The Critical Assessment of Oxidative Stress Parameters as Potential Biomarkers of Carbon Monoxide Poisoning
Abstract
:1. Introduction
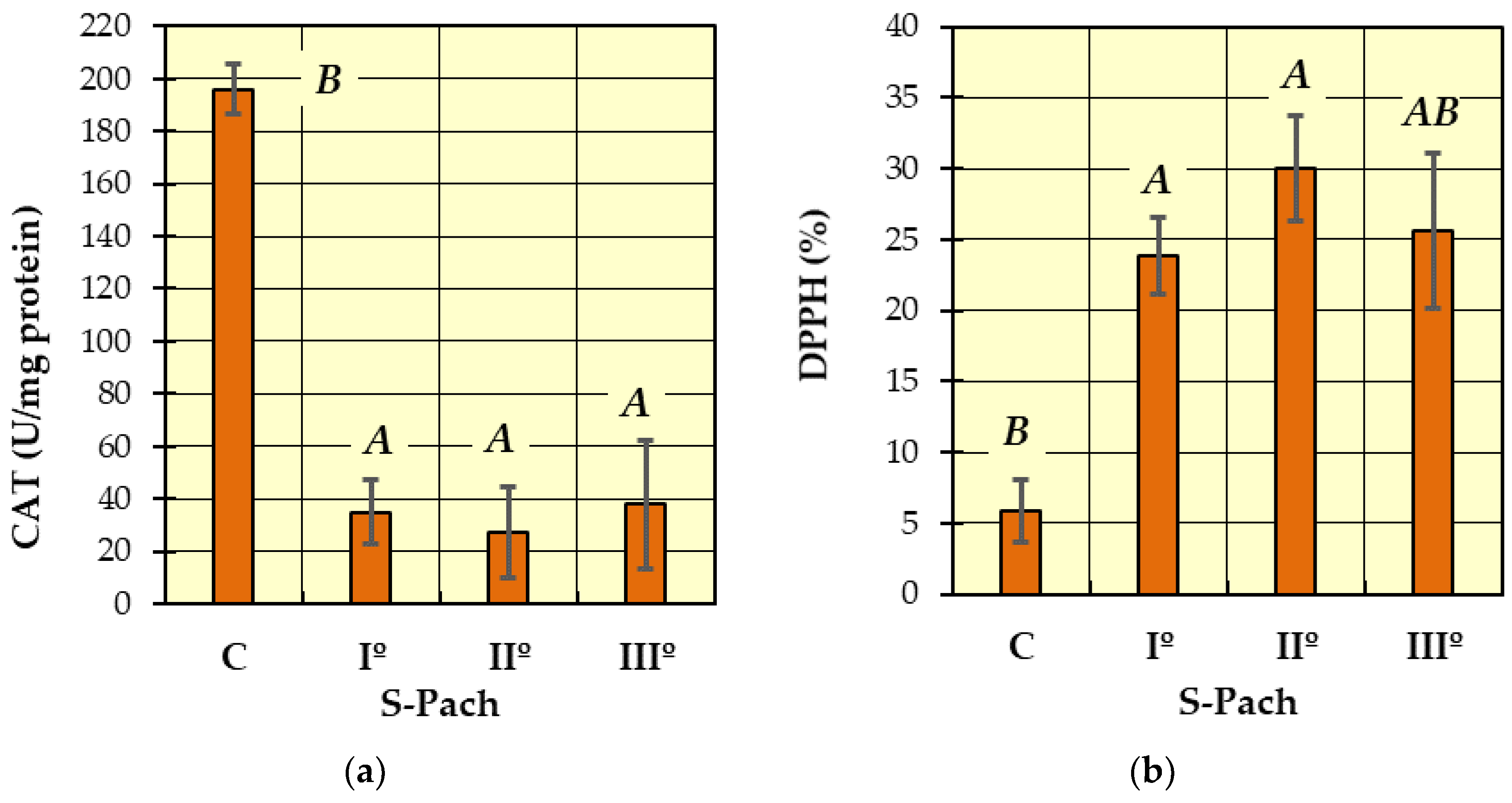
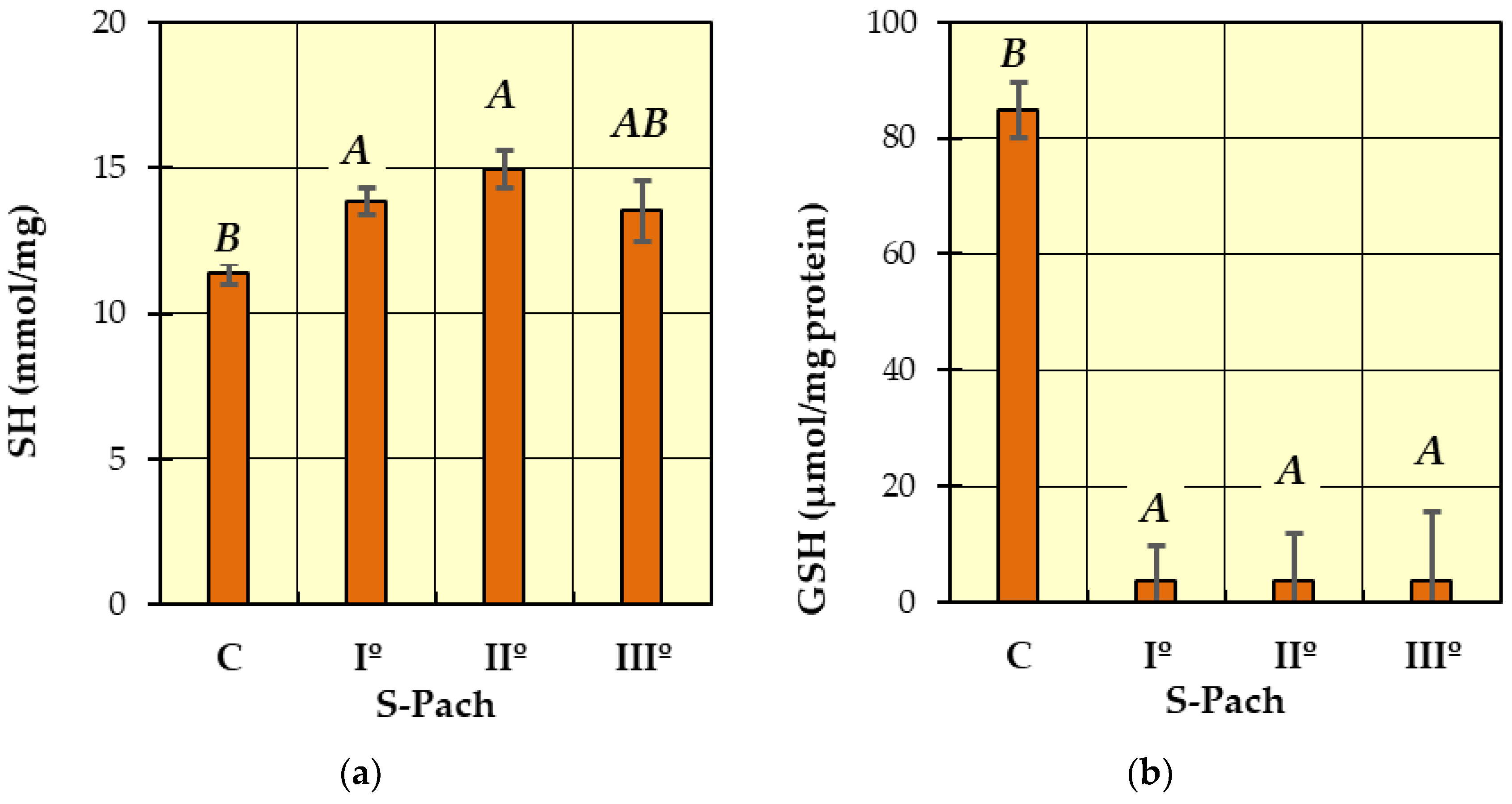
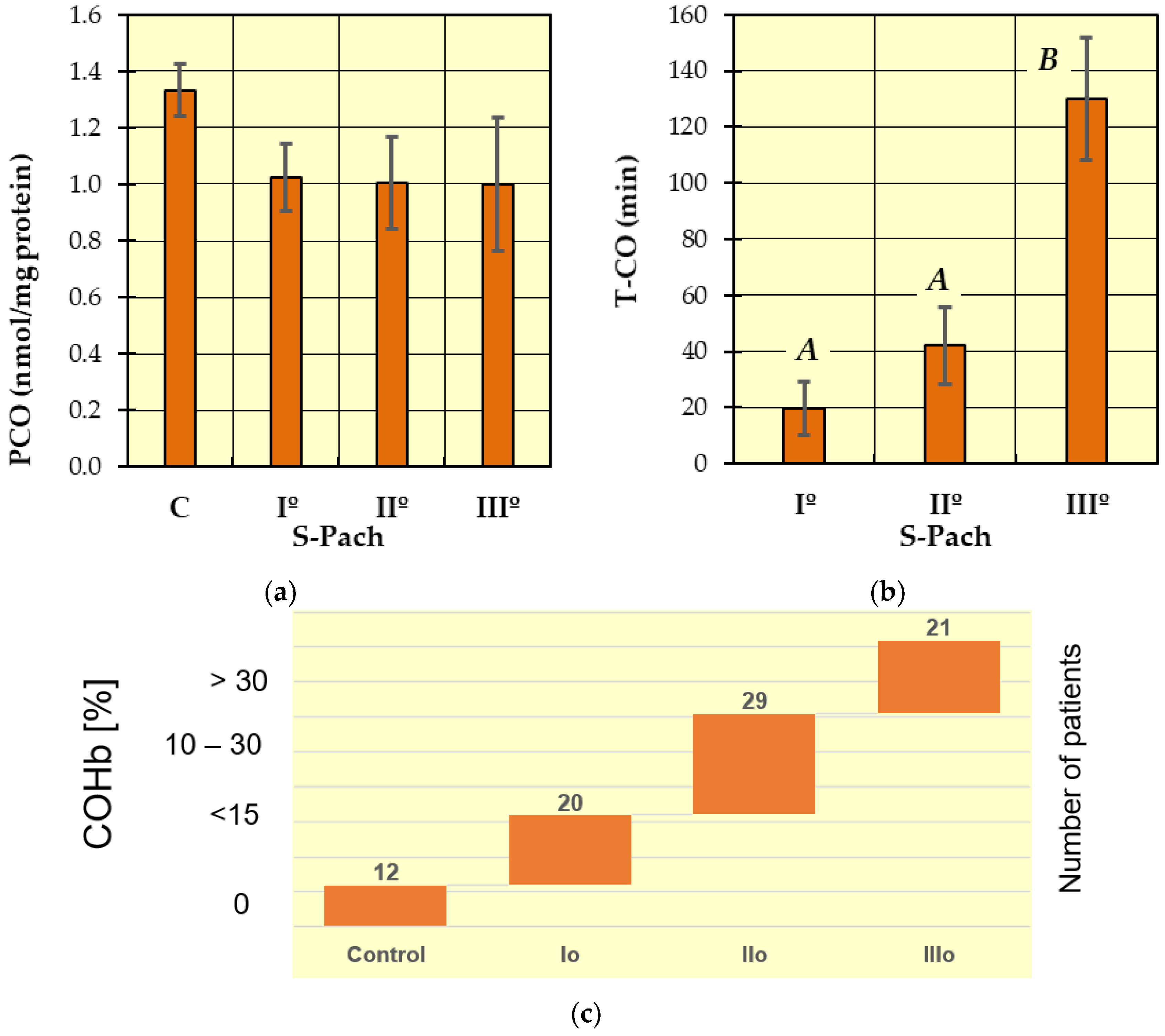
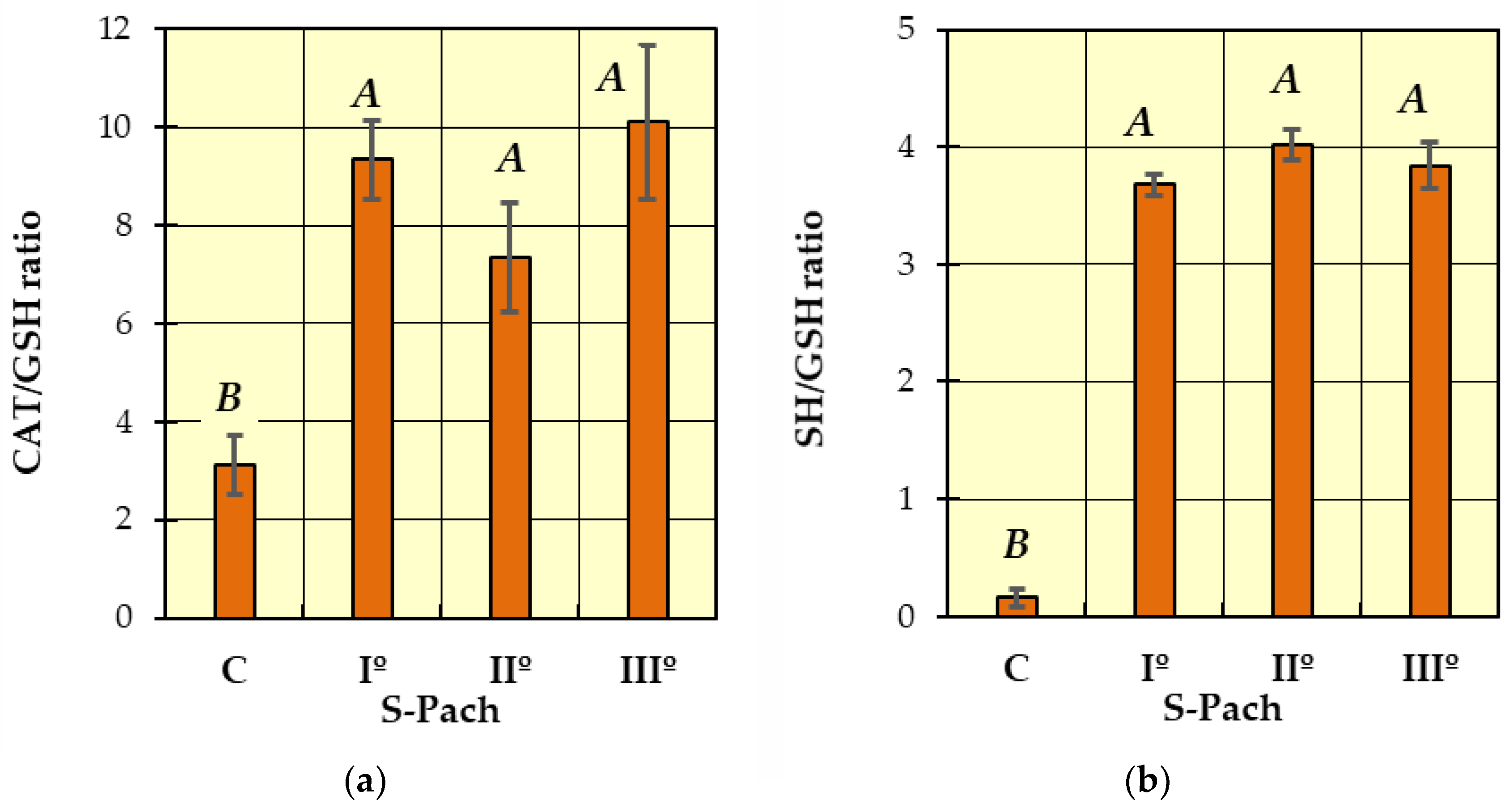
2. Results and Discussion
- (1)
- (2)
- During intense treatment of CO poisoning with oxygen therapy at very high concentrations of free oxygen, it is suspected that the antioxidant systems are eventually overwhelmed, and the rate of cell damage exceeds the capacity of the systems that prevent or repair it.
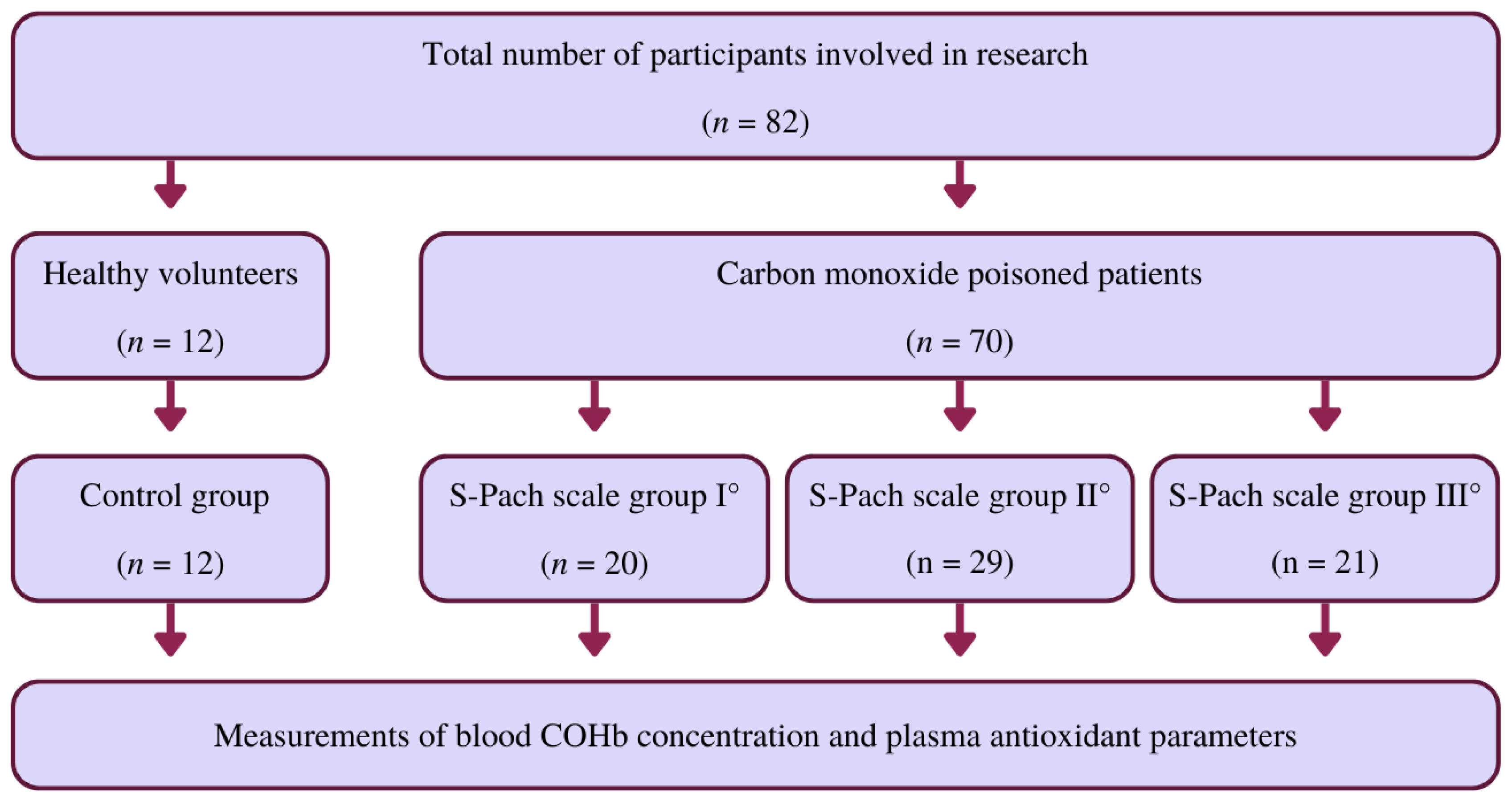
3. Materials and Methods
3.1. Materials
3.2. Measurement of Oxidative-Stress-Related Parameters
3.2.1. Measurement of Catalase (CAT)
3.2.2. Measurement of 2,2-Diphenyl-1-picryl-hydrazyl (DPPH)
3.2.3. Sulfhydryl Groups (-SH)
3.2.4. Measurement of Reduced Glutathione (GSH)
3.2.5. Measurement of the Carbonyl Group (=CO Derivates)
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Świderska, A.; Sein Anand, J. Wybrane zagadnienia dotyczące ostrych zatruć ksenobiotykami w Polsce w 2010 roku. Przegl. Lek. 2012, 69, 409–414. [Google Scholar]
- Nelson, L.; Goldfrank, L.R. (Eds.) Goldfrank’s Toxicologic Emergencies, 9th ed.; McGraw-Hill Medical: New York, NY, USA, 2011; ISBN 978-0-07-160593-9. [Google Scholar]
- Kao, L.W.; Nañagas, K.A. Toxicity Associated with Carbon Monoxide. Clin. Lab. Med. 2006, 26, 99–125. [Google Scholar] [CrossRef]
- Myers, R.A.; Snyder, S.K.; Emhoff, T.A. Subacute Sequelae of Carbon Monoxide Poisoning. Ann. Emerg. Med. 1985, 14, 1163–1167. [Google Scholar] [CrossRef]
- Choi, Y.K.; Por, E.D.; Kwon, Y.-G.; Kim, Y.-M. Regulation of ROS Production and Vascular Function by Carbon Monoxide. Oxidative Med. Cell. Longev. 2012, 2012, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queiroga, C.S.F.; Almeida, A.S.; Vieira, H.L.A. Carbon Monoxide Targeting Mitochondria. Biochem. Res. Int. 2012, 2012, 749845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.L.; Bouillaud, F.; Almeida, A.S.; Vieira, H.L.; Ouidja, M.O.; Dubois-Randé, J.-L.; Foresti, R.; Motterlini, R. Carbon Monoxide Reverses the Metabolic Adaptation of Microglia Cells to an Inflammatory Stimulus. Free Radic. Biol. Med. 2017, 104, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Erecinska, M.; Wagner, M. Mitochondrial Responses to Carbon Monoxide Toxicity. Ann. N. Y. Acad. Sci. 1970, 174, 193–204. [Google Scholar] [CrossRef]
- Thom, S.R.; Ohnishi, S.T.; Ischiropoulos, H. Nitric Oxide Released by Platelets Inhibits Neutrophil B2 Integrin Function Following Acute Carbon Monoxide Poisoning. Toxicol. Appl. Pharmacol. 1994, 128, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Wright, J. Chronic and Occult Carbon Monoxide Poisoning: We Don’t Know What We’re Missing. Emerg. Med. J. 2002, 19, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Min, S.K. A Brain Syndrome Associated with Delayed Neuropsychiatric Sequelae Following Acute Carbon Monoxide Intoxication. Acta Psychiatr. Scand. 1986, 73, 80–86. [Google Scholar] [CrossRef]
- Mathieu, D.; Nolf, M.; Durocher, A.; Saulnier, F.; Frimat, P.; Furon, D.; Wattel, F. Acute Carbon Monoxide Poisoning Risk of Late Sequelae and Treatment by Hyperbaric Oxygen. J. Toxicol. Clin. Toxicol. 1985, 23, 315–324. [Google Scholar] [CrossRef]
- Neubauer, R.A.; Neubauer, V.; Nu, A.K.C.; Maxfield, W.S. Treatment of Late Neurologic Sequelae of Carbon Monoxide Poisoning with Hyperbaric Oxygenation: A Case Series. J. Am. Physicians Surg. 2006, 11, 56–59. [Google Scholar]
- Mannaioni, P.F.; Vannacci, A.; Masini, E. Carbon Monoxide: The Bad and the Good Side of the Coin, from Neuronal Death to Anti-Inflammatory Activity. Inflamm. Res. 2006, 55, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Persson, H.E.; Sjöberg, G.K.; Haines, J.A.; de Garbino, J.P. Poisoning Severity Score. Grading of Acute Poisoning. J. Toxicol. Clin. Toxicol. 1998, 36, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Pach, J.; Persson, H.; Sancewicz-Pach, K.; Groszek, B. Comparison between the poisoning severity score and specific grading scales used at the Department of Clinical Toxicology in Krakow. Przegl. Lek. 1999, 56, 401–408. [Google Scholar] [PubMed]
- Thom, S.R.; Keim, L.W. Carbon Monoxide Poisoning: A Review Epidemiology, Pathophysiology, Clinical Findings, and Treatment Options Including Hyperbaric Oxygen Therapy. J. Toxicol. Clin. Toxicol. 1989, 27, 141–156. [Google Scholar] [CrossRef]
- Gorman, D.; Drewry, A.; Huang, Y.L.; Sames, C. The Clinical Toxicology of Carbon Monoxide. Toxicology 2003, 187, 25–38. [Google Scholar] [CrossRef]
- O’Driscoll, B.R.; Howard, L.S.; Davison, A.G. British Thoracic Society BTS Guideline for Emergency Oxygen Use in Adult Patients. Thorax 2008, 63 (Suppl. S6), vi1-68. [Google Scholar] [CrossRef] [Green Version]
- Fridovich, I. Oxygen Toxicity: A Radical Explanation. J. Exp. Biol. 1998, 201, 1203–1209. [Google Scholar] [CrossRef]
- Piantadosi, C.A. Carbon Monoxide, Reactive Oxygen Signaling, and Oxidative Stress. Free Radic. Biol. Med. 2008, 45, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Bryan, C.L.; Jenkinson, S.G. Oxygen Toxicity. Clin. Chest Med. 1988, 9, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D.; Piantadosi, C.A. Recovery of Energy Metabolism in Rat Brain after Carbon Monoxide Hypoxia. J. Clin. Invest. 1992, 89, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Skrzydlewska, E.; Farbiszewski, R.; Gacko, M. Wpływ oksydacyjnej modyfikacji białek na równowagę proteazy-antyproteazy i proteolizę komórkową. Postępy Hig. Med. Doświadczalnej 1997, 51, 443–456. [Google Scholar]
- Kallstrom, T.J. American Association for Respiratory Care (AARC) Clinical Practice Guideline: Oxygen Therapy for Adults in the Acute Care Facility—2002 Revision & Update. Respir. Care 2002, 47, 717–720. [Google Scholar]
- Abilés, J.; de la Cruz, A.P.; Castaño, J.; Rodríguez-Elvira, M.; Aguayo, E.; Moreno-Torres, R.; Llopis, J.; Aranda, P.; Argüelles, S.; Ayala, A.; et al. Oxidative Stress Is Increased in Critically Ill Patients According to Antioxidant Vitamins Intake, Independent of Severity: A Cohort Study. Crit. Care 2006, 10, R146. [Google Scholar] [CrossRef] [Green Version]
- Thérond, P.; Bonnefont-Rousselot, D.; Davit-Spraul, A.; Conti, M.; Legrand, A. Biomarkers of Oxidative Stress: An Analytical Approach. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 373–384. [Google Scholar] [CrossRef]
- Kavakli, H.S.; Erel, O.; Delice, O.; Gormez, G.; Isikoglu, S.; Tanriverdi, F. Oxidative Stress Increases in Carbon Monoxide Poisoning Patients. Hum. Exp. Toxicol. 2011, 30, 160–164. [Google Scholar] [CrossRef]
- Angelova, P.R.; Myers, I.; Abramov, A.Y. Carbon Monoxide Neurotoxicity Is Triggered by Oxidative Stress Induced by ROS Production from Three Distinct Cellular Sources. Redox Biol. 2023, 60, 102598. [Google Scholar] [CrossRef]
- Li, S.; Yan, T.; Yang, J.Q.; Oberley, T.D.; Oberley, L.W. The Role of Cellular Glutathione Peroxidase Redox Regulation in the Suppression of Tumor Cell Growth by Manganese Superoxide Dismutase. Cancer Res. 2000, 60, 3927–3939. [Google Scholar]
- Pamplona, R.; Costantini, D. Molecular and Structural Antioxidant Defenses against Oxidative Stress in Animals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R843–R863. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-871747-8. [Google Scholar]
- Góth, L. Lipid and Carbohydrate Metabolism in Acatalasemia. Clin. Chem. 2000, 46, 564–566. [Google Scholar] [CrossRef] [Green Version]
- L’Ecuyer, T.; Allebban, Z.; Thomas, R.; Vander Heide, R. Glutathione S-Transferase Overexpression Protects against Anthracycline-Induced H9C2 Cell Death. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2057–H2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balcerczyk, A.; Bartosz, G. Thiols Are Main Determinants of Total Antioxidant Capacity of Cellular Homogenates. Free. Radic. Res. 2003, 37, 537–541. [Google Scholar] [CrossRef] [PubMed]
- de Zwart, L.L.; Meerman, J.H.; Commandeur, J.N.; Vermeulen, N.P. Biomarkers of Free Radical Damage Applications in Experimental Animals and in Humans. Free Radic. Biol. Med. 1999, 26, 202–226. [Google Scholar] [CrossRef] [PubMed]
- Kadiiska, M.B.; Gladen, B.C.; Baird, D.D.; Germolec, D.; Graham, L.B.; Parker, C.E.; Nyska, A.; Wachsman, J.T.; Ames, B.N.; Basu, S.; et al. Biomarkers of Oxidative Stress Study II: Are Oxidation Products of Lipids, Proteins, and DNA Markers of CCl4 Poisoning? Free Radic. Biol. Med. 2005, 38, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in Vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. ISBN 978-0-12-182005-3. [Google Scholar]
- Annegowda, H.; Anwar, L.; Mordi, M.; Ramanathan, S.; Mansor, S. Influence of Sonication on the Phenolic Content and Antioxidant Activity of Terminalia Catappa L. Leaves. Pharmacogn. Res. 2010, 2, 368. [Google Scholar] [CrossRef] [Green Version]
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicrylhydrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Levine, R.L.; Williams, J.A.; Stadtman, E.P.; Shacter, E. Carbonyl Assays for Determination of Oxidatively Modified Proteins. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1994; Volume 233, pp. 346–357. ISBN 978-0-12-182134-0. [Google Scholar]
- Verma, S.P.; Quiroz-Ruiz, A. Critical Values for Six Dixon Tests for Outliers in Normal Samples up to Sizes 100, and Applications in Science and Engineering. Rev. Mex. Cienc. Geol. 2006, 23, 133–161. [Google Scholar]
- Francik, R.; Kryczyk-Kozioł, J.; Krośniak, M.; Francik, S.; Hebda, T.; Pedryc, N.; Knapczyk, A.; Berköz, M.; Ślipek, Z. The Influence of Organic Vanadium Complexes on an Antioxidant Profile in Adipose Tissue in Wistar Rats. Materials 2022, 15, 1952. [Google Scholar] [CrossRef]
- Francik, S.; Knapik, P.; Łapczyńska-Kordon, B.; Francik, R.; Ślipek, Z. Values of Selected Strength Parameters of Miscanthus × Giganteus Stalk Depending on Water Content and Internode Number. Materials 2022, 15, 1480. [Google Scholar] [CrossRef] [PubMed]
- Jówko, E.; Długołęcka, B.; Cieśliński, I.; Kotowska, J. Polymorphisms in Genes Encoding VDR, CALCR and Antioxidant Enzymes as Predictors of Bone Tissue Condition in Young, Healthy Men. Int. J. Mol. Sci. 2023, 24, 3373. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Morabito, R.; Spinelli, S.; Trichilo, V.; Loddo, S.; Sarikas, A.; Dossena, S.; Marino, A. D-Galactose Decreases Anion Exchange Capability through Band 3 Protein in Human Erythrocytes. Antioxidants 2020, 9, 689. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Basile, N.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Oxidation Stress as a Mechanism of Aging in Human Erythrocytes: Protective Effect of Quercetin. Int. J. Mol. Sci. 2022, 23, 7781. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Acai (Euterpe Oleracea) Extract Protects Human Erythrocytes from Age-Related Oxidative Stress. Cells 2022, 11, 2391. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Antioxidant Activity of Quercetin in a H2O2-Induced Oxidative Stress Model in Red Blood Cells: Functional Role of Band 3 Protein. Int. J. Mol. Sci. 2022, 23, 991. [Google Scholar] [CrossRef] [PubMed]
- Zawiślak, A.; Francik, R.; Francik, S.; Knapczyk, A. Impact of Drying Conditions on Antioxidant Activity of Red Clover (Trifolium Pratense), Sweet Violet (Viola Odorata) and Elderberry Flowers (Sambucus Nigra). Materials 2022, 15, 3317. [Google Scholar] [CrossRef]
| Points | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Age (years) | <29 | 30–39 | 40–49 | >50 |
| CO exposition time (min) | <30 | 31–60 | 61–120 | >120 |
| Neurological injury degree | I° - No consciousness disturbances and other neurological changes in physical examination. | II° - Consciousness disturbances (somnolence and agitation); - Hyperreflexia; - Positive Babinski reflex; - Tonic–clonic convulsions; - Increased muscular tone. | III° - Unconsciousness. | IV° - Loss of consciousness; - Hyperreflexia; - Positive Babinski reflex; - Tonic–clonic convulsions; - Decreased muscle tone; - Bradyreflexia. |
| COHb serum level (%) | 0 | <15 | 15–30 | >30 |
| Lactate serum level (mmol/L) | 1.0–1.78 | 1.8–3.6 | 3.7–5.4 | >5.5 |
| The severity of CO poisoning: I° light 1–4 points, II° medium 5–8 points, III° sever > 9 points | ||||
| Source of Variation | SS 1 | df 1 | MS 1 | F 1 | p 1 | Significant | |
|---|---|---|---|---|---|---|---|
| CAT | S-Pach 2 | 530530 | 3 | 176843 | 50.194 | <0.001 | Yes |
| DPPH | S-Pach | 8352 | 3 | 2784 | 15.597 | <0.001 | Yes |
| SH | S-Pach | 169.1 | 3 | 56.4 | 10.338 | <0.001 | Yes |
| GSH | S-Pach | 132439 | 3 | 44146 | 52.231 | <0.001 | Yes |
| =CO derivates | S-Pach | 2.051 | 3 | 0.684 | 2.030 | 0.117 | No |
| CAT/GSH | S-Pach | 701.6 | 3 | 233.9 | 15.929 | <0.001 | Yes |
| SH/GSH | S-Pach | 269.3 | 3 | 89.8 | 404.347 | <0.001 | Yes |
| T-CO | S-Pach | 40945 | 2 | 20473 | 10.794 | <0.001 | Yes |
| Control | I°—Pach | II°—Pach | III°—Pach | |
|---|---|---|---|---|
| CAT (U/mg protein) | 196 ± 82.2 | 35.0 ± 18.3 | 27.3 ± 9.6 | 37.8 ± 22.4 |
| DPPH (%) | 5.9 ± 6.5 | 23.9 ± 16.6 | 30.1 ± 20.5 | 25.6 ± 11.4 |
| SH (mmol/mg protein) | 11.4 ± 2.4 | 13.8 ± 2.6 | 14.9 ± 1.6 | 13.5 ± 1.1 |
| GSH (μmol/mg protein) | 84.8 ± 41.9 | 3.77 ± 0.2 | 3.73 ± 0.16 | 3.75 ± 0.14 |
| =CO derivates (nmol/mg protein) | 1.333 ± 0.57 | 1.025 ± 0.58 | 1.005 ± 0.58 | 1.001 ± 0.66 |
| CAT/GSH ratio | 3.12 ± 2.96 | 9.34 ± 4.88 | 7.35 ± 2.62 | 10.1 ± 6.08 |
| SH/GSH ratio | 0.158 ± 0.086 | 3.678 ± 0.692 | 4.022 ± 0.523 | 3.842 ± 0.711 |
| T-CO (min) | - | 19.8 ± 7.0 | 42 ± 37 | 130 ± 125.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hydzik, P.; Francik, R.; Francik, S.; Gomółka, E.; Eker, E.D.; Krośniak, M.; Noga, M.; Jurowski, K. The Critical Assessment of Oxidative Stress Parameters as Potential Biomarkers of Carbon Monoxide Poisoning. Int. J. Mol. Sci. 2023, 24, 10784. https://doi.org/10.3390/ijms241310784
Hydzik P, Francik R, Francik S, Gomółka E, Eker ED, Krośniak M, Noga M, Jurowski K. The Critical Assessment of Oxidative Stress Parameters as Potential Biomarkers of Carbon Monoxide Poisoning. International Journal of Molecular Sciences. 2023; 24(13):10784. https://doi.org/10.3390/ijms241310784
Chicago/Turabian StyleHydzik, Piotr, Renata Francik, Sławomir Francik, Ewa Gomółka, Ebru Derici Eker, Mirosław Krośniak, Maciej Noga, and Kamil Jurowski. 2023. "The Critical Assessment of Oxidative Stress Parameters as Potential Biomarkers of Carbon Monoxide Poisoning" International Journal of Molecular Sciences 24, no. 13: 10784. https://doi.org/10.3390/ijms241310784
APA StyleHydzik, P., Francik, R., Francik, S., Gomółka, E., Eker, E. D., Krośniak, M., Noga, M., & Jurowski, K. (2023). The Critical Assessment of Oxidative Stress Parameters as Potential Biomarkers of Carbon Monoxide Poisoning. International Journal of Molecular Sciences, 24(13), 10784. https://doi.org/10.3390/ijms241310784








