | 10 | 14 | ||
| Distant metastases | ||||||
| pM0 | 20 | 58 | 1.000 # | 26 | 32 | 0.729 & |
| pM1 | 4 | 14 | 7 | 7 | ||
| Stage | ||||||
| pTNM I or II | 11 | 43 | 0.235 * | 20 | 32 | 0.888 * |
| pTNM III or IV | 13 | 29 | 13 | 16 | ||
| Lymphocyte infiltration | ||||||
| absent | 15 | 39 | 0.517 * | 17 | 22 | 0.781 * |
| present | 9 | 32 | 15 | 17 | ||
| Venous invasion | ||||||
| absent | 9 | 29 | 0.810 * | 13 | 16 | 0.888 * |
| present | 15 | 43 | 20 | 23 | ||
| Feature | ABCG2 Gene Expression Level | |||
|---|---|---|---|---|
| Min. | Median | Max. | p-Value * | |
| Age | ||||
| up to 60 y | 0.63 | 0.03 | 17.64 | 0.718 |
| over 60 y | 0.68 | 0.02 | 14.63 | |
| Sex | ||||
| female | 0.67 | 0.02 | 16.38 | 0.403 |
| male | 0.64 | 0.03 | 17.64 | |
| Tumour localization | ||||
| rectum | 0.66 | 0.04 | 14.63 | 0.911 |
| colon | 0.67 | 0.02 | 17.64 | |
| Histological type | ||||
| adenocarcinoma tubulare | 0.63 | 0.02 | 17.64 | 0.694 |
| adenocarcinoma mucinosum | 0.93 | 0.12 | 6.58 | |
| Histological grade | ||||
| G1 or G2 | 0.63 | 0.04 | 16.38 | 0.362 |
| G3 | 0.83 | 0.02 | 17.64 | |
| Depth of tumour invasion | ||||
| pT1 or pT2 | 0.61 | 0.04 | 14.63 | 0.655 |
| pT3 or pT4 | 0.67 | 0.02 | 17.64 | |
| Lymph node metastasis | ||||
| pN0 | 0.65 | 0.03 | 17.64 | 0.773 |
| pN1 and pN2 | 0.65 | 0.02 | 16.38 | |
| Distant metastases | ||||
| pM0 | 0.69 | 0.03 | 17.64 | 0.149 |
| pM1 | 0.55 | 0.02 | 8.58 | |
| Stage | ||||
| pTNM I or II | 0.67 | 0.03 | 17.64 | 0.799 |
| pTNM III or IV | 0.65 | 0.02 | 16.38 | |
| Lymphocyte infiltration | ||||
| absent | 0.78 | 0.04 | 16.38 | 0.228 |
| present | 0.58 | 0.02 | 14.60 | |
| Venous invasion | ||||
| absent | 0.67 | 0.03 | 17.64 | 0.798 |
| present | 0.65 | 0.02 | 11.19 | |
| Feature | Overall Survival | |
|---|---|---|
| Number of Deaths (%) | p-Value * | |
| Age | ||
| up to 60 y | 22 (48.9) | 0.288 |
| over 60 y | 30 (60.0) | |
| Sex | ||
| female | 27 (54.0) | 0.763 |
| male | 25 (55.6) | |
| Tumour localization | ||
| rectum | 24 (68.6) | 0.141 |
| colon | 28 (47.5) | |
| Histological type | ||
| adenocarcinoma tubulare | 45 (54.9) | 0.915 |
| adenocarcinoma mucinosum | 7 (53.9) | |
| Histological grade | ||
| G1 or G2 | 33 (50.0) | 0.112 |
| G3 | 19 (65.5) | |
| Depth of tumour invasion | ||
| pT1 or pT2 | 12 (41.4) | 0.041 |
| pT3 or pT4 | 40 (61.6) | |
| Lymph node metastasis | ||
| pN0 | 23 (41.8) | 0.001 |
| pN1 and pN2 | 25 (71.4) | |
| Distant metastases | ||
| pM0 | 34 (44.2) | <0.001 |
| pM1 | 18 (100.0) | |
| Stage | ||
| pTNM I or II | 22 (40.7) | <0.001 |
| pTNM III or IV | 30 (73.2) | |
| Lymphocyte infiltration | ||
| absent | 34 (63.0) | 0.036 |
| present | 17 (42.5) | |
| Venous invasion | ||
| absent | 17 (44.7) | 0.070 |
| present | 35 (61.4) | |
| ABCG2 protein expression | ||
| absent | 10 (43.5) | 0.236 |
| present | 42 (58.3) | |
| low | 24 (72.7) | 0.077 |
| high | 18 (46.2) | |
| ABCG2 expression level | ||
| low | 36 (63.2) | 0.080 |
| high | 14 (42.4) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sałagacka-Kubiak, A.; Zawada, D.; Saed, L.; Kordek, R.; Jeleń, A.; Balcerczak, E. ABCG2 Gene and ABCG2 Protein Expression in Colorectal Cancer—In Silico and Wet Analysis. Int. J. Mol. Sci. 2023, 24, 10539. https://doi.org/10.3390/ijms241310539
Sałagacka-Kubiak A, Zawada D, Saed L, Kordek R, Jeleń A, Balcerczak E. ABCG2 Gene and ABCG2 Protein Expression in Colorectal Cancer—In Silico and Wet Analysis. International Journal of Molecular Sciences. 2023; 24(13):10539. https://doi.org/10.3390/ijms241310539
Chicago/Turabian StyleSałagacka-Kubiak, Aleksandra, Dawid Zawada, Lias Saed, Radzisław Kordek, Agnieszka Jeleń, and Ewa Balcerczak. 2023. "ABCG2 Gene and ABCG2 Protein Expression in Colorectal Cancer—In Silico and Wet Analysis" International Journal of Molecular Sciences 24, no. 13: 10539. https://doi.org/10.3390/ijms241310539
APA StyleSałagacka-Kubiak, A., Zawada, D., Saed, L., Kordek, R., Jeleń, A., & Balcerczak, E. (2023). ABCG2 Gene and ABCG2 Protein Expression in Colorectal Cancer—In Silico and Wet Analysis. International Journal of Molecular Sciences, 24(13), 10539. https://doi.org/10.3390/ijms241310539





