Discovery of Novel Pentacyclic Triterpene Acid Amide Derivatives as Excellent Antimicrobial Agents Dependent on Generation of Reactive Oxygen Species
Abstract
:1. Introduction
2. Results and Discussion
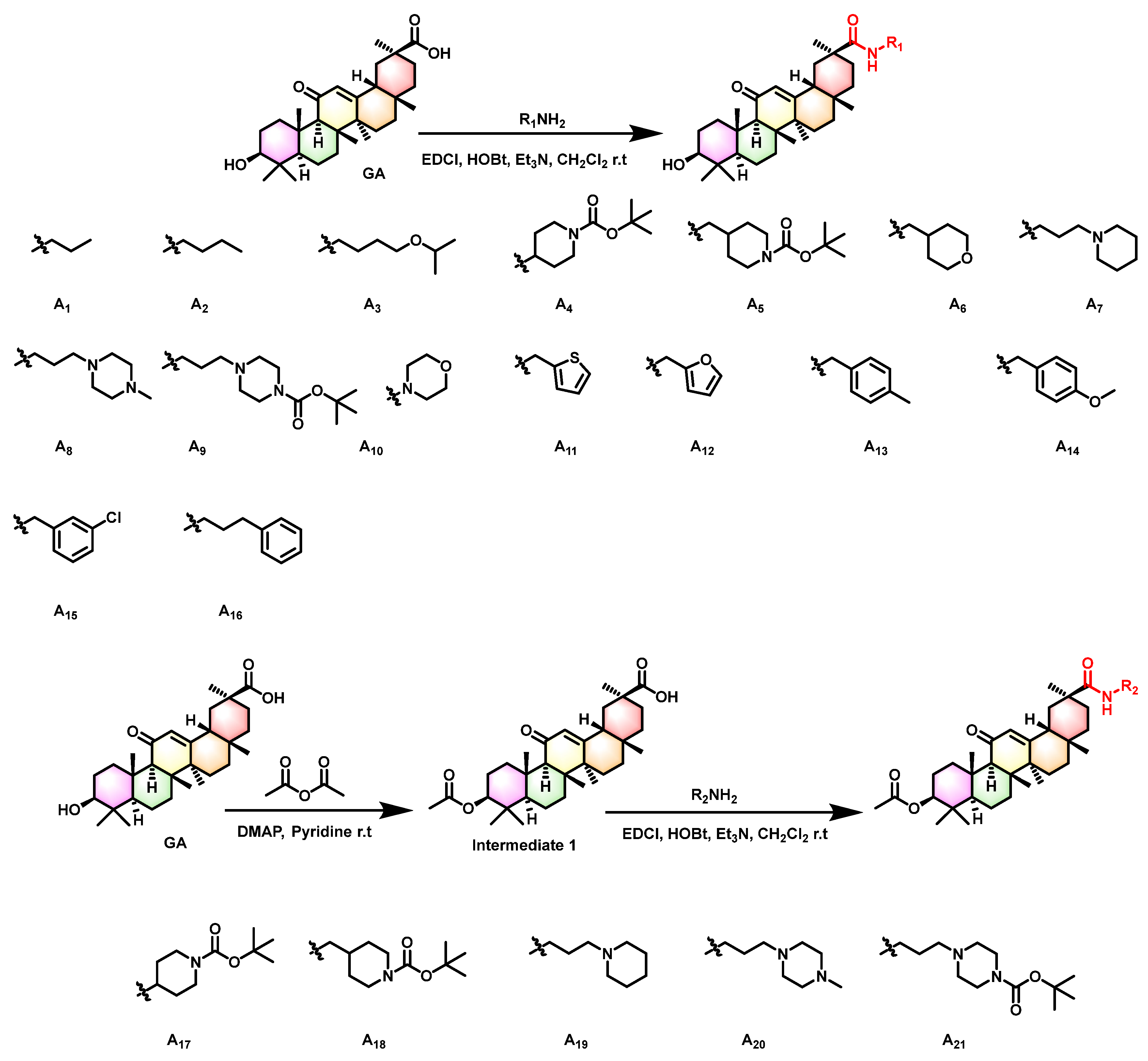
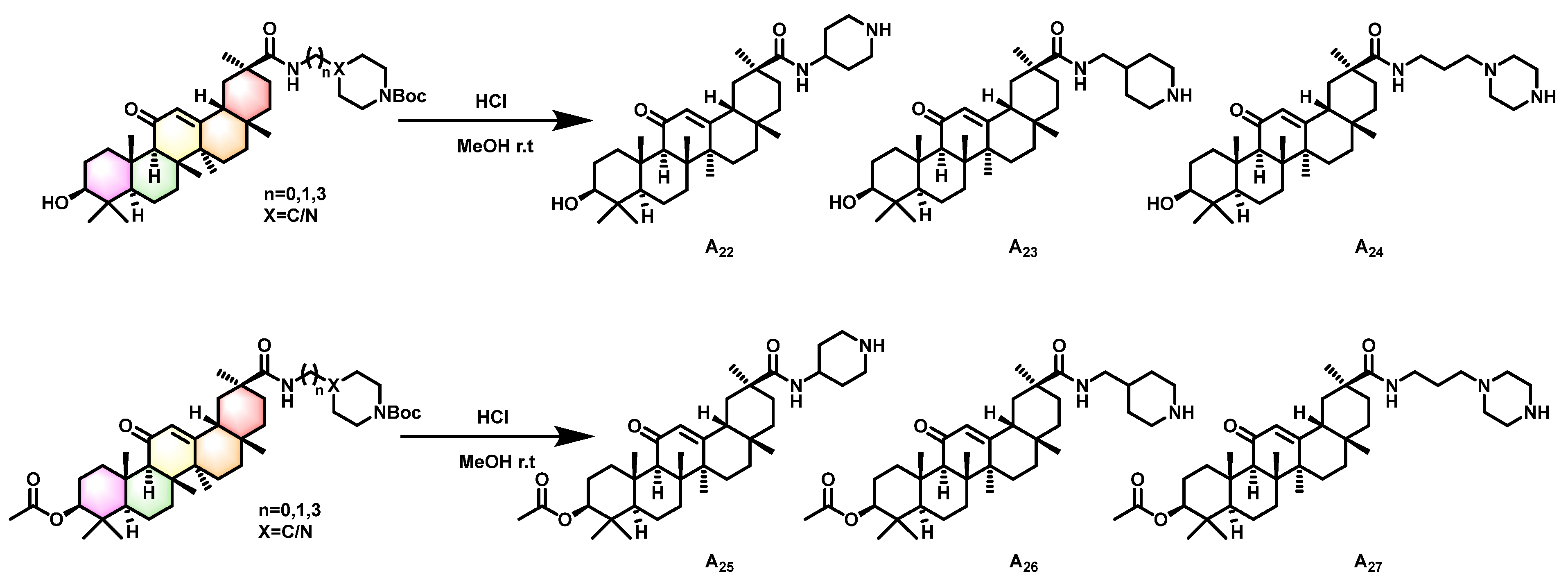
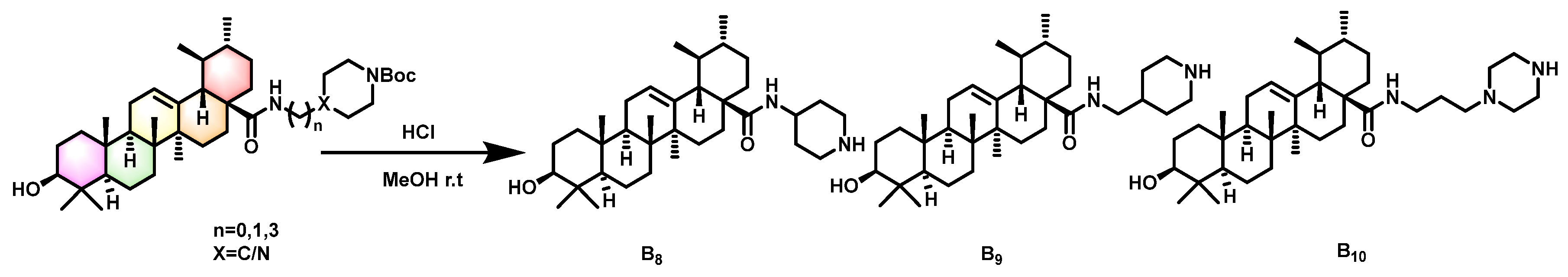
2.1. Synthesis of Title Compounds
2.2. Results of Anti-Xoo and Anti-Xac of the Title Compounds In Vitro
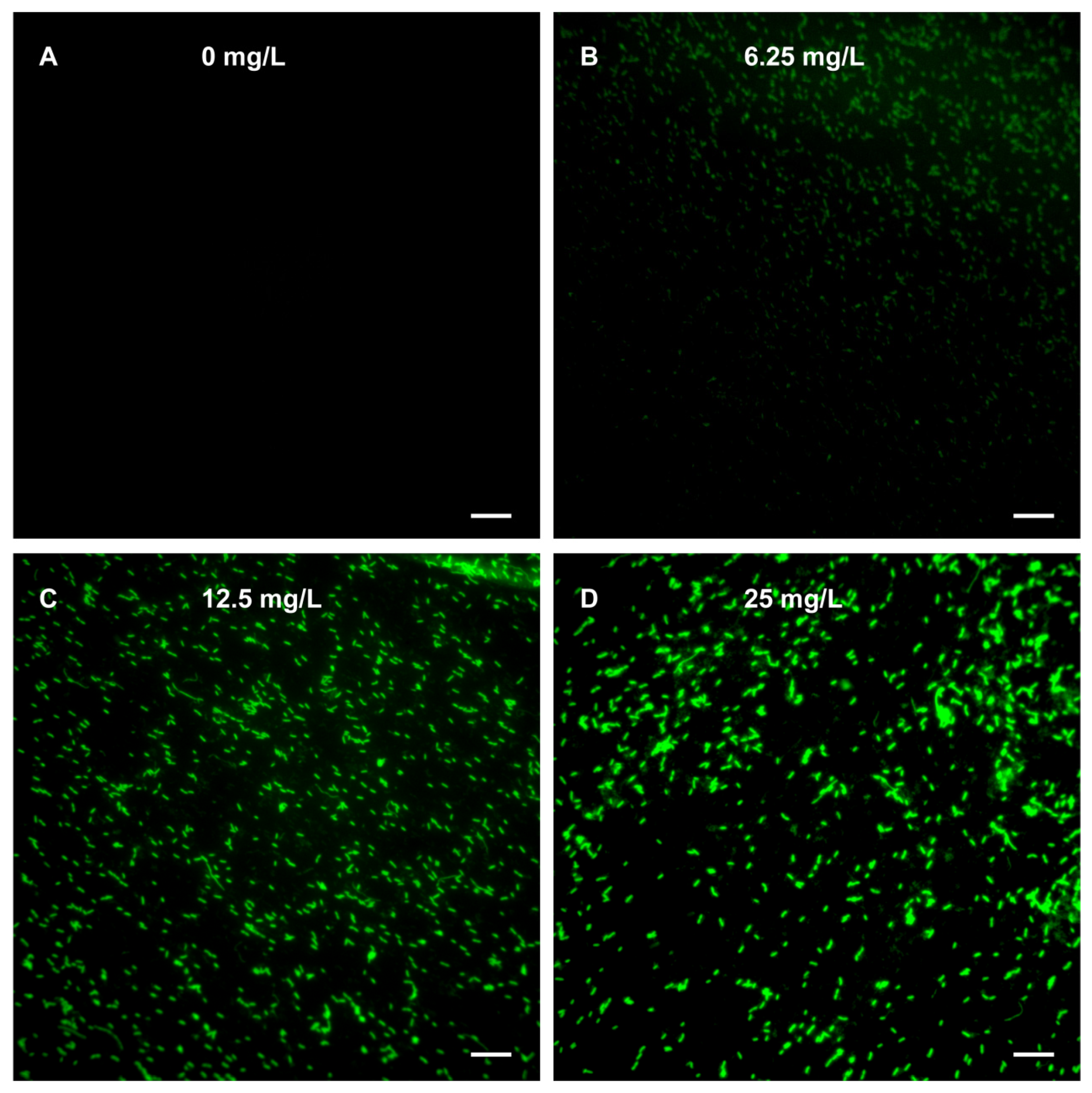
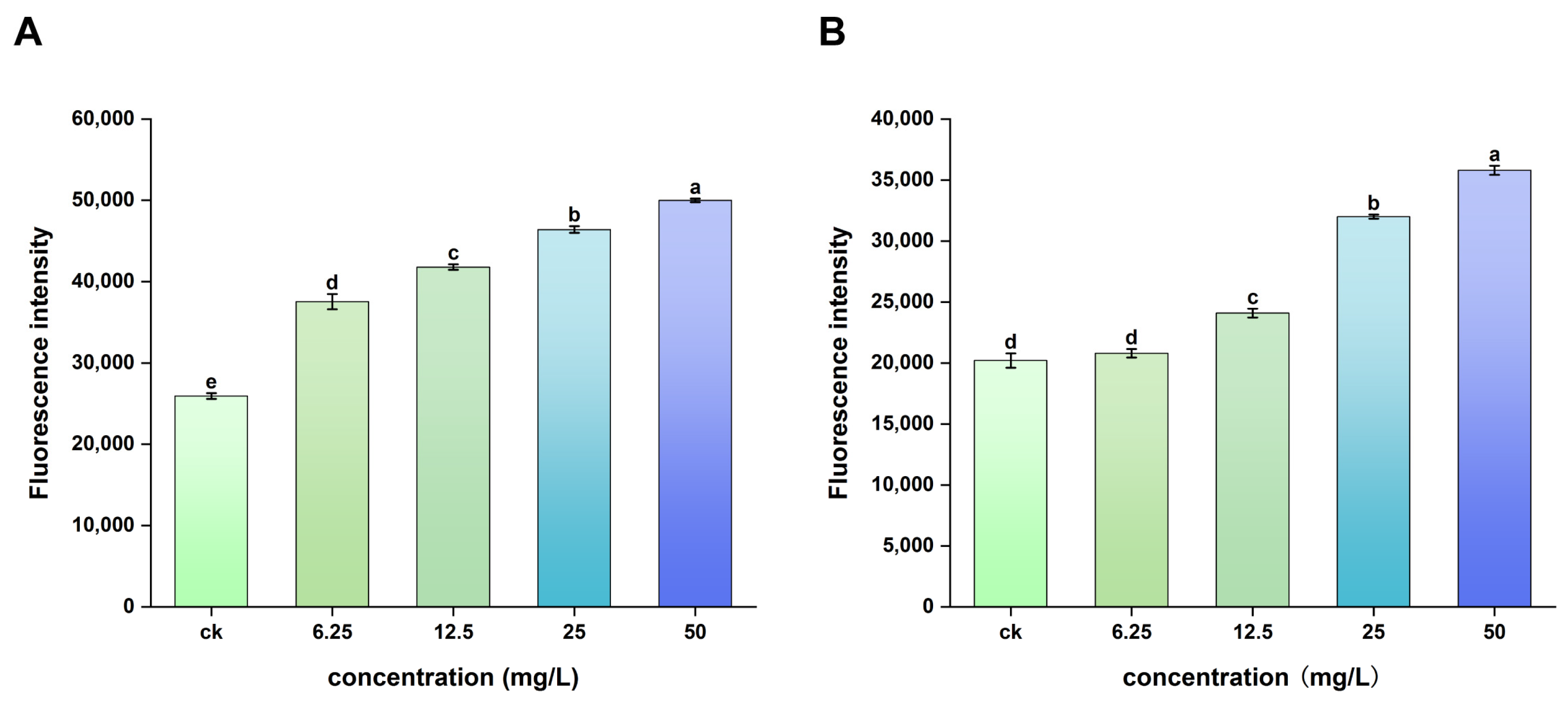
2.3. ROS Fluorescence Imaging
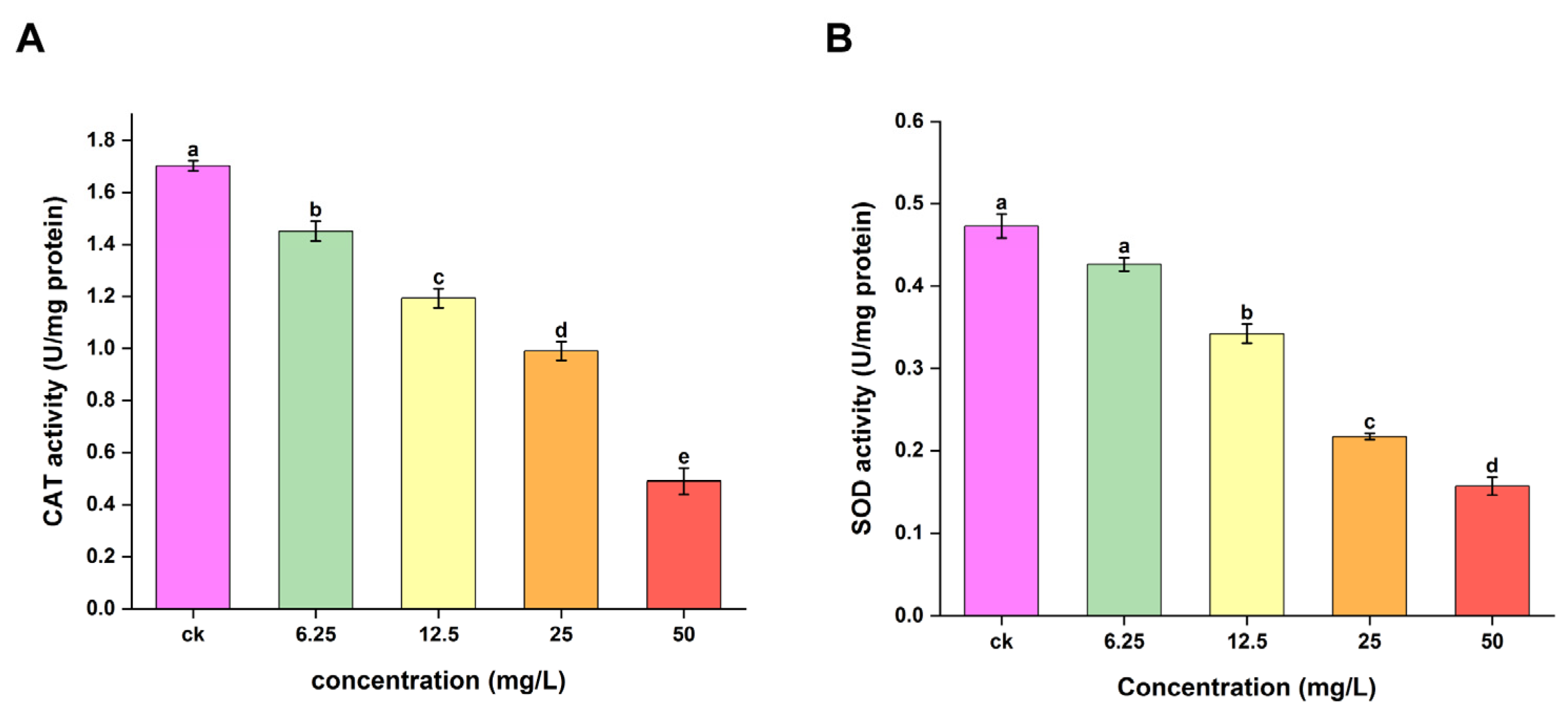
2.4. CAT and SOD Enzyme Activity Assay Results
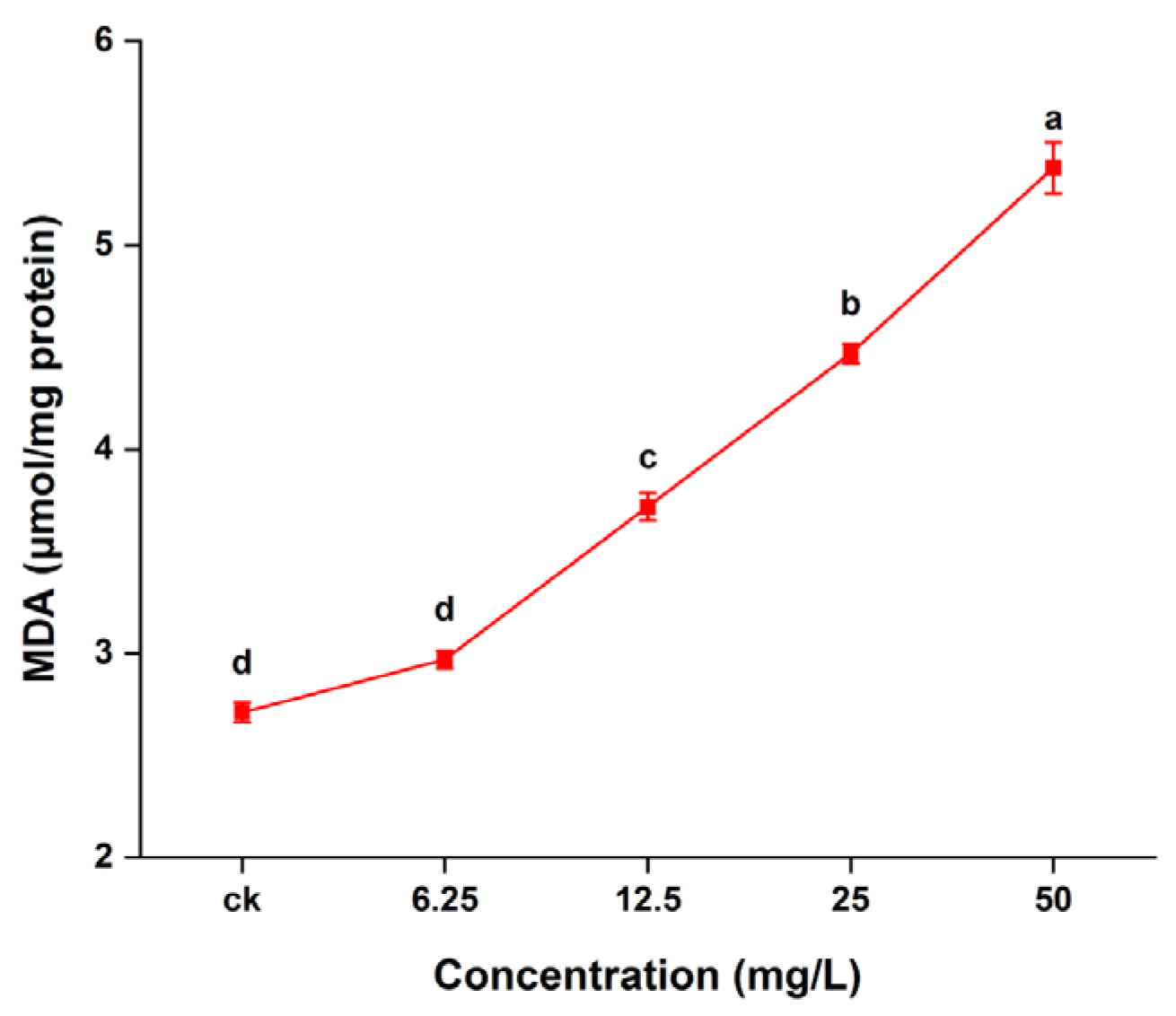
2.5. Lipid Peroxidation
2.6. H2O2 Plate Assay Results
2.7. Cell Membrane Damage Detection Results
2.8. In Vivo Anti-Xoo Effect of Compound A22
2.9. In Vivo Anti-Xac Effect of Compound A22
3. Materials and Methods
3.1. Instruments and Chemical Substances
3.2. General Protocols
3.3. ROS Detection Assay
3.4. Lipid Peroxidation Assay
3.5. Plate Assay
3.6. Assay of Outer Membrane Damage
3.7. Study of Inner Membrane Permeabilization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.B.; Zhang, P.; Li, M.S.; Shakoor, N.; Adeel, M.; Zhou, P.F.; Guo, M.L.; Jiang, Y.Q.; Zhao, W.C.; Lou, B.Z.; et al. Application and mechanisms of metal-based nanoparticles in the control of bacterial and fungal crop diseases. Pest Manag. Sci. 2023, 79, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Feng, Y.M.; Zhang, J.R.; Xiao, W.L.; Liu, S.S.; Zhou, X.; Zhang, H.; Wang, P.Y.; Liu, L.W.; Yang, S. Resistance-driven innovations in the discovery of bactericides: Novel triclosan derivatives decorating isopropanolamine moiety as promising anti-biofilm agents against destructive plant bacterial diseases. Pest Manag. Sci. 2023, 79, 2443–2455. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ye, H.J.; Xiang, H.M.; Zhou, X.; Wang, P.Y.; Liu, S.S.; Yang, B.X.; Yang, H.B.; Liu, L.W.; Yang, S. Photo-Stimuli Smart Supramolecular Self-Assembly of Azobenzene/β-Cyclodextrin Inclusion Complex for Controlling Plant Bacterial Diseases. Adv. Funct. Mater. 2023. [Google Scholar] [CrossRef]
- Qi, P.Y.; Wang, N.; Zhang, T.H.; Feng, Y.M.; Zhou, X.; Zeng, D.; Meng, J.; Liu, L.W.; Jin, L.H.; Yang, S. Anti-Virulence Strategy of Novel Dehydroabietic Acid Derivatives: Design, Synthesis, and Antibacterial Evaluation. Int. J. Mol. Sci. 2023, 24, 2897. [Google Scholar] [CrossRef]
- Xie, J.; Long, Z.Q.; Chen, A.Q.; Ding, Y.G.; Liu, S.T.; Zhou, X.; Liu, L.W.; Yang, S. Novel Sulfonamide Derivatives Containing a Piperidine Moiety as New Bactericide Leads for Managing Plant Bacterial Diseases. Int. J. Mol. Sci. 2023, 24, 5861. [Google Scholar] [CrossRef]
- Chu, P.L.; Feng, Y.M.; Long, Z.Q.; Xiao, W.L.; Ji, J.; Zhou, X.; Qi, P.Y.; Zhang, T.H.; Zhang, H.; Liu, L.W.; et al. Novel Benzothiazole Derivatives as Potential Anti-Quorum Sensing Agents for Managing Plant Bacterial Diseases: Synthesis, Antibacterial Activity Assessment, and SAR Study. J. Agric. Food Chem. 2023, 71, 6525–6540. [Google Scholar] [CrossRef]
- Qi, P.Y.; Zhang, T.H.; Wang, N.; Feng, Y.M.; Zeng, D.; Shao, W.B.; Meng, J.; Liu, L.W.; Jin, L.H.; Zhang, H.; et al. Natural Products-Based Botanical Bactericides Discovery: Novel Abietic Acid Derivatives as Anti-Virulence Agents for Plant Disease Management. J. Agric. Food Chem. 2023, 71, 5463–5475. [Google Scholar] [CrossRef]
- Hirooka, T.; Ishii, H. Chemical control of plant diseases. J. Gen. Plant Pathol. 2013, 79, 390–401. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Bruhl, C.A.; Imfeld, G.; Knabel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmuller, A.; et al. Fungicides: An Overlooked Pesticide Class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef]
- Rangasamy, K.; Athiappan, M.; Devarajan, N.; Parray, J.A.; Shameem, N.; Aruljothi, K.N.; Hashem, A.; Alqarawi, A.A.; Abd Allah, E.F. Cloning and Expression of the Organophosphate Pesticide-Degrading alpha-beta Hydrolase Gene in Plasmid pMK-07 to Confer Cross-Resistance to Antibiotics. BioMed Res. Int. 2018, 2018, 1535209. [Google Scholar] [CrossRef] [Green Version]
- Dorosh, O.; Fernandes, V.C.; Moreira, M.M.; Delerue-Matos, C. Occurrence of pesticides and environmental contaminants in vineyards: Case study of Portuguese grapevine canes. Sci. Total Environ. 2021, 791, 148395. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [Green Version]
- Dryden, M. Reactive oxygen species: A novel antimicrobial. Int. J. Antimicrob. Agents 2018, 51, 299–303. [Google Scholar] [CrossRef]
- Dryden, M.; Cooke, J.; Salib, R.; Holding, R.; Pender, S.L.F.; Brooks, J. Hot topics in reactive oxygen therapy: Antimicrobial and immunological mechanisms, safety and clinical applications. J. Glob. Antimicrob. Resist. 2017, 8, 194–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagnasco, D.; Paggiaro, P.; Latorre, M.; Folli, C.; Testino, E.; Bassi, A.; Milanese, M.; Heffler, E.; Manfredi, A.; Riccio, A.M.; et al. Severe asthma: One disease and multiple definitions. World Allergy Organ J. 2021, 14, 100606. [Google Scholar] [CrossRef] [PubMed]
- Verrillo, M.; Parisi, M.; Savy, D.; Caiazzo, G.; Di Caprio, R.; Luciano, M.A.; Cacciapuoti, S.; Fabbrocini, G.; Piccolo, A. Antiflammatory activity and potential dermatological applications of characterized humic acids from a lignite and a green compost. Sci. Rep. 2022, 12, 2152. [Google Scholar] [CrossRef]
- Dryden, M.; Tawse, C.; Adams, J.; Saeed, K.; Cooke, J. The use of Surgihoney to prevent or eradicate bacterial colonisation in dressing oncology long vascular lines. J. Wound. Care 2014, 23, 338–341. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Cao, L.J.; Xiang, X.; Wu, X.Z.; Ma, L.; Chen, F.; Cao, S.J.; Cheng, C.; Deng, D.W.; Qiu, L. ROS-Catalytic Transition-Metal-Based Enzymatic Nanoagents for Tumor and Bacterial Eradication. Adv. Funct. Mater. 2021, 32, 2107530. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Jafari, S.M.; Salehabadi, A.; Nafchi, A.M.; Uthaya Kumar, U.S.; Khalil, H.P.S.A. Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci. Tech. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Li, G.; Lou, H.X. Strategies to diversify natural products for drug discovery. Med. Res. Rev. 2018, 38, 1255–1294. [Google Scholar] [CrossRef]
- Mohamed, E.H.; Alghamdi, Y.S.; Mostafa Abdel-Hafez, S.; Soliman, M.M.; Alotaibi, S.H.; Hassan, M.Y.; Hany, N.A.D.; Amer, H.H. Susceptibility Assessment of Multidrug Resistant Bacteria to Natural Products. Dose-Response 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Yin, M.C.; Chan, K.C. Nonenzymatic Antioxidative and Antiglycative Effects of Oleanolic Acid and Ursolic Acid. J. Agric. Food Chem. 2007, 55, 7177–7181. [Google Scholar] [CrossRef]
- Zhao, W.B.; Zhao, Z.M.; Ma, Y.; Li, A.P.; Zhang, Z.J.; Hu, Y.M.; Zhou, Y.; Wang, R.; Luo, X.F.; Zhang, B.Q.; et al. Antifungal activity and preliminary mechanism of pristimerin against Sclerotinia sclerotiorum. Ind. Crops Prod. 2022, 185, 115124. [Google Scholar] [CrossRef]
- Sheng, L.X.; Huang, J.Y.; Liu, C.M.; Zhang, J.Z.; Cheng, K.G. Synthesis of oleanolic acid/ursolic acid/glycyrrhetinic acid-hydrogen sulfide donor hybrids and their antitumor activity. Med. Chem. Res. 2019, 28, 1212–1222. [Google Scholar] [CrossRef]
- Zhou, J.X.; Wink, M. Evidence for Anti-Inflammatory Activity of Isoliquiritigenin, 18β-Glycyrrhetinic Acid, Ursolic Acid, and the Traditional Chinese Medicine Plants Glycyrrhiza glabra and Eriobotrya japonica, at the Molecular Level. Medicines 2019, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.L.; Liu, H.W.; Yang, Y.H.; He, J.J.; Yang, B.X.; Yang, L.L.; Yang, S. Novel 18β-glycyrrhetinic acid amide derivatives show dual-acting capabilities for control of plant bacterial diseases through ROS-mediated antibacterial efficiency and activation of plant defense responses. J. Integr. Agric. 2022. [Google Scholar] [CrossRef]
- He, J.J.; Li, T.; Liu, H.W.; Yang, L.L.; Yang, Y.H.; Tao, Q.Q.; Zhou, X.; Wang, P.Y.; Yang, S. Ion exchange pattern-based 18β-glycyrrhetinic acid containing pyridinium salts derivatives as novel antibacterial agents with low toxicity. Arab. J. Chem. 2023, 16, 104771. [Google Scholar] [CrossRef]
- Wang, P.Y.; Xiang, M.; Luo, M.; Liu, H.W.; Zhou, X.; Wu, Z.B.; Liu, L.W.; Li, Z.; Yang, S. Novel piperazine-tailored ursolic acid hybrids as significant antibacterial agents targeting phytopathogens Xanthomonas oryzae pv. oryzae and X. axonopodis pv. citri probably directed by activation of apoptosis. Pest Manag. Sci. 2020, 76, 2746–2754. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.H.; Wu, F.R.; Zhou, H.; Xu, D.; Xu, G. Synthesis and Biological Activity of Novel Pyrazol-5-yl-benzamide Derivatives as Potential Succinate Dehydrogenase Inhibitors. J. Agric. Food Chem. 2021, 69, 5746–5754. [Google Scholar] [CrossRef]
- Sun, R.F.; Wang, Z.W.; Li, Y.Q.; Xiong, L.X.; Liu, Y.X.; Wang, Q.M. Design, synthesis, and insecticidal evaluation of new benzoylureas containing amide and sulfonate groups based on the sulfonylurea receptor protein binding site for diflubenzuron and glibenclamide. J. Agric. Food Chem. 2013, 61, 517–522. [Google Scholar] [CrossRef]
- Hamm, P.C. Discovery, Development, and Current Status of the Chloroacetamide Herbicides. Weed Sci. 2017, 22, 541–545. [Google Scholar] [CrossRef]
- Xiang, M.; Zhou, X.; Luo, T.R.; Wang, P.Y.; Liu, L.W.; Li, Z.; Wu, Z.B.; Yang, S. Design, Synthesis, Antibacterial Evaluation, and Induced Apoptotic Behaviors of Epimeric and Chiral 18β-Glycyrrhetinic Acid Ester Derivatives with an Isopropanolamine Bridge against Phytopathogens. J. Agric. Food Chem. 2019, 67, 13212–13220. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Q.Y.; Zhong, Y.Y.; Cui, X.P.; Jiang, Z.Y.; Wu, P.P.; Zheng, X.; Zhang, K.; Zhao, S.Q. Synthesis, anti-microbial and anti-inflammatory activities of 18β-glycyrrhetinic acid derivatives. Bioorg. Chem. 2020, 101, 103985–103995. [Google Scholar] [CrossRef]
- Usmani, Y.; Ahmed, A.; Faizi, S.; Versiani, M.A.; Shamshad, S.; Khan, S.; Simjee, S.U. Antimicrobial and biofilm inhibiting potential of an amide derivative [N-(2′, 4′-dinitrophenyl)-3β-hydroxyurs-12-en-28-carbonamide] of ursolic acid by modulating membrane potential and quorum sensing against colistin resistant Acinetobacter baumannii. Microb. Pathog. 2021, 157, 104997–105009. [Google Scholar] [CrossRef]
- Gu, W.; Hao, Y.; Zhang, G.; Wang, S.F.; Miao, T.T.; Zhang, K.P. Synthesis, in vitro antimicrobial and cytotoxic activities of new carbazole derivatives of ursolic acid. Bioorg. Med. Chem. Lett. 2015, 25, 554–557. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, Y.H.; Ding, Y.; Meng, J.; Wang, Z.C.; Wang, P.Y. Antibacterial Activity of Novel 18β-Glycyrrhetinic Hydrazide or Amide Derivatives. Chem. Res. Chin. Univ. 2021, 37, 662–667. [Google Scholar] [CrossRef]
- Kocsis, B.; Domokos, J.; Szabo, D. Chemical structure and pharmacokinetics of novel quinolone agents represented by avarofloxacin, delafloxacin, finafloxacin, zabofloxacin and nemonoxacin. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Xu, Y.R.; Yang, Y.; Yao, X.; Fu, Y.; Wang, Y.; Liu, Y.J.; Wang, X.Z. Evaluation of the Anticancer Activity and Mechanism Studies of Glycyrrhetic Acid Derivatives toward HeLa Cells. Molecules 2023, 28, 3164. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Wu, W.Y.; Li, A.L.; Liu, Q.S.; Sun, Y.; Gu, W. Synthesis, anticancer evaluation and mechanism studies of novel indolequinone derivatives of ursolic acid. Bioorgan. Chem. 2021, 109, 104705. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.M.; Meng, J.; Shao, W.B.; Zeng, D.; Ji, J.; Wang, P.Y.; Zhou, X.; Qi, P.Y.; Liu, L.W.; Yang, S. Plant protein-based self-assembling core–shell nanocarrier for effectively controlling plant viruses: Evidence for nanoparticle delivery behavior, plant growth promotion, and plant resistance induction. Chem. Eng. J. 2023, 464. [Google Scholar] [CrossRef]
- Zhang, S.; He, Y.D.; Sen, B.; Wang, G.Y. Reactive oxygen species and their applications toward enhanced lipid accumulation in oleaginous microorganisms. Bioresour. Technol. 2020, 307, 123234. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.C.; Zhang, P.L.; Kumar, K.V.; Li, S.; Geng, R.X.; Zhou, C.H. Discovery of unique thiazolidinone-conjugated coumarins as novel broad spectrum antibacterial agents. Eur. J. Med. Chem. 2022, 232, 114192. [Google Scholar] [CrossRef] [PubMed]
- Tymon, T.M.; Miller, E.P.; Gonzales, J.L.; Raab, A.; Kupper, F.C.; Carrano, C.J. Some aspects of the iodine metabolism of the giant kelp Macrocystis pyrifera (phaeophyceae). J. Inorg. Biochem. 2017, 177, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Ransy, C.; Vaz, C.; Lombes, A.; Bouillaud, F. Use of H2O2 to Cause Oxidative Stress, the Catalase Issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar] [CrossRef] [PubMed]
- Bajbouj, K.; Shafarin, J.; Abdalla, M.Y.; Ahmad, I.M.; Hamad, M. Estrogen-induced disruption of intracellular iron metabolism leads to oxidative stress, membrane damage, and cell cycle arrest in MCF-7 cells. Tumor Biol. 2017, 39, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Choi, U.; Lee, C.R. Antimicrobial Agents That Inhibit the Outer Membrane Assembly Machines of Gram-Negative Bacteria. J. Microbiol. Biotechnol. 2019, 29, 1–10. [Google Scholar] [CrossRef]
- Liu, S.S.; Zeng, D.; Zhang, T.H.; Hu, J.H.; Yang, B.X.; Yang, J.; Zhou, X.; Wang, P.Y.; Liu, L.W.; Wu, Z.B.; et al. Novel spiro[chromanone-2,4′-piperidine]-4-one derivatives as potential inhibitors of fatty acid synthesis in pathogens: Design, synthesis, and biological evaluation. Eur. J. Med. Chem. 2023, 250, 115215. [Google Scholar] [CrossRef]
- Li, F.F.; Zhao, W.H.; Tangadanchu, V.K.R.; Meng, J.P.; Zhou, C.H. Discovery of novel phenylhydrazone-based oxindole-thiolazoles as potent antibacterial agents toward Pseudomonas aeruginosa. Eur. J. Med. Chem. 2022, 239, 114521. [Google Scholar] [CrossRef]
- Huang, X.; Liu, H.W.; Long, Z.Q.; Li, Z.X.; Zhu, J.J.; Wang, P.Y.; Qi, P.Y.; Liu, L.W.; Yang, S. Rational Optimization of 1,2,3-Triazole-Tailored Carbazoles As Prospective Antibacterial Alternatives with Significant In Vivo Control Efficiency and Unique Mode of Action. J. Agric. Food Chem. 2021, 69, 4615–4627. [Google Scholar] [CrossRef]
- Tao, Q.Q.; Liu, L.W.; Wang, P.Y.; Long, Q.S.; Zhao, Y.L.; Jin, L.H.; Xu, W.M.; Chen, Y.; Li, Z.; Yang, S. Synthesis and In Vitro and In Vivo Biological Activity Evaluation and Quantitative Proteome Profiling of Oxadiazoles Bearing Flexible Heterocyclic Patterns. J. Agric. Food Chem. 2019, 67, 7626–7639. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Huang, X.; Liu, L.W.; Wang, P.Y.; Long, Q.S.; Tao, Q.Q.; Li, Z.; Yang, S. Identification of Racemic and Chiral Carbazole Derivatives Containing an Isopropanolamine Linker as Prospective Surrogates against Plant Pathogenic Bacteria: In Vitro and In Vivo Assays and Quantitative Proteomics. J. Agric. Food Chem. 2019, 67, 7512–7525. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.X.; Li, Z.X.; Liu, S.S.; Yang, J.; Wang, P.Y.; Liu, H.W.; Zhou, X.; Liu, L.W.; Wu, Z.B.; Yang, S. Novel cinnamic acid derivatives as a versatile tool for developing agrochemicals for controlling plant virus and bacterial diseases by enhancing plant defense responses. Pest Manag. Sci. 2023, 79, 2556–2570. [Google Scholar] [CrossRef]
- Busch, C.J.; Binder, C.J. Malondialdehyde epitopes as mediators of sterile inflammation. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2017, 1862, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhao, X.Z.; Liu, F.; Cao, Y.; Wang, B.; Wang, X.Y.; Yin, M.; Wang, Q.Z.; Feng, X. Crucial role of oxidative stress in bactericidal effect of parthenolide against Xanthomonas oryzae pv. oryzae. Pest Manag. Sci. 2018, 74, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Su, S.S.; Liu, H.W.; Zhang, J.R.; Qi, P.Y.; Ding, Y.; Zhang, L.; Yang, L.L.; Liu, L.W.; Zhou, X.; Yang, S. Discovery and structure–activity relationship studies of novel tetrahydro-β-carboline derivatives as apoptosis initiators for treating bacterial infections1. J. Integr. Agric. 2023; in press. [Google Scholar] [CrossRef]
- Ning, Y.W.; Yan, A.H.; Yang, K.; Wang, Z.X.; Li, X.F.; Jia, Y.M. Antibacterial activity of phenyllactic acid against Listeria monocytogenes and Escherichia coli by dual mechanisms. Food Chem. 2017, 228, 533–540. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.C.; Bi, Y.; Wang, T.L.; Dong, Y.P.; Yang, Q.; Zhang, T.T. 2-Phenylethyl Isothiocyanate Exerts Antifungal Activity against Alternaria alternata by Affecting Membrane Integrity and Mycotoxin Production. Toxins 2020, 12, 124. [Google Scholar] [CrossRef] [Green Version]




| No. | Xoo, Inhibition (%) | Xac, Inhibition (%) | ||
|---|---|---|---|---|
| 100 mg L−1 | 50 mg L−1 | 100 mg L−1 | 50 mg L−1 | |
| A1 | 14.01 ± 0.48 | 0 | 27.39 ± 1.82 | 16.97 ± 0.35 |
| A2 | 25.05 ± 0.48 | 16.59 ± 0.34 | 24.82 ± 0.83 | 10.78 ± 1.18 |
| A3 | 28.35 ± 0.62 | 16.76 ± 0.43 | 24.10 ± 1.74 | 14.89 ± 0.16 |
| A4 | 25.82 ± 1.48 | 25.07 ± 1.22 | 13.59 ± 0.68 | 11.34 ± 0.11 |
| A5 | 14.53 ± 0.61 | 0 | 12.44 ± 0.54 | 0 |
| A6 | 0 | 0 | 18.32 ± 1.02 | 0 |
| A7 | 100 | 100 | 100 | 100 |
| A8 | 26.20 ± 0.69 | 4.80 ± 1.54 | 54.97 ± 0.54 | 42.66 ± 0.43 |
| A9 | 49.01 ± 1.31 | 43.22 ± 2.16 | 45.65 ± 1.10 | 14.51 ± 1.69 |
| A10 | 11.48 ± 0.40 | 0 | 14.11 ± 0.56 | 0 |
| A11 | 26.76 ± 0.49 | 18.08 ± 0.32 | 25.43 ± 0.90 | 13.23 ± 0.53 |
| A12 | 14.34 ± 1.15 | 0 | 26.48 ± 0.74 | 19.86 ± 0.20 |
| A13 | 0 | 0 | 28.08 ± 0.38 | 19.93 ± 0.96 |
| A14 | 12.47 ± 0.73 | 0 | 25.92 ± 0.65 | 17.41 ± 0.37 |
| A15 | 27.53 ± 1.17 | 26.81 ± 0.37 | 18.38 ± 0.64 | 13.92 ± 0.58 |
| A16 | 20.93 ± 1.42 | 11.10 ± 1.63 | 24.76 ± 1.12 | 19.37 ± 2.40 |
| A17 | 20.46 ± 0.57 | 13.41 ± 0.29 | 17.31 ± 0.65 | 11.64 ± 0.64 |
| A18 | 22.37 ± 0.34 | 16.54 ± 0.42 | 19.15 ± 0.74 | 14.62 ± 0.44 |
| A19 | 100 | 100 | 54.42 ± 1.13 | 46.86 ± 0.63 |
| A20 | 83.86 ± 0.95 | 46.38 ± 0.32 | 53.20 ± 0.90 | 42.96 ± 1.53 |
| A21 | 12.58 ± 0.14 | 0 | 26.99 ± 0.87 | 4.51 ± 0.84 |
| B1 | 65.71 ± 0.54 | 50.05 ± 0.46 | 36.92 ± 1.03 | 0 |
| B2 | 39.61 ± 0.17 | 23.37 ± 0.26 | 33.21 ± 0.54 | 26.42 ± 0.70 |
| B3 | 35.99 ± 0.35 | 17.63 ± 0.19 | 24.64 ± 0.46 | 16.78 ± 0.34 |
| B4 | 100 | 96.15 ± 0.55 | 100 | 100 |
| B5 | 24.25 ± 0.46 | 13.63 ± 0.28 | 20.15 ± 0.32 | 16.22 ± 0.16 |
| B6 | 100 | 100 | 100 | 100 |
| B7 | 47.25 ± 0.29 | 47.25 ± 0.29 | 33.89 ± 0.75 | 23.44 ± 0.56 |
| BT | 100 | 98.32 ± 0.41 | 37.91 ± 0.77 | 25.11 ± 0.39 |
| TC | 45.85 ± 0.43 | 23.14 ± 0.73 | 43.91 ± 0.11 | 24.12 ± 0.49 |
| No. | Xoo, Inhibition (%) | Xac, Inhibition (%) | ||
|---|---|---|---|---|
| 100 mg L−1 | 50 mg L−1 | 100 mg L−1 | 50 mg L−1 | |
| A22 | 100 | 100 | 100 | 100 |
| A23 | 100 | 100 | 100 | 100 |
| A24 | 100 | 100 | 100 | 100 |
| A25 | 100 | 100 | 100 | 100 |
| A26 | 100 | 100 | 100 | 100 |
| A27 | 72.76 ± 0.75 | 49.29 ± 0.90 | 73.31 ± 1.78 | 63.56 ± 0.82 |
| B8 | 100 | 100 | 100 | 100 |
| B9 | 100 | 100 | 100 | 100 |
| B10 | 100 | 100 | 100 | 100 |
| BT | 100 | 98.32 ± 0.41 | 37.91 ± 0.77 | 25.11 ± 0.39 |
| TC | 45.85 ± 0.43 | 23.14 ± 0.73 | 43.91 ± 0.11 | 24.12 ± 0.49 |
| No. | Xoo | Xac | ||
|---|---|---|---|---|
| Regression Equation | EC50 (mg L−1) | Regression Equation | EC50 (mg L−1) | |
| A7 | y = 3.5452x + 0.3240 | 20.84 ± 0.13 | y = 1.7082x + 3.9066 | 4.37 ± 0.04 |
| A8 | >100 | y = 1.2135x + 2.6610 | 84.62 ± 2.12 | |
| A19 | y = 1.8221x + 2.4622 | 24.71 ± 0.36 | y = 0.9400x + 3.3694 | 54.29 ± 1.04 |
| A20 | y = 1.8413x + 1.9751 | 43.93 ± 014 | y= 1.1593x + 2.9623 | 57.24 ± 0.91 |
| A22 | y = 7.2107x + 1.2254 | 3.34 ± 0.10 | y = 1.7068x + 4.0860 | 3.30 ± 0.05 |
| A23 | y = 5.6216x + 0.4634 | 6.41 ± 0.09 | y = 4.5234x + 2.2644 | 4.03 ± 0.12 |
| A24 | y = 3.9871x + 1.4228 | 7.89 ± 0.52 | y = 2.6194x + 3.1286 | 5.18 ± 0.05 |
| A25 | y = 4.7663x + 1.6672 | 5.00 ± 0.04 | y = 4.8039x + 0.2598 | 9.70 ± 0.19 |
| A26 | y = 5.0248x + 1.9465 | 4.05 ± 0.03 | y = 2.4930x + 3.2891 | 4.86 ± 019 |
| A27 | y = 2.1043x + 1.7011 | 36.96 ± 0.39 | y= 1.2455x + 3.1459 | 30.81 ± 0.97 |
| B1 | y = 0.9824x + 3.4146 | 41.10 ± 0.47 | >100 | |
| B4 | y = 3.0673x + 2.1599 | 8.43 ± 0.03 | y = 2.7998x + 3.0624 | 4.92 ± 0.10 |
| B6 | y = 6.1769x − 1.1890 | 10.05 ± 0.20 | y = 3.4013x + 2.6057 | 5.06 ± 0.06 |
| B8 | y = 5.0369x + 0.1523 | 9.17 ± 0.08 | y = 2.9158x + 3.0058 | 4.82 ± 0.03 |
| B9 | y = 1.3787x + 3.9682 | 5.60 ± 0.03 | y = 4.7978x + 1.7313 | 4.80 ± 0.05 |
| B10 | y = 4.3891x + 2.1653 | 4.42 ± 0.12 | y = 3.1919x + 2.9068 | 4.53 ± 0.07 |
| BT | y = 2.0170x + 2.0704 | 28.34 ± 0.39 | y = 2.6471x − 0.3129 | 101.64 ± 0.76 |
| TC | y = 2.0774x + 06817 | 119.87 ± 3.47 | y = 3.7668x − 2.2971 | 86.54 ± 0.18 |
| Chemicals | Curative Activity | Protective Activity | ||||
|---|---|---|---|---|---|---|
| Morbidity (%) | Disease Index (%) | Control Efficiency (%) b | Morbidity (%) | Disease Index (%) | Control Efficiency (%) b | |
| A22 | 100 | 46.67 | 44.74 B | 100 | 42.22 | 50.44 B |
| A22-OPO | 100 | 33.33 | 59.65 A | 100 | 35.56 | 58.26 A |
| A22-OSi | 100 | 34.07 | 60.53 A | 100 | 31.85 | 62.61 A |
| BT | 100 | 51.11 | 39.47 C | 100 | 50.37 | 40.87 C |
| TC | 100 | 51.85 | 38.60 C | 100 | 53.33 | 37.39 C |
| CK a | 100 | 84.44 | 100 | 85.19 | ||
| Chemicals | Protective Efficiency (%) a | Curative Efficiency (%) a |
|---|---|---|
| A22 | 84.37 ± 10.13 A | 58.86 ± 17.08 A |
| TC | 74.44 ± 14.63 B | 51.51 ± 7.49 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Chen, K.; Wang, G.; Liu, H.; Shao, L.; Zhou, X.; Liu, L.; Yang, S. Discovery of Novel Pentacyclic Triterpene Acid Amide Derivatives as Excellent Antimicrobial Agents Dependent on Generation of Reactive Oxygen Species. Int. J. Mol. Sci. 2023, 24, 10566. https://doi.org/10.3390/ijms241310566
Yang Y, Chen K, Wang G, Liu H, Shao L, Zhou X, Liu L, Yang S. Discovery of Novel Pentacyclic Triterpene Acid Amide Derivatives as Excellent Antimicrobial Agents Dependent on Generation of Reactive Oxygen Species. International Journal of Molecular Sciences. 2023; 24(13):10566. https://doi.org/10.3390/ijms241310566
Chicago/Turabian StyleYang, Yihong, Kunlun Chen, Guangdi Wang, Hongwu Liu, Lihui Shao, Xiang Zhou, Liwei Liu, and Song Yang. 2023. "Discovery of Novel Pentacyclic Triterpene Acid Amide Derivatives as Excellent Antimicrobial Agents Dependent on Generation of Reactive Oxygen Species" International Journal of Molecular Sciences 24, no. 13: 10566. https://doi.org/10.3390/ijms241310566







