Production and Characterization of Cellulose Nanocrystals from Eucalyptus Dissolving Pulp Using Endoglucanases from Myceliophthora thermophila
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of EDP
2.2. Expression and Purification of Different EGs Derived from M. thermophila
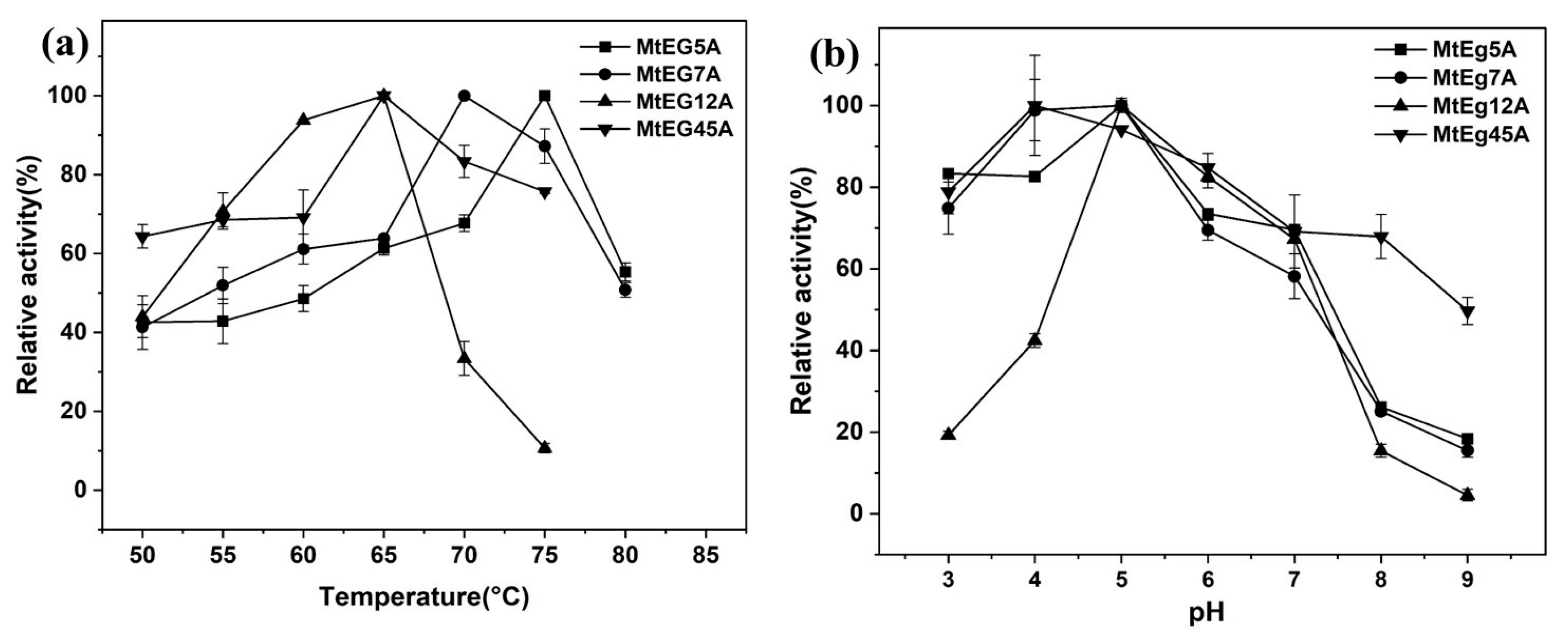
2.3. Enzymatic Properties of Different EGs from M. thermophila
2.4. Effect of Different EGs and EG Combinations on the Preparation of CNCs
2.5. Characteristics of CNCs
2.5.1. Morphological Observation
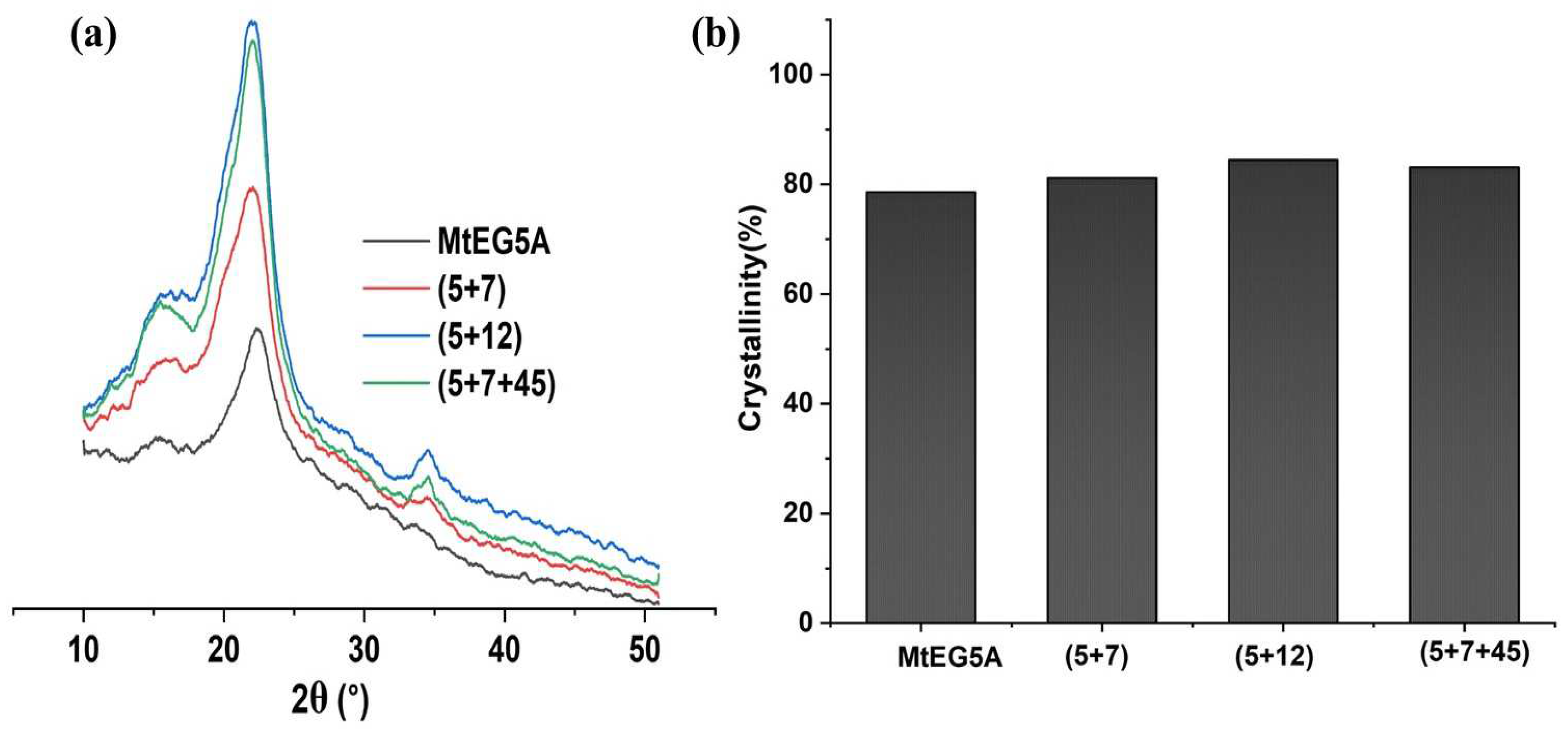
2.5.2. XRD and Crystallinity Analysis
2.5.3. FTIR Analysis
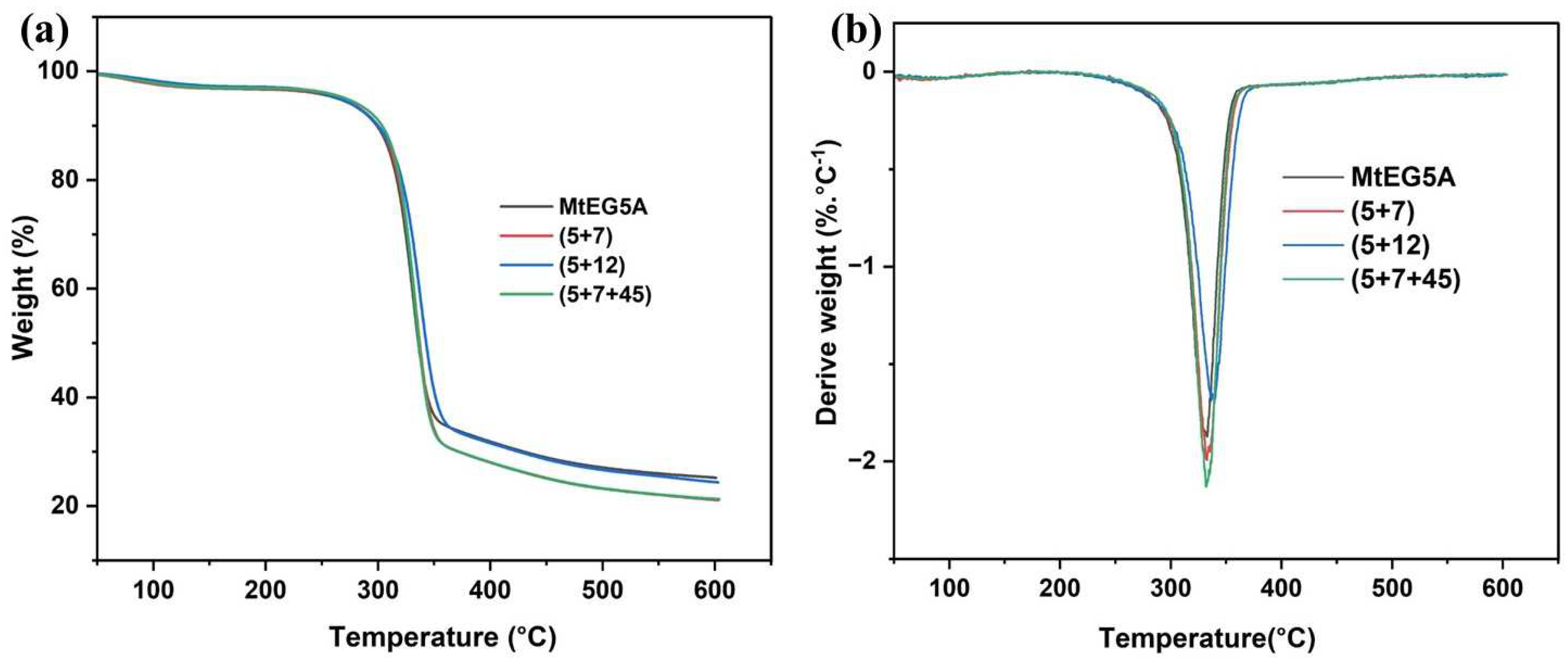
2.5.4. TG Analysis
3. Materials and Methods
3.1. Materials and Strains
3.2. Construction of Engineering Strains for Heterologous Expression of EGs
3.3. Enzyme Production
3.4. Effect of Temperature and pH on Enzymatic Activity
3.5. CNC Preparation
3.6. Characterization of CNCs
3.6.1. Dynamic Light Scattering (DLS)
3.6.2. TEM Analysis
3.6.3. SEM Observation
3.6.4. XRD Analysis
3.6.5. FT-IR Analysis
3.6.6. Thermogravimetric Analysis (TGA)
3.7. Analytical Method
3.7.1. Chemical Compositions of EDP
3.7.2. Protein Concentration
3.7.3. Enzyme Activity Assay
3.7.4. CNC Yield
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in Cellulose Nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Nasir, M.; Hashim, R.; Sulaiman, O.; Asim, M. Nanocellulose: Preparation Methods and Applications. In Cellulose-Reinforced Nanofibre Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 261–276. ISBN 978-0-08-100957-4. [Google Scholar]
- Ferrer, A.; Pal, L.; Hubbe, M. Nanocellulose in Packaging: Advances in Barrier Layer Technologies. Ind. Crops Prod. 2017, 95, 574–582. [Google Scholar] [CrossRef]
- Leite, L.S.F.; Pham, C.; Bilatto, S.; Azeredo, H.M.C.; Cranston, E.D.; Moreira, F.K.; Mattoso, L.H.C.; Bras, J. Effect of Tannic Acid and Cellulose Nanocrystals on Antioxidant and Antimicrobial Properties of Gelatin Films. ACS Sustain. Chem. Eng. 2021, 9, 8539–8549. [Google Scholar] [CrossRef]
- Kaboorani, A.; Auclair, N.; Riedl, B.; Landry, V. Physical and Morphological Properties of UV-Cured Cellulose Nanocrystal (CNC) Based Nanocomposite Coatings for Wood Furniture. Prog. Org. Coat. 2016, 93, 17–22. [Google Scholar] [CrossRef]
- Klemm, D.; Petzold-Welcke, K.; Kramer, F.; Richter, T.; Raddatz, V.; Fried, W.; Nietzsche, S.; Bellmann, T.; Fischer, D. Biotech Nanocellulose: A Review on Progress in Product Design and Today’s State of Technical and Medical Applications. Carbohydr. Polym. 2021, 254, 117313. [Google Scholar] [CrossRef] [PubMed]
- Squinca, P.; Bilatto, S.; Badino, A.C.; Farinas, C.S. Nanocellulose Production in Future Biorefineries: An Integrated Approach Using Tailor-Made Enzymes. ACS Sustain. Chem. Eng. 2020, 8, 2277–2286. [Google Scholar] [CrossRef]
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Emerging Technologies for the Production of Nanocellulose from Lignocellulosic Biomass. Carbohydr. Polym. 2022, 285, 119258. [Google Scholar] [CrossRef]
- ISO/TS 20477; Nanotechnologies—Vocabulary for Cellulose Nanomaterial. International Organization for Standardization: Geneva, Switzerland, 2023.
- Tang, Y.; Yang, H.; Vignolini, S. Recent Progress in Production Methods for Cellulose Nanocrystals: Leading to More Sustainable Processes. Adv. Sustain. Syst. 2022, 6, 2100100. [Google Scholar] [CrossRef]
- Tong, X.; He, Z.; Zheng, L.; Pande, H.; Ni, Y. Enzymatic Treatment Processes for the Production of Cellulose Nanomaterials: A Review. Carbohydr. Polym. 2023, 299, 120199. [Google Scholar] [CrossRef]
- Yu, H.; Abdalkarim, S.Y.H.; Zhang, H.; Wang, C.; Tam, K.C. Simple Process To Produce High-Yield Cellulose Nanocrystals Using Recyclable Citric/Hydrochloric Acids. ACS Sustain. Chem. Eng. 2019, 7, 4912–4923. [Google Scholar] [CrossRef]
- Pacheco, C.M.; Bustos A, C.; Reyes, G. Cellulose Nanocrystals from Blueberry Pruning Residues Isolated by Ionic Liquids and TEMPO-Oxidation Combined with Mechanical Disintegration. J. Dispers. Sci. Technol. 2020, 41, 1731–1741. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Isolation of Oxidized Nanocellulose from Rice Straw Using the Ammonium Persulfate Method. Cellulose 2018, 25, 2143–2149. [Google Scholar] [CrossRef]
- Arantes, V.; Dias, I.K.R.; Berto, G.L.; Pereira, B.; Marotti, B.S.; Nogueira, C.F.O. The Current Status of the Enzyme-Mediated Isolation and Functionalization of Nanocelluloses: Production, Properties, Techno-Economics, and Opportunities. Cellulose 2020, 27, 10571–10630. [Google Scholar] [CrossRef]
- Cebreiros, F.; Seiler, S.; Dalli, S.S.; Lareo, C.; Saddler, J. Enhancing Cellulose Nanofibrillation of Eucalyptus Kraft Pulp by Combining Enzymatic and Mechanical Pretreatments. Cellulose 2021, 28, 189–206. [Google Scholar] [CrossRef]
- Yang, T.; Li, X.; Guo, Y.; Peng, S.; Liu, G.; Zhao, J. Effect of Endoglucanases from Different Glycoside Hydrolase Families on Enzymatic Preparation of Cellulose Nanocrystal. Ind. Crops Prod. 2020, 155, 112755. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Y.; Chang, J.-S.; Lee, D.-J. Enzymes and Enzymatic Mechanisms in Enzymatic Degradation of Lignocellulosic Biomass: A Mini-Review. Bioresour. Technol. 2023, 367, 128252. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Deng, X.-Y.; Shen, W.-H.; Jia, M.-Y. Preparation and Characterization of the Spherical Nanosized Cellulose by the Enzymatic Hydrolysis of Pulp Fibers. Carbohydr. Polym. 2018, 181, 879–884. [Google Scholar] [CrossRef]
- De Aguiar, J.; Bondancia, T.J.; Claro, P.I.C.; Mattoso, L.H.C.; Farinas, C.S.; Marconcini, J.M. Enzymatic Deconstruction of Sugarcane Bagasse and Straw to Obtain Cellulose Nanomaterials. ACS Sustain. Chem. Eng. 2020, 8, 2287–2299. [Google Scholar] [CrossRef]
- Siqueira, G.A.; Dias, I.K.R.; Arantes, V. Exploring the Action of Endoglucanases on Bleached Eucalyptus Kraft Pulp as Potential Catalyst for Isolation of Cellulose Nanocrystals. Int. J. Biol. Macromol. 2019, 133, 1249–1259. [Google Scholar] [CrossRef]
- Wang, W.; Mozuch, M.D.; Sabo, R.C.; Kersten, P.; Zhu, J.Y.; Jin, Y. Production of Cellulose Nanofibrils from Bleached Eucalyptus Fibers by Hyperthermostable Endoglucanase Treatment and Subsequent Microfluidization. Cellulose 2015, 22, 351–361. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Pignon, F.; Belgacem, M.N. Morphological Properties of Nanofibrillated Cellulose Produced Using Wet Grinding as an Ultimate Fibrillation Process. J. Mater. Sci. 2015, 50, 531–541. [Google Scholar] [CrossRef]
- Xu, Y.; Salmi, J.; Kloser, E.; Perrin, F.; Grosse, S.; Denault, J.; Lau, P.C.K. Feasibility of Nanocrystalline Cellulose Production by Endoglucanase Treatment of Natural Bast Fibers. Ind. Crops Prod. 2013, 51, 381–384. [Google Scholar] [CrossRef]
- Karnaouri, A.; Topakas, E.; Antonopoulou, I.; Christakopoulos, P. Genomic Insights into the Fungal Lignocellulolytic System of Myceliophthora thermophila. Front. Microbiol. 2014, 5, 281. [Google Scholar] [CrossRef] [PubMed]
- Karnaouri, A.; Muraleedharan, M.N.; Dimarogona, M.; Topakas, E.; Rova, U.; Sandgren, M.; Christakopoulos, P. Recombinant Expression of Thermostable Processive MtEG5 Endoglucanase and Its Synergism with MtLPMO from Myceliophthora thermophila during the Hydrolysis of Lignocellulosic Substrates. Biotechnol. Biofuels 2017, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Karnaouri, A.C.; Topakas, E.; Christakopoulos, P. Cloning, Expression, and Characterization of a Thermostable GH7 Endoglucanase from Myceliophthora thermophila Capable of High-Consistency Enzymatic Liquefaction. Appl. Microbiol. Biotechnol. 2014, 98, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Berto, G.L.; Velasco, J.; Tasso Cabos Ribeiro, C.; Zanphorlin, L.M.; Noronha Domingues, M.; Tyago Murakami, M.; Polikarpov, I.; De Oliveira, L.C.; Ferraz, A.; Segato, F. Functional Characterization and Comparative Analysis of Two Heterologous Endoglucanases from Diverging Subfamilies of Glycosyl Hydrolase Family 45. Enzym. Microb. Technol. 2019, 120, 23–35. [Google Scholar] [CrossRef]
- Amengual, N.G.; Csarman, F.; Wohlschlager, L.; Ludwig, R. Expression and Characterization of a Family 45 Glycosyl Hydrolase from Fomitopsis pinicola and Comparison to Phanerochaete chrysosporium Cel45A. Enzym. Microb. Technol. 2022, 156, 110000. [Google Scholar] [CrossRef]
- Karlsson, J.; Momcilovic, D.; Wittgren, B.; Schülein, M.; Tjerneld, F.; Brinkmalm, G. Enzymatic Degradation of Carboxymethyl Cellulose Hydrolyzed by the Endoglucanases Cel5A, Cel7B, and Cel45A from Humicola insolens and Cel7B, Cel12A and Cel45A core from Trichoderma reesei: Enzymatic Degradation of Carboxymethyl Cellulose. Biopolymers 2002, 63, 32–40. [Google Scholar] [CrossRef]
- Waghmare, P.R.; Waghmare, P.P.; Gao, L.; Sun, W.; Qin, Y.; Liu, G.; Qu, Y. Efficient Constitutive Expression of Cellulolytic Enzymes in Penicillium oxalicum for Improved Efficiency of Lignocellulose Degradation. J. Microbiol. Biotechnol. 2021, 31, 740–746. [Google Scholar] [CrossRef]
- Blumhoff, M.; Steiger, M.G.; Marx, H.; Mattanovich, D.; Sauer, M. Six Novel Constitutive Promoters for Metabolic Engineering of Aspergillus niger. Appl. Microbiol. Biotechnol. 2013, 97, 259–267. [Google Scholar] [CrossRef]
- Vlasenko, E.; Schülein, M.; Cherry, J.; Xu, F. Substrate Specificity of Family 5, 6, 7, 9, 12, and 45 Endoglucanases. Bioresour. Technol. 2010, 101, 2405–2411. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Zhou, J.-B.; Yi, X.-N.; Wang, Y.-M.; Xue, Y.-P.; Chen, D.-S.; Cheng, X.-P.; Li, M.; Wang, H.-Y.; Chen, K.-Q.; et al. Heterologous Expression and Biochemical Characterization of a Thermostable Endo-β-1,4-Glucanase from Colletotrichum orchidophilum. Bioprocess Biosyst. Eng. 2021, 44, 67–79. [Google Scholar] [CrossRef]
- Segato, F.; Dias, B.; Berto, G.L.; De Oliveira, D.M.; De Souza, F.H.M.; Citadini, A.P.; Murakami, M.T.; Damásio, A.R.L.; Squina, F.M.; Polikarpov, I. Cloning, Heterologous Expression and Biochemical Characterization of a Non-Specific Endoglucanase Family 12 from Aspergillus terreus NIH2624. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2017, 1865, 395–403. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Sabo, R.; Luo, X. Integrated Production of Nano-Fibrillated Cellulose and Cellulosic Biofuel (Ethanol) by Enzymatic Fractionation of Wood Fibers. Green Chem. 2011, 13, 1339–1344. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, S.; Ge, S.; Xiong, L.; Sun, Q. Green Preparation and Characterization of Size-Controlled Nanocrystalline Cellulose via Ultrasonic-Assisted Enzymatic Hydrolysis. Ind. Crops Prod. 2016, 83, 346–352. [Google Scholar] [CrossRef]
- Filson, P.B.; Dawson-Andoh, B.E.; Schwegler-Berry, D. Enzymatic-Mediated Production of Cellulose Nanocrystals from Recycled Pulp. Green Chem. 2009, 11, 1808. [Google Scholar] [CrossRef]
- Bondancia, T.J.; Batista, G.; De Aguiar, J.; Lorevice, M.V.; Cruz, A.J.G.; Marconcini, J.M.; Mattoso, L.H.C.; Farinas, C.S. Cellulose Nanocrystals from Sugar Cane Bagasse Using Organic and/or Inorganic Acids: Techno-Economic Analysis and Life Cycle Assessment. ACS Sustain. Chem. Eng. 2022, 10, 4660–4676. [Google Scholar] [CrossRef]
- Zhu, P.; Feng, L.; Ding, Z.; Bai, X. Preparation of Spherical Cellulose Nanocrystals from Microcrystalline Cellulose by Mixed Acid Hydrolysis with Different Pretreatment Routes. Int. J. Mol. Sci. 2022, 23, 10764. [Google Scholar] [CrossRef]
- Gupta, V.; Ramakanth, D.; Verma, C.; Maji, P.K.; Gaikwad, K.K. Isolation and Characterization of Cellulose Nanocrystals from Amla (Phyllanthus emblica) Pomace. Biomass Convers. Biorefin. 2021, 1–12. [Google Scholar] [CrossRef]
- Uma Maheswari, C.; Obi Reddy, K.; Muzenda, E.; Guduri, B.R.; Varada Rajulu, A. Extraction and Characterization of Cellulose Microfibrils from Agricultural Residue—Cocos nucifera L. Biomass Bioenergy 2012, 46, 555–563. [Google Scholar] [CrossRef]
- Waghmare, P.R.; Kadam, A.A.; Saratale, G.D.; Govindwar, S.P. Enzymatic Hydrolysis and Characterization of Waste Lignocellulosic Biomass Produced after Dye Bioremediation under Solid State Fermentation. Bioresour. Technol. 2014, 168, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.O.; Maheswari, C.U.; Shukla, M.; Rajulu, A.V. Chemical Composition and Structural Characterization of Napier Grass Fibers. Mater. Lett. 2012, 67, 35–38. [Google Scholar] [CrossRef]
- Zope, G.; Goswami, A.; Kulkarni, S. Isolation and Characterization of Cellulose Nanocrystals Produced by Acid Hydrolysis from Banana Pseudostem. BioNanoScience 2022, 12, 463–471. [Google Scholar] [CrossRef]
- Onkarappa, H.S.; Prakash, G.K.; Pujar, G.H.; Rajith Kumar, C.R.; Radha; Latha, M.S.; Betageri, V.S. Synthesis and Characterization of Nanocellulose Using Renewable Resources through Ionic Liquid Medium. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 035001. [Google Scholar] [CrossRef]
- Han, X.; Bi, R.; Khatri, V.; Oguzlu, H.; Takada, M.; Jiang, J.; Jiang, F.; Bao, J.; Saddler, J.N. Use of Endoglucanase and Accessory Enzymes to Facilitate Mechanical Pulp Nanofibrillation. ACS Sustain. Chem. Eng. 2021, 9, 1406–1413. [Google Scholar] [CrossRef]
- Yang, T.; Li, X.; Guo, Y.; Zhao, J.; Qu, Y. Preparation of Nanocellulose Crystal from Bleached Pulp with an Engineering Cellulase and Co-Production of Ethanol. Carbohydr. Polym. 2023, 301, 120291. [Google Scholar] [CrossRef]
- Martínez-Cervera, S.; Salvador, A.; Sanz, T. Comparison of Different Polyols as Total Sucrose Replacers in Muffins: Thermal, Rheological, Texture and Acceptability Properties. Food Hydrocoll. 2014, 35, 1–8. [Google Scholar] [CrossRef]
- Berto, G.L.; Mattos, B.D.; Rojas, O.J.; Arantes, V. Single-Step Fiber Pretreatment with Monocomponent Endoglucanase: Defibrillation Energy and Cellulose Nanofibril Quality. ACS Sustain. Chem. Eng. 2021, 9, 2260–2270. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, H.; Zattera, A. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef]
- Hu, J.; Tian, D.; Renneckar, S.; Saddler, J.N. Enzyme Mediated Nanofibrillation of Cellulose by the Synergistic Actions of an Endoglucanase, Lytic Polysaccharide Monooxygenase (LPMO) and Xylanase. Sci. Rep. 2018, 8, 3195. [Google Scholar] [CrossRef]
- Topakas, E.; Moukouli, M.; Dimarogona, M.; Christakopoulos, P. Expression, Characterization and Structural Modelling of a Feruloyl Esterase from the Thermophilic Fungus Myceliophthora Thermophila. Appl. Microbiol. Biotechnol. 2012, 94, 399–411. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Peng, S.; Cao, Q.; Qin, Y.; Li, X.; Liu, G.; Qu, Y. An Aldonolactonase AltA from Penicillium oxalicum Mitigates the Inhibition of β-Glucosidase during Lignocellulose Biodegradation. Appl. Microbiol. Biotechnol. 2017, 101, 3627–3636. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose Crystallinity Index: Measurement Techniques and Their Impact on Interpreting Cellulase Performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Jia, C.; Chen, L.; Shao, Z.; Agarwal, U.P.; Hu, L.; Zhu, J.Y. Using a Fully Recyclable Dicarboxylic Acid for Producing Dispersible and Thermally Stable Cellulose Nanomaterials from Different Cellulosic Sources. Cellulose 2017, 24, 2483–2498. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass (LAP); Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, April 2008. [Google Scholar]
- Miller, A.B. Primary Prevention of Cancer: Needs and Opportunities for Research. Environ. Health Perspect. 1995, 103, 313–317. [Google Scholar] [CrossRef]


| Cellulose | Hemicellulose | Lignin | Others |
|---|---|---|---|
| 91.45 ± 0.12 | 2.23 ± 0.02 | 0.72 ± 0.03 | 5.08 ± 0.13 |
| Enzyme | GH Family | Accession No. | Predicated Molecular Weight (kDa) | CBM | Glycosylation | Specific Activity (U/mg) | |
|---|---|---|---|---|---|---|---|
| N | O | ||||||
| MtEG5A | 5 | Mycth_86753 | 42.39 | CBM | 3 | 17 | 4.194 |
| MtEG7A | 7 | Mycth_111372 | 48.67 | CBM | 2 | 16 | 3.60 |
| MtEG12A | 12 | Mycth_109444 | 27.11 | - | - | - | 1.47 |
| MtEG45A | 45 | Mycth_76901 | 23.64 | - | - | 3 | 2.51 |
| Enzyme Used in Preparation | Length * (nm) | Width * (nm) | Average Apparent Size (nm) ** | CNCs Yield (%) |
|---|---|---|---|---|
| MtEG5A | 825 ± 108 | 79 ± 16 | 696 ± 49 | 5.33 ± 0.2 |
| (5+7) | 386 ± 85 | 45 ± 13 | 476 ± 52 | 6.40 ± 0.16 |
| (5+12) | 466 ± 199 | 47 ± 7 | 633 ± 29 | 5.67 ± 1.1 |
| (5+7+45) | 609 ± 103 | 40 ± 5 | 670 ± 35 | 8.80 ± 1.2 |
| Sample | Tonset (°C) | Tmax (°C) | Residue at 600 °C (%) |
|---|---|---|---|
| MtEG5A | 246.58 | 330.14 | 27.74 |
| (5+7) | 262.31 | 333.09 | 25.51 |
| (5+12) | 257.25 | 340.32 | 27.07 |
| (5+7+45) | 263.95 | 338.18 | 24.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waghmare, P.; Xu, N.; Waghmare, P.; Liu, G.; Qu, Y.; Li, X.; Zhao, J. Production and Characterization of Cellulose Nanocrystals from Eucalyptus Dissolving Pulp Using Endoglucanases from Myceliophthora thermophila. Int. J. Mol. Sci. 2023, 24, 10676. https://doi.org/10.3390/ijms241310676
Waghmare P, Xu N, Waghmare P, Liu G, Qu Y, Li X, Zhao J. Production and Characterization of Cellulose Nanocrystals from Eucalyptus Dissolving Pulp Using Endoglucanases from Myceliophthora thermophila. International Journal of Molecular Sciences. 2023; 24(13):10676. https://doi.org/10.3390/ijms241310676
Chicago/Turabian StyleWaghmare, Pratima, Nuo Xu, Pankajkumar Waghmare, Guodong Liu, Yinbo Qu, Xuezhi Li, and Jian Zhao. 2023. "Production and Characterization of Cellulose Nanocrystals from Eucalyptus Dissolving Pulp Using Endoglucanases from Myceliophthora thermophila" International Journal of Molecular Sciences 24, no. 13: 10676. https://doi.org/10.3390/ijms241310676
APA StyleWaghmare, P., Xu, N., Waghmare, P., Liu, G., Qu, Y., Li, X., & Zhao, J. (2023). Production and Characterization of Cellulose Nanocrystals from Eucalyptus Dissolving Pulp Using Endoglucanases from Myceliophthora thermophila. International Journal of Molecular Sciences, 24(13), 10676. https://doi.org/10.3390/ijms241310676







