Physiological Significance of the Heterogeneous Distribution of Zeaxanthin and Lutein in the Retina of the Human Eye
Abstract
1. Introduction
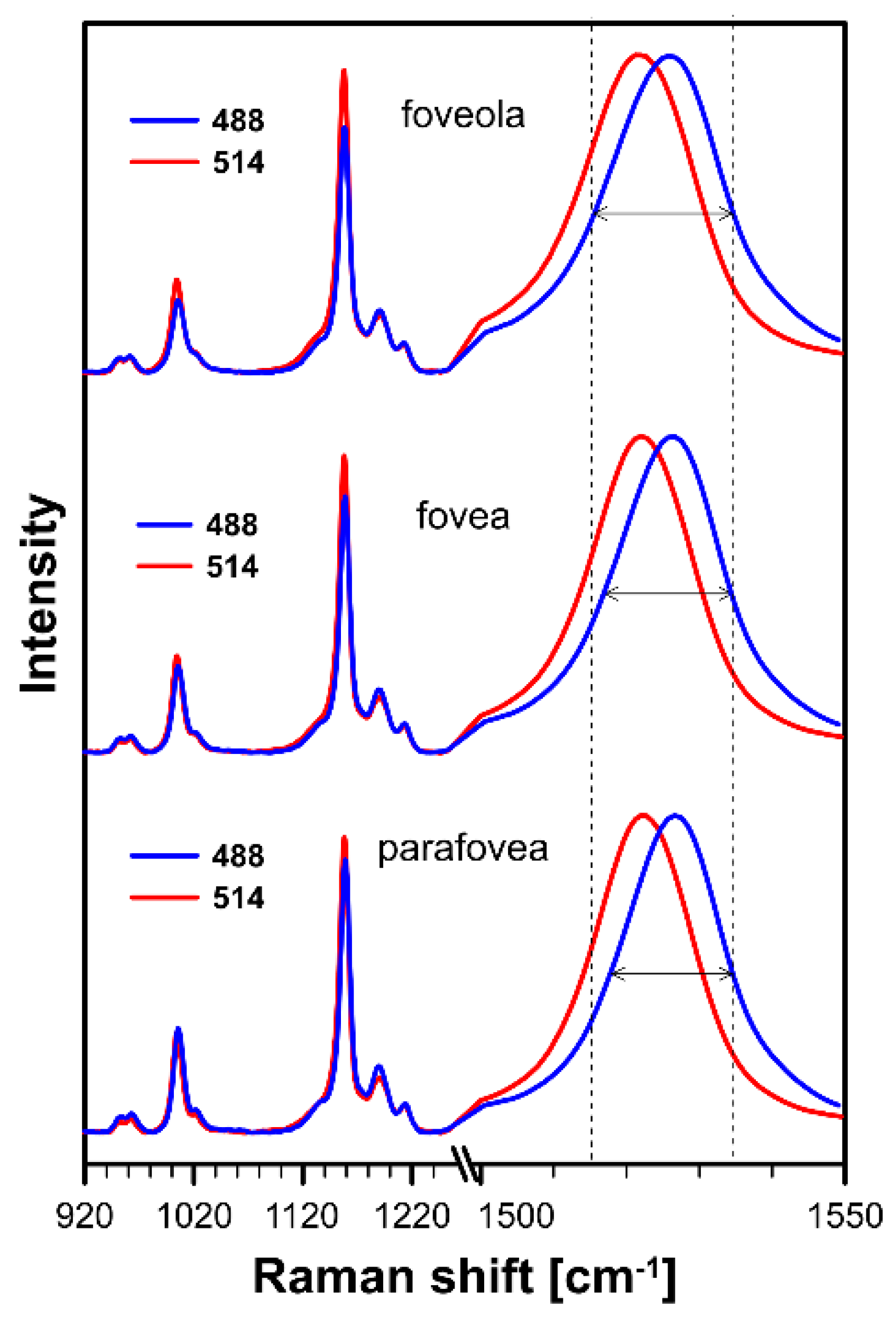
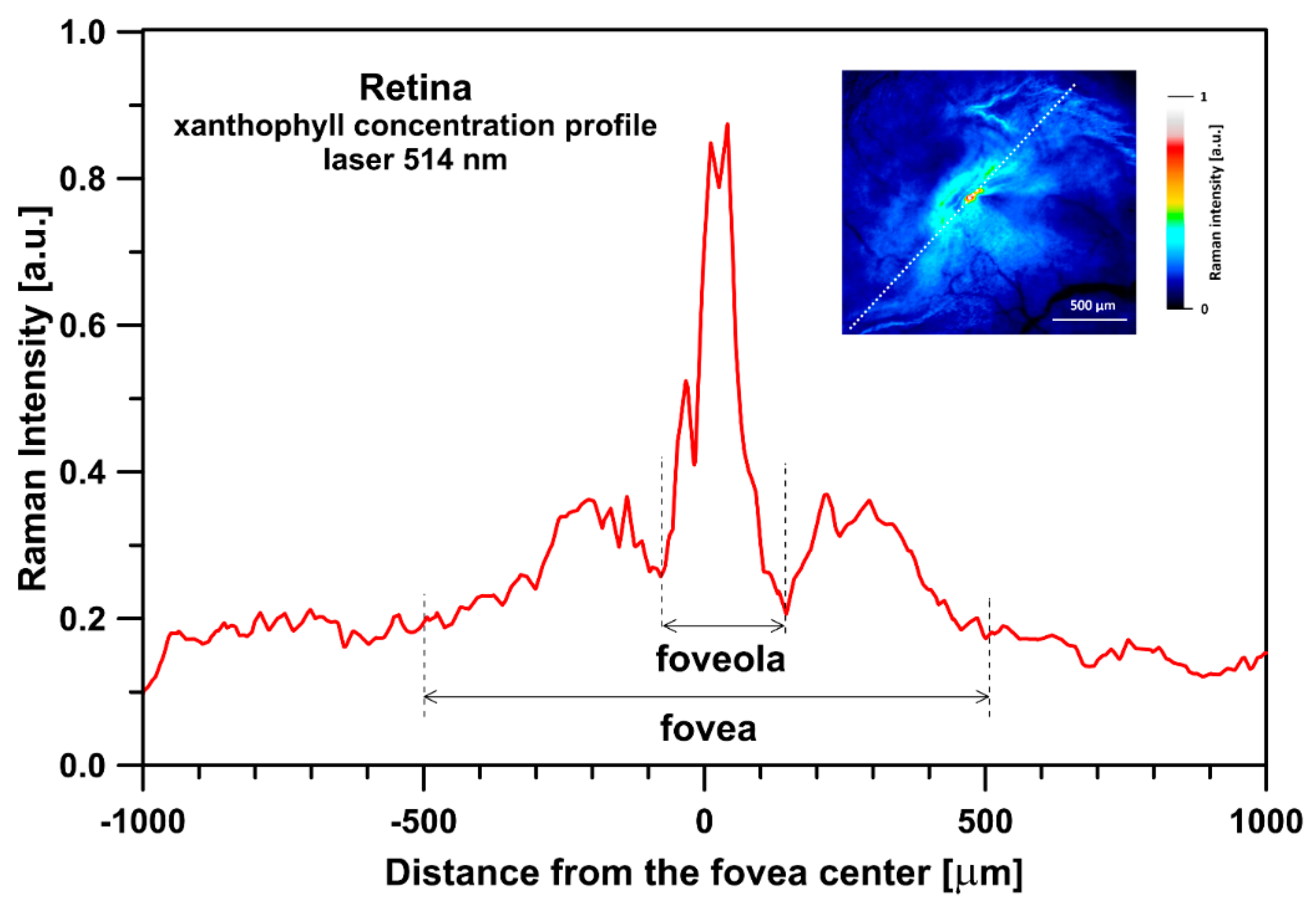
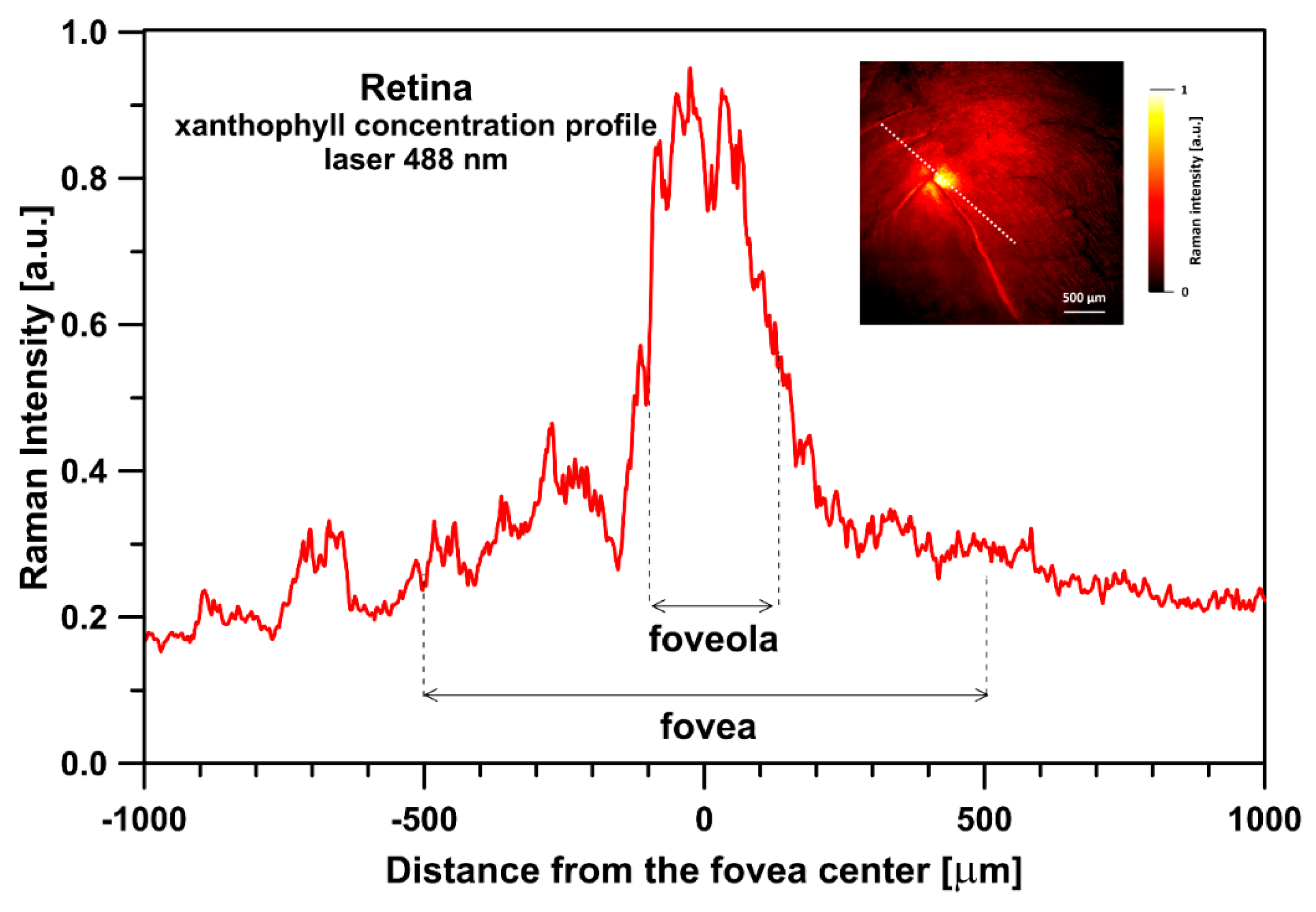
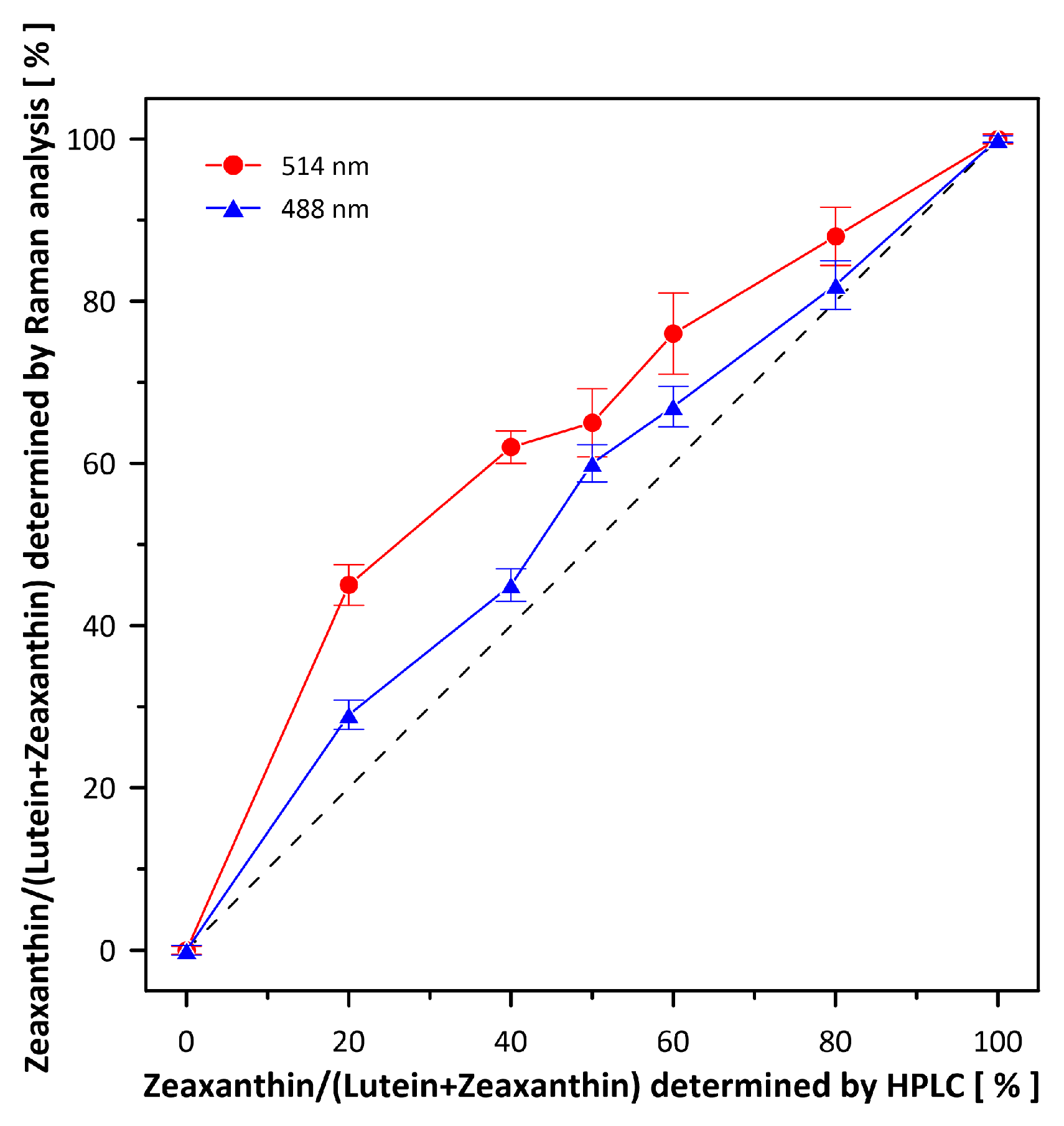
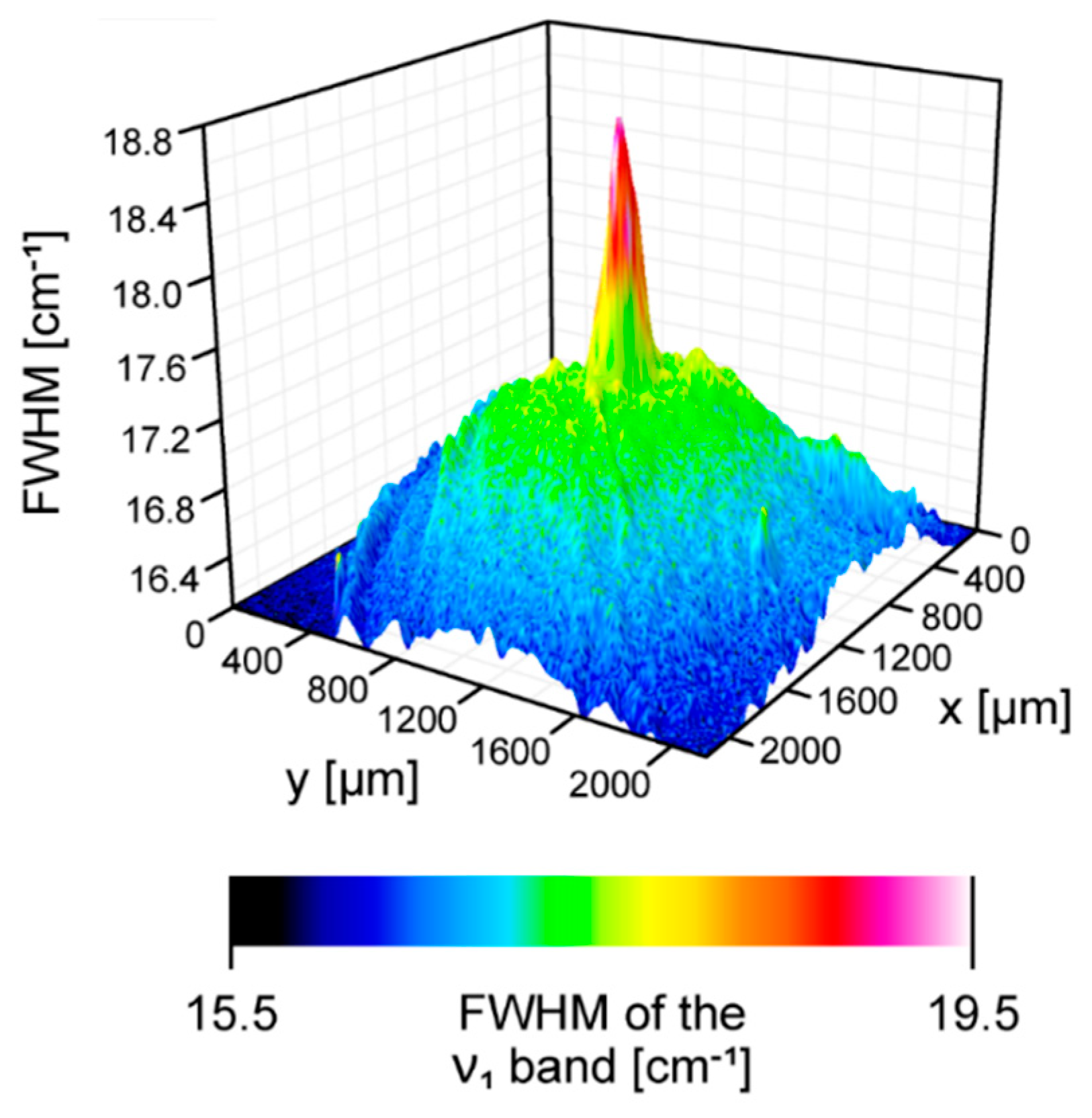
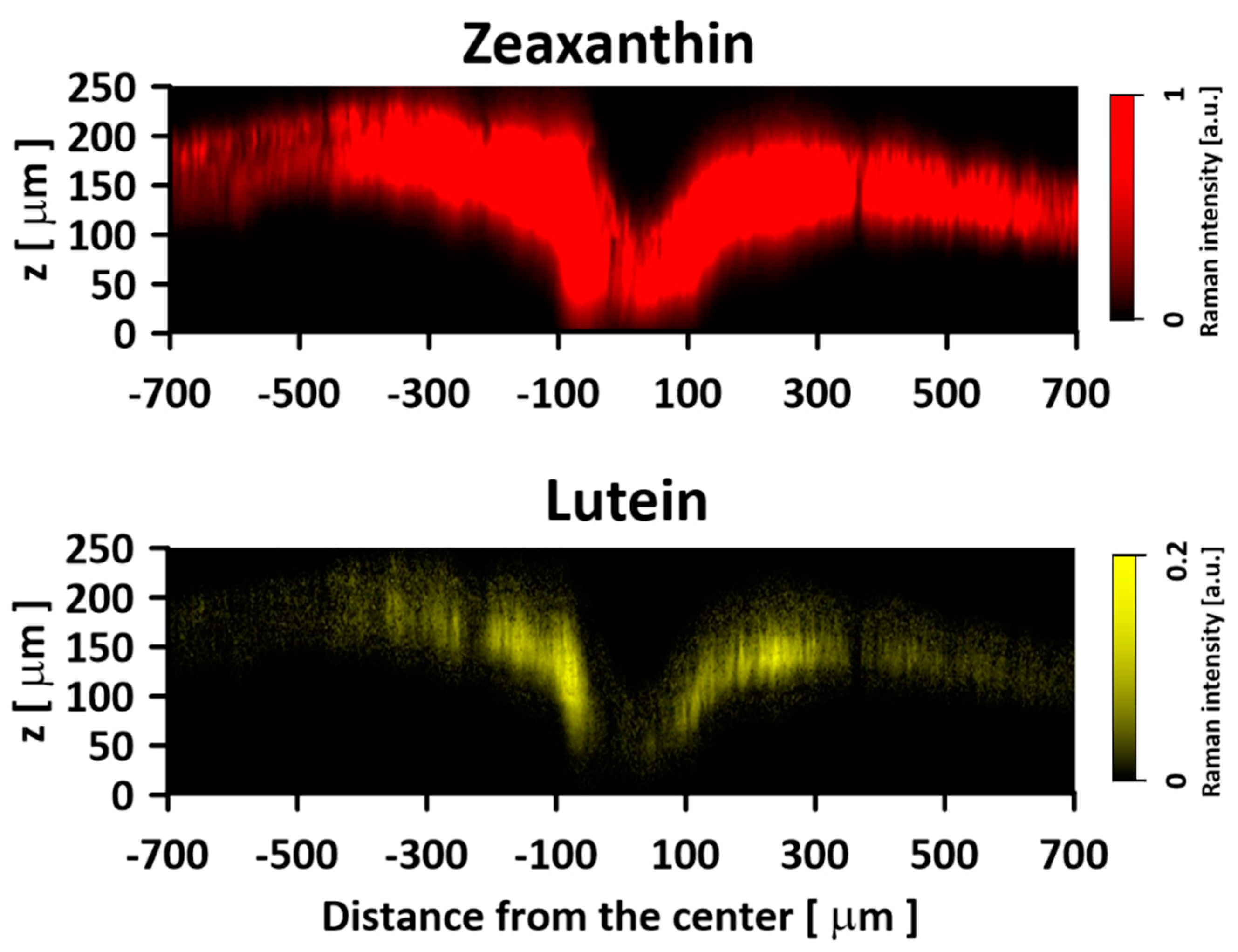
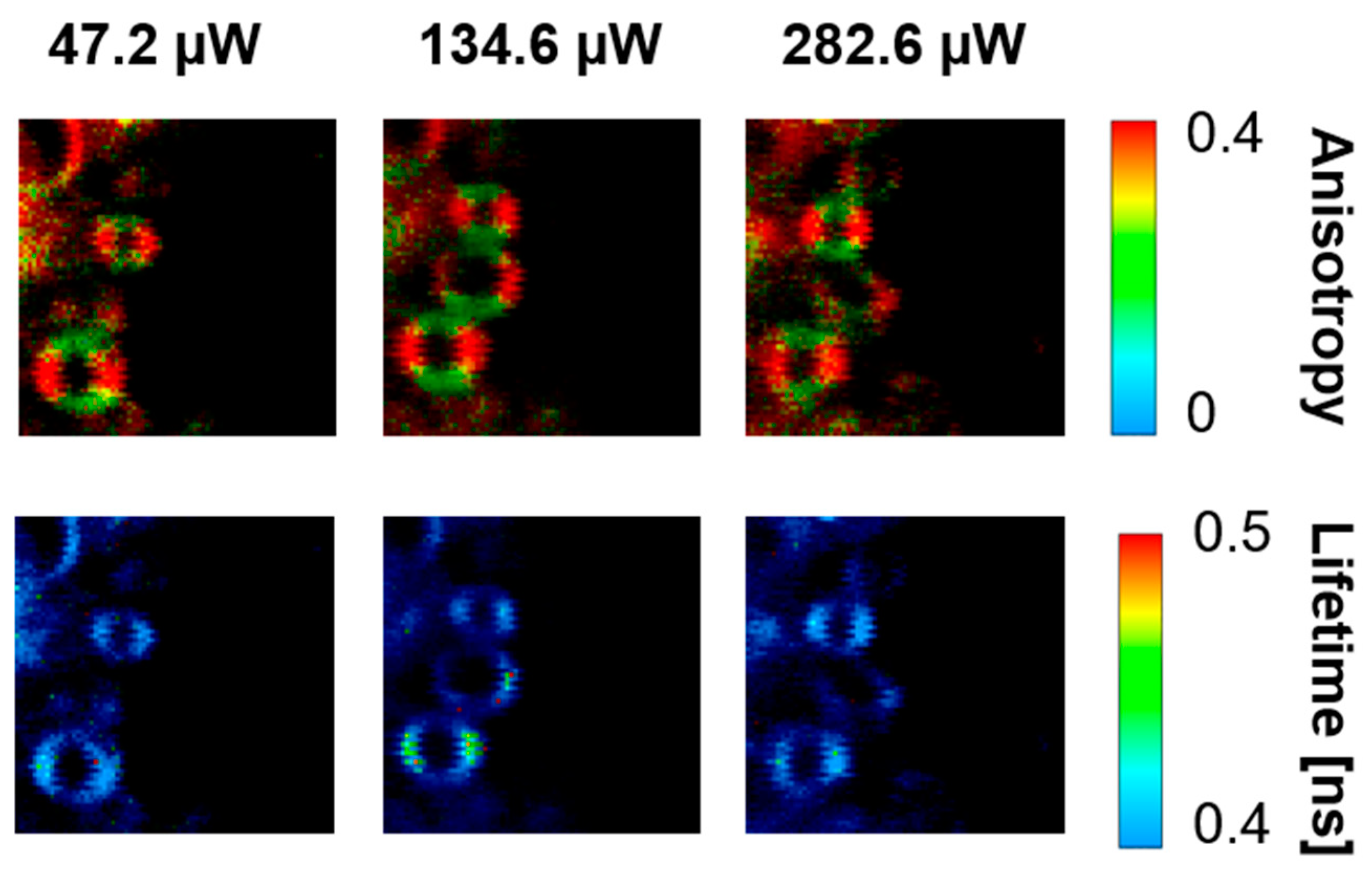
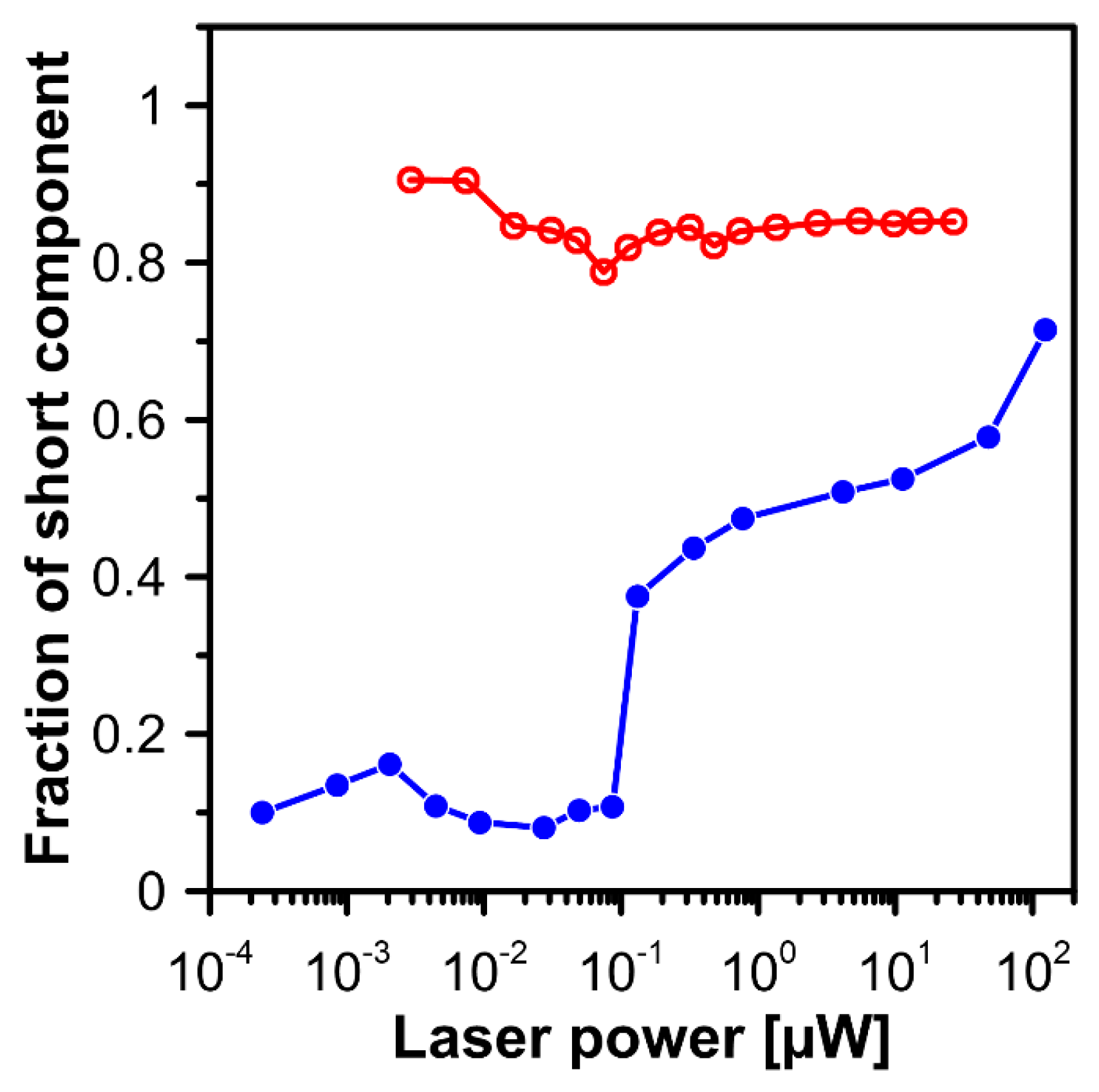
2. Results and Discussion
3. Materials and Methods
3.1. Retina Preparation
3.2. Raman Imaging
3.3. Fluorescence Lifetime Imaging Microscopy (FLIM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernstein, P.S.; Li, B.X.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Vorobyev, M. Ecology and evolution of primate colour vision. Clin. Exp. Optom. 2004, 87, 230–238. [Google Scholar] [CrossRef]
- Snodderly, D.; Brown, P.K.; Delori, F.C.; Auran, J.D. The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Investig. Opthalmology Vis. Sci. 1984, 25, 660–673. [Google Scholar]
- Bone, R.A.; Landrum, J.T.; Fernandez, L.; Tarsis, S.L. Analysis of the macular pigment by HPLC: Retinal distribution and age study. Investig. Ophthalmol. Vis. Sci. 1988, 29, 843–849. [Google Scholar]
- Bhosale, P.; Bernstein, P.S.; Zhao, D.Y. HPLC Measurement of Ocular Carotenoid Levels in Human Donor Eyes in the Lutein Supplementation Era. Invest. Opthalmol. Vis. Sci. 2007, 48, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, R.; Calvo, C.M.; Conrady, C.D.; Bernstein, P.S. What do we know about the macular pigment in AMD: The past, the present, and the future. Eye 2018, 32, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Widomska, J.; Gruszecki, W.I.; Subczynski, W.K. Factors Differentiating the Antioxidant Activity of Macular Xanthophylls in the Human Eye Retina. Antioxidants 2021, 10, 601. [Google Scholar] [CrossRef]
- Snodderly, D.M.; Auran, J.D.; Delori, F.C. The Macular Pigment. II. Spatial-Distribution in Primate Retinas. Invest. Opthalmol. Vis. Sci. 1984, 25, 674–685. [Google Scholar]
- Li, B.; George, E.W.; Rognon, G.T.; Gorusupudi, A.; Ranganathan, A.; Chang, F.-Y.; Shi, L.; Frederick, J.M.; Bernstein, P.S. Imaging lutein and zeaxanthin in the human retina with confocal resonance Raman microscopy. Proc. Natl. Acad. Sci. USA 2020, 117, 12352–12358. [Google Scholar] [CrossRef]
- Luchowski, R.; Grudzinski, W.; Welc, R.; Pinto, M.M.M.; Sek, A.; Ostrowski, J.; Nierzwicki, L.; Chodnicki, P.; Wieczor, M.; Sowinski, K.; et al. Light-Modulated Sunscreen Mechanism in the Retina of the Human Eye. J. Phys. Chem. B 2021, 125, 6090–6102. [Google Scholar] [CrossRef]
- Winn, B.; Whitaker, D.; Elliott, D.B.; Phillips, N.J. Factors affecting light-adapted pupil size in normal human subjects. Investig. Opthalmol. Vis. Sci. 1994, 35, 1132–1137. [Google Scholar]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef]
- Sęk, A.; Welc, R.; Mendes-Pinto, M.; Reszczyńska, E.; Grudzinski, W.; Luchowski, R.; Gruszecki, W. Raman spectroscopy analysis of molecular configuration forms of the macular xanthophylls. J. Raman Spectrosc. 2020, 51, 635–641. [Google Scholar] [CrossRef]
- Arteni, A.-A.; Fradot, M.; Galzerano, D.; Mendes-Pinto, M.M.; Sahel, J.-A.; Picaud, S.; Robert, B.; Pascal, A.A. Structure and Conformation of the Carotenoids in Human Retinal Macular Pigment. PLoS ONE 2015, 10, e0135779. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, F.; Tasumi, M.; Miyazawa, T. Excitation Profile of Resonance Raman Effect of Beta-Carotene. J. Mol. Spectrosc. 1974, 50, 286–303. [Google Scholar] [CrossRef]
- Li, B.; George, E.W.; Vachali, P.; Chang, F.-Y.; Gorusupudi, A.; Arunkumar, R.; Giauque, N.A.; Wan, Z.; Frederick, J.M.; Bernstein, P.S. Mechanism for the selective uptake of macular carotenoids mediated by the HDL cholesterol receptor SR-BI. Exp. Eye Res. 2023, 229, 109429. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.; Larson, A.J.; Frederick, J.M.; Southwick, K.; Thulin, C.D.; Bernstein, P.S. Identification and Characterization of a Pi Isoform of Glutathione S-Transferase (GSTP1) as a Zeaxanthin-binding Protein in the Macula of the Human Eye. J. Biol. Chem. 2004, 279, 49447–49454. [Google Scholar] [CrossRef]
- Bhosale, P.; Li, B.X.; Sharifzadeh, M.; Gellermann, W.; Frederick, J.M.; Tsuchida, K.; Bernstein, P.S. Purification and Partial Characterization of a Lutein-Binding Protein from Human Retina. Biochemistry 2009, 48, 4798–4807. [Google Scholar] [CrossRef]
- Reszczynska, E.; Welc, R.; Grudzinski, W.; Trebacz, K.; Gruszecki, W.I. Carotenoid binding to proteins: Modeling pigment transport to lipid membranes. Arch. Biochem. Biophys. 2015, 584, 125–133. [Google Scholar] [CrossRef]
- Grudzinski, W.; Nierzwicki, L.; Welc, R.; Reszczynska, E.; Luchowski, R.; Czub, J.; Gruszecki, W.I. Localization and Orien-tation of Xanthophylls in a Lipid Bilayer. Sci. Rep. 2017, 7, 9619. [Google Scholar] [CrossRef]
- Makuch, K.; Hryc, J.; Markiewicz, M.; Pasenkiewicz-Gierula, M. Lutein and Zeaxanthin in the Lipid Bilayer–Similarities and Differences Revealed by Computational Studies. Front. Mol. Biosci. 2021, 8, 768449. [Google Scholar] [CrossRef] [PubMed]
- Makuch, K.; Markiewicz, M.; Pasenkiewicz-Gierula, M. Asymmetric Spontaneous Intercalation of Lutein into a Phospholipid Bilayer, a Computational Study. Comput. Struct. Biotechnol. J. 2019, 17, 516–526. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grudzinski, W.; Luchowski, R.; Ostrowski, J.; Sęk, A.; Mendes Pinto, M.M.; Welc-Stanowska, R.; Zubik-Duda, M.; Teresiński, G.; Rejdak, R.; Gruszecki, W.I. Physiological Significance of the Heterogeneous Distribution of Zeaxanthin and Lutein in the Retina of the Human Eye. Int. J. Mol. Sci. 2023, 24, 10702. https://doi.org/10.3390/ijms241310702
Grudzinski W, Luchowski R, Ostrowski J, Sęk A, Mendes Pinto MM, Welc-Stanowska R, Zubik-Duda M, Teresiński G, Rejdak R, Gruszecki WI. Physiological Significance of the Heterogeneous Distribution of Zeaxanthin and Lutein in the Retina of the Human Eye. International Journal of Molecular Sciences. 2023; 24(13):10702. https://doi.org/10.3390/ijms241310702
Chicago/Turabian StyleGrudzinski, Wojciech, Rafal Luchowski, Jan Ostrowski, Alicja Sęk, Maria Manuela Mendes Pinto, Renata Welc-Stanowska, Monika Zubik-Duda, Grzegorz Teresiński, Robert Rejdak, and Wieslaw I. Gruszecki. 2023. "Physiological Significance of the Heterogeneous Distribution of Zeaxanthin and Lutein in the Retina of the Human Eye" International Journal of Molecular Sciences 24, no. 13: 10702. https://doi.org/10.3390/ijms241310702
APA StyleGrudzinski, W., Luchowski, R., Ostrowski, J., Sęk, A., Mendes Pinto, M. M., Welc-Stanowska, R., Zubik-Duda, M., Teresiński, G., Rejdak, R., & Gruszecki, W. I. (2023). Physiological Significance of the Heterogeneous Distribution of Zeaxanthin and Lutein in the Retina of the Human Eye. International Journal of Molecular Sciences, 24(13), 10702. https://doi.org/10.3390/ijms241310702







