The Role of SOCS3 in Regulating Meat Quality in Jinhua Pigs
Abstract
1. Introduction
2. Results
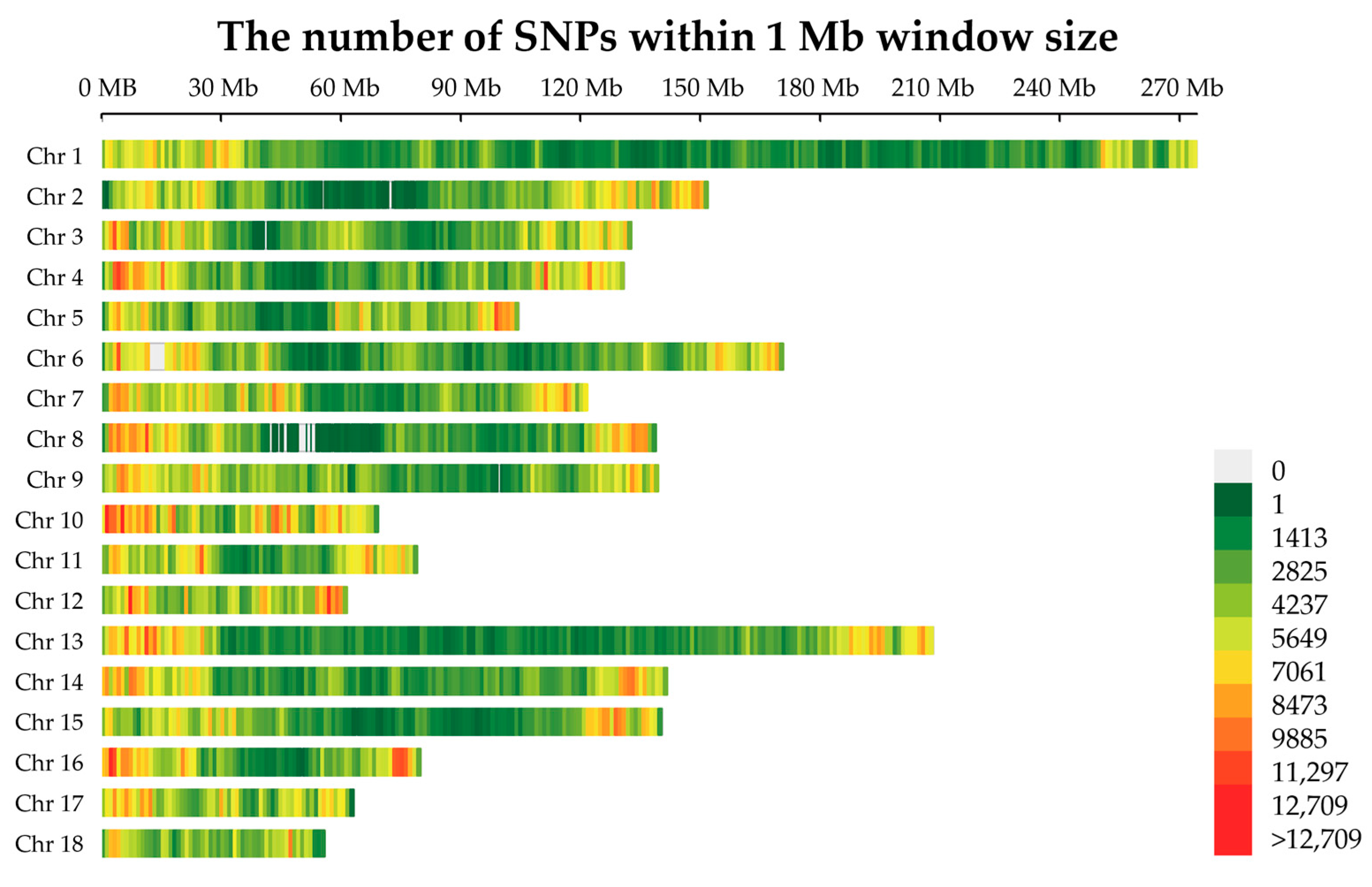
2.1. Genetic Population Structure Analysis in Jinhua Pigs
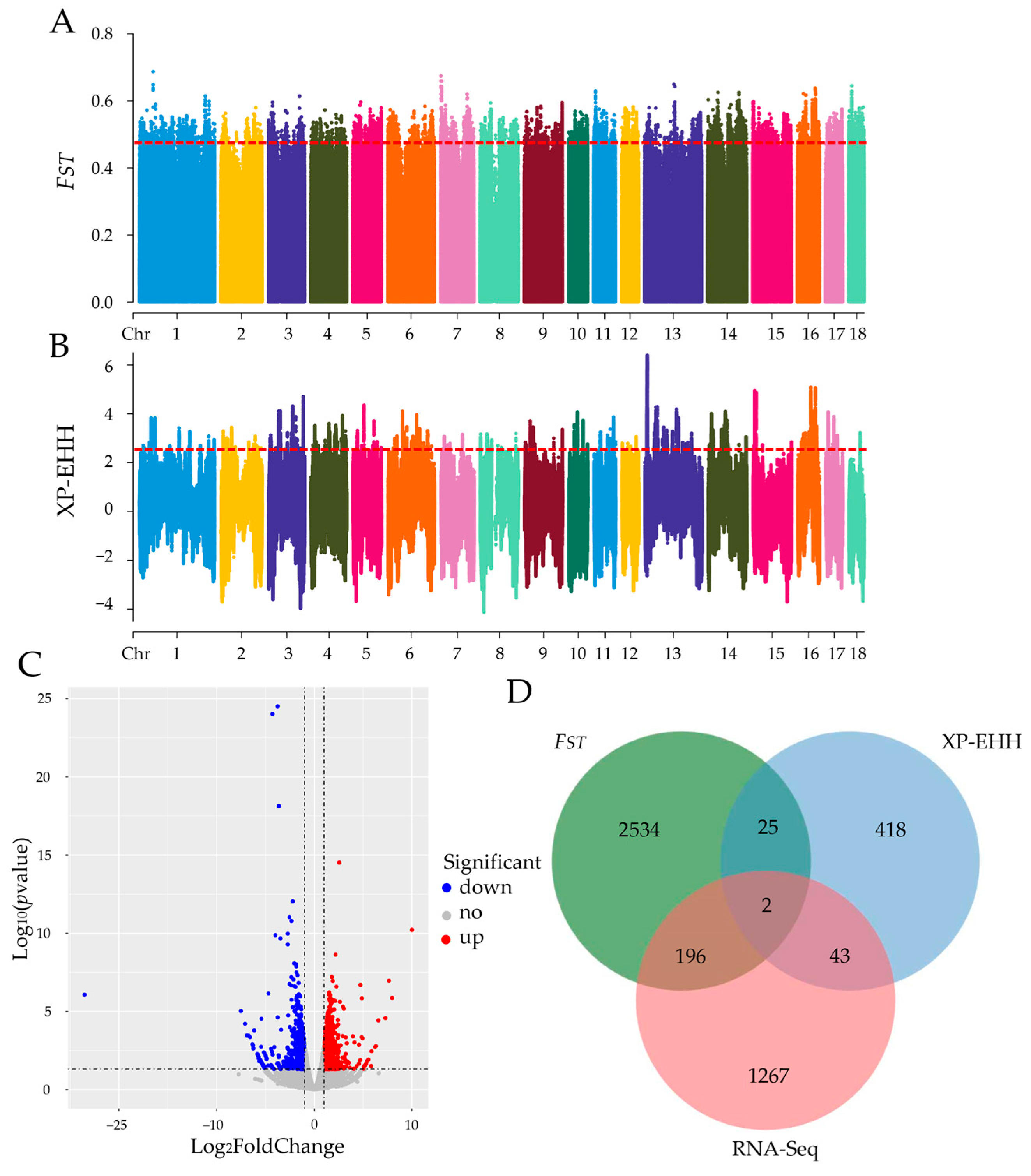
2.2. Screening for Candidate Genes Based on Selection Signature and RNA-Seq
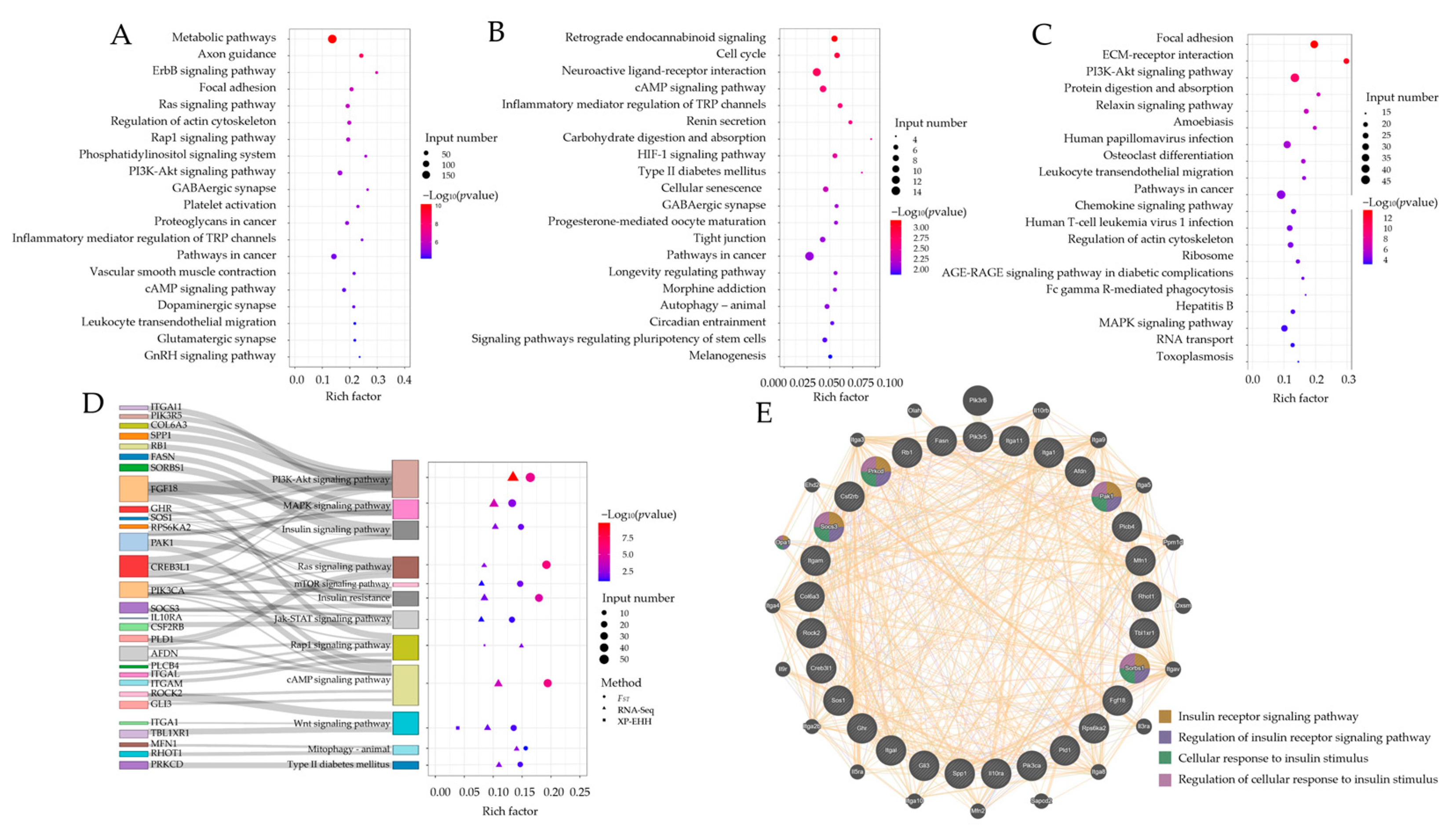
2.3. Functional Analysis of Candidate Genes
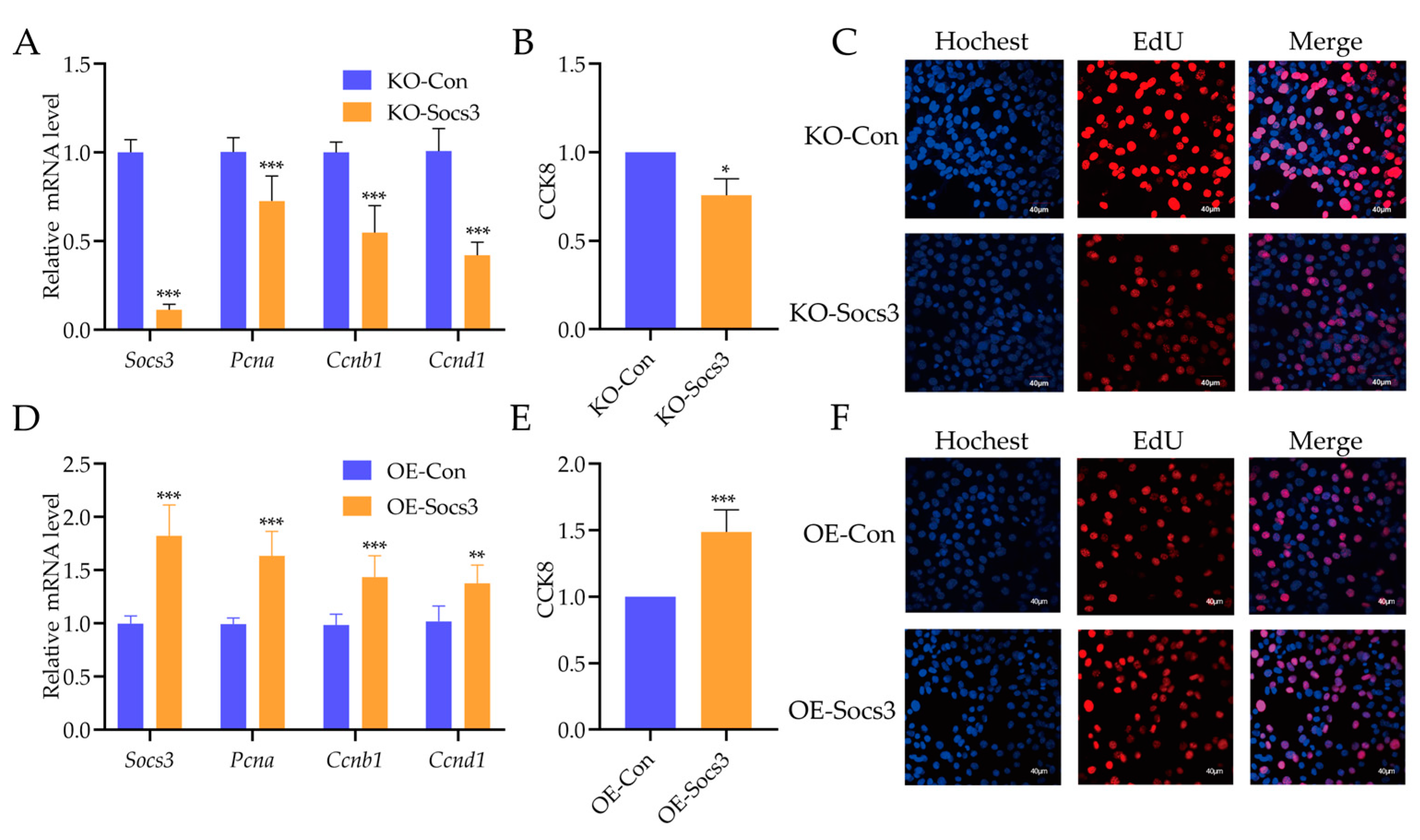
2.4. Effects of Socs3 on the Proliferation Ability of Myoblasts
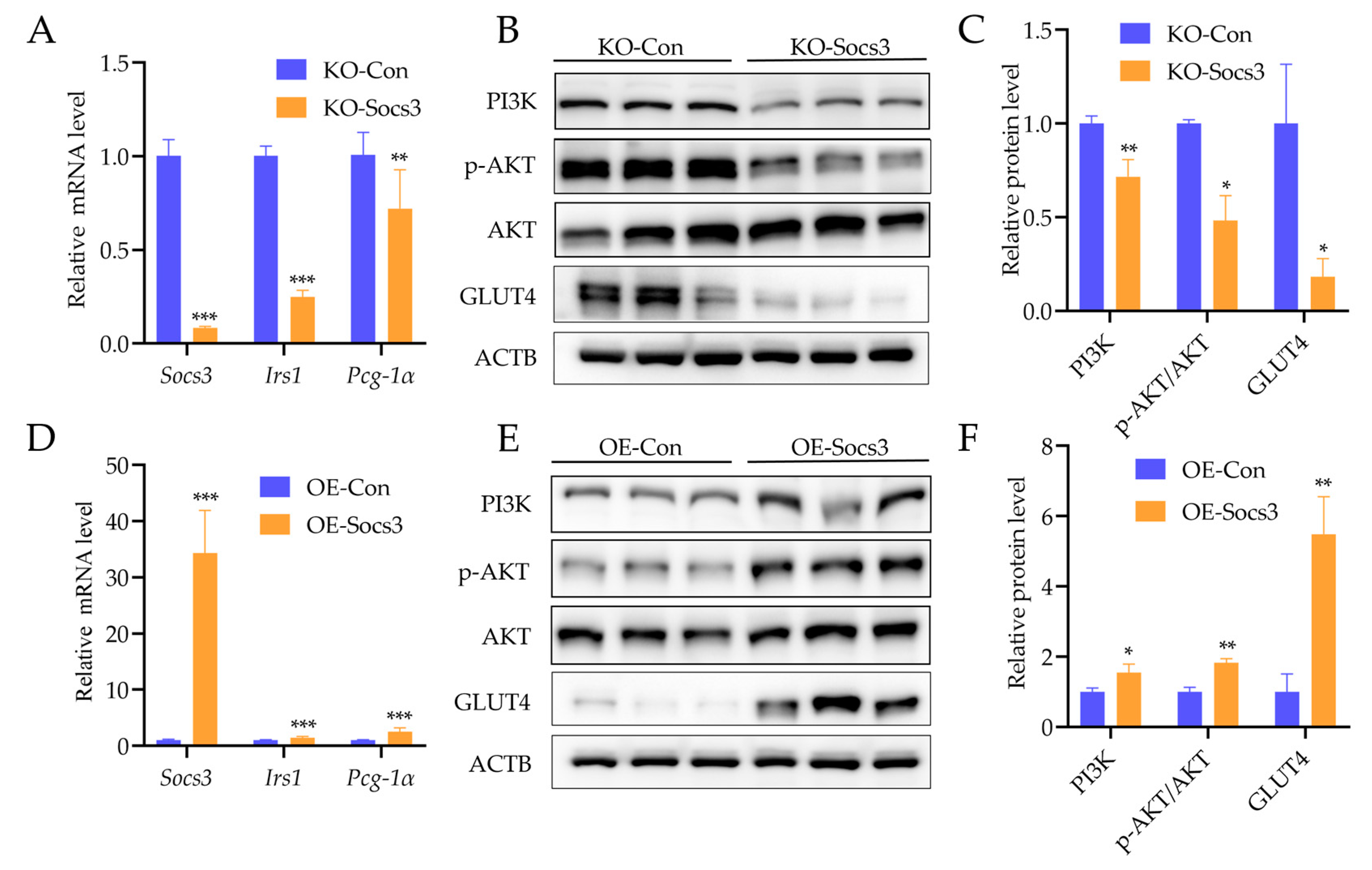
2.5. Socs3 Is Necessary for Glucose Uptake of Myotubes Cells
3. Discussion
4. Materials and Methods
4.1. Genotype and Quality Control
4.2. Population Structure
4.3. Selective Signatures Analysis and RNA-Seq Analysis
4.4. Functional Annotation
4.5. Cell Culture and Treatment
4.6. SOCS3 Knockdown and Overexpression
4.7. Cell Proliferation and Cell Cycle Assay
4.8. Quantitative Real-Time PCR
4.9. Western Blot
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Sun, H.; Chen, Q.; Zhang, X.; Wang, Q.; Pan, Y. A genome scan for selection signatures in Taihu pig breeds using next-generation sequencing. Anim. Int. J. Anim. Biosci. 2019, 13, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, P.; Wang, S.; Ji, X.; Chen, D.; Jiang, A.; Xiao, W.; Gu, Y.; Jiang, Y.; Zeng, Y.; et al. Genome-wide DNA methylation analysis in Chinese Chenghua and Yorkshire pigs. BMC Genom. Data 2021, 22, 21. [Google Scholar] [CrossRef]
- Shi, Y.; Fan, S.; Wu, M.; Zuo, Z.; Li, X.; Jiang, L.; Shen, Q.; Xu, P.; Zeng, L.; Zhou, Y.; et al. YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression. Nat. Commun. 2019, 10, 4892. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Z.; Zhang, Z.; Xiao, Q.; Mawed, S.; Xu, Z.; Zhang, X.; Yang, H.; Zhu, M.; Xue, M.; et al. Genomic signatures reveal selection of characteristics within and between Meishan pig populations. Anim. Genet. 2018, 49, 119–126. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, H.; Zhang, Z.; Zhao, Q.; Olasege, B.S.; Li, Q.; Yue, Y.; Ma, P.; Zhang, X.; Wang, Q.; et al. Assessment of Autozygosity Derived From Runs of Homozygosity in Jinhua Pigs Disclosed by Sequencing Data. Front. Genet. 2019, 10, 274. [Google Scholar] [CrossRef]
- Noidad, S.; Limsupavanich, R.; Suwonsichon, S.; Chaosap, C. Effect of visual marbling levels in pork loins on meat quality and Thai consumer acceptance and purchase intent. Asian-Australas. J. Anim. Sci. 2019, 32, 1923–1932. [Google Scholar] [CrossRef]
- Gjerlaug-Enger, E.; Aass, L.; Odegård, J.; Vangen, O. Genetic parameters of meat quality traits in two pig breeds measured by rapid methods. Anim. Int. J. Anim. Biosci. 2010, 4, 1832–1843. [Google Scholar] [CrossRef]
- Suzuki, K.; Irie, M.; Kadowaki, H.; Shibata, T.; Kumagai, M.; Nishida, A. Genetic parameter estimates of meat quality traits in Duroc pigs selected for average daily gain, longissimus muscle area, backfat thickness, and intramuscular fat content. J. Anim. Sci. 2005, 83, 2058–2065. [Google Scholar] [CrossRef]
- Lonergan, S.M.; Huff-Lonergan, E.; Rowe, L.J.; Kuhlers, D.L.; Jungst, S.B. Selection for lean growth efficiency in Duroc pigs influences pork quality. J. Anim. Sci. 2001, 79, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.R.; Baas, T.J.; Stalder, K.J.; Mabry, J.W. Effect of long-term selection for increased leanness on meat and eating quality traits in Duroc swine. J. Anim. Sci. 2006, 84, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Sant’anna, A.C.; Valente, T.D.S.; Magalhães, A.F.B.; Espigolan, R.; Ceballos, M.C.; de Albuquerque, L.G.; Paranhos da Costa, M.J.R. Relationships between temperament, meat quality, and carcass traits in Nellore cattle1. J. Anim. Sci. 2019, 97, 4721–4731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Gong, H.; Cui, L.; Zhang, W.; Ma, J.; Chen, C.; Ai, H.; Xiao, S.; Huang, L.; et al. Genetic correlation of fatty acid composition with growth, carcass, fat deposition and meat quality traits based on GWAS data in six pig populations. Meat Sci. 2019, 150, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Bérard, J.; Kreuzer, M.; Bee, G. Effect of litter size and birth weight on growth, carcass and pork quality, and their relationship to postmortem proteolysis. J. Anim. Sci. 2008, 86, 2357–2368. [Google Scholar] [CrossRef]
- Ma, J.; Yang, J.; Zhou, L.; Ren, J.; Liu, X.; Zhang, H.; Yang, B.; Zhang, Z.; Ma, H.; Xie, X.; et al. A splice mutation in the PHKG1 gene causes high glycogen content and low meat quality in pig skeletal muscle. PLoS Genet. 2014, 10, e1004710. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, D.; Bastiaansen, J.; Malek, M.; Helm, J.; Woollard, J.; Plastow, G.; Rothschild, M. Evidence for new alleles in the protein kinase adenosine monophosphate-activated gamma(3)-subunit gene associated with low glycogen content in pig skeletal muscle and improved meat quality. Genetics 2001, 159, 1151–1162. [Google Scholar] [CrossRef]
- Milan, D.; Jeon, J.T.; Looft, C.; Amarger, V.; Robic, A.; Thelander, M.; Rogel-Gaillard, C.; Paul, S.; Iannuccelli, N.; Rask, L.; et al. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science 2000, 288, 1248–1251. [Google Scholar] [CrossRef]
- Park, J.E.; Seo, Y.; Han, J.S. HM-chromanone, a component of Portulaca oleracea L., stimulates glucose uptake and glycogen synthesis in skeletal muscle cell. Phytomed. Int. J. Phytother. Phytopharm. 2021, 83, 153473. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, S.; Zhang, K.; Zhan, H.; Peng, X.; Xie, S.; Li, X.; Zhao, S.; Ma, Y. Identifying Selection Signatures for Backfat Thickness in Yorkshire Pigs Highlights New Regions Affecting Fat Metabolism. Genes 2019, 10, 254. [Google Scholar] [CrossRef]
- Diao, S.; Huang, S.; Chen, Z.; Teng, J.; Ma, Y.; Yuan, X.; Chen, Z.; Zhang, H.; Li, J.; Zhang, Z. Genome-Wide Signatures of Selection Detection in Three South China Indigenous Pigs. Genes 2019, 10, 346. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Z.; Yuan, Z.; Lo, L.J.; Chen, J.; Wang, Y.; Peng, J. Distinctive genes determine different intramuscular fat and muscle fiber ratios of the longissimus dorsi muscles in Jinhua and landrace pigs. PLoS ONE 2013, 8, e53181. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Luo, C.; Wang, M.; Guo, L.; Chen, X.; Li, Z.; Zheng, M.; Folaniyi, B.S.; Luo, W.; Shu, D.; et al. Genome diversity of Chinese indigenous chicken and the selective signatures in Chinese gamecock chicken. Sci. Rep. 2020, 10, 14532. [Google Scholar] [CrossRef]
- Tan, X.; Liu, R.; Li, W.; Zheng, M.; Zhu, D.; Liu, D.; Feng, F.; Li, Q.; Liu, L.; Wen, J.; et al. Assessment the effect of genomic selection and detection of selective signature in broilers. Poult. Sci. 2022, 101, 101856. [Google Scholar] [CrossRef]
- Duarte, I.N.H.; Bessa, A.F.O.; Rola, L.D.; Genuíno, M.V.H.; Rocha, I.M.; Marcondes, C.R.; Regitano, L.C.A.; Munari, D.P.; Berry, D.P.; Buzanskas, M.E. Cross-population selection signatures in Canchim composite beef cattle. PLoS ONE 2022, 17, e0264279. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, H.; Liu, Y.; Sun, L.; Chen, N.; Jiang, F.; You, W.; Yang, Z.; Zhang, B.; Song, E.; et al. Assessing Genomic Diversity and Selective Pressures in Bohai Black Cattle Using Whole-Genome Sequencing Data. Animals 2022, 12, 665. [Google Scholar] [CrossRef]
- Vatsiou, A.I.; Bazin, E.; Gaggiotti, O.E. Detection of selective sweeps in structured populations: A comparison of recent methods. Mol. Ecol. 2016, 25, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Kang, Y.J.; Sung, B.; Jang, J.Y.; Hwang, N.L.; Oh, H.J.; Ahn, Y.R.; Kim, H.J.; Shin, J.H.; Yoo, M.A.; et al. Folic acid is necessary for proliferation and differentiation of C2C12 myoblasts. J. Cell. Physiol. 2018, 233, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yuan, L.; Chen, G.; Chen, X.; Yang, X.; Fan, T.; Sun, C.; Fan, D.; Chen, Z. Deciphering Obesity-Related Gene Clusters Unearths SOCS3 Immune Infiltrates and 5mC/m6A Modifiers in Ossification of Ligamentum Flavum Pathogenesis. Front. Endocrinol. 2022, 13, 861567. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Tong, J.; Zhu, S.; Batista, P.J.; Duffy, E.E.; Zhao, J.; Bailis, W.; Cao, G.; Kroehling, L.; Chen, Y.; et al. m6A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature 2017, 548, 338–342. [Google Scholar] [CrossRef]
- Phielix, E.; Begovatz, P.; Gancheva, S.; Bierwagen, A.; Kornips, E.; Schaart, G.; Hesselink, M.K.C.; Schrauwen, P.; Roden, M. Athletes feature greater rates of muscle glucose transport and glycogen synthesis during lipid infusion. JCI Insight 2019, 4, e127928. [Google Scholar] [CrossRef]
- Yang, H.; Li, H.; Song, Y.; Sui, Y.; Du, Z.; Zhang, G. Bombyxin II Regulates Glucose Absorption and Glycogen Synthesis through the PI3K Signaling Pathway in HepG2 Cells. BioMed Res. Int. 2021, 2021, 6639232. [Google Scholar] [CrossRef]
- Pedroso, J.A.B.; Ramos-Lobo, A.M.; Donato, J., Jr. SOCS3 as a future target to treat metabolic disorders. Hormones 2019, 18, 127–136. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Pereira, D.F.; Kappel, V.D.; Folador, P.; Figueiredo Mdos, S.; Pizzolatti, M.G.; Silva, F.R. Insulin signaling: A potential signaling pathway for the stimulatory effect of kaempferitrin on glucose uptake in skeletal muscle. Eur. J. Pharmacol. 2013, 712, 1–7. [Google Scholar] [CrossRef]
- Di Camillo, B.; Eduati, F.; Nair, S.K.; Avogaro, A.; Toffolo, G.M. Leucine modulates dynamic phosphorylation events in insulin signaling pathway and enhances insulin-dependent glycogen synthesis in human skeletal muscle cells. BMC Cell Biol. 2014, 15, 9. [Google Scholar] [CrossRef]
- Ye, J.; Zheng, R.; Wang, Q.; Liao, L.; Ying, Y.; Lu, H.; Cianflone, K.; Ning, Q.; Luo, X. Downregulating SOCS3 with siRNA ameliorates insulin signaling and glucose metabolism in hepatocytes of IUGR rats with catch-up growth. Pediatr. Res. 2012, 72, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Palanivel, R.; Fullerton, M.D.; Galic, S.; Honeyman, J.; Hewitt, K.A.; Jorgensen, S.B.; Steinberg, G.R. Reduced Socs3 expression in adipose tissue protects female mice against obesity-induced insulin resistance. Diabetologia 2012, 55, 3083–3093. [Google Scholar] [CrossRef] [PubMed]
- Montori-Grau, M.; Aguilar-Recarte, D.; Zarei, M.; Pizarro-Delgado, J.; Palomer, X.; Vázquez-Carrera, M. Endoplasmic reticulum stress downregulates PGC-1α in skeletal muscle through ATF4 and an mTOR-mediated reduction of CRTC2. Cell Commun. Signal. CCS 2022, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sun, W.; Luo, G.; Wu, L.; Xu, G.; Hou, D.; Hou, Y.; Guo, X.; Mu, X.; Qin, L.; et al. Panax notoginseng saponins alleviate skeletal muscle insulin resistance by regulating the IRS1-PI3K-AKT signaling pathway and GLUT4 expression. FEBS Open Bio 2019, 9, 1008–1019. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Chen, T.; Xiong, J.L.; Wu, D.; Xi, Q.Y.; Luo, J.Y.; Sun, J.J.; Zhang, Y.L. miR-146b Inhibits Glucose Consumption by Targeting IRS1 Gene in Porcine Primary Adipocytes. Int. J. Mol. Sci. 2018, 19, 783. [Google Scholar] [CrossRef]
- Li, W.; Liang, X.; Zeng, Z.; Yu, K.; Zhan, S.; Su, Q.; Yan, Y.; Mansai, H.; Qiao, W.; Yang, Q.; et al. Simvastatin inhibits glucose uptake activity and GLUT4 translocation through suppression of the IR/IRS-1/Akt signaling in C2C12 myotubes. Biomed. Pharmacother. Biomed. Pharmacother. 2016, 83, 194–200. [Google Scholar] [CrossRef]
- Yang, B.; Cui, L.; Perez-Enciso, M.; Traspov, A.; Crooijmans, R.; Zinovieva, N.; Schook, L.B.; Archibald, A.; Gatphayak, K.; Knorr, C.; et al. Genome-wide SNP data unveils the globalization of domesticated pigs. Genet. Sel. Evol. GSE 2017, 49, 71. [Google Scholar] [CrossRef]
- Browning, B.L.; Zhou, Y.; Browning, S.R. A One-Penny Imputed Genome from Next-Generation Reference Panels. Am. J. Hum. Genet. 2018, 103, 338–348. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Chen, Z.; Sun, J.; Cao, C.; Wu, F.; Xu, Z.; Zhao, W.; Sun, H.; Guo, L.; et al. PHARP: A pig haplotype reference panel for genotype imputation. Sci. Rep. 2022, 12, 12645. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Sabeti, P.C.; Varilly, P.; Fry, B.; Lohmueller, J.; Hostetter, E.; Cotsapas, C.; Xie, X.; Byrne, E.H.; McCarroll, S.A.; Gaudet, R.; et al. Genome-wide detection and characterization of positive selection in human populations. Nature 2007, 449, 913–918. [Google Scholar] [CrossRef]
- Szpiech, Z.A.; Hernandez, R.D. selscan: An efficient multithreaded program to perform EHH-based scans for positive selection. Mol. Biol. Evol. 2014, 31, 2824–2827. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Chen, Z.; Zhang, Z.; Wang, Z.; Zhang, Z.; Wang, Q.; Pan, Y. The Role of SOCS3 in Regulating Meat Quality in Jinhua Pigs. Int. J. Mol. Sci. 2023, 24, 10593. https://doi.org/10.3390/ijms241310593
Wu F, Chen Z, Zhang Z, Wang Z, Zhang Z, Wang Q, Pan Y. The Role of SOCS3 in Regulating Meat Quality in Jinhua Pigs. International Journal of Molecular Sciences. 2023; 24(13):10593. https://doi.org/10.3390/ijms241310593
Chicago/Turabian StyleWu, Fen, Zitao Chen, Zhenyang Zhang, Zhen Wang, Zhe Zhang, Qishan Wang, and Yuchun Pan. 2023. "The Role of SOCS3 in Regulating Meat Quality in Jinhua Pigs" International Journal of Molecular Sciences 24, no. 13: 10593. https://doi.org/10.3390/ijms241310593
APA StyleWu, F., Chen, Z., Zhang, Z., Wang, Z., Zhang, Z., Wang, Q., & Pan, Y. (2023). The Role of SOCS3 in Regulating Meat Quality in Jinhua Pigs. International Journal of Molecular Sciences, 24(13), 10593. https://doi.org/10.3390/ijms241310593







