Plasma Metabolomic Profiling after Feeding Dried Distiller’s Grains with Solubles in Different Cattle Breeds
Abstract
:1. Introduction
2. Results
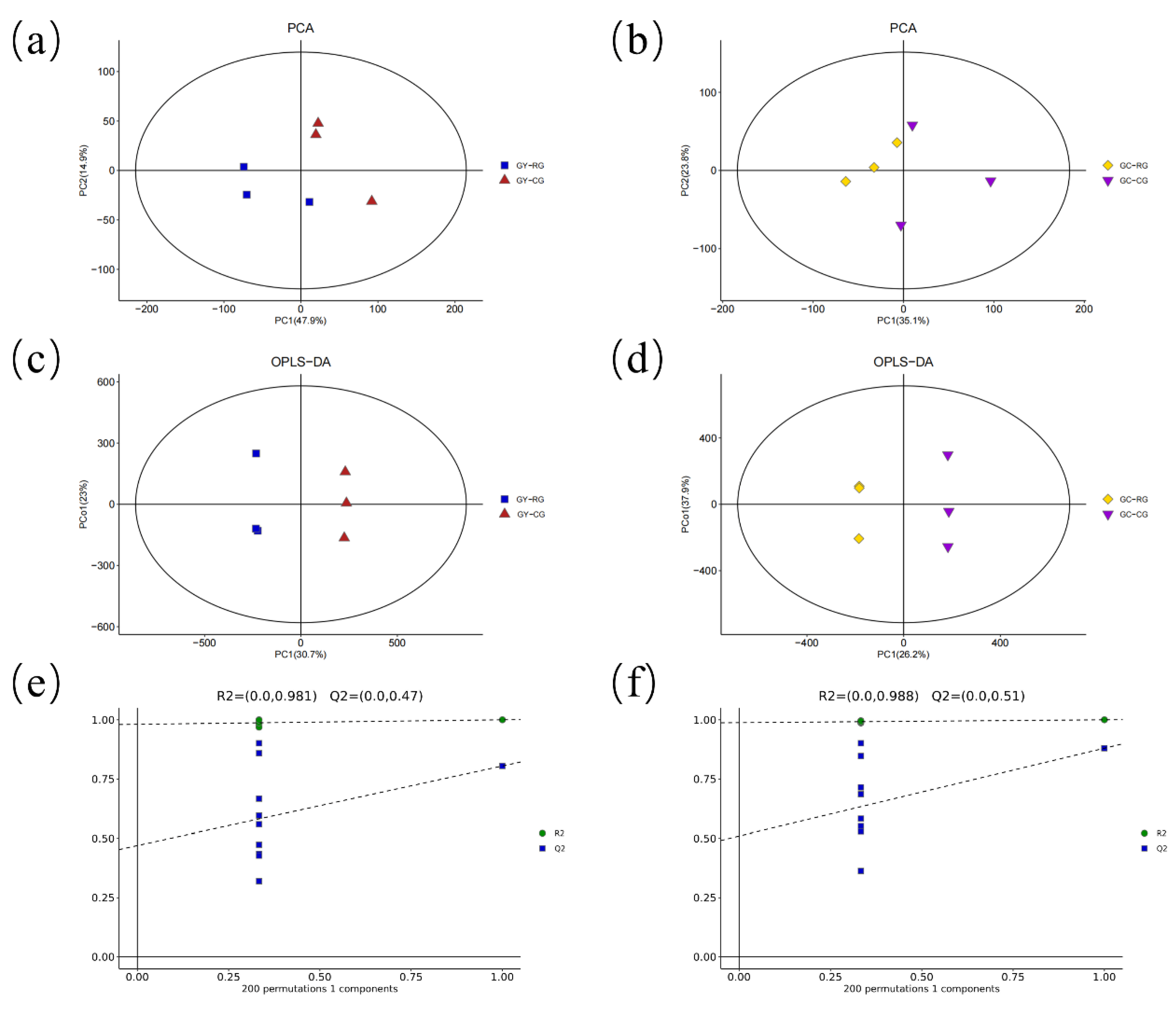
2.1. Quality Control of Untargeted Metabolic Profiling
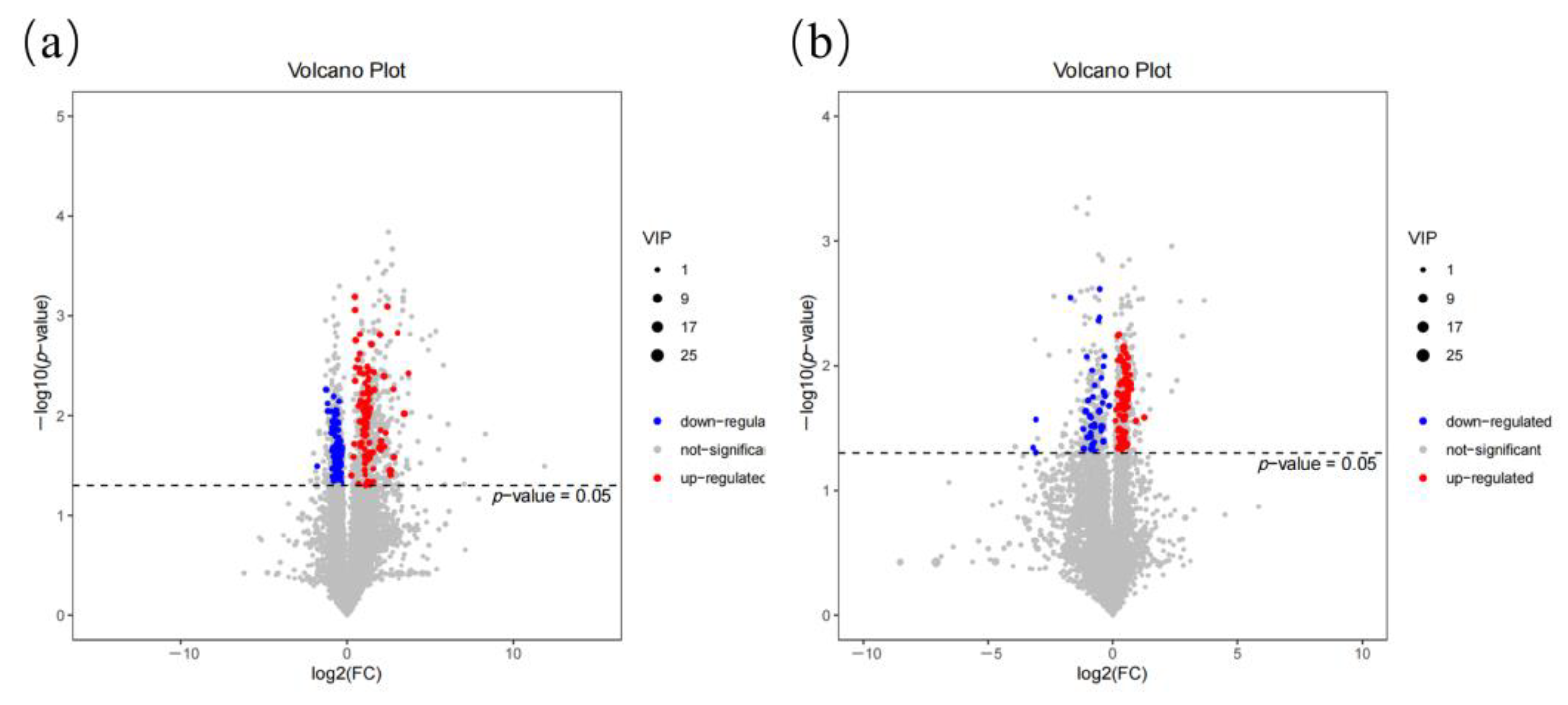
2.2. Differential Metabolite Screening
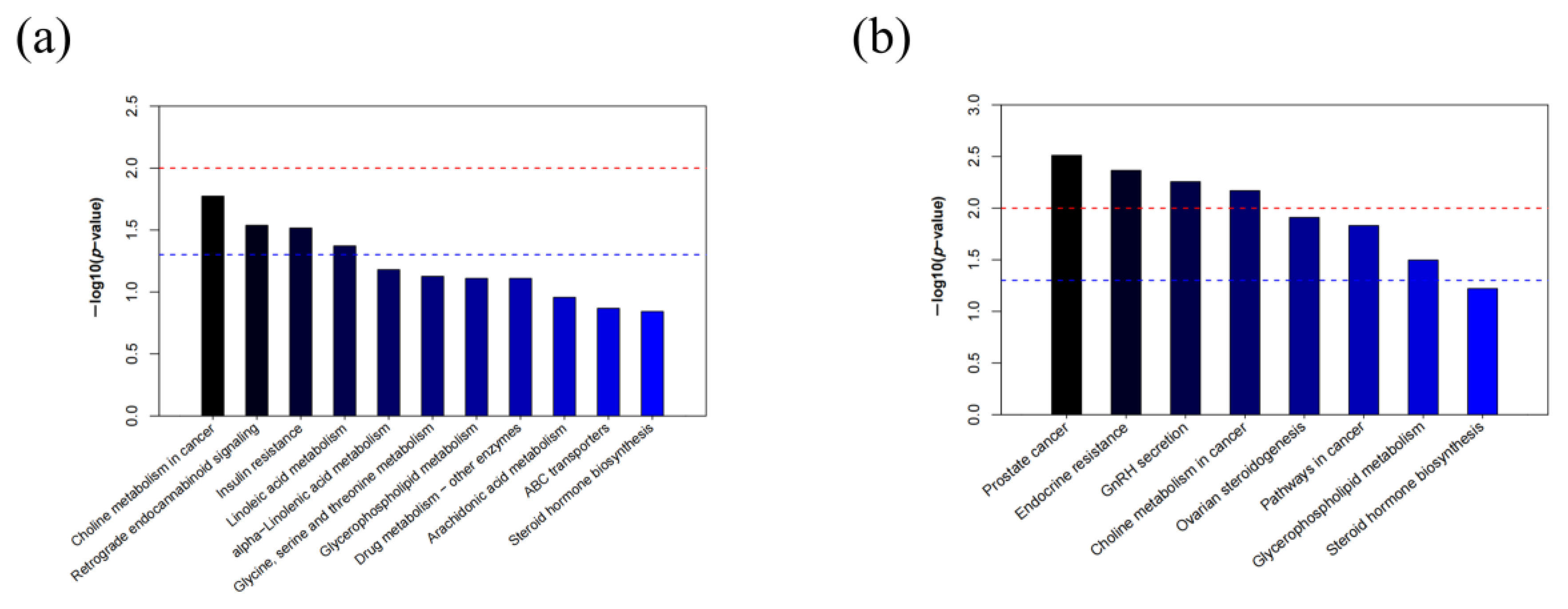
2.3. Metabolic Pathways Analysis
3. Discussion
4. Materials and Methods
4.1. Subjects Studied
4.2. Sample Preparation
4.3. LC–MS Analysis
4.4. Data Processing and Analysis
4.5. Putative Identification of Important Metabolites
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ao, T.; Ran, Y.; Chen, Y.; Li, R.; Luo, Y.; Liu, X.; Li, D. Effect of viscosity on process stability and microbial community composition during anaerobic mesophilic digestion of Maotai-flavored distiller’s grains. Bioresour. Technol. 2020, 297, 122460. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Z.; Wan, F.; Li, A. Potential for exploitation of distiller’s grains in grain-saving beef cattle industry. Chin. J. Anim. Sci. 2010, 46, 58–61. [Google Scholar]
- Liu, K. Chemical composition of distillers grains, a review. J. Agric. Food Chem. 2011, 59, 1508–1526. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Robe, J.; Grigsby, Z.; Rathert-Williams, A.; Major, M.; Lalman, D.L.; Foote, A.P.; Tedeschi, L.O.; Beck, P.A. Effects of supplementation rate of an extruded dried distillers’ grains cube fed to growing heifers on voluntary intake and digestibility of bermudagrass hay. J. Anim. Sci. 2022, 100, skac097. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, M.; Lei, H.; Li, H.; Deng, X. Research progress of comprehensive feed utilization of distiller’s grains. Agric. Technol. Serv. 2023, 40, 88–90. [Google Scholar]
- Zhang, S.Z.; Penner, G.B.; Yang, W.Z.; Oba, M. Effects of partially replacing barley silage or barley grain with dried distillers grains with solubles on rumen fermentation and milk production of lactating dairy cows. J. Dairy Sci. 2010, 93, 3231–3242. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Chen, J.; Zhu, L.; Guo, T.; Qin, D.; Hu, Z.; Han, S.; Wang, J.; Matias, F.B.; Wen, L.; et al. Oryzanol alleviates high fat and cholesterol diet-induced hypercholesterolemia associated with the modulation of the gut microbiota in hamsters. Food Funct. 2022, 13, 4486–4501. [Google Scholar] [CrossRef]
- Kou, J.; He, C.; Cui, L.; Zhang, Z.; Wang, W.; Tan, L.; Liu, D.; Zheng, W.; Gu, W.; Xia, N. Discovery of Potential Biomarkers for Postmenopausal Osteoporosis Based on Untargeted GC/LC-MS. Front. Endocrinol. 2022, 13, 849076. [Google Scholar] [CrossRef]
- Velenosi, T.J.; Hennop, A.; Feere, D.A.; Tieu, A.; Kucey, A.S.; Kyriacou, P.; McCuaig, L.E.; Nevison, S.E.; Kerr, M.A.; Urquhart, B.L. Untargeted plasma and tissue metabolomics in rats with chronic kidney disease given AST-120. Sci. Rep. 2016, 6, 22526. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, C.C.; Balzano-Nogueira, L.; Bisinotto, D.Z.; Ruiz, A.R.; Duarte, G.A.; Conesa, A.; Galvao, K.N.; Bisinotto, R.S. Differences in uterine and serum metabolome associated with metritis in dairy cows. J. Dairy Sci. 2023, 106, 3525–3536. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Hou, X.; Hu, C.; Zhang, L.; Wang, S.; Zhang, Q.; Shi, K. Multi-Channel Metabolomics Analysis Identifies Novel Metabolite Biomarkers for the Early Detection of Fatty Liver Disease in Dairy Cows. Cells 2022, 11, 2883. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Hu, R.; Peng, Q.; Xue, B.; Wang, L. Comparison of carcass characteristics and meat quality between Simmental crossbred cattle, cattle-yaks and Xuanhan yellow cattle. J. Sci. Food Agric. 2021, 101, 3927–3932. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, C.; Guo, Y.; Wang, C.; Wu, G.; Huang, W.; Yang, R.; Zhang, Y. Effect of Maotai distillers’grains on amino acid content of Guanling cattle and crossbred cattle. Biotechnology 2022, 32, 605–610. [Google Scholar]
- Zhang, Y. Feed utilization of distiller’s grains and its effect on livestock production and economic benefits. Feed Res. 2021, 44, 157–160. [Google Scholar]
- Dai, Q.; Ma, J.; Cao, G.; Hu, R.; Zhu, Y.; Li, G.; Zou, H.; Wang, Z.; Peng, Q.; Xue, B.; et al. Comparative study of growth performance, nutrient digestibility, and ruminal and fecal bacterial community between yaks and cattle-yaks raised by stall-feeding. AMB Express 2021, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, A.P.; Hazari, P.P.; Prakash, S.; Sethi, P.; Kaushik, A.; Roy, B.G.; Kathait, S.; Singh, B.; Mishra, A.K. [(99m)Tc]Tc-DTPA-Bis(cholineethylamine) as an Oncologic Tracer for the Detection of Choline Transporter (ChT) and Choline Kinase (ChK) Expression in Cancer. ACS Omega 2022, 7, 12509–12523. [Google Scholar] [CrossRef]
- Sonkar, K.; Ayyappan, V.; Tressler, C.M.; Adelaja, O.; Cai, R.; Cheng, M.; Glunde, K. Focus on the glycerophosphocholine pathway in choline phospholipid metabolism of cancer. NMR Biomed. 2019, 32, e4112. [Google Scholar] [CrossRef]
- Shekher, A.; Tiwari, A.K.; Awasthee, N.; Verma, S.S.; Dixit, V.K.; Sinha, N.; Gupta, S.C.; Puneet. Genes involved in phosphatidylcholine biosynthesis correlate with nuclear factor-kappaB in biliary tract cancer patients: Evidence from (1)H NMR and computational analyses. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158970. [Google Scholar] [CrossRef]
- Knuplez, E.; Marsche, G. An Updated Review of Pro- and Anti-Inflammatory Properties of Plasma Lysophosphatidylcholines in the Vascular System. Int. J. Mol. Sci. 2020, 21, 4501. [Google Scholar] [CrossRef]
- Darwish, T.A.; Luks, E.; Moraes, G.; Yepuri, N.R.; Holden, P.J.; James, M. Synthesis of deuterated [D32]oleic acid and its phospholipid derivative [D64 ]dioleoyl-sn-glycero-3-phosphocholine. J. Label. Comp. Radiopharm. 2013, 56, 520–529. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Y.; Song, X.; Jia, Q.; Zhang, X.; Qian, Y.; Qiu, J. Analysis of glycerophospholipid metabolism after exposure to PCB153 in PC12 cells through targeted lipidomics by UHPLC-MS/MS. Ecotoxicol. Env. Saf. 2019, 169, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Silanikove, N.; Rauch-Cohen, A.; Shapiro, F.; Arieli, A.; Merin, U.; Leitner, G. Lipopolysaccharide challenge of the mammary gland in cows induces nitrosative stress that impairs milk oxidative stability. Animal 2012, 6, 1451–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavangira, V.; Sordillo, L.M. Role of lipid mediators in the regulation of oxidative stress and inflammatory responses in dairy cattle. Res.Vet. Sci. 2018, 116, 4–14. [Google Scholar] [CrossRef]
- Gnott, M.; Vogel, L.; Kroger-Koch, C.; Dannenberger, D.; Tuchscherer, A.; Troscher, A.; Trevisi, E.; Stefaniak, T.; Bajzert, J.; Starke, A.; et al. Changes in fatty acids in plasma and association with the inflammatory response in dairy cows abomasally infused with essential fatty acids and conjugated linoleic acid during late and early lactation. J. Dairy Sci. 2020, 103, 11889–11910. [Google Scholar] [CrossRef]
- James, M.J.; Gibson, R.A.; Cleland, L.G. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am. J. Clin. Nutr. 2000, 71, 343S–348S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.Q.; Ma, X.W.; Dong, X.Y.; Tao, Z.R.; Lu, L.Z.; Zou, X.T. Effects of parental dietary linoleic acid on growth performance, antioxidant capacity, and lipid metabolism in domestic pigeons (Columba livia). Poult. Sci. 2020, 99, 1471–1482. [Google Scholar] [CrossRef]
- Lv, H.; Ren, D.; Yan, W.; Wang, Y.; Liu, H.; Shen, M. Linoleic acid inhibits Lactobacillus activity by destroying cell membrane and affecting normal metabolism. J. Sci. Food Agric. 2020, 100, 2057–2064. [Google Scholar] [CrossRef]
- Shrestha, N.; Vidimce, J.; Holland, O.J.; Cuffe, J.S.M.; Beck, B.R.; Perkins, A.V.; McAinch, A.J.; Hryciw, D.H. Maternal and Postnatal High Linoleic Acid Diet Impacts Lipid Metabolism in Adult Rat Offspring in a Sex-Specific Manner. Int. J. Mol. Sci. 2021, 22, 2946. [Google Scholar] [CrossRef]
- Draycott, S.A.V.; Elmes, M.J.; Muhlhausler, B.S.; Langley-Evans, S. Omega-6: Omega-3 Fatty Acid Ratio and Total Fat Content of the Maternal Diet Alter Offspring Growth and Fat Deposition in the Rat. Nutrients 2020, 12, 2505. [Google Scholar] [CrossRef] [PubMed]
- Gabbs, M.; Zahradka, P.; Taylor, C.G.; Aukema, H.M. Time Course and Sex Effects of alpha-Linolenic Acid-Rich and DHA-Rich Supplements on Human Plasma Oxylipins: A Randomized Double-Blind Crossover Trial. J. Nutr. 2021, 151, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Fortin, E.; Blouin, R.; Lapointe, J.; Petit, H.V.; Palin, M.F. Linoleic acid, alpha-linolenic acid and enterolactone affect lipid oxidation and expression of lipid metabolism and antioxidant-related genes in hepatic tissue of dairy cows. Br. J. Nutr. 2017, 117, 1199–1211. [Google Scholar] [CrossRef] [Green Version]
- Filipcev, B.; Kojic, J.; Krulj, J.; Bodroza-Solarov, M.; Ilic, N. Betaine in Cereal Grains and Grain-Based Products. Foods 2018, 7, 49. [Google Scholar] [CrossRef] [Green Version]
- Craig, S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghoneem, W.M.A.; El-Tanany, R.R.A. Impact of natural betaine supplementation on rumen fermentation and productive performance of lactating Damascus goats. Trop. Anim. Health Prod. 2023, 55, 123. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.R.; Poddar, N.K.; Dar, T.A.; Kumar, R.; Ahmad, F. Protein and DNA destabilization by osmolytes: The other side of the coin. Life Sci. 2011, 88, 117–125. [Google Scholar] [CrossRef]
- Ji, Y.; Fan, X.; Zhang, Y.; Li, J.; Dai, Z.; Wu, Z. Glycine regulates mucosal immunity and the intestinal microbial composition in weaned piglets. Amino. Acids 2022, 54, 385–398. [Google Scholar] [CrossRef]
- Anzmann, A.F.; Pinto, S.; Busa, V.; Carlson, J.; McRitchie, S.; Sumner, S.; Pandey, A.; Vernon, H.J. Multi-omics studies in cellular models of methylmalonic acidemia and propionic acidemia reveal dysregulation of serine metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165538. [Google Scholar] [CrossRef]
- Loriot, Y.; Fizazi, K.; de Bono, J.S.; Forer, D.; Hirmand, M.; Scher, H.I. Enzalutamide in castration-resistant prostate cancer patients with visceral disease in the liver and/or lung: Outcomes from the randomized controlled phase 3 AFFIRM trial. Cancer 2017, 123, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Baratchian, M.; Tiwari, R.; Khalighi, S.; Chakravarthy, A.; Yuan, W.; Berk, M.; Li, J.; Guerinot, A.; de Bono, J.; Makarov, V.; et al. H3K9 methylation drives resistance to androgen receptor-antagonist therapy in prostate cancer. Proc. Natl. Acad. Sci. USA 2022, 119, e2114324119. [Google Scholar] [CrossRef] [PubMed]
- Stincic, T.L.; Kelly, M.J. Estrogenic regulation of reproduction and energy homeostasis by a triumvirate of hypothalamic arcuate neurons. J. Neuroendocr. 2022, 34, e13145. [Google Scholar] [CrossRef] [PubMed]
- Emons, G.; Grundker, C. The Role of Gonadotropin-Releasing Hormone (GnRH) in Endometrial Cancer. Cells 2021, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- D’Occhio, M.J.; Aspden, W.J. Endocrine and reproductive responses of male and female cattle to agonists of gonadotrophin-releasing hormone. J. Reprod. Fertil. Suppl. 1999, 54, 101–114. [Google Scholar] [CrossRef]
- Gallab, R.S.; Hassanein, E.M.; Rashad, A.M.A.; El-Shereif, A.A. Maximizing the reproductive performances of anestrus dairy buffalo cows using GnRH analogue-loaded chitosan nanoparticles during the low breeding season. Anim. Reprod. Sci. 2022, 244, 107044. [Google Scholar] [CrossRef]
| GY-RG Compared with GY-CG | ||||||
|---|---|---|---|---|---|---|
| Nos. | Metabolites | VIP | p-Value | FC | Ion Mode | Super-Class |
| 1 | 6-Methylmercaptopurine | 17.849 | 0.026 | 2.338 | neg | Organoheterocyclic compounds |
| 2 | Benzaldehyde, p-amino-, thiosemicarbazone | 6.266 | 0.021 | 3.992 | neg | Unclassified |
| 3 | C16 Sphinganine | 6.238 | 0.023 | 0.720 | pos | Lipids and lipid-like molecules |
| 4 | PC(20:0/18:3(6Z,9Z,12Z)) | 5.615 | 0.021 | 0.79 | pos | Lipids and lipid-like molecules |
| 5 | 6-Thioxanthine | 4.608 | 0.035 | 6.028 | neg | Unclassified |
| 6 | Sodium tetradecyl sulfate | 3.125 | 0.035 | 0.674 | neg | Organic acids and derivatives |
| 7 | Betaine | 2.644 | 0.029 | 0.705 | pos | Organic acids and derivatives |
| 8 | Nuatigenin 3-beta-D-glucopyranoside | 2.555 | 0.012 | 0.594 | neg | Lipids and lipid-like molecules |
| 9 | Ethyl 1-(propylthio)propyl disulfide | 2.553 | 0.018 | 4.114 | neg | Organosulfur compounds |
| 10 | Diethyl disulfide | 2.164 | 0.0134 | 2.073 | neg | Organosulfur compounds |
| 11 | Norfloxacin | 1.906 | 0.021 | 0.551 | pos | Organoheterocyclic compounds |
| 12 | L-Acetylcarnitine | 1.780 | 0.012 | 0.565 | pos | Lipids and lipid-like molecules |
| 13 | Scillirosidin | 1.773 | 0.025 | 0.598 | pos | Lipids and lipid-like molecules |
| 14 | PC(18:2(9Z,12Z)/20:1(11Z)) | 1.573 | 0.0424 | 0.754 | pos | Lipids and lipid-like molecules |
| 15 | 4-Ethynylaniline | 1.484 | 0.049 | 2.151 | pos | Unclassified |
| 16 | 2E,4E,6Z-Nonatrienal | 1.454 | 0.0221 | 4.081 | neg | Lipids and lipid-like molecules |
| 17 | CP 339818 | 1.426 | 0.013 | 0.527 | pos | Unclassified |
| 18 | 5-(Methylthio)-2-[(methylthio)methyl]-2-pentenal | 1.413 | 0.004 | 2.592 | neg | Organic oxygen compounds |
| 19 | PC(18:1(11Z)/18:3(6Z,9Z,12Z)) | 1.402 | 0.037 | 0.659 | neg | Lipids and lipid-like molecules |
| 20 | O-Cresol | 1.400 | 0.042 | 0.645 | pos | Benzenoids |
| 21 | N-oleoyl glutamic acid | 1.316 | 0.011 | 0.667 | pos | Lipids and lipid-like molecules |
| 22 | 5,7-octadienoic acid | 1.226 | 0.0148 | 4.995 | pos | Lipids and lipid-like molecules |
| 23 | Palmatine | 1.206 | 0.008 | 0.441 | pos | Unclassified |
| 24 | 11-Dehydrocorticosterone | 1.191 | 0.032 | 0.287 | pos | Lipids and lipid-like molecules |
| 25 | Armillaripin | 1.182 | 0.029 | 0.590 | pos | Lipids and lipid-like molecules |
| 26 | 2,3-dinor Thromboxane B1 | 1.141 | 0.014 | 4.058 | neg | Unclassified |
| 27 | 8-Hydroxyguanine | 1.127 | 0.012 | 0.630 | pos | Organoheterocyclic compounds |
| 28 | 2E,7-Octadienoic acid | 1.046 | 0.020 | 4.785 | pos | Lipids and lipid-like molecules |
| 29 | (7R)-7-(5-Carboxy-5-oxopentanoyl)aminocephalosporinate | 1.033 | 0.008 | 2.377 | pos | Organoheterocyclic compounds |
| GC-RG Compared with GC-CG | ||||||
| Nos. | Metabolites | VIP | p-Value | FC | Ion Mode | Super-Class |
| 1 | LysoPC(P-18:1(9Z)) | 5.757 | 0.043 | 1.458 | pos | Unclassified |
| 2 | LysoPC(22:5(4Z,7Z,10Z,13Z,16Z)) | 3.239 | 0.046 | 0.554 | neg | Lipids and lipid-like molecules |
| 3 | Capsidiol | 2.862 | 0.043 | 0.565 | pos | Lipids and lipid-like molecules |
| 4 | PS(17:2(9Z,12Z)/20:0) | 2.721 | 0.023 | 0.472 | neg | Lipids and lipid-like molecules |
| 5 | SM(d18:1/16:1) | 2.368 | 0.037 | 0.494 | pos | Lipids and lipid-like molecules |
| 6 | PC(15:0/22:2(13Z,16Z)) | 2.095 | 0.013 | 1.417 | pos | Lipids and lipid-like molecules |
| 7 | Testosterone | 1.660 | 0.026 | 0.541 | pos | Lipids and lipid-like molecules |
| 8 | Monocyclic botryococcane | 1.133 | 0.041 | 1.228 | pos | Lipids and lipid-like molecules |
| 9 | PC(16:0/O-1:0) | 1.064 | 0.033 | 1.271 | pos | Unclassified |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, T.; Xu, D.; Zhu, M.; Luo, X.; Zhang, R.; He, G.; Chen, Z.; Mei, S.; Zhou, B.; et al. Plasma Metabolomic Profiling after Feeding Dried Distiller’s Grains with Solubles in Different Cattle Breeds. Int. J. Mol. Sci. 2023, 24, 10677. https://doi.org/10.3390/ijms241310677
Zhang J, Zhang T, Xu D, Zhu M, Luo X, Zhang R, He G, Chen Z, Mei S, Zhou B, et al. Plasma Metabolomic Profiling after Feeding Dried Distiller’s Grains with Solubles in Different Cattle Breeds. International Journal of Molecular Sciences. 2023; 24(13):10677. https://doi.org/10.3390/ijms241310677
Chicago/Turabian StyleZhang, Junjie, Tiantian Zhang, Duhan Xu, Mingming Zhu, Xiaofen Luo, Rong Zhang, Guangxia He, Ze Chen, Shihui Mei, Bijun Zhou, and et al. 2023. "Plasma Metabolomic Profiling after Feeding Dried Distiller’s Grains with Solubles in Different Cattle Breeds" International Journal of Molecular Sciences 24, no. 13: 10677. https://doi.org/10.3390/ijms241310677





