Kinetics of Glucoregulatory Peptide Hormones during Hemodialysis with Cellulose Triacetate and Polysulfone Dialyzers in Patients with Diabetes and End-Stage Kidney Disease
Abstract
:1. Introduction
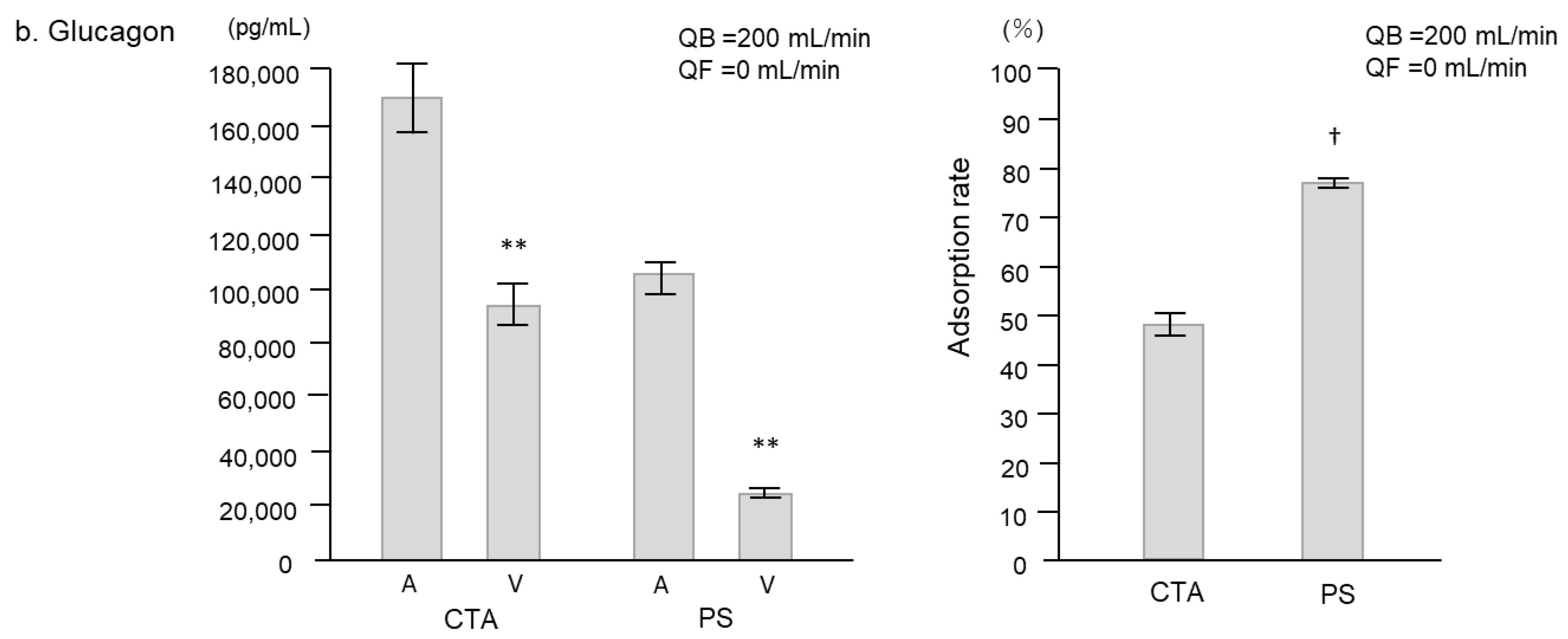
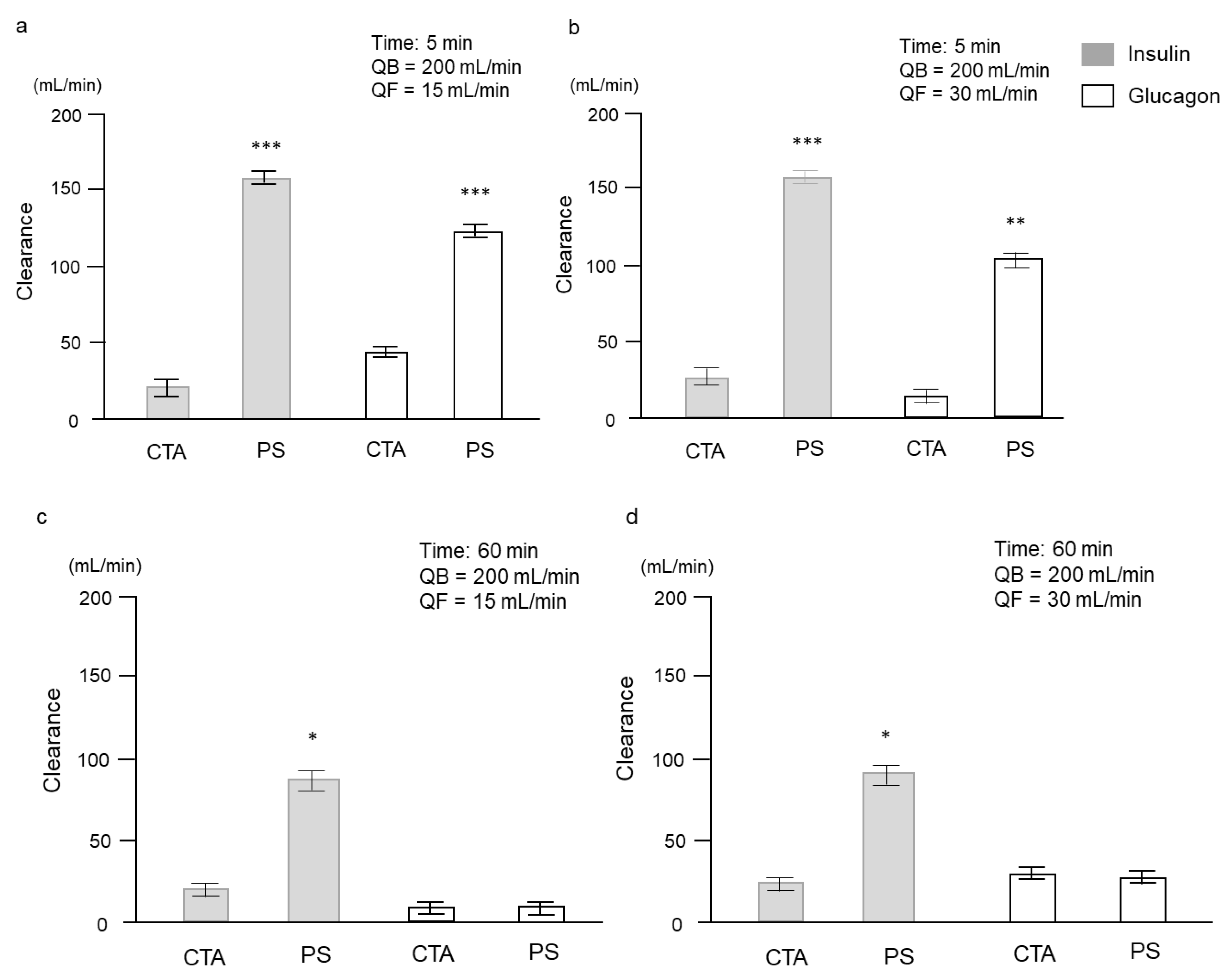
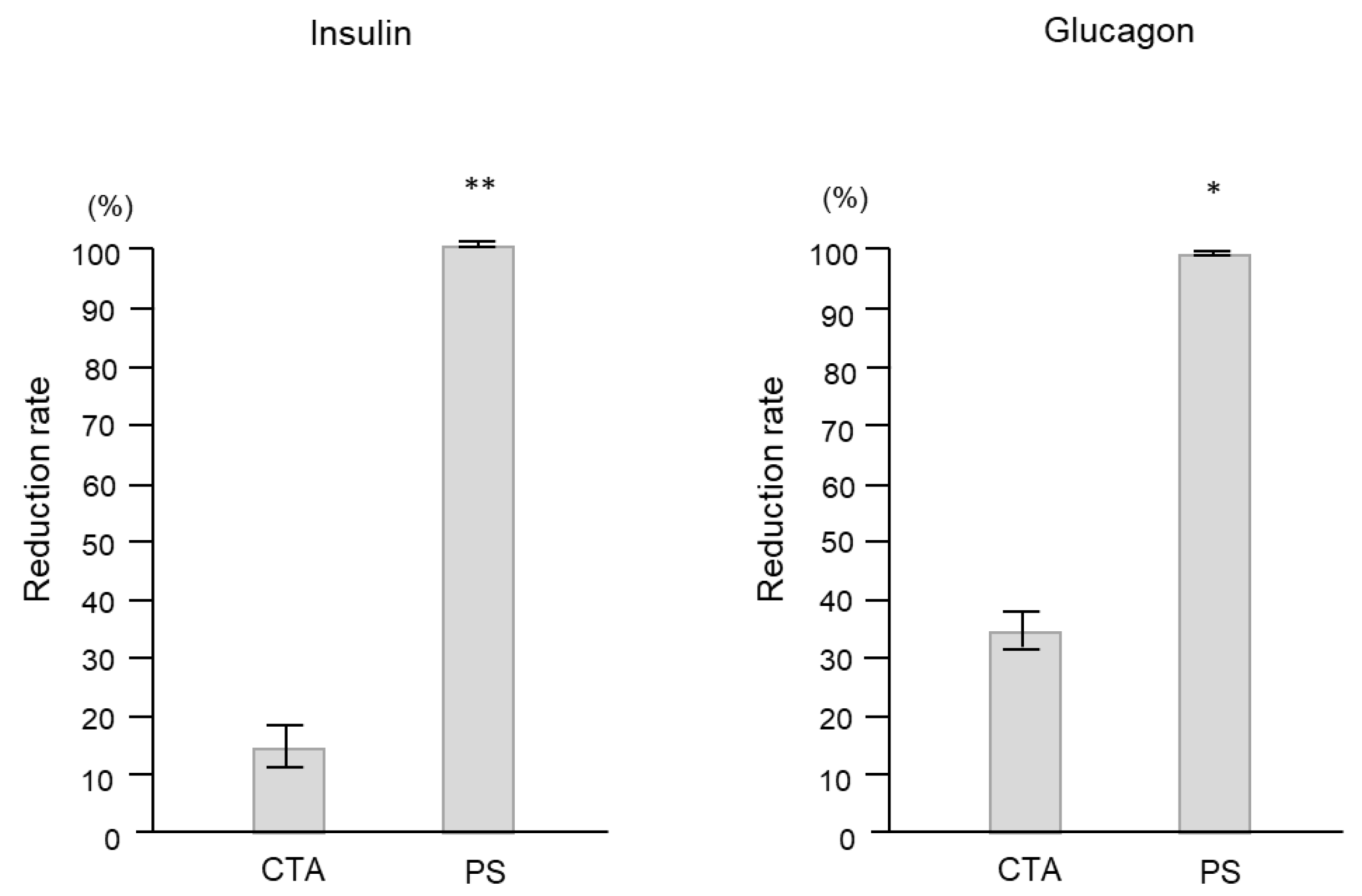
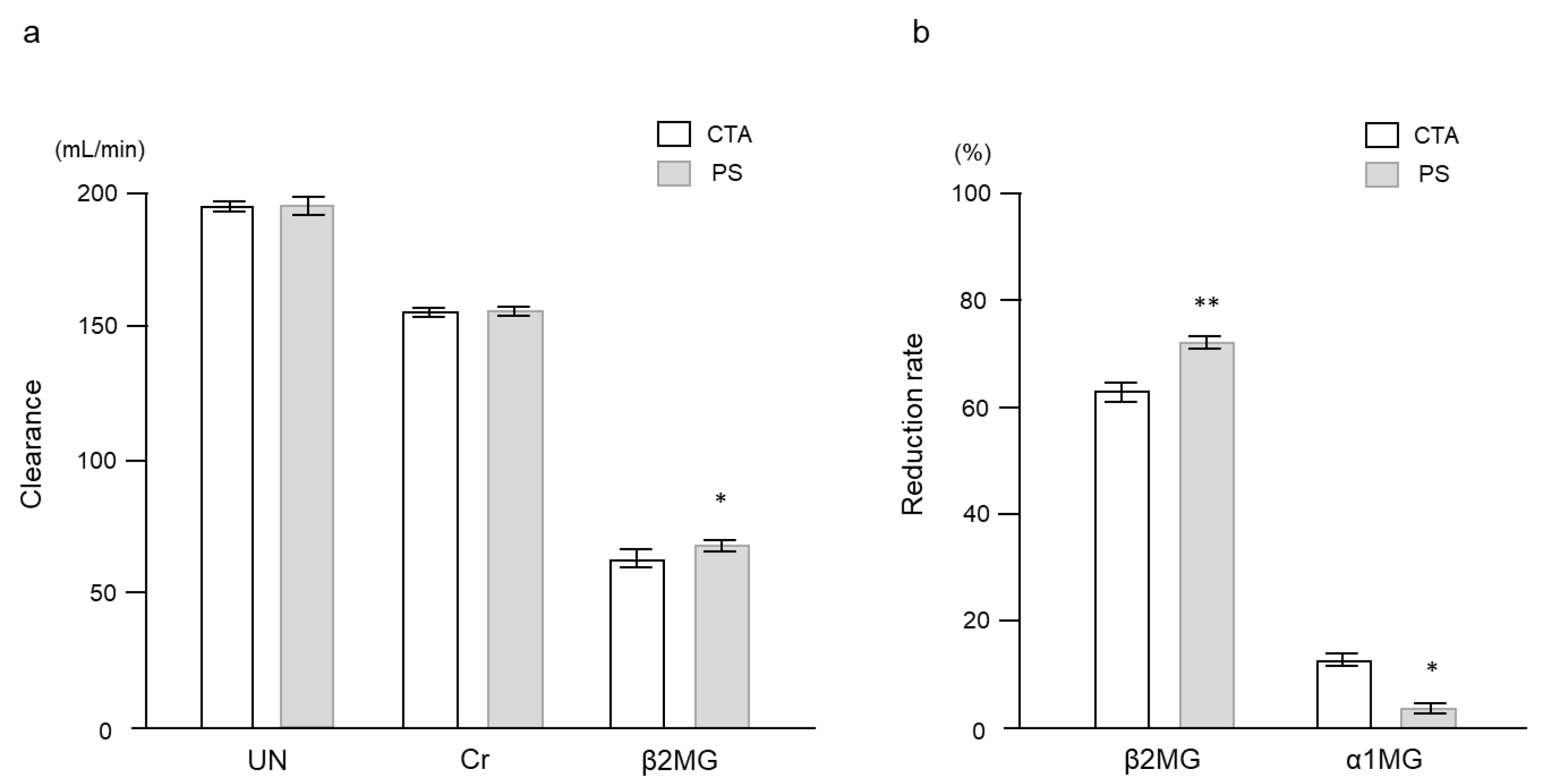
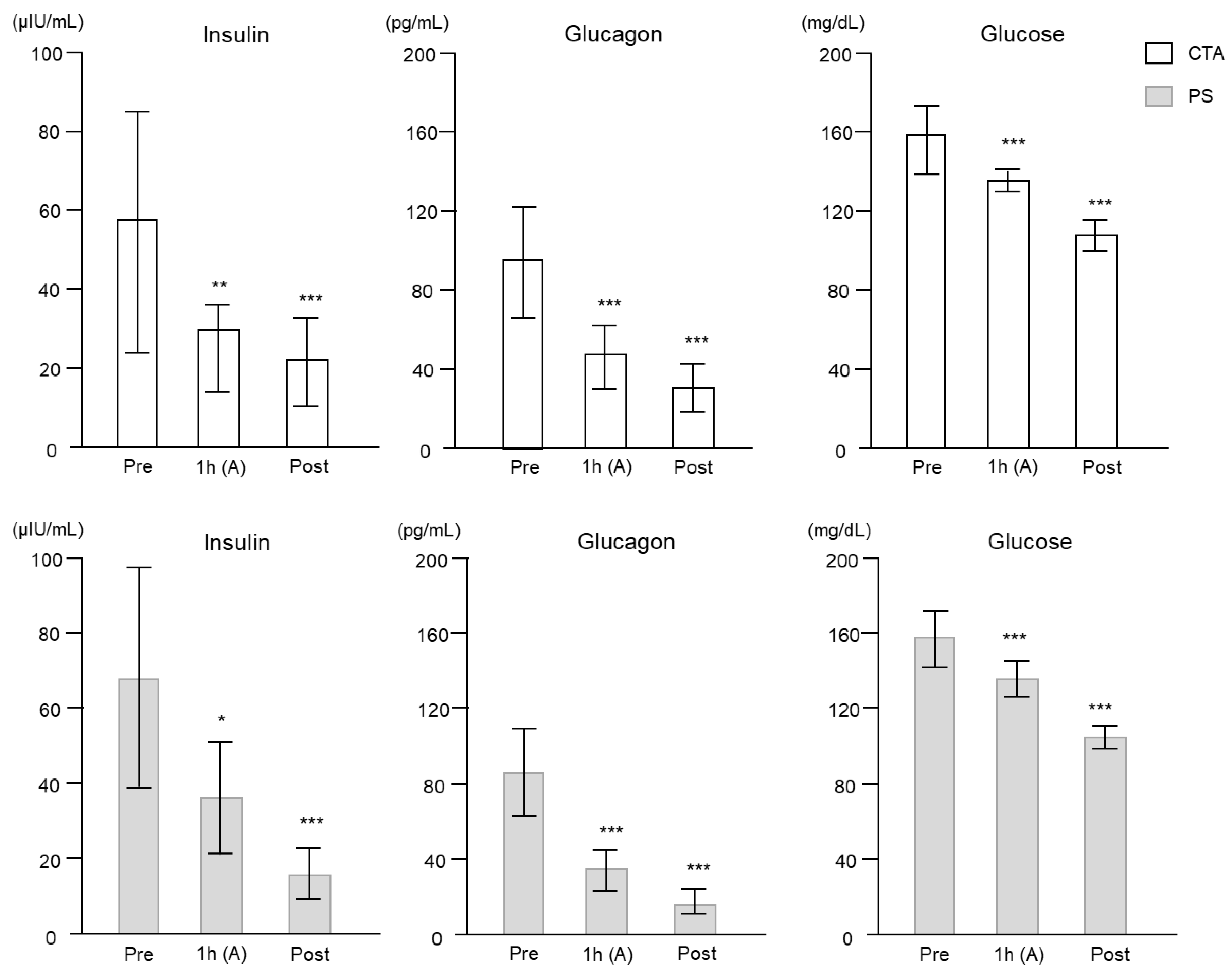
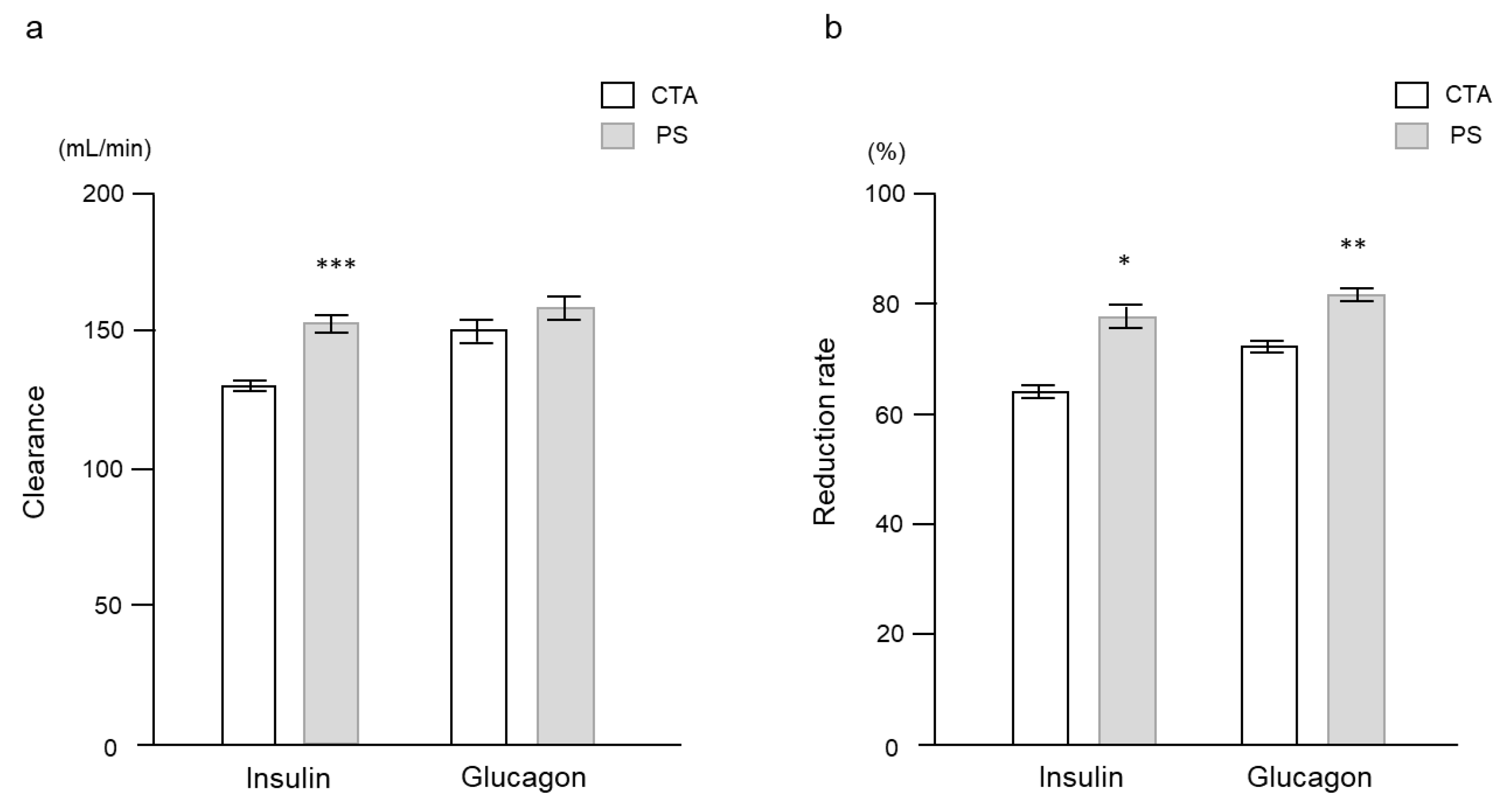
2. Results
2.1. Experimental Study
2.2. Clinical Study
3. Discussion
4. Materials and Methods
4.1. Experimental Study
4.2. Clinical Study
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nitta, K.; Abe, M.; Masakane, I.; Hanafusa, N.; Taniguchi, M.; Hasegawa, T.; Nakai, S.; Wada, A.; Hamano, T.; Hoshino, J.; et al. Annual dialysis data report 2018, JSDT Renal Data Registry: Dialysis fluid quality, hemodialysis and hemodiafiltration, peritoneal dialysis, and diabetes. Ren. Replace. Ther. 2020, 6, 51. [Google Scholar] [CrossRef]
- Nakao, T.; Inaba, M.; Abe, M.; Kaizu, K.; Shima, K.; Babazono, T.; Tomo, T.; Hirakata, H.; Akizawa, T.; Japanese Society for Dialysis Therapy. Best practice for diabetic patients on hemodialysis 2012. Ther. Apher. Dial. 2015, 19 (Suppl. S1), 40–66. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kalantar-Zadeh, K. Haemodialysis-induced hypoglycaemia and glycaemic disarrays. Nat. Rev. Nephrol. 2015, 11, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Okada, K.; Maruyama, T.; Ikeda, K.; Kikuchi, F.; Kaizu, K.; Matsumoto, K. Comparison of the effects of polysulfone and polyester-polymer alloy dialyzers on glycemic control in diabetic patients undergoing hemodialysis. Clin. Nephrol. 2009, 71, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Okada, K.; Ikeda, K.; Matsumoto, S.; Soma, M.; Matsumoto, K. Characterization of insulin adsorption behavior of dialyzer membranes used in hemodialysis. Artif. Organs 2011, 35, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Okada, K.; Maruyama, T.; Inoshita, A.; Ikeda, K.; Uto, E.; Kikuchi, F.; Matsumoto, K. Clinical evaluation of plasma insulin and C-peptide levels with 3 different high-flux dialyzers in diabetic patients on hemodialysis. Int. J. Artif. Organs 2018, 31, 898–904. [Google Scholar] [CrossRef]
- Abe, M.; Masakane, I.; Wada, A.; Nakai, S.; Nitta, K.; Nakamoto, H. Super high-flux membrane dialyzers improve mortality in patients on hemodialysis: A 3-year nationwide cohort study. Clin. Kidney J. 2021, 15, 473–483. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kawanishi, H.; Suzuki, K.; Nakai, S.; Tsuchida, K.; Tabei, S.M.; Akiba, T.; Masakane, I.; Takemoto, Y.; Tomo, T.; et al. Japanese society for dialysis therapy clinical guideline for “Maintenance hemodialysis: Hemodialysis prescriptions”. Ther. Apher. Dial. 2015, 19 (Suppl. S1), 67–92. [Google Scholar] [CrossRef]
- Yamashita, A.C. Mass transfer mechanisms in high performance membrane dialyzers. Contrib. Nephrol. 2011, 173, 95–102. [Google Scholar]
- Abe, M.; Hamano, T.; Wada, A.; Nakai, S.; Masakane, I. High-performance membrane dialyzers andmortality in hemodialysis patients: A 2-year cohort study from the Annual Survey of the Japanese Renal Data Registry. Am. J. Nephrol. 2017, 46, 82–92. [Google Scholar] [CrossRef]
- Mollmann, S.H.; Bukrinsky, J.T.; Frokiaer, S.; Elofsson, U. Adsorption of human insulin and AspB28 insulin on a PTFE-like surface. J. Colloid Interface Sci. 2005, 286, 28–35. [Google Scholar] [CrossRef]
- Matsumoto, H.; Koyama, Y.; Tanioka, A. Interaction of proteins with weak amphoteric charged membrane surfaces: Effect of pH. J. Colloid Interface Sci. 2003, 82, 82–88. [Google Scholar] [CrossRef]
- Mollmann, S.H.; Jorgensen, L.; Bukrinsky, J.T.; Elofsson, U.; Norde, W.; Frokjaer, S. Interfacial adsorption of insulin: Conformational changes and reversibility of adsorption. Eur. J. Pharm. Sci. 2006, 27, 194–204. [Google Scholar] [CrossRef]
- Jimbo, T.; Ramirez, P.; Tanioka, A.; Mafe, S.; Minoura, N. Passive transport of ionic drugs through membranes with pH-dependent fixed charges. J. Colloid Interface Sci. 2000, 225, 447–454. [Google Scholar] [CrossRef]
- Inagaki, O.; Nishian, Y.; Iwaki, R.; Nakagawa, K.; Takamitsu, Y.; Fujita, Y. Adsorption of nafamostat mesilate by hemodialysis membranes. Artif. Organs 1992, 16, 553–558. [Google Scholar] [CrossRef]
- Tian, Q.; Gomersall, C.D.; Leung, P.P.; Choi, G.Y.; Joynt, G.M.; Tan, P.E.; Wong, A.S. The adsorption of vancomycin by polyacrylonitrile, polyamide, and polysulfone hemofilters. Artif. Organs 2008, 32, 81–84. [Google Scholar] [CrossRef]
- Abe, M.; Okada, K.; Matsumoto, K. Plasma insulin and C-peptide concentrations in diabetic patients undergoing hemodialysis: Comparison with five types of high-flux dialyzer membranes. Diabetes Res. Clin. Pract. 2008, 82, e17–e19. [Google Scholar] [CrossRef]
- Zhang, S.; Saito, K.; Matsumoto, H.; Minagawa, M.; Tanioka, A. Characterization of insulin adsorption behavior on amphoteric charged membranes. Polym. J. 2008, 40, 837–841. [Google Scholar] [CrossRef] [Green Version]
- Bromer, W.W.; Boucher, M.E.; Patterson, J.M.; Pekar, A.H.; Frank, B.H. Glucagon structure and function. I. Purification and properties of bovine glucagon and monodesmidoglucagon. J. Biol. Chem. 1972, 247, 2581–2585. [Google Scholar] [CrossRef]
- Chabenne, J.R.; DiMarchi, M.A.; Gelfanov, V.M.; DiMarchi, R.D. Optimization of the native glucagon sequence for medicinal purposes. J. Diabetes Sci. Technol. 2010, 4, 1322–1331. [Google Scholar] [CrossRef] [Green Version]
- Abe, M.; Kizu, K.; Matsumoto, K. Plasma insulin is removed by hemodialysis: Evaluation of the relation between plasma insulin and glucose by using a dialysate with or without glucose. Ther. Apher. Dial. 2007, 11, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Chazot, C.; Shahmir, E.; Matias, B.; Laidlaw, S.; Kopple, J.D. Dialytic nutrition: Provision of amino acids in dialysate during hemodialysis. Kidney Int. 1997, 52, 1663–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kistler, B.M.; Benner, D.; Burrowes, J.D.; Campbell, K.L.; Fouque, D.; Garibotto, G.; Kopple, J.D.; Kovesdy, C.P.; Rhee, C.M.; Steiber, A.; et al. Eating during Hemodialysis Treatment: A Consensus Statement from the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| a | |||||||
| Dialyzers | 5 min, QB = 200 mL/min, QF = 15 mL/min | 5 min, QB = 200 mL/min, QF = 30 mL/min | |||||
| A | V | F | A | V | F | ||
| Insulin (μIU/mL) | CTA | 6010 ± 1767 | 5703 ± 1701 | 3273 ± 1105 | 5830 ± 1769 | 5640 ± 1720 | 5230 ± 1382 |
| PS | 5013 ± 1142 | 854 ± 219 | 0 | 5120 ± 1107 | 712 ± 180 | 0 | |
| Glucagon (pg/mL) | CTA | 164,667 ± 3929 | 118,667 ± 9024 | 35,133 ± 3766 | 148,000 ± 6658 | 136,333 ± 7688 | 86,566 ± 19,766 |
| PS | 82,667 ± 2333 | 29,633 ± 1449 | 1540 ± 329 | 54,367 ± 8888 | 25,333 ± 2669 | 588 ± 43 | |
| b | |||||||
| Dialyzers | 60 min, QB = 200 mL/min, QF = 15 mL/min | 60 min, QB = 200 mL/min, QF = 30 mL/min | |||||
| A | V | F | A | V | F | ||
| Insulin (μIU/mL) | CTA | 5806 ± 1731 | 5596 ± 1640 | 5536 ± 1616 | 5810 ± 1667 | 5696 ± 1664 | 5163 ± 1477 |
| PS | 47.6 ± 7.6 | 29.9 ± 6.1 | 0 | 41.0 ± 6.7 | 27.2 ± 4.3 | 0 | |
| Glucagon (pg/mL) | CTA | 139,333 ± 8950 | 119,000 ± 7371 | 111,500 ± 6331 | 137,667 ± 881 | 128,333 ± 1201 | 112,833 ± 12,657 |
| PS | 2447 ± 160 | 2140 ± 113 | 857 ± 21 | 2347 ± 173 | 2190 ± 146 | 760 ± 24 | |
| n (male/female) | 20 (13/7) |
| Age, years | 70.8 ± 1.1 |
| Body mass index, kg/m2 | 22.7 ± 0.4 |
| Dialysis duration, months | 46 (37–69) |
| History of CVD, n (%) | 5 (25.0) |
| Primary disease of CKD, n (%) | |
| Diabetic nephropathy | 16 (80.0) |
| Chronic glomerulonephritis | 2 (10.0) |
| Nephrosclerosis | 1 (5.0) |
| Other | 1 (5.0) |
| Systolic blood pressure, mmHg | 144 ± 2 |
| Diastolic blood pressure, mmHg | 80 ± 2 |
| Heart rate, bpm | 74 ± 2 |
| Hemoglobin, g/dL | 11.0 ± 0.1 |
| Albumin, g/dL | 3.8 ± 0.1 |
| Serum urea nitrogen, mg/dL | 63 ± 3 |
| Creatinine, mg/dL | 8.8 ± 0.6 |
| Uric acid, mg/dL | 6.9 ± 0.2 |
| Calcium, mg/dL | 8.7 ± 0.1 |
| Phosphate, mg/dL | 5.6 ± 0.1 |
| Casual plasma glucose, mg/dL | 159 ± 5 |
| HbA1c, % | 6.0 ± 0.1 |
| Glycated albumin, % | 20.3 ± 0.3 |
| Medication, n (%) | |
| Insulin therapy | 6 (30.0) |
| DPP-4 inhibitors | 16 (80.0) |
| GLP-1 receptor agonists | 1 (5.0) |
| α-glucosidase inhibitors | 3 (15.0) |
| Diet and exercise therapy alone | 2 (10.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takao, N.; Maruyama, T.; Kobayashi, H.; Kitai, M.; Yoshida, Y.; Takashima, H.; Abe, M. Kinetics of Glucoregulatory Peptide Hormones during Hemodialysis with Cellulose Triacetate and Polysulfone Dialyzers in Patients with Diabetes and End-Stage Kidney Disease. Int. J. Mol. Sci. 2023, 24, 10604. https://doi.org/10.3390/ijms241310604
Takao N, Maruyama T, Kobayashi H, Kitai M, Yoshida Y, Takashima H, Abe M. Kinetics of Glucoregulatory Peptide Hormones during Hemodialysis with Cellulose Triacetate and Polysulfone Dialyzers in Patients with Diabetes and End-Stage Kidney Disease. International Journal of Molecular Sciences. 2023; 24(13):10604. https://doi.org/10.3390/ijms241310604
Chicago/Turabian StyleTakao, Nobuteru, Takashi Maruyama, Hiroki Kobayashi, Maki Kitai, Yoshinori Yoshida, Hiroyuki Takashima, and Masanori Abe. 2023. "Kinetics of Glucoregulatory Peptide Hormones during Hemodialysis with Cellulose Triacetate and Polysulfone Dialyzers in Patients with Diabetes and End-Stage Kidney Disease" International Journal of Molecular Sciences 24, no. 13: 10604. https://doi.org/10.3390/ijms241310604





