Unveiling Angiotensin II and Losartan-Induced Gene Regulatory Networks Using Human Urine-Derived Podocytes
Abstract
1. Introduction
2. Results
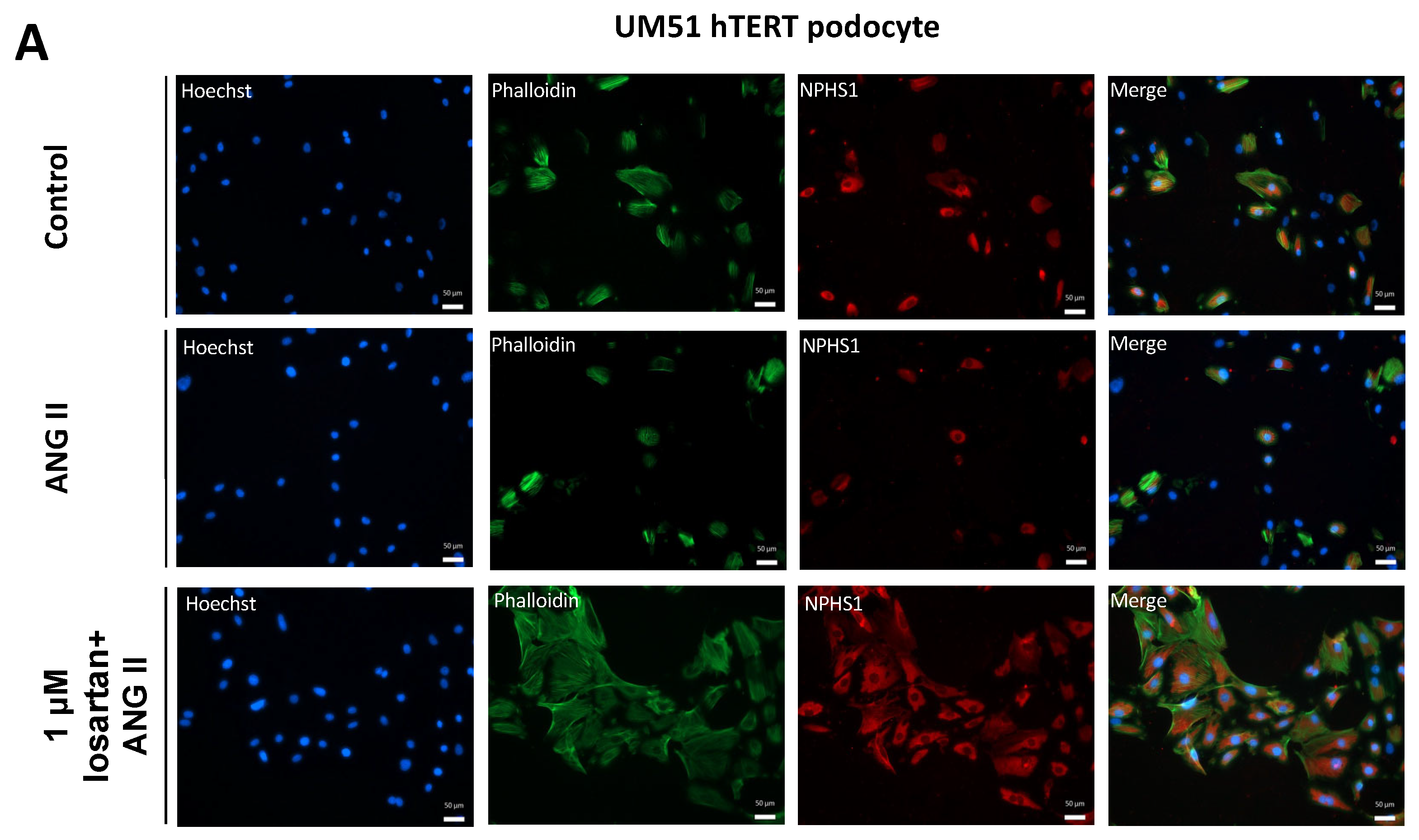
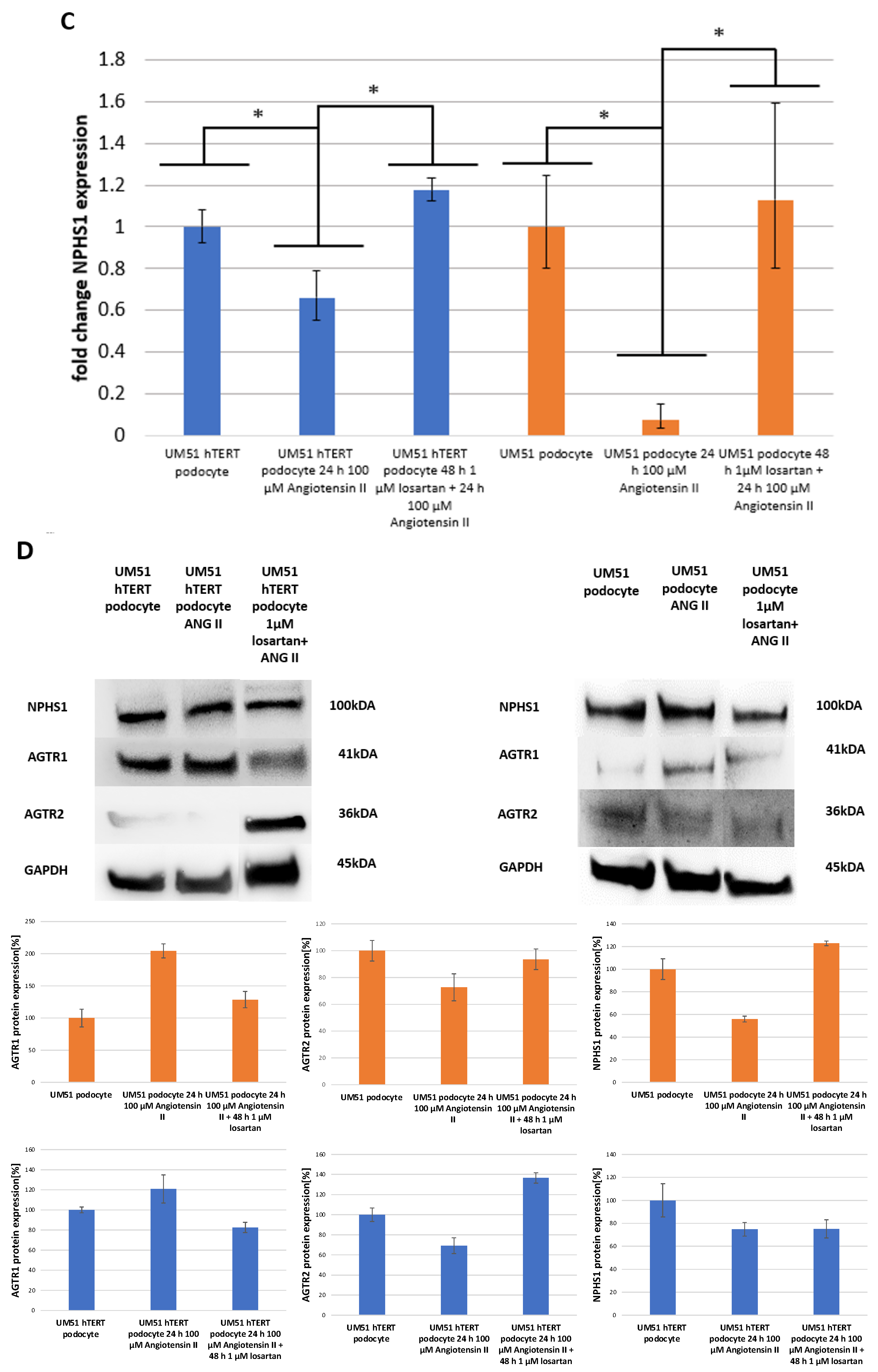
2.1. Losartan Rescues ANG II Induced Down-Regulation of NPHS1 Expression
2.2. The Effect of Angiotensin II and Losartan on the Expression of Angiotensin Receptor Type 1 and 2
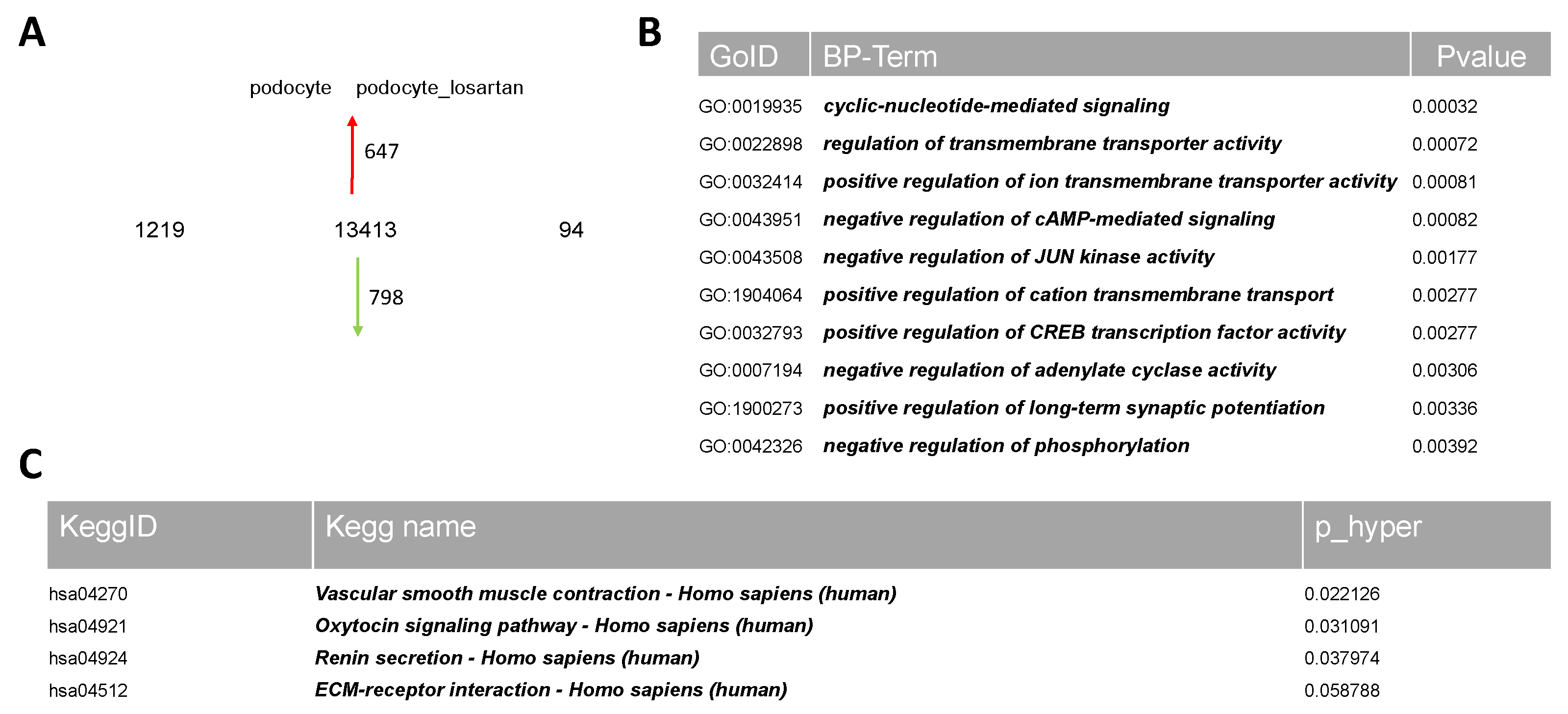
2.3. Comparative Transcriptome Analysis of Urine-Derived Podocytes Treated with and without Losartan
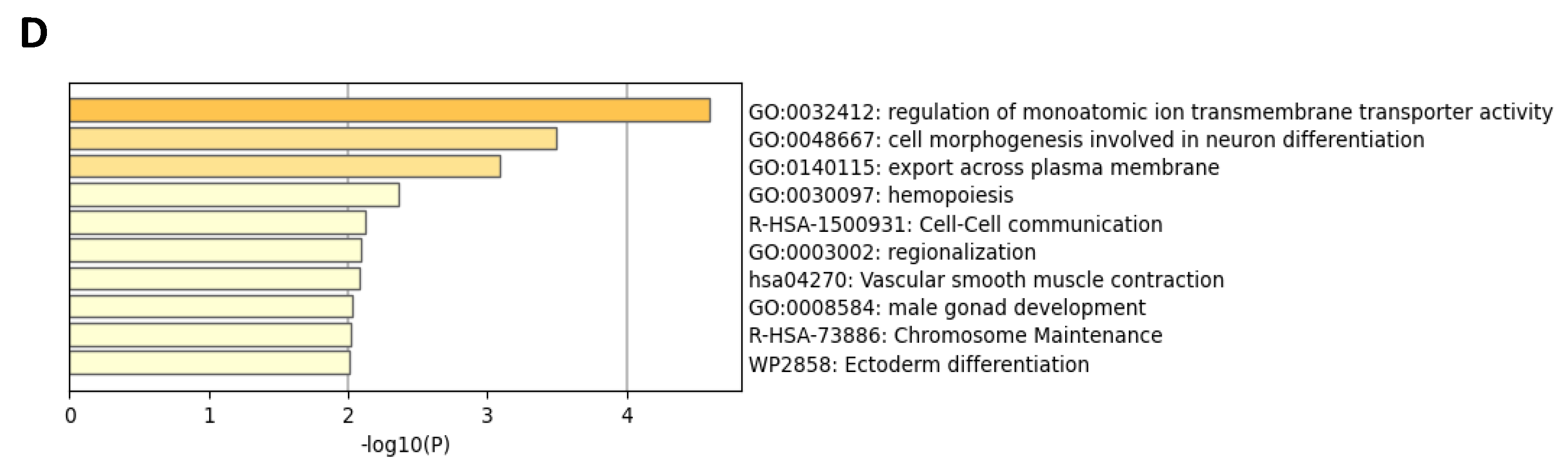
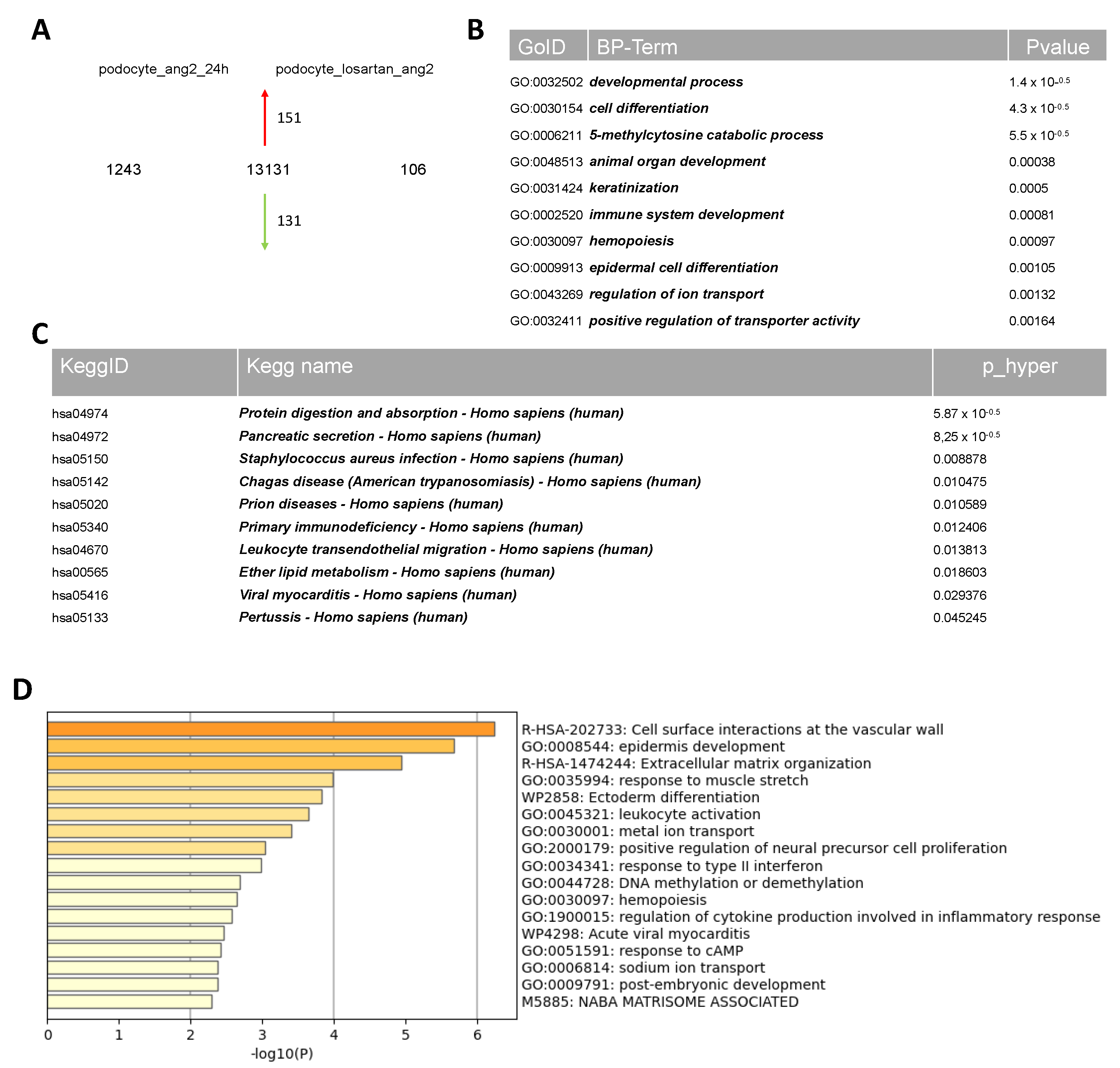
2.4. Comparative Transcriptome Analysis of Urine-Derived Podocytes Treated with Angiotensin II and the Combination of Angiotensin II and Losartan
3. Discussion
4. Materials and Methods
4.1. Cell Culture Conditions
4.2. Immunofluorescence Staining
4.3. Relative Quantification of Podocyte-Associated Gene Expression via Real-Time PCR
4.4. Western Blot Analysis
4.5. Microarray Data Analyses
4.6. Ethics Statement
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, R.F.; Lang, F.; Heckmann, M. Physiologie des Menschen, 31st ed.; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-662-54121-0. [Google Scholar]
- Schwarz, K.; Simons, M.; Reiser, J.; Saleem, M.; Faul, C.; Kriz, W.; Shaw, A.; Holzmann, L.; Mundel, P. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J. Clin. Investig. 2001, 108, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Jones, N. Nephrin Signaling in the Podocyte: An Updated View of Signal Regulation at the Slit Diaphragm and Beyond. Front. Endocrinol. 2018, 9, 302. [Google Scholar] [CrossRef]
- Li, C.; Ruotsalainen, V.; Tryggvason, K.; Shaw, A.; Miner, J. CD2AP is expressed with nephrin in developing podocytes and is found widely in mature kidney and elsewhere. Am. J. Physiol. Renal Physiol. 2000, 279, F785–F792. [Google Scholar] [CrossRef]
- Barreto-Chaves, M.L.; Mello-Aires, M. Effect of luminal angiotensin II and ANP on early and late cortical distal tubule HCO3-reabsorption. Am. J. Physiol. 1996, 271, F977–F984. [Google Scholar] [CrossRef]
- Kwon, T.-H.; Nielsen, J.; Kim, Y.-H.; Knepper, M.A.; Frøkiaer, J.; Nielsen, S. Regulation of sodium transporters in the thick ascending limb of rat kidney: Response to angiotensin II. Am. J. Physiol. 2003, 285, F152–F165. [Google Scholar] [CrossRef] [PubMed]
- Rocque, B.L.; Babayeva, S.; Li, J.; Leung, V.; Nezvitsky, L.; Cybulsky, A.V.; Gros, P.; Torban, E. Deficiency of the Planar Cell Polarity Protein Vangl2 in Podocytes Affects Glomerular Morphogenesis and Increases Susceptibility to Injury. J. Am. Soc. Nephrol. 2015, 26, 576–586. [Google Scholar] [CrossRef]
- Tojo, A.; Tisher, C.C.; Madsen, K.M. Angiotensin II regulates H(+)-ATPase activity in rat cortical collecting duct. Am. J. Physiol. 1994, 267, F1045–F1051. [Google Scholar] [CrossRef]
- Xie, K.; Xu, C.; Zhang, M.; Wang, M.; Min, L.; Qian, C.; Wang, Q.; Ni, Z.; Mou, S.; Dai, H.; et al. Yes-associated protein regulates podocyte cell cycle re-entry and dedifferentiation in adriamycin-induced nephropathy. Cell Death Dis. 2019, 10, 915. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Giebisch, G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am. J. Physiol. 1996, 271, F143–F149. [Google Scholar] [CrossRef] [PubMed]
- Kaschina, E.; Unger, T. Angiotensin AT1/AT2 receptors: Regulation, signalling and function. Blood Press. 2003, 12, 70–88. [Google Scholar] [CrossRef]
- Savoia, C.; Burger, D.; Nishigaki, N.; Montezano, A.; Touyz, R.M. Angiotensin II and the vascular phenotype in hypertension. Camb. Univ. Press 2011, 13, 11. [Google Scholar] [CrossRef]
- Siragy, H.M. AT1 and AT2 Receptor in the Kidney: Role in Health and Disease. Semin. Nephrol. 2004, 24, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Ames, M.; Atkins, C.; Pitt, B. The renin-angiotensin-aldosterone system and its suppression. J. Vet. Intern. Med. 2019, 33, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.; Touyz, A.F.; Dominiczak, R.; Webb, C.; Johns, D.G. Angiotensin receptors: Signaling, vascular pathophysiology, and interactions with ceramide. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H2337–H2365. [Google Scholar] [CrossRef] [PubMed]
- Widdop, R.; Jones, E.; Hannan, R.; Gaspari, T. Angiotensin AT2 receptors: Cardiovascular hope or hype? Br. J. Pharmacol. 2003, 140, 809–824. [Google Scholar] [CrossRef] [PubMed]
- Macconi, D.; Abbate, M.; Morigi, M.; Angioletti, S.; Mister, M.; Buelli, S.; Bonomelli, M.; Mundel, P.; Endlich, K.; Remuzzi, A.; et al. Permselective Dysfunction of Podocyte-Podocyte Contact upon Angiotensin II Unravels the Molecular Target for Renoprotective Intervention. Am. J. Pathol. 2006, 168, 1073–1085. [Google Scholar] [CrossRef]
- Suzuki, K.; Han, G.D.; Miyauchi, N.; Hashimoto, T.; Nakatsue, T.; Fujioka, Y.; Koike, H.; Shimizu, F.; Kawachi, H. Angiotensin II Type 1 and Type 2 Receptors Play Opposite Roles in Regulating the Barrier Function of Kidney Glomerular Capillary Wall. Am. J. Pathol. 2007, 170, 1841–1853. [Google Scholar] [CrossRef]
- Erichsen, L.; Thimm, C.; Bohndorf, M.; Rahman, M.S.; Wruck, W.; Adjaye, J. Activation of the Renin–Angiotensin System Disrupts the Cytoskeletal Architecture of Human Urine-Derived Podocytes. Cells 2022, 11, 1095. [Google Scholar] [CrossRef]
- Remuzzi, G.; Benigni, A.; Remuzzi, A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J. Clin. Investig. 2006, 116, 288–296. [Google Scholar] [CrossRef]
- Currie, G.; Delles, C. Proteinuria and its relation to cardiovascular disease. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 13–24. [Google Scholar] [CrossRef]
- Ferrario, C. Role of Angiotensin II in Cardiovascular Disease—Therapeutic Implications of More Than a Century of Research. JRAAS 2006, 7, 3–145. [Google Scholar] [CrossRef] [PubMed]
- Atlas, S. The Renin-Angiotensin Aldosterone System: Pathophysiological Role and Pharmacologic Inhibition. J. Manag. Care Pharm. 2007, 13, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Wruck, W.; Spitzhorn, L.-S.; Nguyen, L.; Bohndorf, M.; Martins, S.; Asar, F.; Ncube, A.; Erichsen, L.; Graffmann, N.; et al. The FGF, TGFβ and WNT axis Modulate Self-renewal of Human SIX2 + Urine Derived Renal Progenitor Cells. Sci. Rep. 2020, 10, 739. [Google Scholar] [CrossRef]
- Onoda, N.; Kawabata, A.; Hasegawa, K.; Sakakura, M.; Urakawa, I.; Seki, M.; Zenkoh, J.; Suzuki, A.; Suzuki, Y. Spatial and single-cell transcriptome analysis reveals changes in gene expression in response to drug perturbation in rat kidney. DNA Res. 2022, 29, dsac007. [Google Scholar] [CrossRef] [PubMed]
- Komers, R.; Xu, B.; McClelland, A.; Kantharidis, P.; Mittal, A.; Cohen, H.T.; Cohen, D.M. Transcriptome-Based Analysis of Kidney Gene Expression Changes Associated with Diabetes in OVE26 Mice, in the Presence and Absence of Losartan Treatment. PLoS ONE 2014, 9, e96987. [Google Scholar] [CrossRef]
- Omachi, K.; Kaseda, S.; Yokota, T.; Kamura, M.; Teramoto, K.; Kuwazuru, J.; Kojima, H.; Nohara, H.; Koyama, K.; Ohtsuki, S.; et al. Metformin ameliorates the severity of experimental Alport syndrome. Sci. Rep. 2021, 11, 7053. [Google Scholar] [CrossRef]
- Erichsen, L.; Kloss, L.D.F.; Thimm, C.; Bohndorf, M.; Schichel, K.; Wruck, W.; Adjaye, J. Derivation of the immortalized cell line-UM51-PrePodo-hTERT and its responsiveness to Angiotensin II and activation of RAAS. Cells 2023, 12, 342. [Google Scholar] [CrossRef]
- Hypertension. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension#:~:text=For%20most%20people%2C%20the%20goal,vessels%20and%20prevent%20kidney%20damage (accessed on 25 May 2023).
- Crowley, S.D.; Gurley, S.B.; Herrera, M.J.; Ruiz, P.; Griffiths, R.; Kumar, A.P.; Kim, H.-S.; Smithies, O.; Le, T.H.; Coffman, T.M. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc. Natl. Acad. Sci. USA 2006, 47, 17985–17990. [Google Scholar] [CrossRef]
- Sobczuk, P.; Szcylik, C.; Porta, C.; Czarnecka, A.M. Renin angiotensin system deregulation as renal cancer risk factor. Oncol. Lett. 2017, 14, 5059–5068. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Mundel, P. Dual effects of RAS blockade on blood pressure and podocyte function. Curr. Hypertens. Rep. 2007, 9, 403–408. [Google Scholar] [CrossRef]
- Nguyen, L.; Wruck, W.; Erichsen, L.; Graffmann, N.; Adjaye, J. The Nephrotoxin Puromycin Aminonucleoside Induces Injury in Kidney Organoids Differentiated from Induced Pluripotent Stem Cells. Cells 2022, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Lau, R.W.K.; Fisher, C.; Phan, T.K.; Ozkocak, D.C.; Selby, J.; Saini, S.; Mukundan, S.; Wise, A.F.; Savige, J.; Poon, I.K.H.; et al. Modelling X-linked Alport Syndrome With Induced Pluripotent Stem Cell-Derived Podocytes. Kidney Int. Rep. 2021, 6, 2912–2917. [Google Scholar] [CrossRef]
- Ciampi, O.; Iacone, R.; Longaretti, L.; Benedetti, V.; Graf, M.; Magnone, M.C.; Patsch, C.; Xinaris, C.; Remuzzi, G.; Benigni, A.; et al. Generation of functional podocytes from human induced pluripotent stem cells. Stem Cell Res. 2016, 17, 130–139. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho Ribeiro, P.; Oliveira, L.F.; Filho, M.A.; Caldas, H.C. Differentiating Induced Pluripotent Stem Cells into Renal Cells: A New Approach to Treat Kidney Diseases. Stem Cell Res. 2020, 2020, 8894590. [Google Scholar] [CrossRef]
- Yanofsky, S.M.; Dugas, C.M.; Katsurada, A.; Liu, J.; Saifudeen, Z.; El-Dahr, S.S.; Satou, R. Angiotensin II biphasically regulates cell differentiation in human iPSC-derived kidney organoids. Am. J. Physiol. Renal Physiol. 2021, 321, 559–571. [Google Scholar] [CrossRef]
- Rüster, C.; Wolf, G. Renin-angiotensin-aldosterone system and progression of renal disease. J. Am. Soc. Nephrol. 2006, 17, 2985–2991. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, W.; Yang, Y.; Pan, Y.; Yang, Q.; Chen, X.; Singhal, P.C.; Ding, G. IQGAP1 regulates actin cytoskeleton organization in podocytes through interaction with nephrin. Cell Signal 2015, 27, 867–877. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Y.; Liu, Y.; Lian, W.; Chen, X.; Ren, Z.; Wang, H.; Singhal, P.C.; Ding, G. Angiotensin II down-regulates nephrin–Akt signaling and induces podocyte injury: Roleof c-Abl. Mol. Biol. Cell 2016, 27, 197–208. [Google Scholar] [CrossRef]
- Stegbauer, J.; Coffman, T.M. New insights into angiotensin receptor actions: From blood pressure to aging. Curr. Opin. Nephrol. Hypertens. 2011, 20, 84–88. [Google Scholar] [CrossRef]
- Mclntyre, M.; Caffe, S.E.; Michalak, R.A.; Reid, J.L. Losartan, an Orally Active Angiotensin (AT,) Receptor Antagonist: A Review of Its Efficacy and Safety in Essential Hypertension. Pharmacol. Ther. 1997, 74, 181–194. [Google Scholar] [CrossRef]
- Perez-Hernandez, J.; Olivares, M.D.; Forner, M.J.; Chaves, F.J.; Cortes, R.; Redon, J. Urinary dedifferentiated podocytes as a non-invasive biomarker of lupus nephritis. Nephrol. Dial. Transplant. 2016, 31, 780–789. [Google Scholar] [CrossRef]
- Rabow, S.; Hjorth, U.; Schönbeck, S.; Olofsson, P. Effects of oxytocin and anaesthesia on vascular tone in pregnant women: A randomised double-blind placebo-controlled study using non-invasive pulse wave analysis. BMC Pregnancy Childbirth 2018, 18, 453. [Google Scholar] [CrossRef]
- Song, W.; Wang, H.; Wu, Q. Atrial natriuretic peptide in cardiovascular biology and disease (NPPA). Gene 2015, 569, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lartey, J.; Taggart, J.; Robson, S.; Taggart, M. Altered Expression of Human Smooth Muscle Myosin Phosphatase Targeting (MYPT) Isovariants with Pregnancy and Labor. PLoS ONE 2016, 11, e0164352. [Google Scholar] [CrossRef] [PubMed]
- Basosi, R.; Gaggelli, E.; Valensin, G. The effect of binding divalent metal ions on the conformation of human angiotensin-II in solution as probed by nuclear and electron paramagnetic resonance investigations. J. Inorg. Biochem. 1984, 20, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Kanai, A.; Suzuki, Y.; Ogino, H.; Ochi, H. Adrenergic receptor signaling induced by Klf15, a regulator of regeneration enhancer, promotes kidney reconstruction. Proc. Natl. Acad. Sci. USA 2022, 119, e2204338119. [Google Scholar] [CrossRef] [PubMed]
- Farber, M.J.; Rizaldy, R.; Hildebrand, J.D. Shroom2 regulates contractility to control endothelial morphogenesis. Mol. Biol. Cell 2011, 22, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Leibowitz, A.; Yamamoto, N.; Rautureau, Y.; Paradis, P.; Schiffrin, E.L. Angiotensin Type 2 Receptor Agonist Compound 21 Reduces Vascular Injury and Myocardial Fibrosis in Stroke-Prone Spontaneously Hypertensive Rats. Hypertension 2012, 59, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Izuhara, Y.; Nangaku, M.; Inagi, R.; Tominaga, N.; Aizawa, T.; Kurokawa, K.; van Ypersele de Strihou, C.; Miyata, T. Renoprotective Properties of Angiotensin Receptor Blockers beyond Blood Pressure Lowering. J. Am. Soc. Nephrol. 2005, 16, 3631–3641. [Google Scholar] [CrossRef]
- Zhang, H.; Mo, X.; Zhou, Z.; Zhu, Z.; HuangFu, X.; Xu, T.; Wang, A.; Guo, Z. Associations among NPPA gene polymorphisms, serum ANP levels, and hypertension in the Chinese Han population. J. Hum. Hypertens. 2019, 33, 641–647. [Google Scholar] [CrossRef]
- Potter, L.R.; Yoder, A.R.; Flora, D.R.; Antos, L.K.; Dickey, D.M. Natriuretic Peptides: Their Structures, Receptors, Physiologic Functions and Therapeutic Applications. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 341–366. [Google Scholar] [CrossRef]
- Alqarni, S.A.; Bineid, A.; Ahmad, S.F.; Al-Harbi, N.O.; Alqahtani, F.; Ibrahim, K.E.; Ali, N.; Nadeem, A. Blockade of Tyrosine Kinase, LCK Leads to Reduction in Airway Inflammation through Regulation of Pulmonary Th2/Treg Balance and Oxidative Stress in Cockroach Extract-Induced Mouse Model of Allergic Asthma. Metabolites 2022, 12, 793. [Google Scholar] [CrossRef] [PubMed]
- Hall, G.; Wang, L.; Spurrney, R.F. TRPC Channels in Proteinuric Kidney Diseases. Cells 2019, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Polu, K.R.; Möller, C.C.; Kenlan, P.; Altintas, M.M.; Wei, C.; Faul, C.; Herbert, S.; Villegas, I.; Avila-Casadoq, C.; et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 2005, 37, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Kerjaschki, D. Polycation-induced dislocation of slit daiphragms and formation of cell junctions in rat kidney glomeruli: The effects of low temperature, divalent cations, colchicine, and cytochalasin B. Lab. Investig. 1978, 39, 430–440. [Google Scholar] [PubMed]
- Tian, D.; Jacobo, S.M.P.; Billing, D.; Rozkalne, A.; Gage, S.D.; Anagnostou, T.; Pavenstädt, H.; Hsu, H.-H.; Schlondorff, J.; Ramos, A.; et al. Antagonistic Regulation of Actin Dynamics and Cell Motility by TRPC5 and TRPC6 Channels. Sci. Signal. 2011, 3, ra77. [Google Scholar] [CrossRef]
- Kuwahara, K.; Wang, Y.; McAnally, J.; Richardson, J.A.; Bassel-Duby, R.; Hill, J.; Olson, E. TRCP6 fulfills a calcineurin signaling circuit during pathologic carciac remodeling. J. Clin. Investig. 2006, 116, 3114–3126. [Google Scholar] [CrossRef] [PubMed]
- Binz-Lotter, J.; Jüngst, C.; Rinschen, M.M.; Koehler, S.; Zentis, P.; Schauss, A.; Schermer, B.; Benzing, T.; HAckl, M.J. Injured Podocytes Are Sensitized to Angiotensin II–Induced Calcium Signaling. J. Am. Soc. Nephrol. 2020, 31, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.; Donnelly, M.; Merscher-Gomez, S.; Chang, Y.H.; Franz, S.; Delfgaauw, J.; Chang, J.-M.; Choi, H.Y.; Campbell, K.N.; Kim, K.; et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinureic effect of cyclosporine A. Nat. Med. 2008, 14, 931–938. [Google Scholar] [CrossRef]
- Schenk, L.K.; Möller-Kerutt, A.; Klosowski, R.; Wolters, D.; Schaffner-Reckinger, E.; Weide, T.; Pavenstädt, H.; Vollenbröker, B. Angiotensin II regulates phosphorylation of actin-associated proteins in human podocytes. FASEB J. 2017, 31, 5019–5035. [Google Scholar] [CrossRef]
- Namba, Y.; Ito, M.; Shigesada, K.; Maruyama, K. Human T cell L-plastin bundles actin filaments in a calcium-dependent manner. J. Biochem. 1992, 112, 503–507. [Google Scholar] [CrossRef]
- Adapted from “Mechanisms of Cancer-Associated Fibroblast Activation”, by BioRender.com. 2023. Available online: https://app.biorender.com/biorender-templates (accessed on 1 January 2023).
- Miller, R.G., Jr. Beyond Anova: Basics of Applied Statistics; Reissue Edition; Routledge: London, UK, 1997; ISBN 978-0-412-07011-2. [Google Scholar]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.S.; Irizarry, R.A. A frameowrk for oligonucleotide microarray preprocessing. Bioinformatics 2010, 26, 2363–2367. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Liaw, W.H.A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; Schwartz, M. Gplots: Various R Programming Tools for Plotting Data; R Package Version: Vienna, Austria, 2015. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thimm, C.; Erichsen, L.; Wruck, W.; Adjaye, J. Unveiling Angiotensin II and Losartan-Induced Gene Regulatory Networks Using Human Urine-Derived Podocytes. Int. J. Mol. Sci. 2023, 24, 10551. https://doi.org/10.3390/ijms241310551
Thimm C, Erichsen L, Wruck W, Adjaye J. Unveiling Angiotensin II and Losartan-Induced Gene Regulatory Networks Using Human Urine-Derived Podocytes. International Journal of Molecular Sciences. 2023; 24(13):10551. https://doi.org/10.3390/ijms241310551
Chicago/Turabian StyleThimm, Chantelle, Lars Erichsen, Wasco Wruck, and James Adjaye. 2023. "Unveiling Angiotensin II and Losartan-Induced Gene Regulatory Networks Using Human Urine-Derived Podocytes" International Journal of Molecular Sciences 24, no. 13: 10551. https://doi.org/10.3390/ijms241310551
APA StyleThimm, C., Erichsen, L., Wruck, W., & Adjaye, J. (2023). Unveiling Angiotensin II and Losartan-Induced Gene Regulatory Networks Using Human Urine-Derived Podocytes. International Journal of Molecular Sciences, 24(13), 10551. https://doi.org/10.3390/ijms241310551





