Transcriptome-Wide Identification of the GRAS Transcription Factor Family in Pinus massoniana and Its Role in Regulating Development and Stress Response
Abstract
:1. Introduction
2. Results
2.1. Identification and Multiple Sequence Analysis of GRASs in P. massoniana
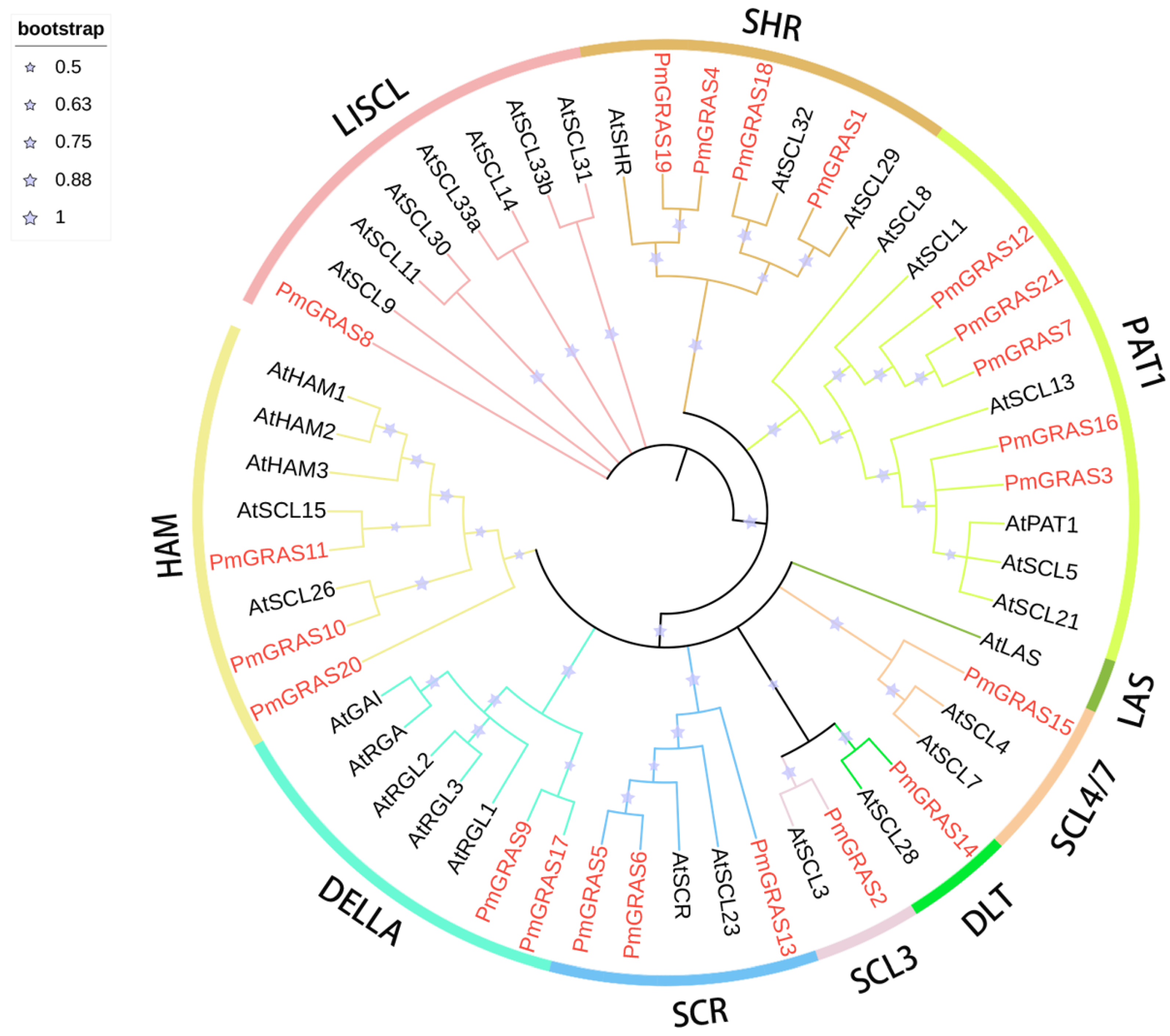
2.2. Phylogenetic Analysis of GRAS Protein Sequences in P. massoniana and A. thaliana
2.3. Analysis of PmGRAS Family Protein Motifs and Gene Structures
2.4. Subcellular Localization Analysis of PmGRASs
2.5. Transcriptome Analysis of Needles of P. massoniana under Drought Stress
2.6. Expression Pattern of PmGRASs under PEG and Mechanical Injury Stress
2.7. Expression Pattern of PmGRASs under Different Hormone Treatments
2.8. Expression Levels of PmGRASs in Different Tissues
2.9. Transcriptional Activity Assays of PmGRAS9
3. Discussion
3.1. PmGRAS Gene Structure and Evolutionary Analysis
3.2. Possible Functions of PmGRAS Members
3.3. Role of PmGRASs in the Development of Different Tissues of P. massoniana
3.4. Subcellular Localization and Transcriptional Activation Analysis of the DELLA Subfamily
4. Materials and Methods
4.1. Plant Materials and Different Treatments
4.2. Identification and Bioinformatics Analysis of GRAS Genes in P. massoniana
4.3. Phylogenetic Analyses and Classifications
4.4. Subcellular Localization and Transcriptional Activity of Candidates
4.5. Transcriptional Pattern Analysis
4.6. RNA Extraction and qRT-PCR Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riano-Pachon, D.M.; Ruzicic, S.; Dreyer, I.; Mueller-Roeber, B. PlnTFDB: An integrative plant transcription factor database. BMC Bioinform. 2007, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.C.; Qiu, W.M.; Jin, K.M.; Lu, Z.C.; Han, X.J.; Zhuo, R.Y.; Liu, X.G.; He, Z.Q. Comprehensive analysis of WRKY gene family in Sedum plumbizincicola responding to cadmium stress. J. Nanjing For. Univ. Nat. 2023, 47, 49–60. [Google Scholar] [CrossRef]
- Hirsch, S.; Oldroyd, G.E. GRAS-domain transcription factors that regulate plant development. Plant Signal. Behav. 2009, 4, 698–700. [Google Scholar] [CrossRef] [Green Version]
- Pysh, L.D.; Wysocka-Diller, J.W.; Camilleri, C.; Bouchez, D.; Benfey, P.N. The GRAS gene family in Arabidopsis: Sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J. 1999, 18, 111–119. [Google Scholar] [CrossRef]
- Richards, D.E.; Peng, J.; Harberd, N.P. Plant GRAS and metazoan STATs: One family? Bioessays 2000, 22, 573–577. [Google Scholar] [CrossRef]
- Sun, X.L.; Jones, W.T.; Rikkerink, E.H.A. GRAS proteins: The versatile roles of intrinsically disordered proteins in plant signalling. Biochem. J. 2012, 442, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.B.; Liu, S.Y.; Liu, D.L.; Guo, F.; Yang, Y.Y.; Dong, T.T.; Zhang, Y.; Ma, C.; Tang, Z.X.; Li, F.F.; et al. Genome-wide survey and expression analysis of GRAS transcription factor family in sweetpotato provides insights into their potential roles in stress response. BMC Plant Biol. 2022, 22, 232. [Google Scholar] [CrossRef]
- Cenci, A.; Rouard, M. Evolutionary analyses of GRAS transcription factors in Angiosperms. Front Plant Sci. 2017, 2, 273. [Google Scholar] [CrossRef] [Green Version]
- Laskar, P.; Bhattacharya, S.; Chaudhuri, A.; Kundu, A. Exploring the GRAS gene family in common bean (Phaseolus vulgaris L.): Characterization, evolutionary relationships, and expression analyses in response to abiotic stresses. Planta 2021, 254, 84. [Google Scholar] [CrossRef]
- Yadav, S.; Yadava, Y.K.; Kohli, D.; Meena, S.; Paul, V.; Jain, P.K. Genome-wide identification and expression analysis of the GRAS gene family in response to drought stress in chickpea (Cicer arietinum L.). 3 Biotech 2022, 12, 64. [Google Scholar] [CrossRef]
- Kumari, P.; Gahlaut, V.; Kaur, E.; Singh, S.; Kumar, S.; Jaiswal, V. Genome-Wide identification of GRAS transcription factors and their potential roles in growth and development of Rose (Rosa chinensis). J. Plant Growth Regul. 2023, 42, 1505–1521. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, Y.; He, Z.H.; Wang, Z.B.; Tian, Z.Z.; Wang, C. Cloning and salt tolerance analysis of BpGRAS1 gene in Betula platyphylla. J. Nanjing For. Univ. Nat. Sci. 2021, 45, 38–46. [Google Scholar] [CrossRef]
- Abarca, D.; Pizarro, A.; Hernández, I.; Sánchez, C.; Solana, S.P.; Amo, A.; Carneros, E.; Díaz-Sala, C. The GRAS gene family in pine: Transcript expression patterns associated with the maturation-related decline of competence to form adventitious roots. BMC Plant Biol. 2014, 14, 354. [Google Scholar] [CrossRef] [Green Version]
- Aoyanagi, T.; Ikeya, S.; Kobayashi, A.; Kozaki, A. Gene regulation via the combination of transcription factors in the INDETERMINATE DOMAIN and GRAS families. Genes 2020, 11, 613. [Google Scholar] [CrossRef]
- Maggio, A.; Barbieri, G.; Raimondi, G.; Pascale, S. Contrasting effects of GA3 treatments on tomato plants exposed to increasing salinity. J. Plant Growth Regul. 2010, 29, 63–72. [Google Scholar] [CrossRef]
- Bolle, C.; Koncz, C.; Chua, N.H. PAT1, a new member of the GRAS family, is involved in phytochrome a signal transduction. Genes Dev. 2000, 14, 1269–1278. [Google Scholar] [CrossRef]
- Di-Laurenzio, L.; Wysocka-Diller, J.; Malamy, J.E.; Pysh, L.; Helariutta, Y.; Freshour, G.; Hahn, M.G.; Feldman, K.A. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 1996, 86, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Gao, F.; Li, T.H.; Chen, T.X.; Zheng, X.B.; Lian, X.D.; Wang, X.B.; Zhang, H.P.; Cheng, J.; Wang, W.; et al. Genome-wide analysis of the GRAS transcription factor gene family in peach (Prunus persica) and ectopic expression of PpeDELLA1 and PpeDELLA2 in Arabidopsis result in dwarf phenotypes. Sci. Hortic. 2022, 298, 0304–4238. [Google Scholar] [CrossRef]
- Greb, T.; Clarenz, O.; Schafer, E.; Muller, D.; Herrero, R.; Schmitz, G.; Theres, K. Molecular analysis of the lateral suppressor gene in Arabidopsis reveals a conserved control mechanism for axillary meristem formation. Genes Dev. 2003, 17, 1175–1187. [Google Scholar] [CrossRef] [Green Version]
- Miyashima, S.; Hashimoto, T.; Nakajima, K. ARGONAUTE1 acts in Arabidopsis root radial pattern formation independently of the SHR/SCR pathway. Plant Cell Physiol. 2009, 50, 626–634. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, M.; Balkunde, R.; Bui, H.; Jackson, D. An aminoacyl tRNA synthetase, OKI1, is required for proper shoot meristem size in Arabidopsis. Plant Cell Physiol. 2019, 60, 2597–2608. [Google Scholar] [CrossRef]
- Würschum, T.; Gross-Hardt, R.; Laux, T. APETALA2 regulates the stem cell niche in the Arabidopsis shoot meristem. Plant Cell 2006, 18, 295–307. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Zhang, B.; Su, T.; Li, P.; Xin, X.; Wang, W.; Zhao, X.; Yu, Y.; Zhang, D.; Yu, S.; et al. BrLAS, a GRAS transcription factor from Brassica rapa, is involved in drought stress tolerance in transgenic Arabidopsis. Front. Plant Sci. 2018, 9, 1792. [Google Scholar] [CrossRef] [Green Version]
- Czikkel, B.E.; Maxwell, D.P. NtGRAS1, a novel stress-induced member of the GRAS family in tobacco, localizes to the nucleus. J Plant Physiol. 2007, 164, 1220–1230. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Li, C.; Li, H.; Zhang, J.; Ye, Z. Silencing GRAS2 reduces fruit weight in tomato. Chin. Bull. Bot. 2018, 60, 498–513. [Google Scholar] [CrossRef] [Green Version]
- Courbier, S. Bundling up: VaPAT1 forms a complex with VaIDD3 to activate cold tolerance in Amur grape calli. Plant Physiol. 2021, 186, 1373–1374. [Google Scholar] [CrossRef]
- Dutta, M.; Saha, A.; Moin, M.; Kirti1, P.B. Genome-wide identification, transcript profiling and bioinformatic analyses of GRAS transcription factor genes in rice. Front. Plant Sci. 2021, 12, 777285. [Google Scholar] [CrossRef]
- Uluisik, S.; Kıyak, A.; Kurt, F.; Filiz, E. STAY-GREEN (SGR) genes in tomato (Solanum lycopersicum): Genome-wide identification, and expression analyses reveal their involvements in ripening and salinity stress responses. Hortic. Environ. Biotechnol. 2022, 63, 557–569. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Lu, X.; Chen, L.; Liu, J.; Wu, H. Identification and Expression Analysis of GRAS Transcription Factors to Elucidate Candidate Genes Related to Stolons, Fruit Ripening and Abiotic Stresses in Woodland Strawberry (Fragaria vesca). Int. J. Mol. Sci. 2019, 20, 4593. [Google Scholar] [CrossRef] [Green Version]
- Ji, K.S.; Xu, L.A.; Wang, D.B. Progresses and achievements of genetic improvement on Masson pine (Pinus massoniana) in China. J. Nanjing For. Univ. Nat. Sci. 2022, 46, 10–22. [Google Scholar] [CrossRef]
- Wu, F.; Zhu, P.H.; Ji, K.S. Response of distribution pattern of Masson's pine to future climate change. J. Nanjing For. Univ. Nat. Sci. 2022, 46, 196–204. [Google Scholar] [CrossRef]
- Liu, X.; Widmer, A. Genome-wide Comparative Analysis of the GRAS Gene Family in Populus, Arabidopsis and Rice. Plant Mol Biol. Rep. 2014, 32, 1129–1145. [Google Scholar] [CrossRef]
- Tyler, L.; Thomas, S.G.; Hu, J.H.; Dill, A.; Alonso, J.M.; Ecker, J.R.; Sun, T.P. DELLA proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol. 2004, 135, 1008–1019. [Google Scholar] [CrossRef] [Green Version]
- Noel, B.T.; Antonio, S.M.; David, A. Regulation of DELLA proteins by Post-translational modifications. Plant Cell Physiol. 2020, 61, 1891–1901. [Google Scholar] [CrossRef]
- Khan, Y.; Xiong, Z.; Zhang, H.; Liu, S.; Yaseen, T.; Hui, T. Expression and roles of GRAS gene family in plant growth, signal transduction, biotic and abiotic stress resistance and symbiosis formation-a review. Plant Biol. 2022, 24, 404–416. [Google Scholar] [CrossRef]
- Grimplet, J.; Agudelo-Romero, P.; Teixeira, R.T.; Martinez-Zapater, J.M.; Fortes, A.M. Structural and functional analysis of the GRAS gene family in grapevine indicates a role of GRAS proteins in the control of development and stress responses. Front. Plant Sci. 2016, 7, 353. [Google Scholar] [CrossRef] [Green Version]
- Locascio, A.; Blázquez, M.A.; Alabadí, D. Genomic analysis of DELLA protein activity. Plant Cell Physiol. 2013, 54, 1229–1237. [Google Scholar] [CrossRef] [Green Version]
- Stuurman, J.; Jäggi, F.; Kuhlemeier, C. Shoot meristem maintenance is controlled by a GRAS-gene mediated signal from differentiating cells. Gene. Dev. 2002, 16, 2213–2218. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Cho, J.S.; Jeon, H.W.; Sangsawang, K.; Shim, D.; Choi, Y.I.; Park, E.J.; Lee, H.; Ko, J.H. Wood transcriptome profiling identifies critical pathway genes of secondary wall biosynthesis and novel regulators for vascular cambium development in Populus. Genes 2019, 10, 690. [Google Scholar] [CrossRef] [Green Version]
- Hakoshima, T. Structural basis of the specific interactions of GRAS family proteins. FEBS Lett. 2018, 592, 489–501. [Google Scholar] [CrossRef] [Green Version]
- Fambrini, M.; Mariotti, L.; Parlanti, S.; Salvini, M.; Pugliesi, C. A GRAS-like gene of sunflower (Helianthus annuus L.) alters the gibberellin content and axillary meristem outgrowth in transgenic Arabidopsis plants. Plant Biol. 2015, 17, 1123–1134. [Google Scholar] [CrossRef]
- Kovacs, S.; Fodor, L.; Domonkos, A.; Ayaydin, F.; Laczi, K.; Rákhely, A.; Kalo, P. Amino acid polymorphisms in the VHIID conserved motif of nodulation signaling pathways 2 distinctly modulate symbiotic signaling and nodule morphogenesis in Medicago truncatula. Front. Plant Sci. 2021, 12, 709857. [Google Scholar] [CrossRef]
- Brand, L.H.; Fischer, N.M.; Harter, K.; Kohlbacher, O.; Wanke, D. Elucidating the evolutionary conserved DNA-binding specifcities of WRKY transcription factors by molecular dynamics and in vitro binding assays. Nucleic Acids Res. 2013, 41, 9764–9778. [Google Scholar] [CrossRef]
- Wen, C.K.; Chang, C. Arabidopsis RGL1 encodes a negative regulator of gibberellin responses. Plant Cell 2002, 14, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Xian, Z.; Kang, X.; Tang, N.; Li, Z. Genome-wide identification, phylogeny and expression analysis of GRAS gene family in tomato. BMC Plant Biol. 2015, 15, 209. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Carol, P.; Richards, D.E.; King, K.E.; Cowling, R.J.; Murphy, J.P.; Harberd, N.P. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev. 1997, 11, 3194–3205. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Wei, X.B.; Lai, D.L.; Yang, H.; Feng, L.; Li, L.; Niu, K.; Chen, L.; Xiang, D.B.; Ruan, J.J.; et al. Genome-wide investigation of the GRAS transcription factor family in foxtail millet (Setaria italica L.). BMC Plant Biol. 2021, 21, 508. [Google Scholar] [CrossRef]
- Torres-Galea, P.; Huang, L.F.; Chua, N.H.; Bolle, C. The GRAS protein SCL13 is a positive regulator of phytochrome-dependent red light signaling, but can also modulate phytochrome a responses. Mol. Genet. Genom. 2006, 276, 13–30. [Google Scholar] [CrossRef]
- Ma, H.S.; Liang, D.; Shuai, P.; Xia, X.L.; Yin, W.L. The salt- and drought-inducible poplar GRAS protein SCL7 confers salt and drought tolerance in Arabidopsis thaliana. J. Exp. Bot. 2010, 61, 4011–4019. [Google Scholar] [CrossRef] [Green Version]
- Fode, B.; Siemsen, T.; Turow, C.; Weigel, R.; Gatz, C. The Arabidopsis GRAS protein SCL14 interacts with class II TGA transcription factors and is essential for the activation of stress-inducible promoters. Plant Cell 2008, 20, 3122–3135. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, S.; Kim, J.; Muñoz, A.; Heckmann, A.B.; Downie, J.A.; Oldroyd, G.E.D. GRAS proteins form a DNA binding complex to induce gene expression during nodulation signaling in Medicago truncatula. Plant Cell 2009, 21, 545–557. [Google Scholar] [CrossRef] [Green Version]
- Heckmann, A.B.; Lombardo, F.; Miwa, H.; Perry, J.A.; Bunnewell, S.; Parniske, M.; Downie, J.A. Lotus japonicus nodulation requires two GRAS domain regulators, one of which is functionally conserved in a non-legume. Plant Physiol. 2006, 142, 1739–1750. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.O.; Chang, K.S.; Kim, I.A.; Lee, M.H.; Lee, S.A.; Song, S.K.; Lim, J. Funneling of gibberellin signaling by the GRAS transcription regulator SCARECROW-LIKE 3 in the Arabidopsis root. Proc. Natl. Acad. Sci. USA 2011, 108, 2166–2171. [Google Scholar] [CrossRef] [Green Version]
- Seemann, C.; Heck, C.; Voß, C.; Schmoll, J.; Enderle, E.; Schwarz, D.; Requena, N. Root cortex development is fine-tuned by the interplay of MIGs, SCL3 and DELLAs during arbuscular mycorrhizal symbiosis. New Phytol. 2022, 233, 848–965. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.W.; Ma, J.J.; Liu, H.M.; Han, F.X.; Li, Y.; Niu, S.H. Gibberellin signaling is required for far-red light-induced shoot elongation in Pinus tabuliformis seedlings. Plant Physiol. 2019, 182, 948–965. [Google Scholar] [CrossRef]
- Heyman, J.; Cools, T.; Canher, B.; Shavialenka, S.; Traas, J.; Vercauteren, I.; Daele, H.V.; Persiau, G.; Jaeger, G.D.; Sugimoto, K.; et al. The heterodimeric transcription factor complex ERF115-PAT1 grants regeneration competence. Nat. Plants 2016, 2, 16165. [Google Scholar] [CrossRef]
- Bolle, C. The role of GRAS proteins in plant signal transduction and development. Planta 2004, 218, 683–692. [Google Scholar] [CrossRef]
- David-Schwartz, R.; Borovsky, Y.; Zemach, H.; Paran, I. CaHAM is autoregulated and regulates CaSTM expression and is required for shoot apical meristem organization in pepper. Plant Sci. 2013, 203, 8–16. [Google Scholar] [CrossRef]
- To, V.T.; Shi, Q.; Zhang, Y.; Shi, J.; Shen, C.; Zhang, D.; Cai, W. Genome-wide analysis of the GRAS gene family in Barley (Hordeum vulgare L.). Genes 2020, 11, 553. [Google Scholar] [CrossRef]
- Ito, T.; Fukazawa, J. SCARECROW-LIKE3 regulates the transcription of gibberellin-related genes by acting as a transcriptional co-repressor of GAI-ASSOCIATED FACTOR1. Plant Mol. Biol. 2021, 105, 463–482. [Google Scholar] [CrossRef]
- Jaiswal, V.; Kakkar, M.; Kumari, P.; Zinta, G.; Gahlaut, V.; Kumar, S. Multifaceted roles of GRAS transcription factors in growth and stress responses in plants. iScience 2022, 25, 105026. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, B.; Song, S.K.; Heo, J.O.; Yu, N.I.; Lee, S.A.; Kim, M.; Kim, D.G.; Sohn, S.O.; Lim, C.E.; et al. Large-scale analysis of the GRAS gene family in Arabidopsis thaliana. Plant Mol. Biol. 2008, 67, 659–670. [Google Scholar] [CrossRef] [Green Version]
- Leone, M.; Keller, M.M.; Cerrudo, I.; Ballaré, C.L. To grow or defend? Low red : Far-red ratios reduce jasmonate sensitivity in Arabidopsis seedlings by promoting DELLA degradation and increasing JAZ10 stability. New Phytol. 2014, 204, 355–367. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of bigbiological data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Lin, L.L.; Hu, A.Q.; Chen, G.; Zhang, J.Y.; Cao, G.Q.; Cao, S.J. Cloning and expression characteristics of ClWRKY44 gene from Cunninghamia lcunninghamia. J. Nanjing For. Univ. Nat. Sci. 2022, 46, 203–209. [Google Scholar] [CrossRef]
- Zhu, P.H.; Ma, Y.Y.; Zhu, L.Z.; Chen, Y.; Li, R.; Ji, K.S. Selection of suitable reference genes in Pinus massoniana Lamb. Under different abiotic stresses for qPCR normalization. Forests 2019, 10, 632. [Google Scholar] [CrossRef] [Green Version]
- Harshitha, R.; Arunraj, D.R. Real-time quantitative PCR: A tool for absolute and relative quantification. Biochem. Mol. Biol. Educ. 2021, 49, 800–812. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Agassin, R.H.; Ji, K. Transcriptome-Wide Identification of the GRAS Transcription Factor Family in Pinus massoniana and Its Role in Regulating Development and Stress Response. Int. J. Mol. Sci. 2023, 24, 10690. https://doi.org/10.3390/ijms241310690
Yang Y, Agassin RH, Ji K. Transcriptome-Wide Identification of the GRAS Transcription Factor Family in Pinus massoniana and Its Role in Regulating Development and Stress Response. International Journal of Molecular Sciences. 2023; 24(13):10690. https://doi.org/10.3390/ijms241310690
Chicago/Turabian StyleYang, Ye, Romaric Hippolyte Agassin, and Kongshu Ji. 2023. "Transcriptome-Wide Identification of the GRAS Transcription Factor Family in Pinus massoniana and Its Role in Regulating Development and Stress Response" International Journal of Molecular Sciences 24, no. 13: 10690. https://doi.org/10.3390/ijms241310690




