Separation of Palladium from Alkaline Cyanide Solutions through Microemulsion Extraction Using Imidazolium Ionic Liquids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Behavior
2.1.1. Influence of Oscillation Time
2.1.2. Influence of Ionic Liquid Concentration
2.1.3. Influence of pH
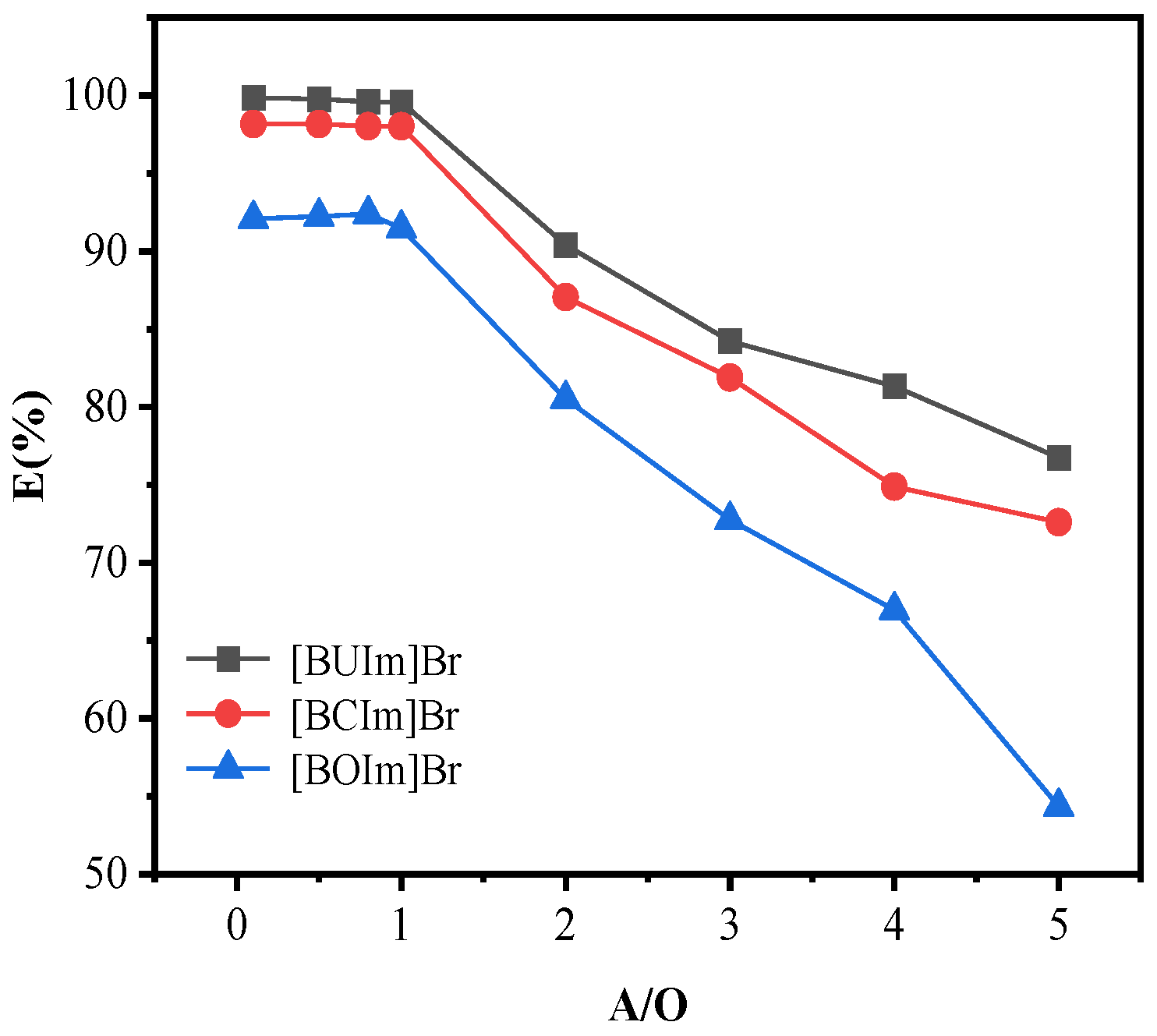
2.1.4. Influence of the Phase Ratio (Water/Organic)
2.1.5. Influence of NaBr Concentration
2.1.6. Stripping of Palladium
2.1.7. Effect of Stripping Extraction Time
2.1.8. Extraction of Palladium from Mixed Metal Solution
2.1.9. Reusability of [BUIm]Br/n-Heptane/n-Pentanol/NaCl Microemulsion System
2.2. Mechanistic Analysis
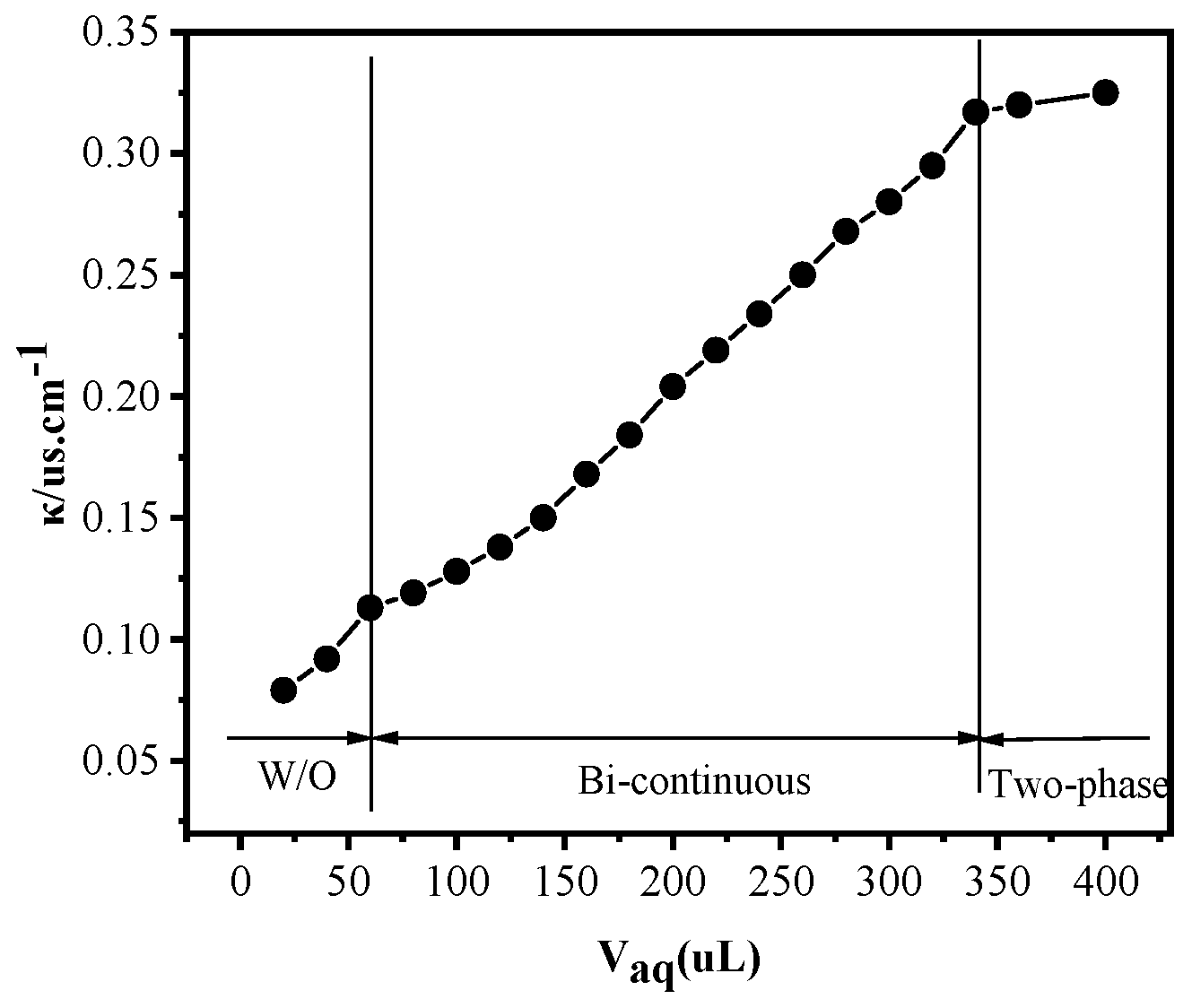
2.2.1. Conductivity of Microemulsions
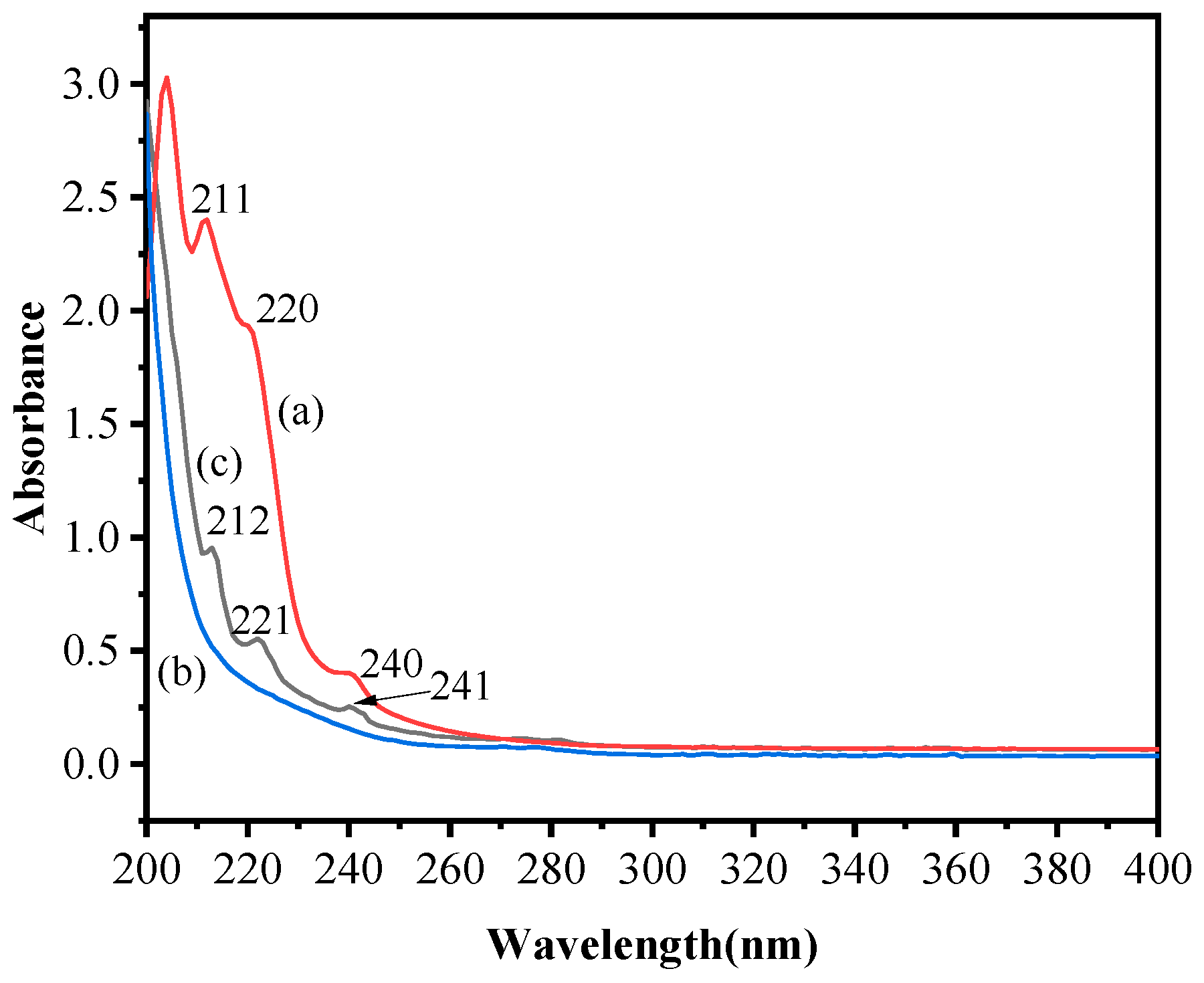
2.2.2. UV Spectra Analysis
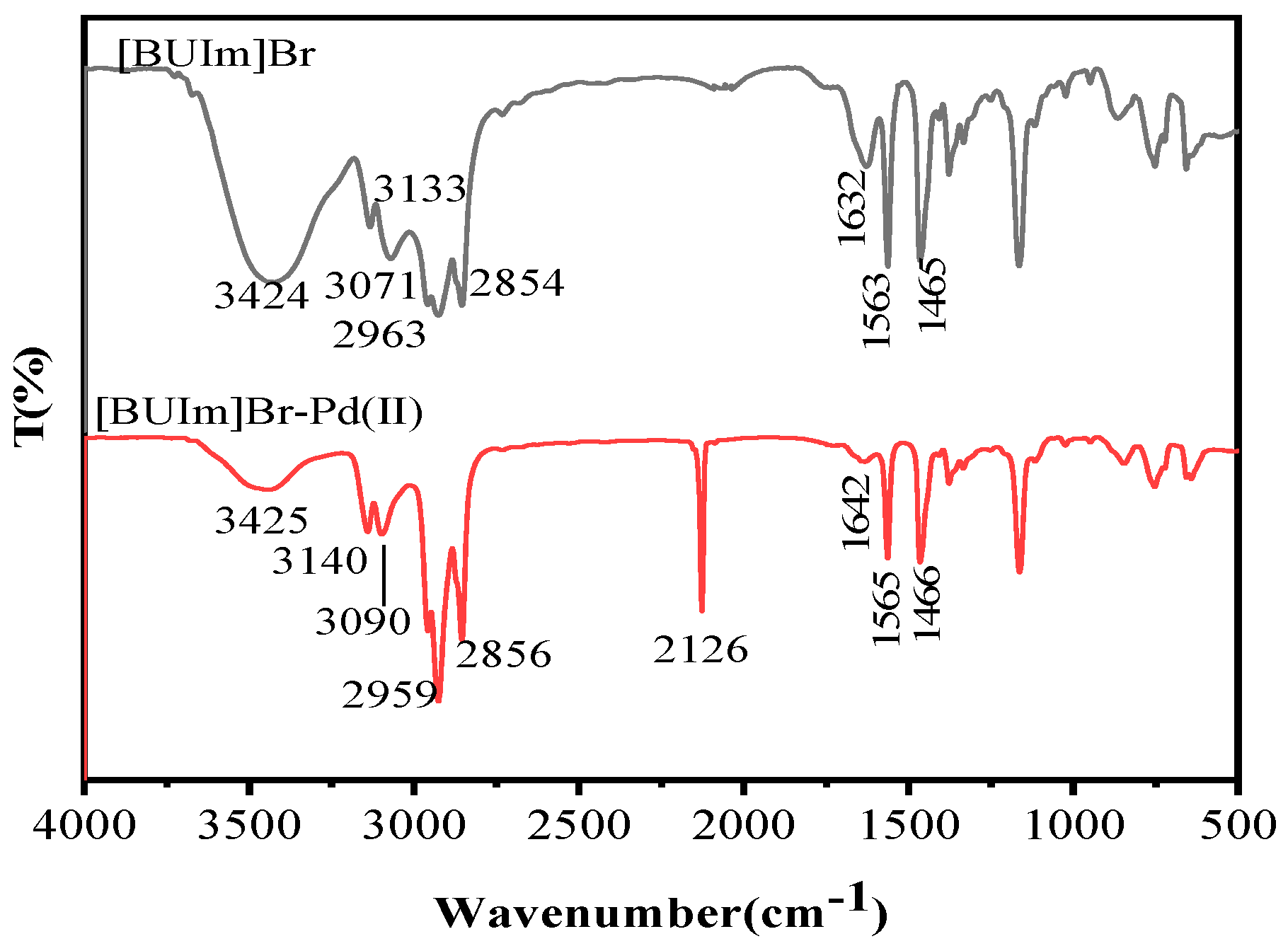
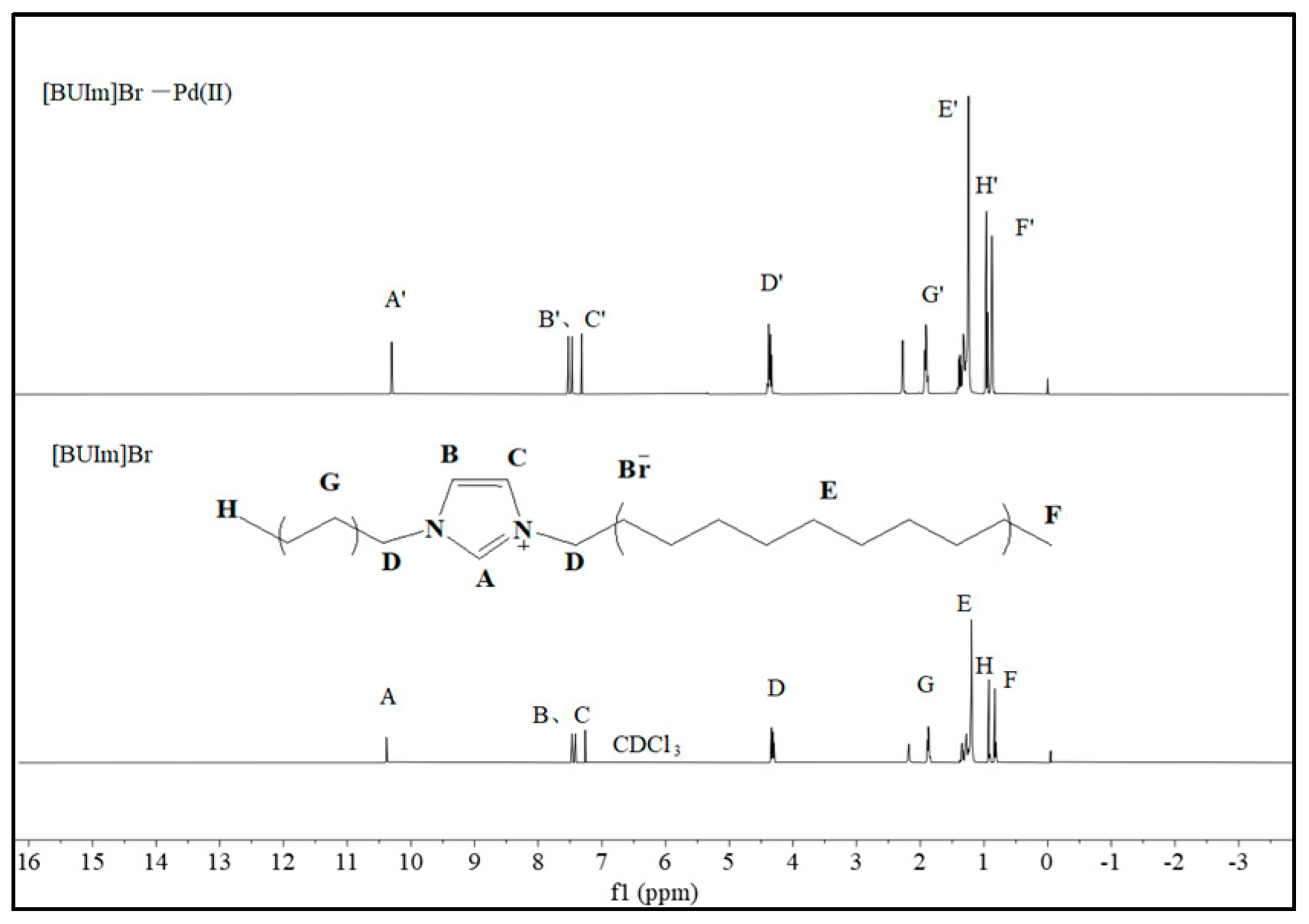
2.2.3. 1H NMR and Infrared Spectra Analysis
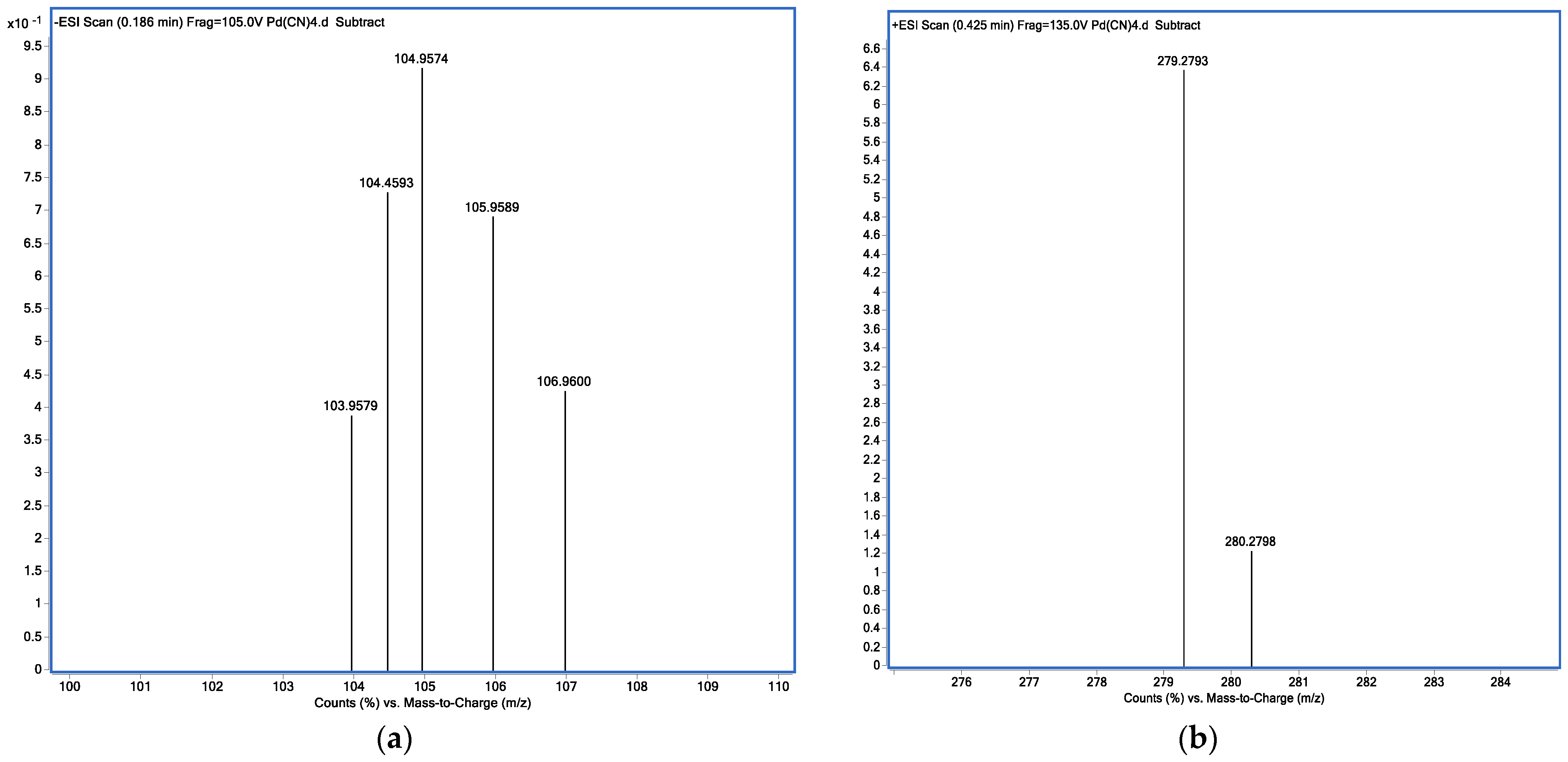
2.2.4. MS Analysis
2.2.5. Job’s Method
2.2.6. Influence of Temperature
2.3. Density Functional Theory (DFT) Study
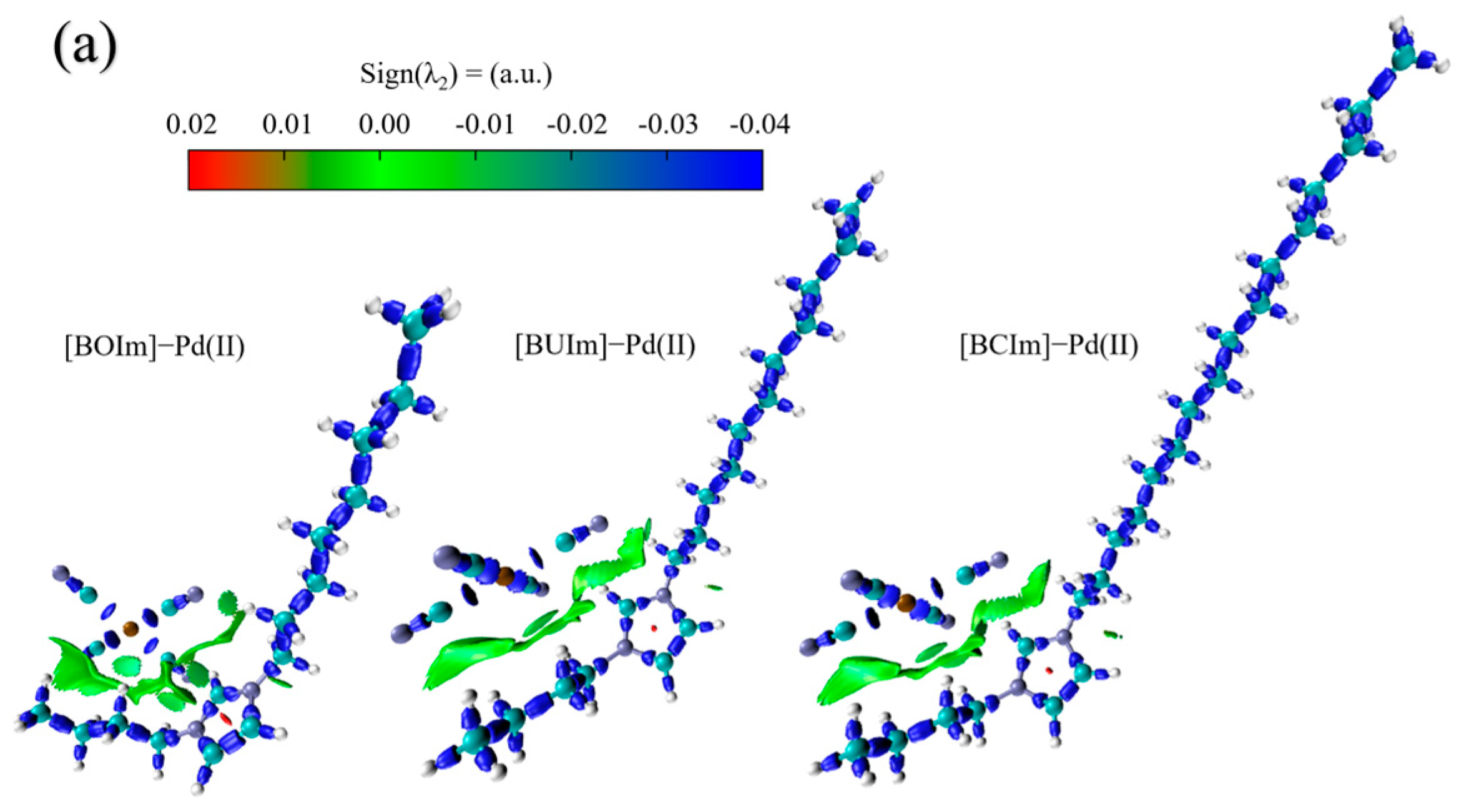
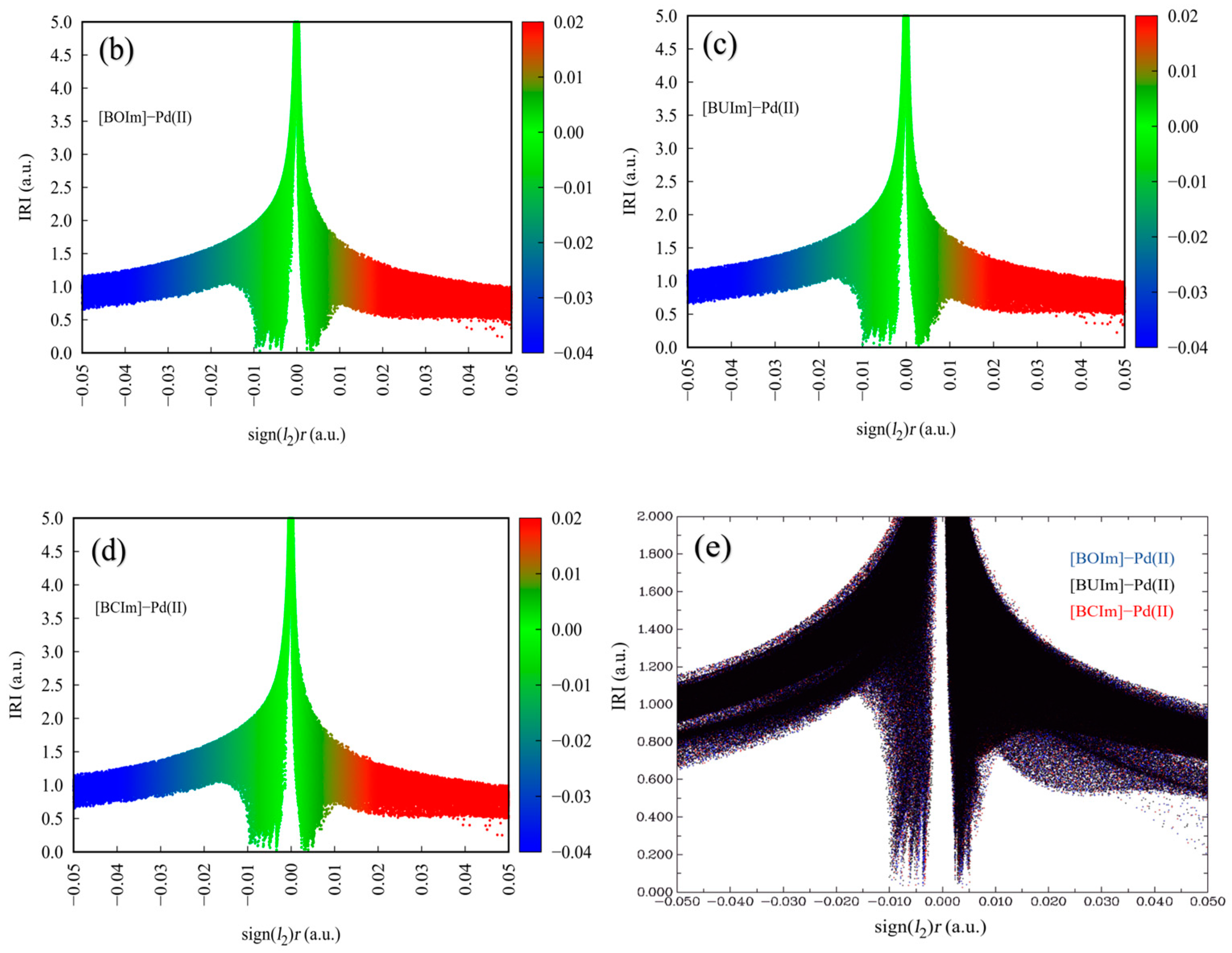
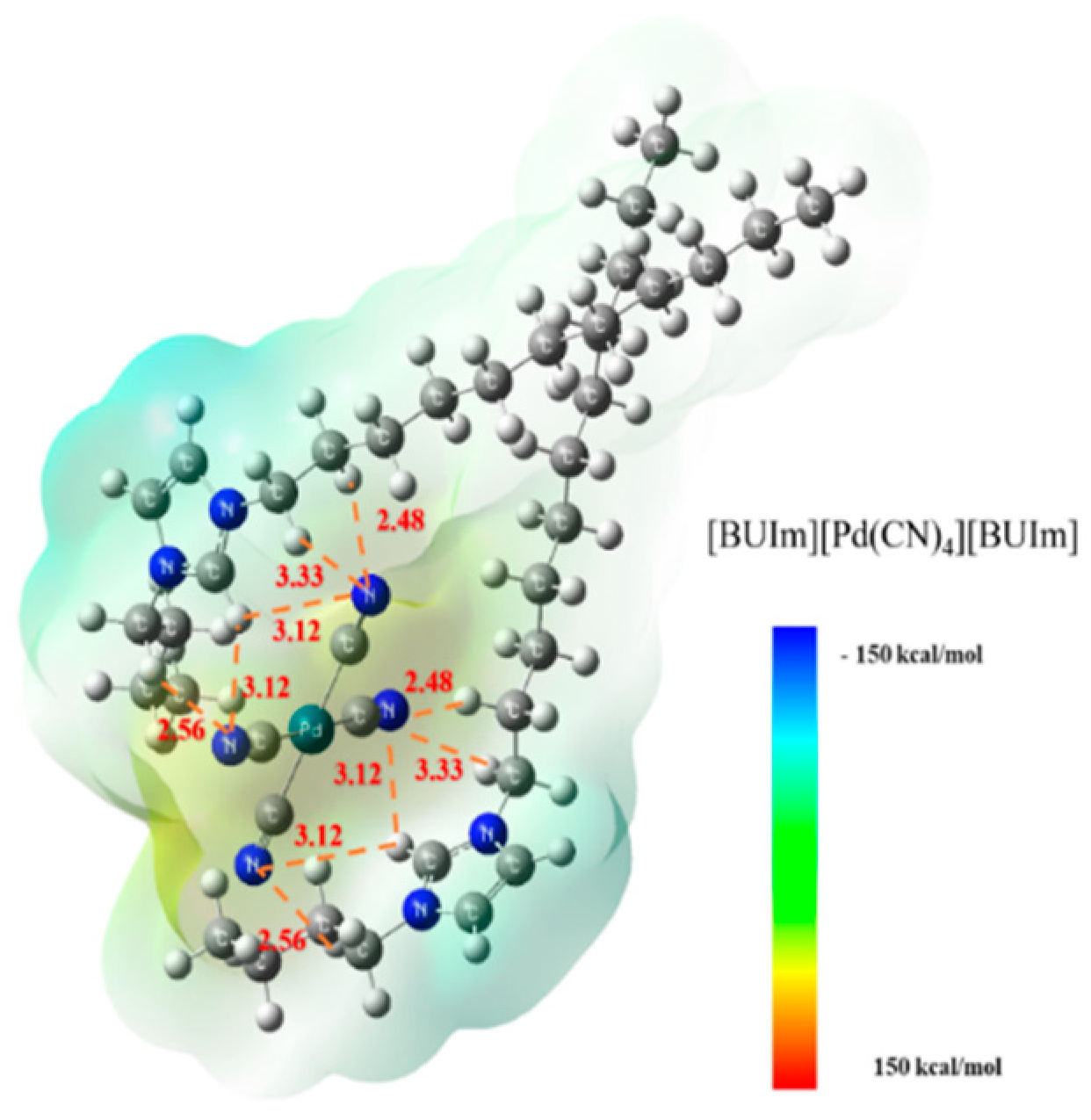
2.3.1. Analysis of Ion Exchange Products

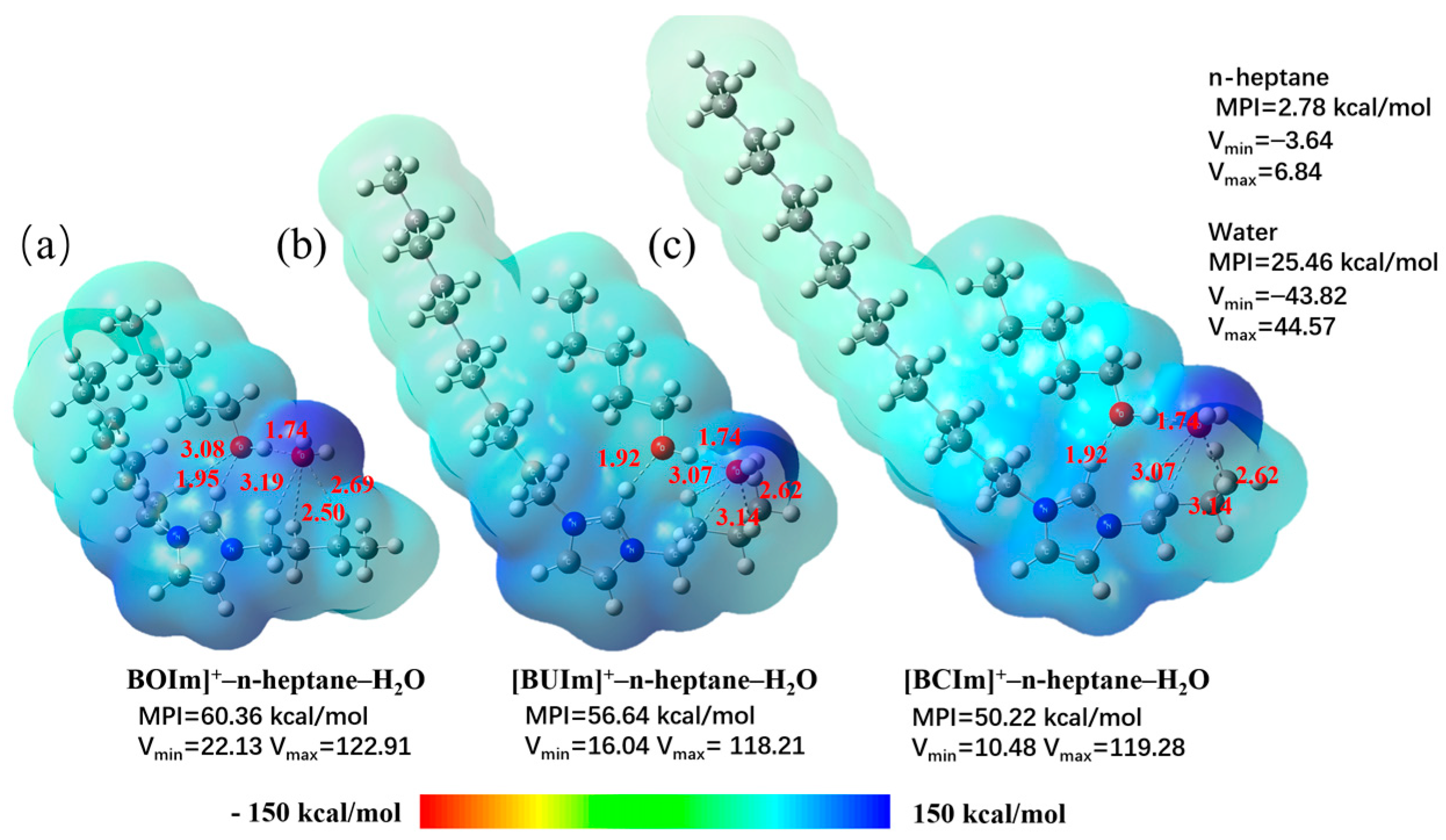
2.3.2. Analysis of Extractant in the Organic Phase
3. Materials and Methods
3.1. Instruments and Reagents
3.2. Synthesis of Ionic Liquids
3.3. Preparation of ILs/n-Heptane/n-Pentanol/NaCl Microemulsion
3.4. Extraction Process
3.5. Recovery of Palladium from the Organic Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, H.; Ding, Y.; Wen, Q.; Liu, B.; Zhang, S. Separation and purification of platinum group metals from aqueous solution: Recent developments and industrial applications. Resour. Conserv. Recycl. 2021, 167, 105417. [Google Scholar] [CrossRef]
- Ilyas, S.; Kim, H.; Srivastava, R.R. Separation of platinum group metals from model chloride solution using phosphonium-based ionic liquid. Sep. Purif. Technol. 2021, 278, 119577. [Google Scholar] [CrossRef]
- Fontana, D.; Pietrantonio, M.; Pucciarmati, S.; Torelli, G.N.; Bonomi, C.; Masi, F. Palladium recovery from monolithic ceramic capacitors by leaching, solvent extraction and reduction. J. Mater. Cycles Waste Manag. 2017, 20, 1199–1206. [Google Scholar] [CrossRef]
- Paiva, A. Recycling of Palladium from Spent Catalysts Using Solvent Extraction—Some Critical Points. Metals 2017, 7, 505. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Wu, S.; Huang, Z.; Chen, J.; Chen, M.J. Separation and recovery of Pd (II) and Pt(II) from cyanide liquors of Pd-Pt flotation concentrate via solvent extraction. J. Chem. Technol. Biotechnol. 2017, 92, 1699–1709. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, M.; Zhong, L.; Ye, Q.; Jiang, S.; Huang, Z. Highly Effective Removal of Metal Cyanide Complexes and Recovery of Palladium Using Quaternary-Ammonium-Functionalized MOFs. Molecules 2018, 23, 2086. [Google Scholar] [CrossRef] [Green Version]
- Miltzarek, G.L.; Sampaio, C.H.; Cortina, J.L. Cyanide recovery in hydrometallurgical plants: Use of synthetic solutions constituted by metallic cyanide complexes. Miner. Eng. 2002, 15, 75–82. [Google Scholar] [CrossRef]
- Miralles, E.; Compañó, R.; Granados, M.; Prat, M.D. Determination of metal-cyanide complexes by ion-interaction chromatography with fluorimetric detection. Anal. Chim. Acta 2000, 403, 197–204. [Google Scholar] [CrossRef]
- Mpinga, C.N.; Bradshaw, S.M.; Akdogan, G.; Snyders, C.A.; Eksteen, J.J. The extraction of Pt, Pd and Au from an alkaline cyanide simulated heap leachate by granular activated carbon. Miner. Eng. 2014, 55, 11–17. [Google Scholar] [CrossRef]
- Mpinga, C.N.; Bradshaw, S.M.; Akdogan, G.; Snyders, C.A.; Eksteen, J.J. Evaluation of the Merrill–Crowe process for the simultaneous removal of platinum, palladium and gold from cyanide leach solutions. Hydrometallurgy 2014, 142, 36–46. [Google Scholar] [CrossRef]
- Finnegan, I.; Toerien, S.; Abbot, L.; Smit, F.; Raubenheimer, H.G. Identification and characterisation of an Acinetobacter sp. capable of assimilation of a range of cyano-metal complexes, free cyanide ions and simple organic nitriles. Appl. Microbiol. Biotechnol. 1991, 36, 142–144. [Google Scholar] [CrossRef]
- Quan, Z.-X.; Bae, J.-W.; Rhee, S.-K.; Cho, Y.-G.; Lee, S.-T. Toxicity and degradation of metal-complexed cyanide by a bacterial consortium under sulfate-reducing conditions. Biotechnol. Lett. 2004, 26, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Yeddou, A.R.; Chergui, S.; Chergui, A.; Halet, F.; Hamza, A.; Nadjemi, B.; Ould-Dris, A.; Belkouch, J. Removal of cyanide in aqueous solution by oxidation with hydrogen peroxide in presence of copper-impregnated activated carbon. Miner. Eng. 2011, 24, 788–793. [Google Scholar] [CrossRef]
- Qi, F.; Yang, B.; Wang, Y.; Mao, R.; Zhao, X. H2O2 Assisted Photoelectrocatalytic Oxidation of Ag-Cyanide Complexes at Metal-free g-C3N4 Photoanode with Simultaneous Ag Recovery. ACS Sustain. Chem. Eng. 2017, 5, 5001–5007. [Google Scholar] [CrossRef]
- Schoeman, E.; Bradshaw, S.M.; Akdogan, G.; Snyders, C.A.; Eksteen, J.J. The extraction of platinum and palladium from a synthetic cyanide heap leach solution with strong base anion exchange resins. Int. J. Miner. Process. 2017, 162, 27–35. [Google Scholar] [CrossRef]
- Leão, V.A.; Ciminelli, V.S.T. Application of Ion Exchange Resins in Gold Hydrometallurgy. A Tool for Cyanide Recycling. Solvent Extr. Ion Exch. 2000, 18, 567–582. [Google Scholar] [CrossRef]
- Dai, X.; Breuer, P.L. Cyanide and copper cyanide recovery by activated carbon. Miner. Eng. 2009, 22, 469–476. [Google Scholar] [CrossRef]
- Aliprandini, P.; Veiga, M.M.; Marshall, B.G.; Scarazzato, T.; Espinosa, D.C.R. Investigation of mercury cyanide adsorption from synthetic wastewater aqueous solution on granular activated carbon. J. Water Process Eng. 2020, 34, 101154. [Google Scholar] [CrossRef]
- Seung-Mok, L.; Diwakar, T. Application of ferrate(VI) in the treatment of industrial wastes containing metal-complexed cyanides: A green treatment. J. Environ. Sci. 2009, 21, 1347–1352. [Google Scholar] [CrossRef]
- Tian, L.; Chen, P.; Jiang, X.H.; Chen, L.S.; Tong, L.L.; Yang, H.Y.; Fan, J.P.; Wu, D.S.; Zou, J.P.; Luo, S.L. Mineralization of cyanides via a novel Electro-Fenton system generating •OH and •O2−. Water Res. 2021, 209, 117890. [Google Scholar] [CrossRef]
- Chen, M.; Ye, Q.; Jiang, S.; Shao, M.; Jin, C.; Huang, Z. Two-Step Elution Recovery of Cyanide Platinum Using Functional Metal Organic Resin. Molecules 2019, 24, 2779. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.Q.; Liu, N.N.; Li, G.P.; Dang, L.T. Characterization of a supported ionic liquid membrane used for the removal of cyanide from wastewater. Water Sci. Technol. 2017, 76, 3142–3149. [Google Scholar] [CrossRef]
- Snyders, C.A.; Bradshaw, S.M.; Akdogan, G.; Eksteen, J.J. The effect of temperature, cyanide and base metals on the adsorption of Pt, Pd and Au onto activated carbon. Hydrometallurgy 2014, 149, 132–142. [Google Scholar] [CrossRef]
- Alaei, R.; Javanshir, S.; Behnamfard, A. Treatment of gold ore cyanidation wastewater by adsorption onto a Hydrotalcite-type anionic clay as a novel adsorbent. J. Environ. Health Sci. Eng. 2020, 18, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Alonso-González, O.; Nava-Alonso, F.; Uribe-Salas, A.; Dreisinger, D. Use of quaternary ammonium salts to remove copper–cyanide complexes by solvent extraction. Miner. Eng. 2010, 23, 765–770. [Google Scholar] [CrossRef]
- Gao, T.-y.; Liu, K.-r.; Han, Q.; Xu, B.-s. Enrichment of copper and recycling of cyanide from copper–cyanide waste by solvent extraction. Int. J. Miner. Metall. Mater. 2016, 23, 1258–1263. [Google Scholar] [CrossRef]
- Ilyas, S.; Kim, H. Recovery of Platinum-Group Metals from an Unconventional Source of Catalytic Converter Using Pressure Cyanide Leaching and Ionic Liquid Extraction. JOM 2022, 74, 1020–1026. [Google Scholar] [CrossRef]
- Yang, X.; Miao, C.; Sun, Y.; Lei, T.; Xie, Q.; Wang, S. Efficient extraction of gold(I) from alkaline aurocyanide solution using green ionic liquid-based aqueous biphasic systems. J. Taiwan Inst. Chem. Eng. 2018, 91, 176–185. [Google Scholar] [CrossRef]
- Barulli, L.; Mezzetta, A.; Brunetti, B.; Guazzelli, L.; Vecchio Ciprioti, S.; Ciccioli, A. Evaporation thermodynamics of the tetraoctylphosphonium bis(trifluoromethansulfonyl)imide([P8888]NTf2) and tetraoctylphosphonium nonafluorobutane-1-sulfonate ([P8888]NFBS) ionic liquids. J. Mol. Liq. 2021, 333, 115892. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Zullo, V.; Iuliano, A.; Guazzelli, L. Sugar-Based Ionic Liquids: Multifaceted Challenges and Intriguing Potential. Molecules 2021, 26, 2052. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Jin, C.; Chen, M.; Fan, M.; Luo, G.; Shao, M.; Huang, Z.; Xie, X. Hydrophobic phosphonium-based ionic liquids as novel extractants for palladium(II) recovery from alkaline cyanide solutions. J. Mol. Liq. 2021, 336, 116358. [Google Scholar] [CrossRef]
- Lu, W.; Lu, Y.; Liu, F.; Shang, K.; Wang, W.; Yang, Y. Extraction of gold(III) from hydrochloric acid solutions by CTAB/n-heptane/iso-amyl alcohol/Na2SO3 microemulsion. J. Hazard. Mater. 2011, 186, 2166–2170. [Google Scholar] [CrossRef]
- Tong, Y.; Han, L.; Yang, Y. Microemulsion Extraction of Gold(III) from Hydrochloric Acid Medium Using Ionic Liquid as Surfactant and Extractant. Ind. Eng. Chem. Res. 2012, 51, 16438–16443. [Google Scholar] [CrossRef]
- Zheng, Y.; Fang, L.; Yan, Y.; Lin, S.; Liu, Z.; Yang, Y. Extraction of palladium (II) by a silicone ionic liquid-based microemulsion system from chloride medium. Sep. Purif. Technol. 2016, 169, 289–295. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Lee, J.-c.; Kim, M.-s.; Kim, S.-k.; Chagnes, A.; Cote, G. Sustainable extraction and separation of precious metals from hydrochloric media using novel ionic liquid-in-water microemulsion. Hydrometallurgy 2017, 171, 344–354. [Google Scholar] [CrossRef]
- Shao, M.; Chen, M.; Fan, M.; Luo, G.; Jin, C.; Huang, Z. Microemulsion system constructed with a new cyano-functionalized ionic liquid for the extraction of Pd (II) and preparation of palladium nanoparticles. Sep. Purif. Technol. 2021, 275, 119198. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Tang, N.; Miao, C.; Wang, Y.; Tang, L.; Wang, S.; Yang, X. Simultaneous extraction and recovery of gold(I) from alkaline solutions using an environmentally benign polymer inclusion membrane with ionic liquid as the carrier. Sep. Purif. Technol. 2019, 222, 136–144. [Google Scholar] [CrossRef]
- He, K.; Tang, J.; Weng, H.; Chen, G.; Wu, Z.; Lin, M. Efficient extraction of precious metal ions by a membrane emulsification circulation extractor. Sep. Purif. Technol. 2019, 213, 93–100. [Google Scholar] [CrossRef]
- Li, X.; He, G.; Zheng, W.; Xiao, G. Study on conductivity property and microstructure of TritonX-100/alkanol/n-heptane/water microemulsion. Colloids Surf. A Physicochem. Eng. Asp. 2010, 360, 150–158. [Google Scholar] [CrossRef]
- Bharatiya, B.; Hassan, P.A.; Sastry, N.V. Formulation of pyridinium based RTIL-in-cyclohexane microemulsions: Investigations on size, conductivity and molecular interactions. J. Mol. Liq. 2016, 218, 586–594. [Google Scholar] [CrossRef]
- Tan, Z.; Zhang, Y.; Dai, C.; Li, L.; Li, Y.; Xie, X.-A. Investigation of AOT/isooctane/water reverse microemulsion system with the presence of different mass ratios of SDS: Conductivity and water solubilization. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129271. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Q.; Xiang, Z.; Yang, Y. Separation of Pt(IV), Pd (II), Ru(III), and Rh(III) from chloride medium using liquid–liquid extraction with mixed imidazolium-based ionic liquids. Sep. Sci. Technol. 2018, 53, 2064–2073. [Google Scholar] [CrossRef]
- Łuczak, J.; Hupka, J. Studies on formation and percolation in ionic liquids/TX-100/water microemulsions. J. Mol. Liq. 2014, 199, 552–558. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Interaction Region Indicator: A Simple Real Space Function Clearly Revealing Both Chemical Bonds and Weak Interactions. Chem. Methods 2021, 1, 231–239. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Chen, Q. Intermolecular interaction characteristics of the all-carboatomic ring, cyclo[18]carbon: Focusing on molecular adsorption and stacking. Carbon 2021, 171, 514–523. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Murray, J.S.; Brinck, T.; Lane, P.; Paulsen, K.; Politzer, P. Statistically-based interaction indices derived from molecular surface electrostatic potentials: A general interaction properties function (GIPF). J. Mol. Struct. Theochem. 1994, 307, 55–64. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring Nature and Predicting Strength of Hydrogen Bonds: A Correlation Analysis Between Atoms-in-Molecules Descriptors, Binding Energies, and Energy Components of Symmetry-Adapted Perturbation Theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.-Y.; Huang, J.; Shen, S.; Wang, C.-J.; Wu, Z.-Y.; Xie, B. Efficient separation of V(V) and Cr(VI) in aqua by microemulsion extraction. Sep. Purif. Technol. 2020, 238, 116409. [Google Scholar] [CrossRef]
- Qi, W.; He, J.; Li, M.; Zhai, M.; Zhao, L. Efficient extraction of rhenium through demulsification of imidazolium ionic liquid-based microemulsions from aqueous solution. Sep. Purif. Technol. 2022, 297, 121574. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, M.; Shen, C.; Li, G.; Su, Y. Continuous Extraction of Gold(III) Using Pyridine Ionic Liquid-Based Water-in-Oil Microemulsion in Microreactors. Ind. Eng. Chem. Res. 2019, 58, 12729–12740. [Google Scholar] [CrossRef]
- Chen, M.; Li, S.; Luo, G.; Fan, M.; Chen, J.; Huang, Z.; Xie, X. Selective recovery of palladium and rhodium by combined extraction and photocatalytic reduction. Sep. Purif. Technol. 2021, 274, 119006. [Google Scholar] [CrossRef]
- Chabba, S.; Vashishat, R.; Kang, T.S.; Mahajan, R.K. Self–aggregation Behavior of Dialkyl Imidazolium based Ionic Liquids in Aqueous Medium: Effect of Alkyl Chain Length. ChemistrySelect 2016, 1, 2458–2470. [Google Scholar] [CrossRef]
- Men, S.; Jin, Y.; Licence, P. Probing the impact of the N3-substituted alkyl chain on the electronic environment of the cation and the anion for 1,3-dialkylimidazolium ionic liquids. Phys. Chem. Chem. Phys. 2020, 22, 17394–17400. [Google Scholar] [CrossRef]








| Reverse Extractant | Extraction Rate (%) | Stripping Extraction (%) |
|---|---|---|
| KBr | 99.7 | 76.1 |
| KI | 99.8 | 91.6 |
| NaOH | 99.8 | 7.9 |
| KSCN | 99.8 | 99.5 |
| E% | S% | R% | ||
|---|---|---|---|---|
| 1 mol·L−1 KCl | 0.5 mol·L−1 KSCN | |||
| Pd(CN)42− | 99.2 | 2.9 | 96.1 | 95.3 |
| Fe(CN)63− | 98.7 | 97.8 | - | 96.5 |
| Co(CN)63− | 98.5 | 98.0 | - | 96.5 |
| Time | Extraction Rate (%) | Recovery Rate (%) |
|---|---|---|
| 1 | 99.2 | 96.5 |
| 2 | 98.5 | 94.8 |
| 3 | 97.3 | 93.2 |
| 4 | 94.8 | 91.6 |
| 5 | 94.8 | 90.7 |
| [BUIm]Br Bands (cm−1) | [BUIm]Br-Pd (II) Bands (cm−1) | Vibration |
|---|---|---|
| 3424 | 3425 | stretching vibration of –OH |
| 3133 | 3140 | =C–H stretching of imidazolium ring |
| 3071 | 3090 | C–H stretching near imidazolium ring |
| 2963 | 2959 | C–H stretching of CH3 |
| 2854 | 2856 | C–H stretching of CH2 |
| - | 2126 | C≡N stretching vibration of Pd(CN)42− |
| 1632 | 1642 | C=C stretching of imidazolium ring |
| 1563 | 1565 | C=N stretching of imidazolium ring |
| 1465 | 1466 | C–H stretching of CH2 |
| Temperature (K) | ΔG (KJ mol−1) | ΔH (KJ mol−1) | ΔS (J mol−1 K−1) |
|---|---|---|---|
| 298 | −19.10 | −38.37 | −64.82 |
| 303 | −18.71 | ||
| 308 | −18.32 | ||
| 313 | −18.17 | ||
| 318 | −17.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Liu, C.; Xu, X.; Wu, Y.; Chen, M.; Huang, Z. Separation of Palladium from Alkaline Cyanide Solutions through Microemulsion Extraction Using Imidazolium Ionic Liquids. Int. J. Mol. Sci. 2023, 24, 10709. https://doi.org/10.3390/ijms241310709
Deng H, Liu C, Xu X, Wu Y, Chen M, Huang Z. Separation of Palladium from Alkaline Cyanide Solutions through Microemulsion Extraction Using Imidazolium Ionic Liquids. International Journal of Molecular Sciences. 2023; 24(13):10709. https://doi.org/10.3390/ijms241310709
Chicago/Turabian StyleDeng, Hui, Chali Liu, Xin Xu, Yuanyuan Wu, Muhan Chen, and Zhangjie Huang. 2023. "Separation of Palladium from Alkaline Cyanide Solutions through Microemulsion Extraction Using Imidazolium Ionic Liquids" International Journal of Molecular Sciences 24, no. 13: 10709. https://doi.org/10.3390/ijms241310709




