In Vitro/In Vivo Hepatoprotective and Antioxidant Effects of Defatted Extract and a Phenolic Fraction Obtained from Phlomis Tuberosa
Abstract
:1. Introduction
2. Results
2.1. Preparation of E and FF and Phytochemical Analysis
2.1.1. Total Flavonoid Content
2.1.2. Identification of Compounds in E and FF
2.1.3. Validation of the Quantitative Analysis Method and Assay of Verbascoside
Specificity
Limit of Quantification and Limit of Detection
Linearity/Accuracy
Precision
Application of the Developed and Validated Method
2.1.4. Total Polyphenols as Gallic Acid Equivalents (GAE)
2.2. Pharmacological Evaluation
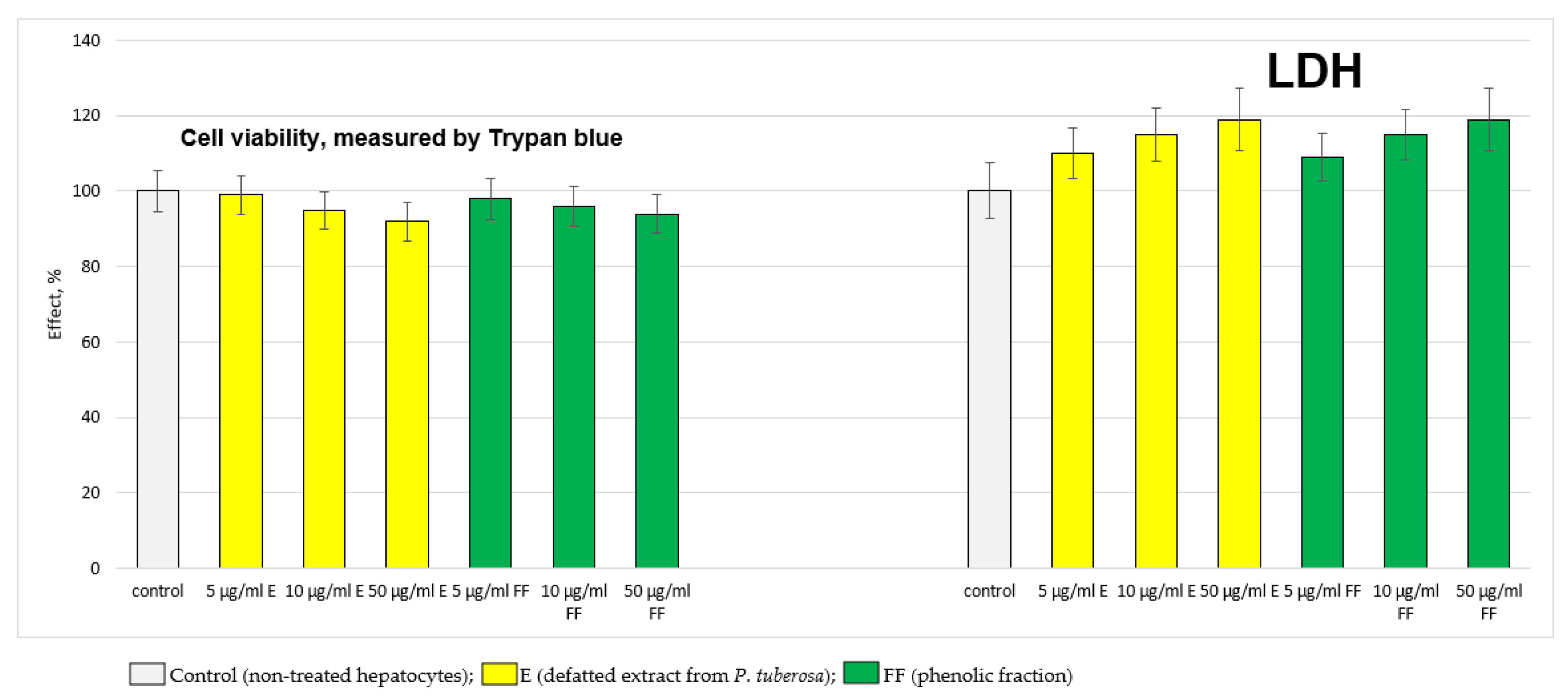
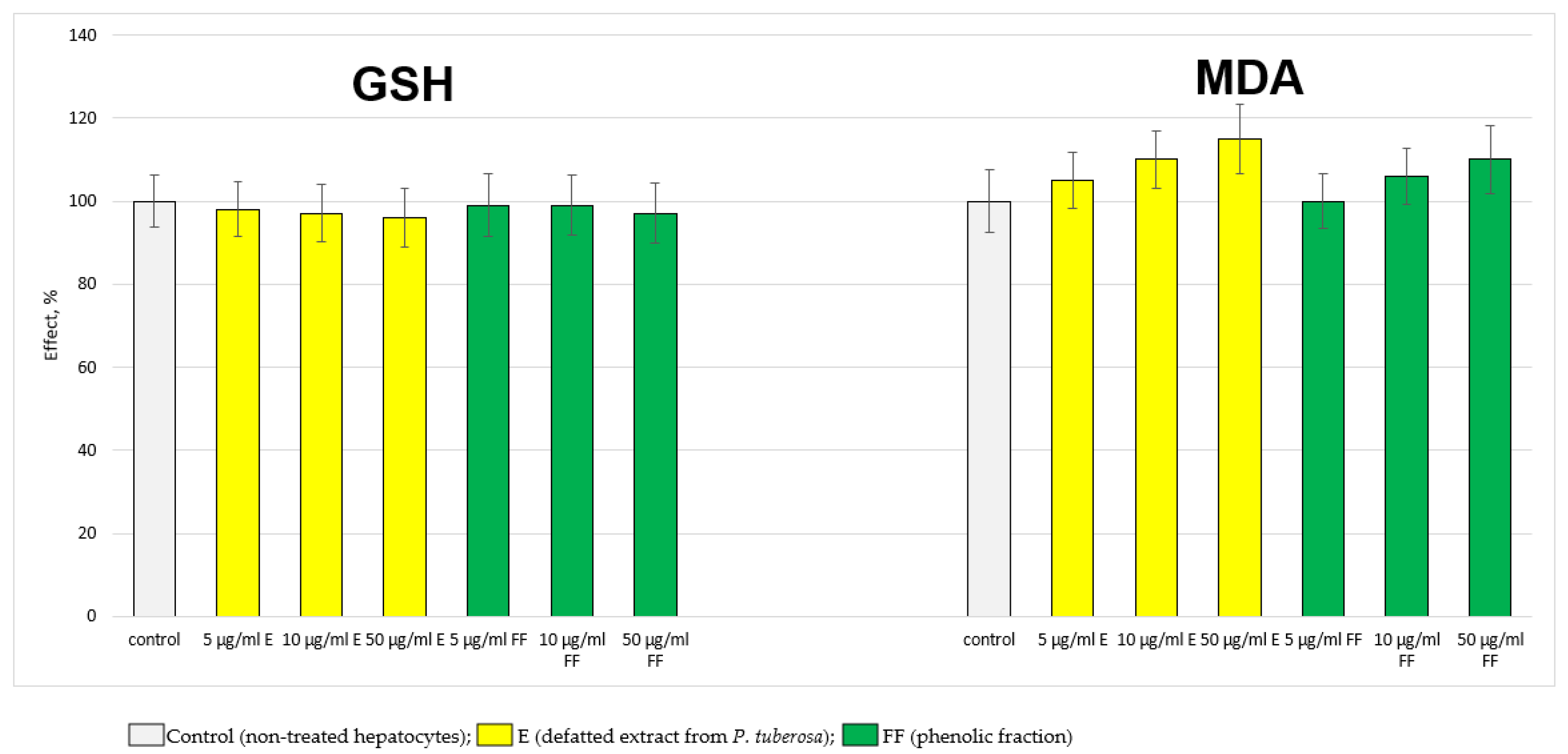
2.2.1. Results from the In Vitro Experiments on Hepatocytes
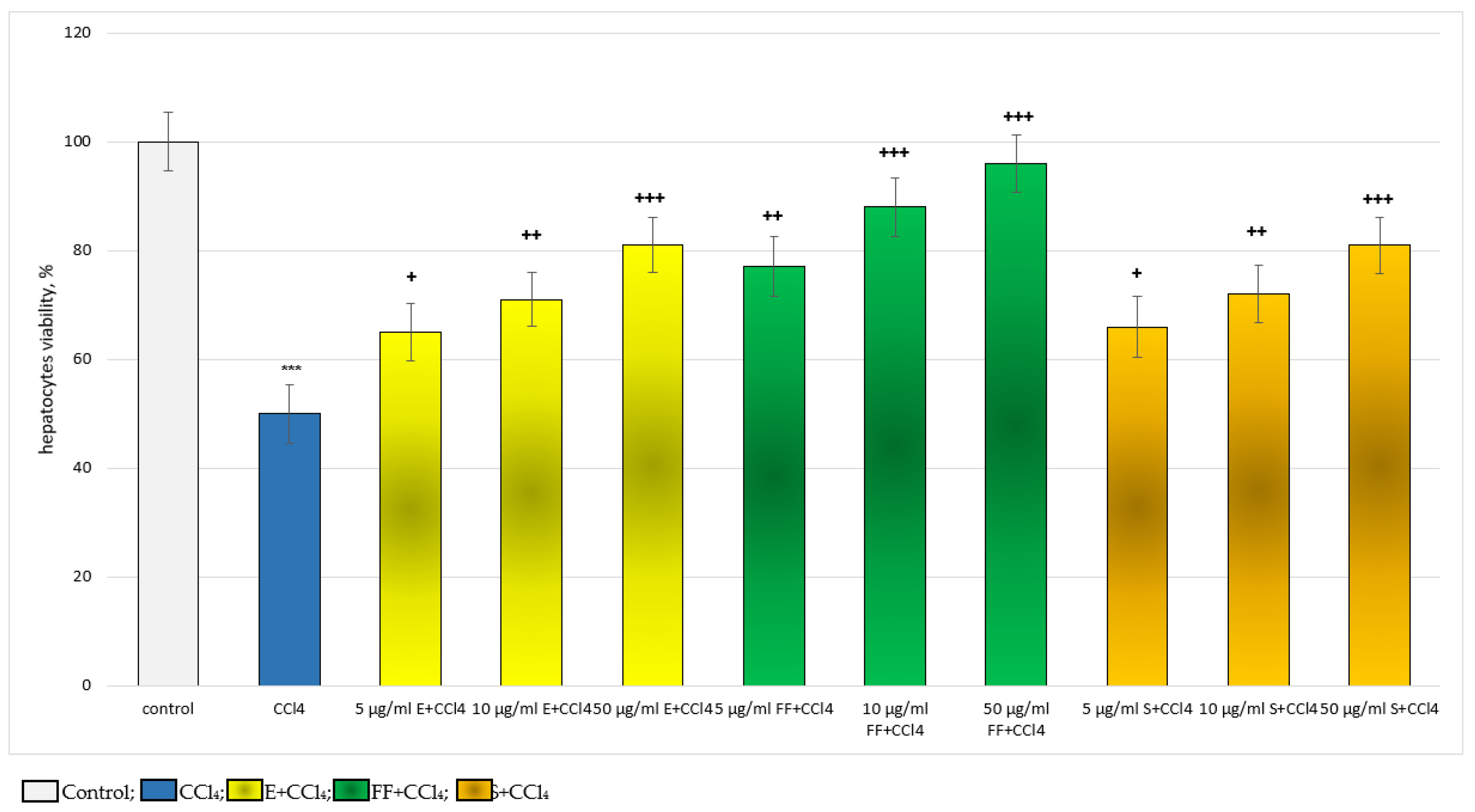
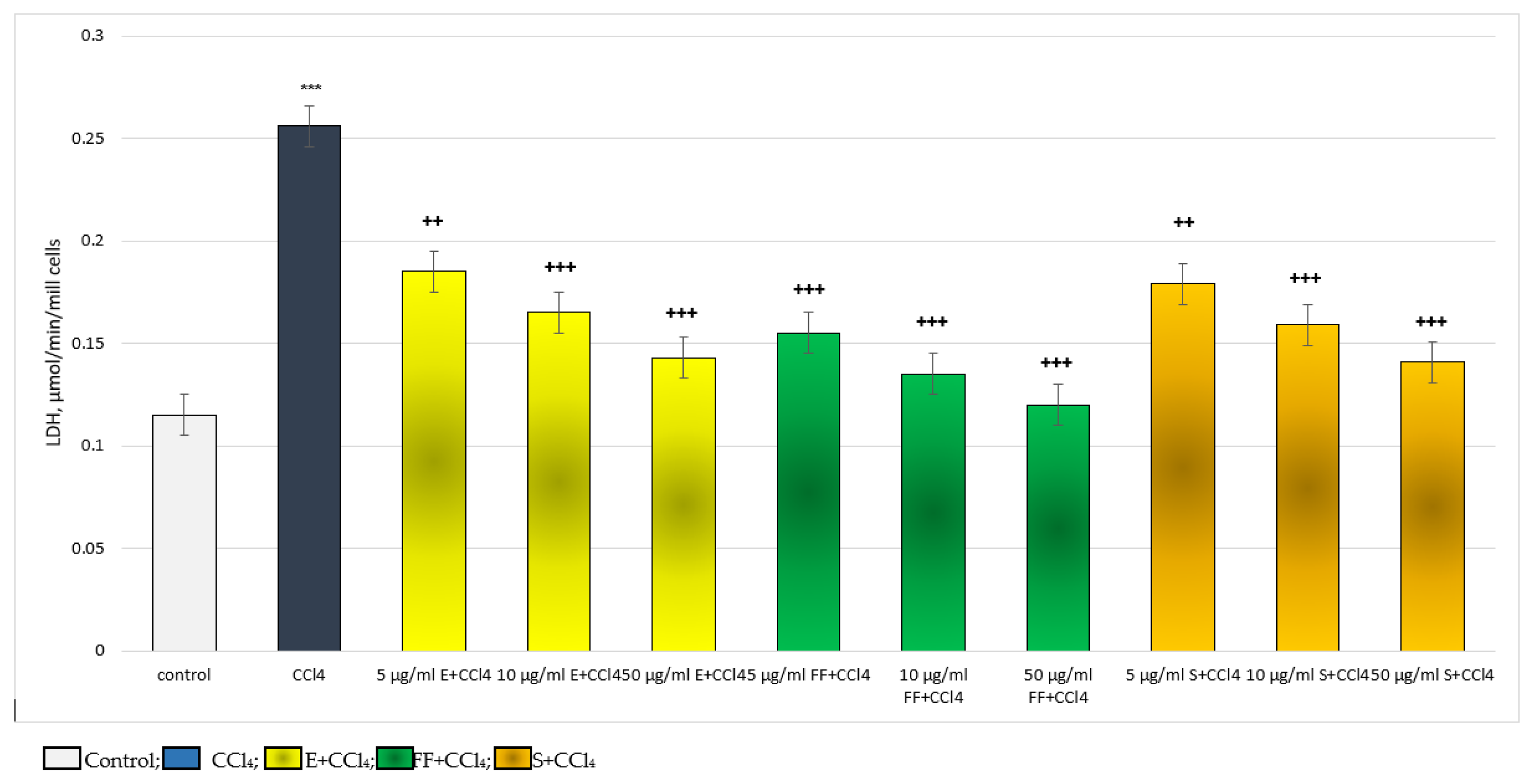
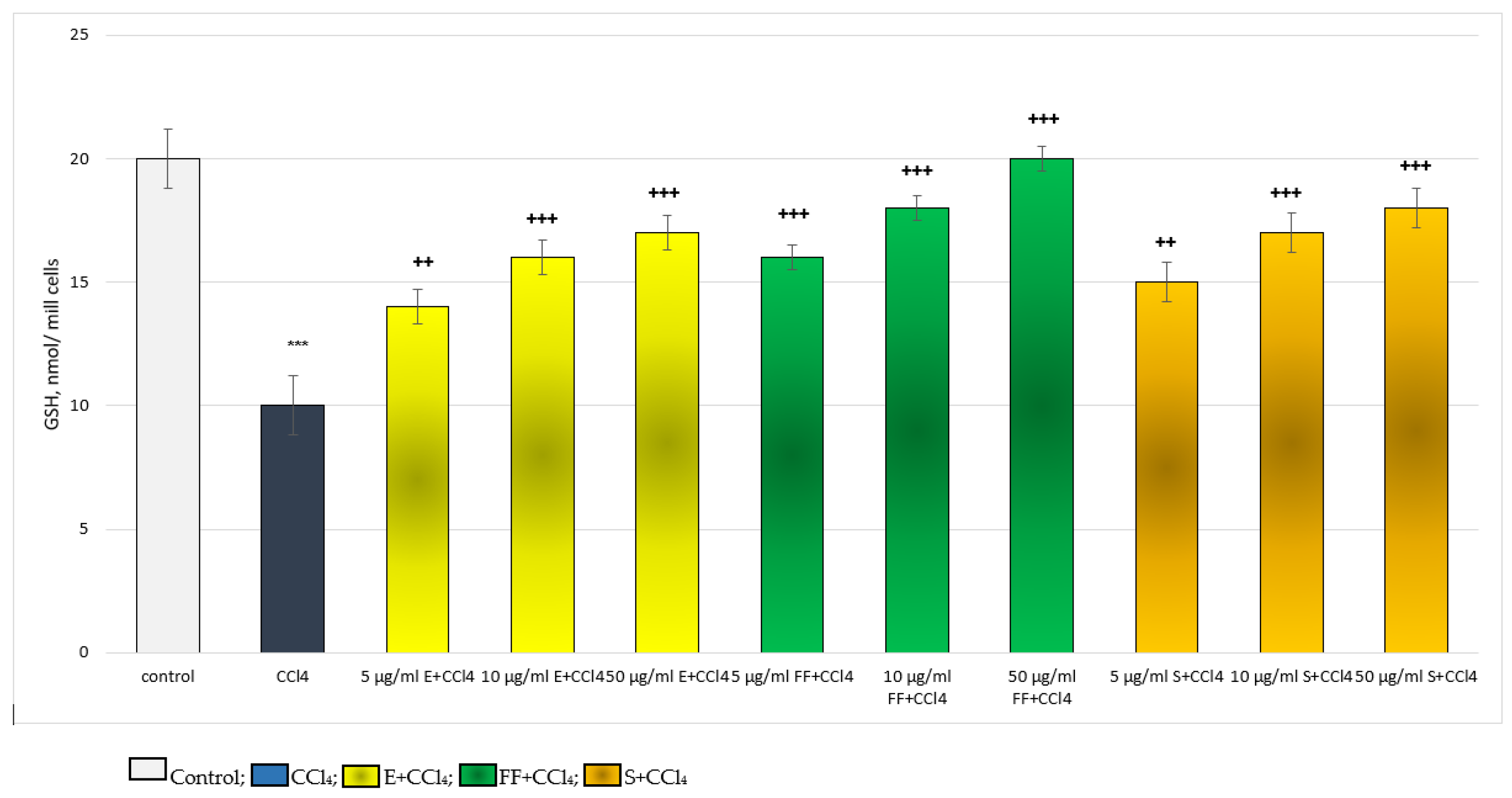
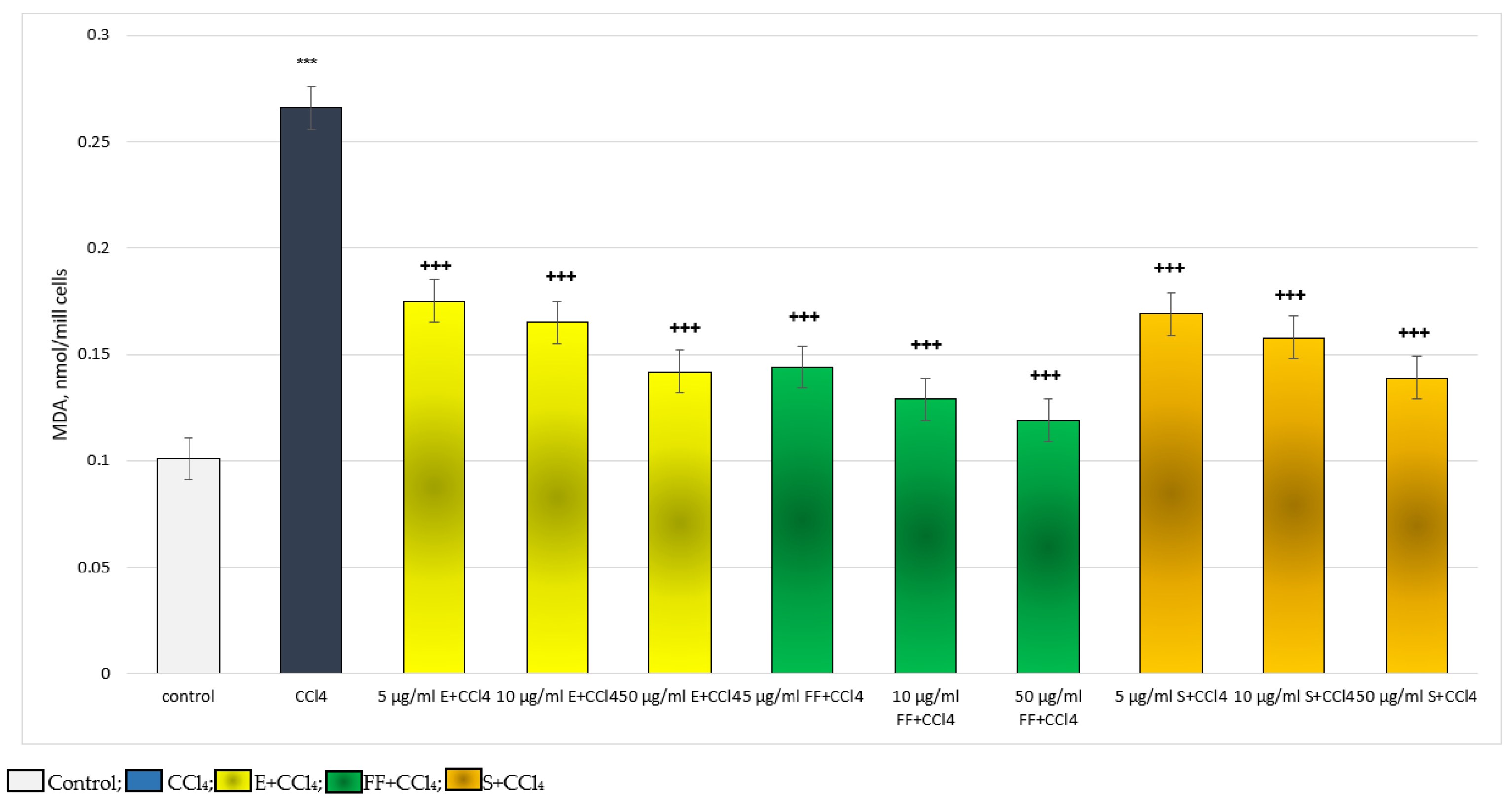
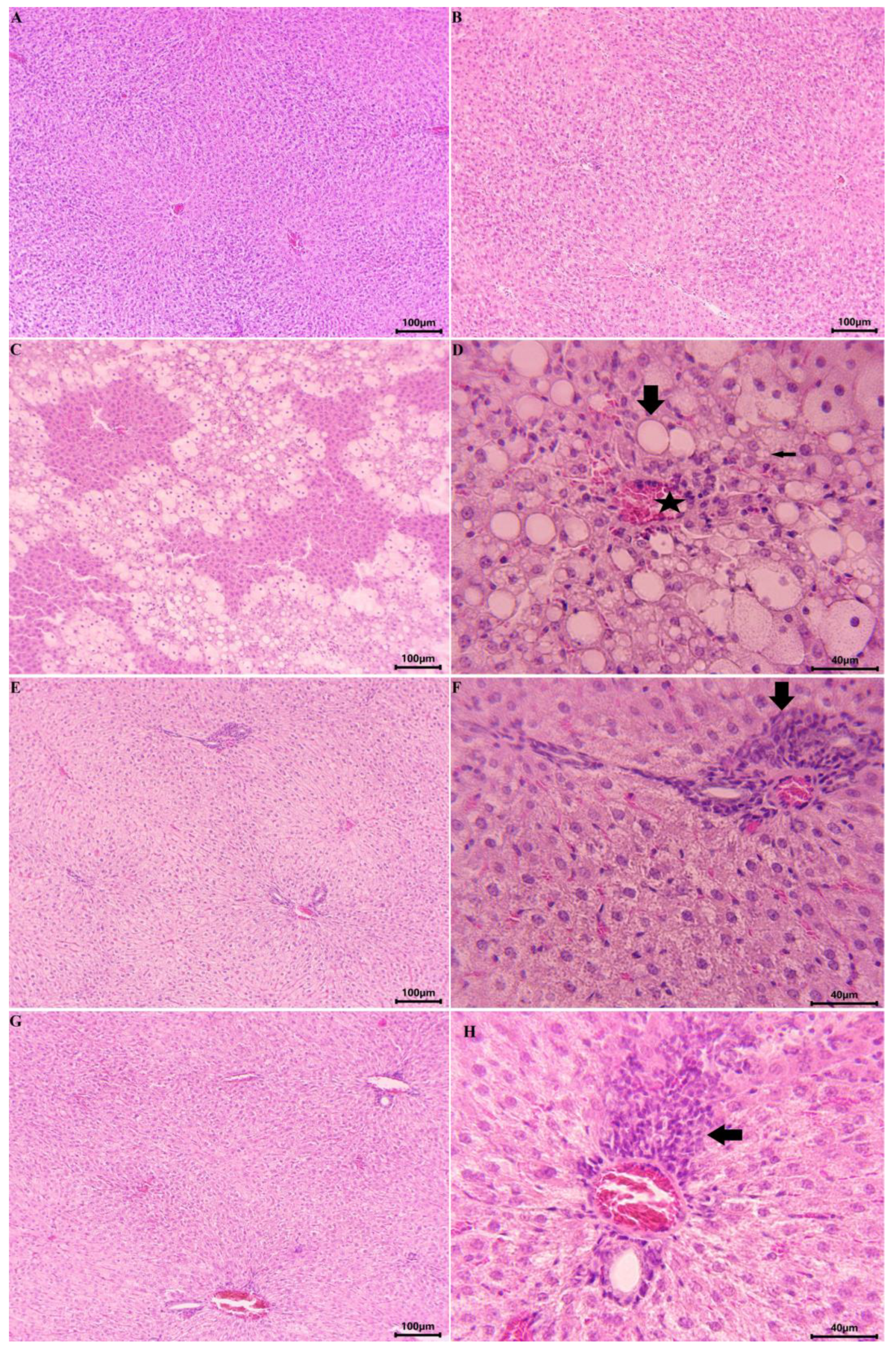
2.2.2. Results from the In Vivo Experiment in Rats
3. Discussion
4. Materials and Methods
4.1. Plant Material, Extraction, Preparation of the Phenolic Fraction and Phytochemical Analysis
4.2. Animals
4.3. Isolation and Incubation of Rat Hepatocytes
4.4. Incubation of Hepatocytes
4.5. Determination of Hepatocyte Viability by Trypan Blue Test
4.6. Determination of the Activity of the Lactate Dehydrogenase Enzyme
4.7. Determination of the Level of GSH
4.8. Determination of MDA Production
4.9. Design of the In Vivo Experiments:
- Ist group: Control (NaCl 0.9% 14 days)
- IInd group: Phlomis tuberosa extract (E) (100 mg/kg p.o./14 days) [46]
- IIIrd group: CCl4 (10% solution, administered once on the 7th day; 1.25 mL/kg p.o.) [47]
- IVth group: E (100 mg/kg p.o./14 days) in combination with CCl4 (10% solution, administered once on the 7th day; 1.25 mL/kg p.o.)
- Vth group: Silymarin (S) (100 mg/kg p.o./14 days) in combination with CCl4 (10% solution, administered once on the 7th day; 1.25 mL/kg p.o.)
4.10. MDA and GSH Measurement in Liver Homogenate
4.11. Analysis of ASAT and ALAT
4.12. Histopathological Examination
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amor, I.L.-B.; Boubaker, J.; Sgaier, M.B.; Skandrani, I.; Bhouri, W.; Neffati, A.; Kilani, S.; Bouhlel, I.; Ghedira, K.; Chekir-Ghedira, L. Phytochemistry and Biological Activities of Phlomis Species. J. Ethnopharmacol. 2009, 125, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Couladis, M.; Tzakou, O.; Verykokidou, E.; Harvala, C. Screening of Some Greek Aromatic Plants for Antioxidant Activity. Phytother. Res. 2003, 17, 194–195. [Google Scholar] [CrossRef] [PubMed]
- Delazar, A.; Sabzevari, A.; Mojarrab, M.; Nazemiyeh, H.; Esnaashari, S.; Nahar, L.; Razavi, S.M.; Sarker, S.D. Free-Radical-Scavenging Principles from Phlomis Caucasica. J. Nat. Med. 2008, 62, 464–466. [Google Scholar] [CrossRef]
- Sarkhail, P.; Amin, G.; Surmaghi, M.H.S.; Shafiee, A. Composition of the Volatile Oils of Phlomis Lanceolata Boiss. & Hohen., Phlomis Anisodonta Boiss. and Phlomis Bruguieri Desf. from Iran. Flavour Fragr. J. 2005, 20, 327–329. [Google Scholar] [CrossRef]
- Simeonova, R.; Kondeva-Burdina, M.; Vitcheva, V.; Mitcheva, M. Some in Vitro/in Vivo Chemically-Induced Experimental Models of Liver Oxidative Stress in Rats. Biomed. Res. Int. 2014, 2014, 6. [Google Scholar] [CrossRef] [Green Version]
- McGregor, D.; Lang, M. Carbon Tetrachloride: Genetic Effects and Other Modes of Action. Mutat. Res. Genet. Toxicol. 1996, 366, 181–195. [Google Scholar]
- Shkondrov, A.; Ivanova, A.; Kondeva-Burdina, M.; Krasteva, I. Essential Oil in Phlomis Tuberosa. Pharmacia 2023, 70, 61–65. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Dudareva, L.V.; Tankhaeva, L.M. Chemical Composition of Essential Oils from Galeopsis Bifida and Phlomoides Tuberosa. Chem. Nat. Compd. 2010, 46, 316–318. [Google Scholar] [CrossRef]
- Shkondrov, A.; Krasteva, I.; Pavlova, D.; Zdraveva, P. Determination of Flavonoids in Related Astragalus Species (Sect. Incani) Occurring in Bulgaria. Comptes. Rendus. L’Academie Bulg. Des Sci. 2017, 70, 363–366. [Google Scholar]
- Trullols, E.; Ruisánchez, I.; Rius, F.X. Validation of Qualitative Analytical Methods. TrAC Trends Anal. Chem. 2004, 23, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.C.; Saraswat, P. Analytical Method Verification, Method Revalidation, and Method Transfer; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Andersen, O.M.; Markham, K.R. Flavonoids: Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Guideline, I.C.H.H.T. Validation of Analytical Procedures: Text and Methodology Q2 (R1). In Proceedings of the International conference on harmonization, Geneva, Switzerland, November 2005; pp. 11–12. [Google Scholar]
- Siddiqui, N.; Rauf, A.; Latif, A.; Mahmood, Z. Spectrophotometric Determination of the Total Phenolic Content, Spectral and Fluorescence Study of the Herbal Unani Drug Gul-e-Zoofa (Nepeta Bracteata Benth). J. Taibah Univ. Med. Sci. 2017, 12, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Fau, D.; Berson, A.; Eugene, D.; Fromenty, B.; Fisch, C.; Pessayre, D. Mechanism for the Hepatotoxicity of the Antiandrogen, Nilutamide. Evidence Suggesting That Redox Cycling of This Nitroaromatic Drug Leads to Oxidative Stress in Isolated Hepatocytes. J. Pharmacol. Exp. Ther. 1992, 263, 69–77. [Google Scholar]
- Timbrell, J. Factors Affecting Metabolism and Disposition. Toxic Responses to Foreign Compounds—Direct Toxic Action: Tissue Lessions. In Principles of Biochemical Toxicology, 3rd ed.; Timbrell, J., Ed.; Taylor and Francis: Abingdon, UK, 2000; pp. 175–180. [Google Scholar]
- Weber, L.W.D.; Boll, M.; Stampfl, A. Hepatotoxicity and Mechanism of Action of Haloalkanes: Carbon Tetrachloride as a Toxicological Model. Crit. Rev. Toxicol. 2003, 33, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Dianzani, M.U. Lipid Peroxidation and Cancer: A Critical Reconsideration. Tumori J. 1989, 75, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Sridar, C.; Goosen, T.C.; Kent, U.M.; Williams, J.A.; Hollenberg, P.F. Silybin Inactivates Cytochromes P450 3A4 and 2C9 and Inhibits Major Hepatic Glucuronosyltransferases. Drug Metab. Dispos. 2004, 32, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Muriel, P.; Mourelle, M. Prevention by Silymarin of Membrane Alterations in Acute CCl4 Liver Damage. J. Appl. Toxicol. 1990, 10, 275–279. [Google Scholar] [CrossRef]
- Valenzuela, A.; Garrido, A. Biochemical Bases of the Pharmacological Action of the Flavonoid Silymarin and of Its Structural Isomer Silibinin. Biol. Res. 1994, 27, 105–112. [Google Scholar]
- Chen, P.-N.; Hsieh, Y.-S.; Chiou, H.-L.; Chu, S.-C. Silibinin Inhibits Cell Invasion through Inactivation of Both PI3K-Akt and MAPK Signaling Pathways. Chem. Biol. Interact. 2005, 156, 141–150. [Google Scholar] [CrossRef]
- Wu, L.; Georgiev, M.I.; Cao, H.; Nahar, L.; El-Seedi, H.R.; Sarker, S.D.; Xiao, J.; Lu, B. Therapeutic Potential of Phenylethanoid Glycosides: A Systematic Review. Med. Res. Rev. 2020, 40, 2605–2649. [Google Scholar] [CrossRef]
- Gu, H.; Gu, X.; Xu, Q.; Kang, W. Antioxidant Activity in Vitro and Hepatoprotective Effect of Phlomis Maximowiczii in Vivo. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasheed, M.U.; Naqvi, S.A.R.; Rasool, N.; Shah, S.A.A.; Zakaria, Z.A. Anti-Diabetic and Cytotoxic Evaluation of Phlomis Stewartii Plant Phytochemicals on Cigarette Smoke Inhalation and Alloxan-Induced Diabetes in Wistar Rats. Metabolites 2022, 12, 1133. [Google Scholar] [CrossRef] [PubMed]
- Sarkhail, P.; Abdollahi, M.; Fadayevatan, S.; Shafiee, A.; Mohammadirad, A.; Dehghan, G.; Esmaily, H.; Amin, G. Effect of Phlomis Persica on Glucose Levels and Hepatic Enzymatic Antioxidants in Streptozotocin-Induced Diabetic Rats. Pharmacogn. Mag. 2010, 6, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkhail, P.; Rahmanipour, S.; Fadyevatan, S.; Mohammadirad, A.; Dehghan, G.; Amin, G.; Shafiee, A.; Abdollahi, M. Antidiabetic Effect of Phlomis Anisodonta: Effects on Hepatic Cells Lipid Peroxidation and Antioxidant Enzymes in Experimental Diabetes. Pharmacol. Res. 2007, 56, 261–266. [Google Scholar] [CrossRef]
- Stojkovic, D.; Drakulic, D.; Dias, M.I.; Zengin, G.; Barros, L.; Ivanov, M.; Gašic, U.; Rajcevic, N.; Stevanovic, M.; Ferreira, I.C.F.R.; et al. Phlomis Fruticosa L. Exerts in Vitro Antineurodegenerative and Antioxidant Activities and Induces Prooxidant Effect in Glioblastoma Cell Line. EXCLI J. 2022, 21, 387–399. [Google Scholar] [CrossRef]
- Stojković, D.; Gašić, U.; Drakulić, D.; Zengin, G.; Stevanović, M.; Rajčević, N.; Soković, M. Chemical Profiling, Antimicrobial, Anti-Enzymatic, and Cytotoxic Properties of Phlomis Fruticosa L. J. Pharm. Biomed. Anal. 2021, 195, 113884. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Orlando, G.; Di Simone, S.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Politi, M.; Tirillini, B.; et al. Protective Effects Induced by Alcoholic Phlomis Fruticosa and Phlomis Herba-Venti Extracts in Isolated Rat Colon: Focus on Antioxidant, Anti-Inflammatory, and Antimicrobial Activities in Vitro. Phytother. Res. 2019, 33, 2387–2400. [Google Scholar] [CrossRef]
- Alpay, M.; Dulger, G.; Sahin, I.E.; Dulger, B. Evaluating Antimicrobial and Antioxidant Capacity of Endemic Phlomis Russeliana from Turkey and Its Antiproliferative Effect on Human Caco-2 Cell Lines. An. Acad. Bras. Cienc. 2019, 91, e20180404. [Google Scholar] [CrossRef] [Green Version]
- Usmanov, D.; Yusupova, U.; Syrov, V.; Ramazonov, N.; Rasulev, B. Iridoid Glucosides and Triterpene Acids from Phlomis Linearifolia, Growing in Uzbekistan and Its Hepatoprotective Activity. Nat. Prod. Res. 2021, 35, 2449–2453. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Zeljković, S.Ć. Chemical Composition and Antioxidant Activity of Phlomis Leucophracta, an Endemic Species from Turkey. Nat. Prod. Res. 2020, 34, 851–854. [Google Scholar] [CrossRef]
- Zaabat, N.; Hay, A.-E.; Michalet, S.; Skandrani, I.; Chekir-Ghedira, L.; Dijoux-Franca, M.-G.; Akkal, S. Chemical Composition, Antioxidant, Genotoxique and Antigenotoxic Potentials of Phlomis Bovei De Noé Aerial Parts. Iran. J. Pharm. Res. 2020, 19, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Dellai, A.; Mansour, H.B.; Limem, I.; Bouhlel, I.; Sghaier, M.B.; Boubaker, J.; Ghedira, K.; Chekir-Ghedira, L. Screening of Antimutagenicity via Antioxidant Activity in Different Extracts from the Flowers of Phlomis Crinita Cav. Ssp Mauritanica Munby from the Center of Tunisia. Drug Chem. Toxicol. 2009, 32, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, B.; Riaz, N.; Saleem, M.; Naveed, M.A.; Ashraf, M.; Alam, U.; Rafiq, H.M.; Tareen, R.B.; Jabbar, A. Isolation of Natural Compounds from Phlomis Stewartii Showing α-Glucosidase Inhibitory Activity. Phytochemistry 2013, 96, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Li, M.; Han, K.; Ma, Q.; Wang, J.; Qiu, Y.; Yu, L.; He, X.; Liu, D.; Lv, H. Phenylethanoid Glycosides of Phlomis Younghusbandii Mukerjee Ameliorate Acute Hypobaric Hypoxia-Induced Brain Impairment in Rats. Mol. Immunol. 2019, 108, 81–88. [Google Scholar] [CrossRef]
- Limem, I.; Bouhlel, I.; Bouchemi, M.; Kilani, S.; Boubaker, J.; Ben-Sghaier, M.; Skandrani, I.; Behouri, W.; Neffati, A.; Ghedira, K.; et al. Phlomis Mauritanica Extracts Reduce the Xanthine Oxidase Activity, Scavenge the Superoxide Anions, and Inhibit the Aflatoxin B1-, Sodium Azide-, and 4-Nitrophenyldiamine-Induced Mutagenicity in Bacteria. J. Med. Food 2010, 13, 717–724. [Google Scholar] [CrossRef]
- Bader, A.; Tuccinardi, T.; Granchi, C.; Martinelli, A.; Macchia, M.; Minutolo, F.; De Tommasi, N.; Braca, A. Phenylpropanoids and Flavonoids from Phlomis Kurdica as Inhibitors of Human Lactate Dehydrogenase. Phytochemistry 2015, 116, 262–268. [Google Scholar] [CrossRef] [Green Version]
- López, V.; Jäger, A.K.; Akerreta, S.; Cavero, R.Y.; Calvo, M.I. Antioxidant Activity and Phenylpropanoids of Phlomis lychnitis L.: A Traditional Herbal Tea. Plant Foods Hum. Nutr. 2010, 65, 179–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z. Phenolic Composition and Antioxidant Activities of Two Phlomis Species: A Correlation Study. Comptes Rendus Biol. 2009, 332, 816–826. [Google Scholar] [CrossRef]
- Markham, K.R. Techniques of Flavonoid Identification; Academic Press London: London, UK, 1982; Volume 31. [Google Scholar]
- Swartz, M.E.; Krull, I.S. Analytical Method Development and Validation; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Mitcheva, M.; Kondeva, M.; Vitcheva, V.; Nedialkov, P.; Kitanov, G. Effect of Benzophenones from Hypericum Annulatum on Carbon Tetrachloride-Induced Toxicity in Freshly Isolated Rat Hepatocytes. Redox Rep. 2006, 11, 3–8. [Google Scholar] [CrossRef]
- Abdelghffar, E.A.R.; El-Nashar, H.A.S.; Fayez, S.; Obaid, W.A.; Eldahshan, O.A. Ameliorative Effect of Oregano (Origanum lulgare) versus Silymarin in Experimentally Induced Hepatic Encephalopathy. Sci. Rep. 2022, 12, 17854. [Google Scholar] [CrossRef]
- Ahn, T.-H.; Yang, Y.-S.; Lee, J.-C.; Moon, C.-J.; Kim, S.-H.; Jun, W.; Park, S.-C.; Kim, J.-C. Ameliorative Effects of Pycnogenol® on Carbon Tetrachloride-Induced Hepatic Oxidative Damage in Rats. Phyther. Res. 2007, 21, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Qiu, A.; Guan, J.; Shi, Z. Antioxidant and Protective Effect of an Oleanolic Acid-Enriched Extract of A. Deliciosa Root on Carbon Tetrachloride Induced Rat Liver Injury. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. S1), 169–173. [Google Scholar] [PubMed]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 5th ed.; Churchill Livingstone Publications: Edinburgh, UK, 2002. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondeva-Burdina, M.; Shkondrov, A.; Popov, G.; Manov, V.; Krasteva, I. In Vitro/In Vivo Hepatoprotective and Antioxidant Effects of Defatted Extract and a Phenolic Fraction Obtained from Phlomis Tuberosa. Int. J. Mol. Sci. 2023, 24, 10631. https://doi.org/10.3390/ijms241310631
Kondeva-Burdina M, Shkondrov A, Popov G, Manov V, Krasteva I. In Vitro/In Vivo Hepatoprotective and Antioxidant Effects of Defatted Extract and a Phenolic Fraction Obtained from Phlomis Tuberosa. International Journal of Molecular Sciences. 2023; 24(13):10631. https://doi.org/10.3390/ijms241310631
Chicago/Turabian StyleKondeva-Burdina, Magdalena, Aleksandar Shkondrov, Georgi Popov, Vasil Manov, and Ilina Krasteva. 2023. "In Vitro/In Vivo Hepatoprotective and Antioxidant Effects of Defatted Extract and a Phenolic Fraction Obtained from Phlomis Tuberosa" International Journal of Molecular Sciences 24, no. 13: 10631. https://doi.org/10.3390/ijms241310631






