Accelerated Bone Loss in Transgenic Mice Expressing Constitutively Active TGF-β Receptor Type I
Abstract
:1. Introduction
2. Results
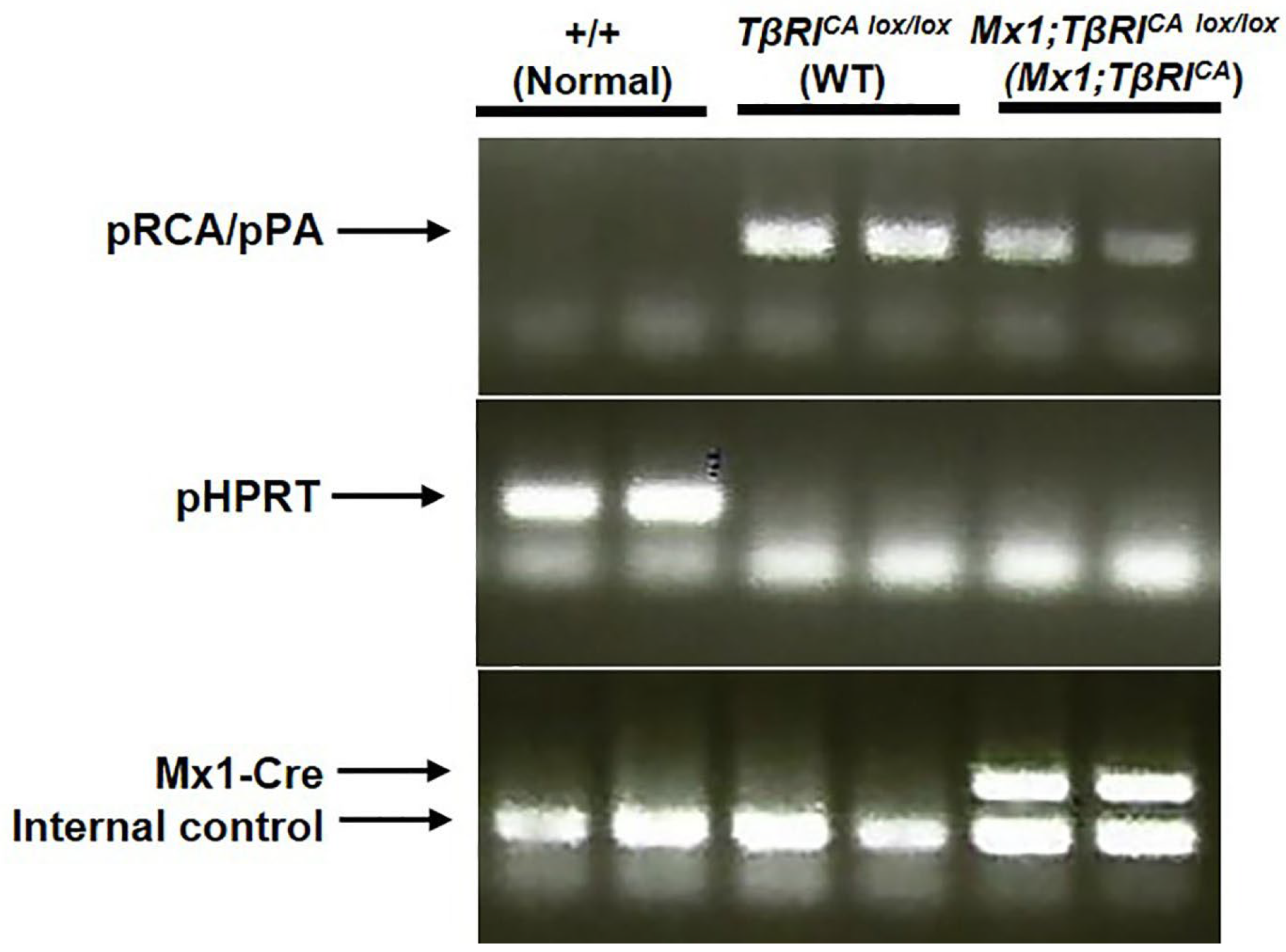
2.1. Generation of Mx1;TβRICA Mice
2.2. Mx1;TβRICA Mice Decreases Serum Calcium and Increases Serum PTH Levels
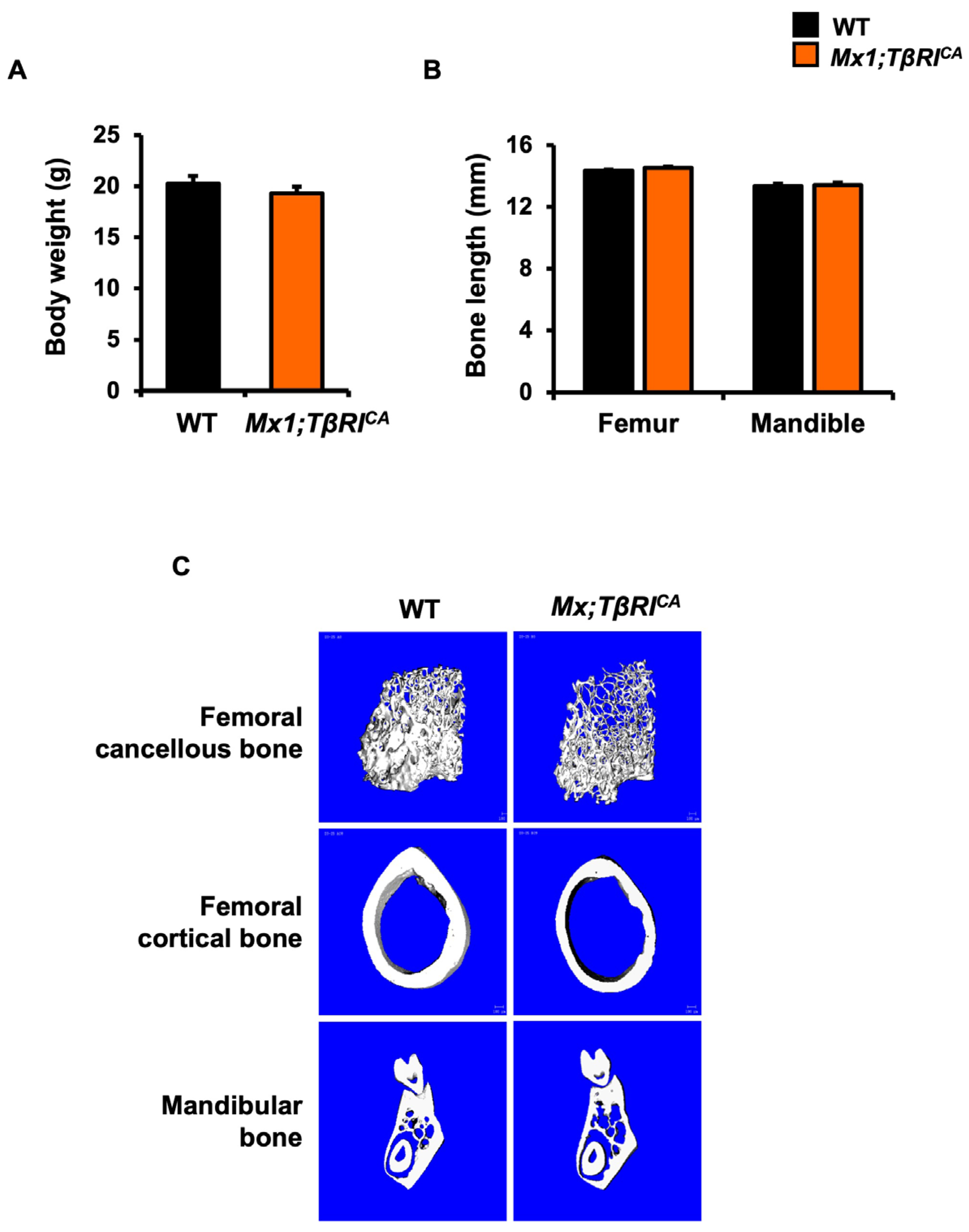
2.3. Mx1;TβRICA Mice Have Cancellous and Cortical Bone Loss in Femurs and Mandibles
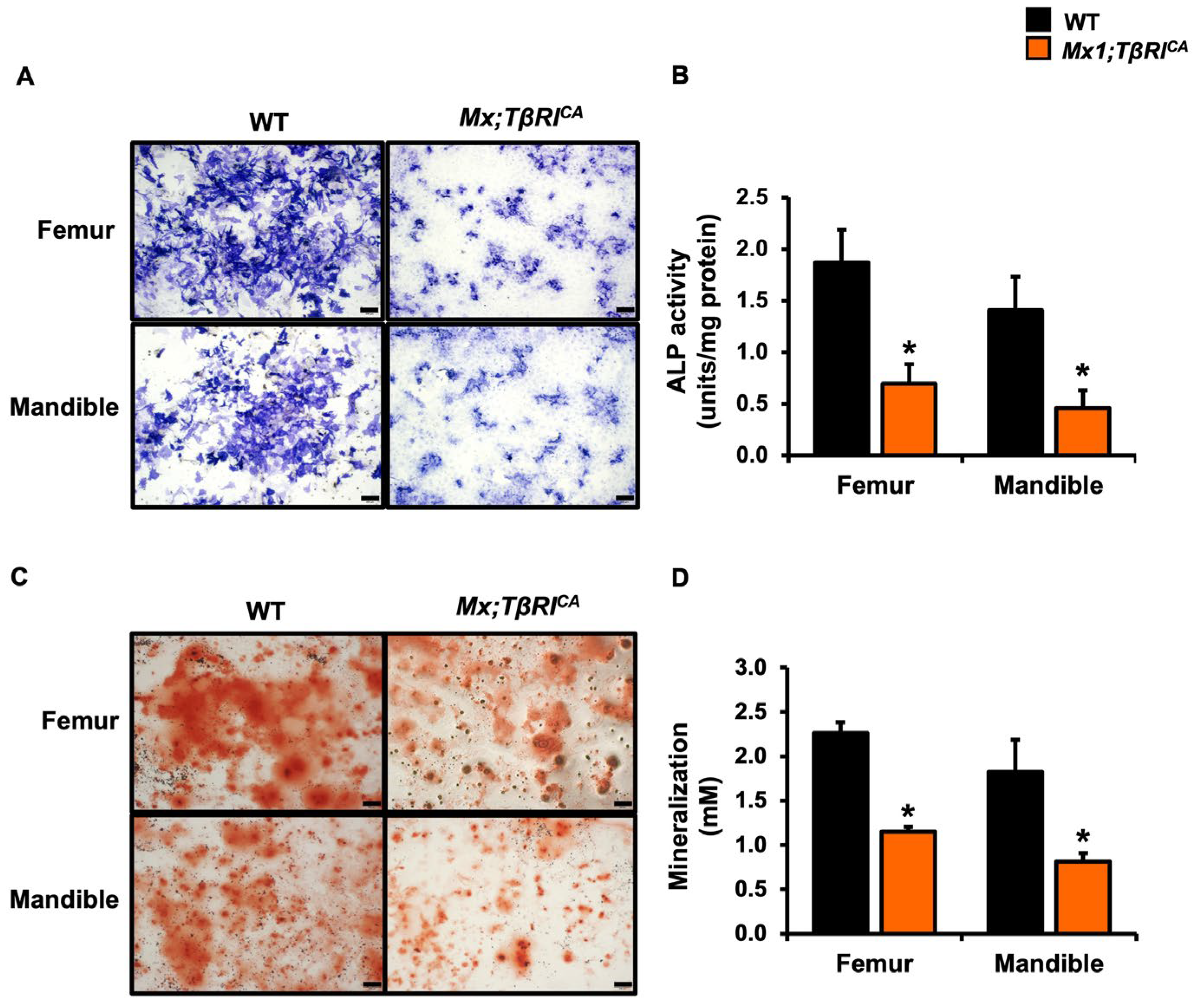
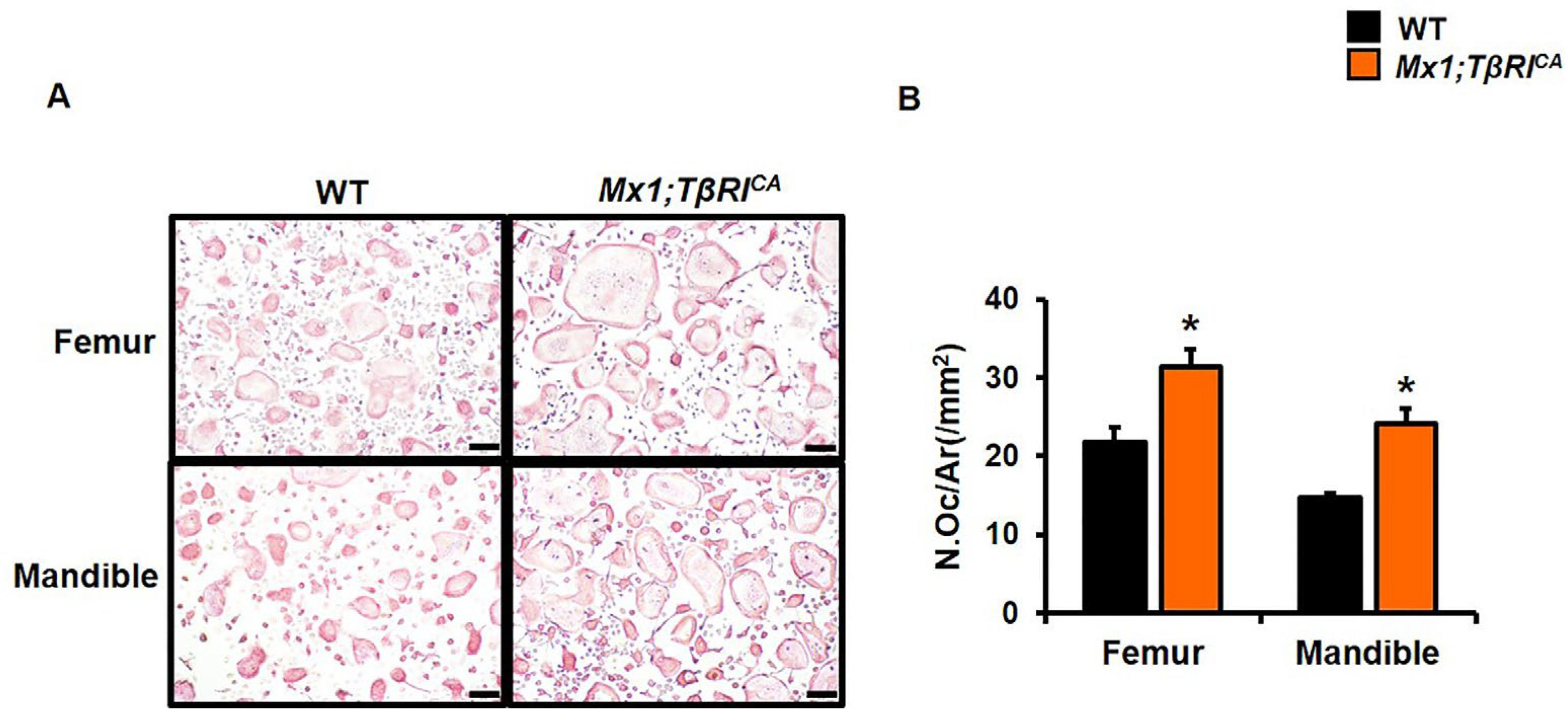
2.4. Mx1;TβRICA Mice Had Decreased Bone Formation and Increased Bone Resorption
2.5. Mx1;TβRICA Mice Have Decreased Osteoblast Differentiation and Increased Osteoclast Differentiation
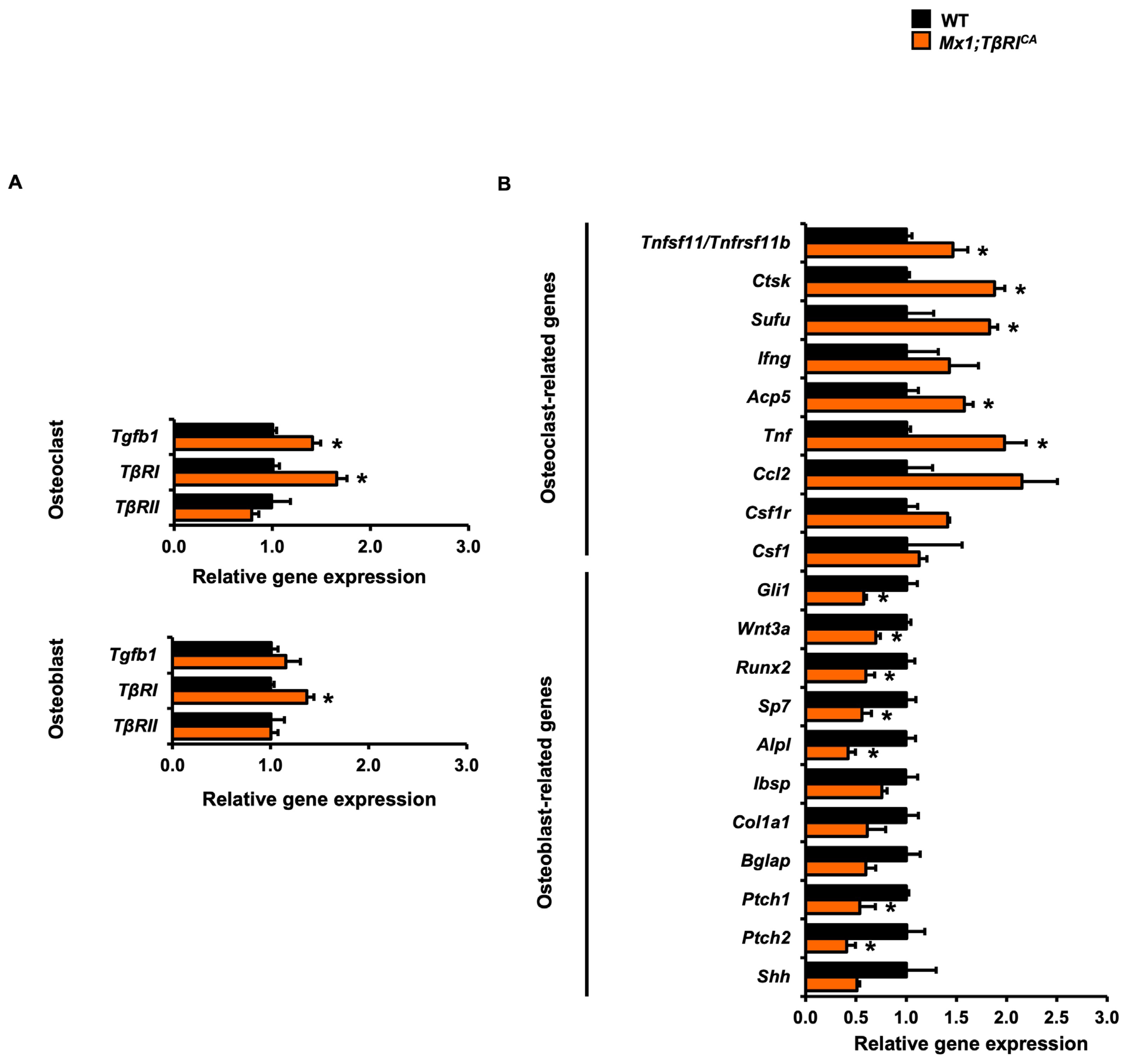
2.6. Mx1;TβRICA Mice Have Decreased Osteoblast and Increased Osteoclast-Related Gene Expression
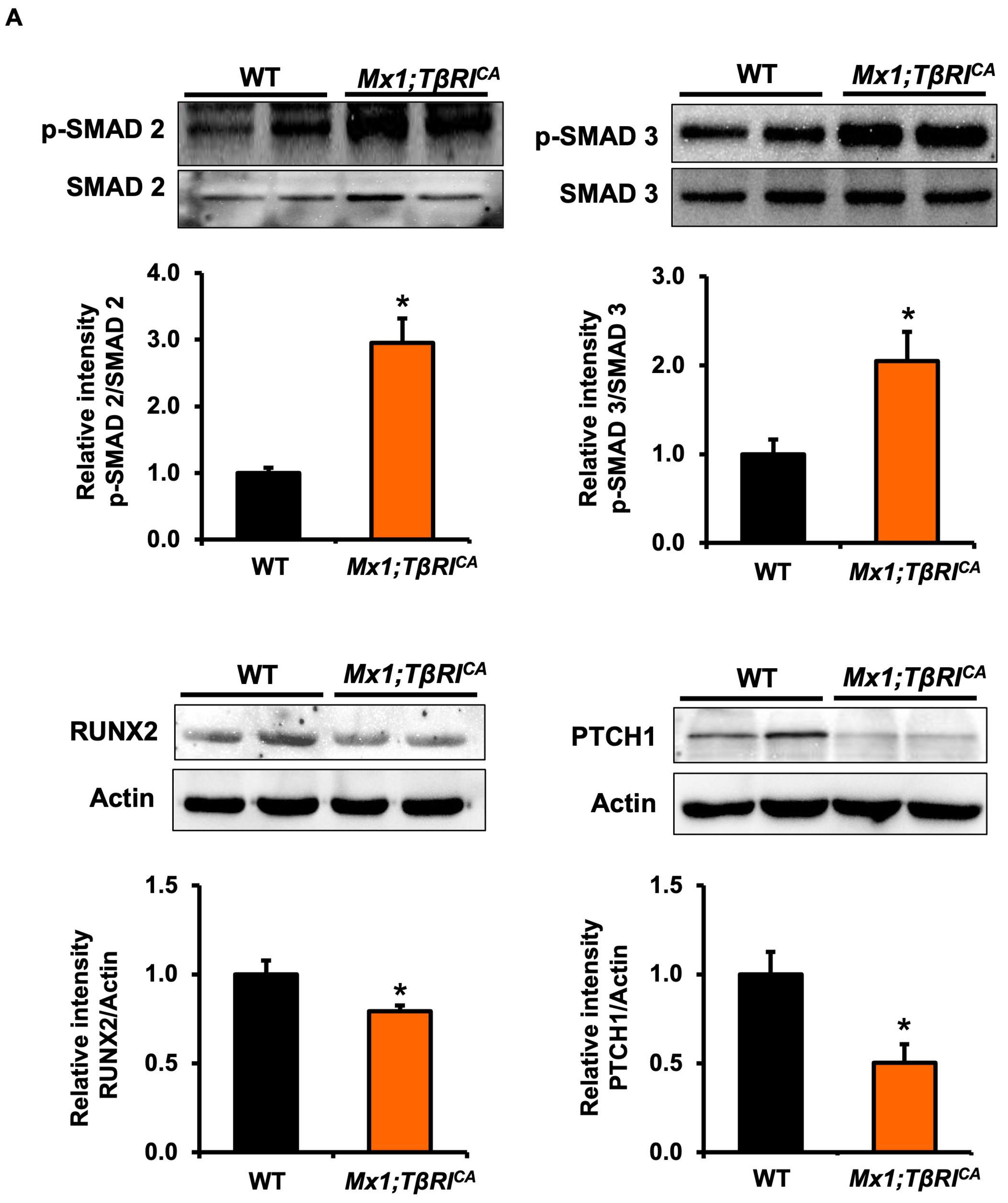
2.7. Mx1;TβRICA Mice Have Increased TGF-β Signaling Leading to Decreases in RUNX2 and PTCH1 and Increases in CTSK Protein Levels
2.8. Mx1;TβRICA Mice Have Decreased Bone Hardness in Tibia
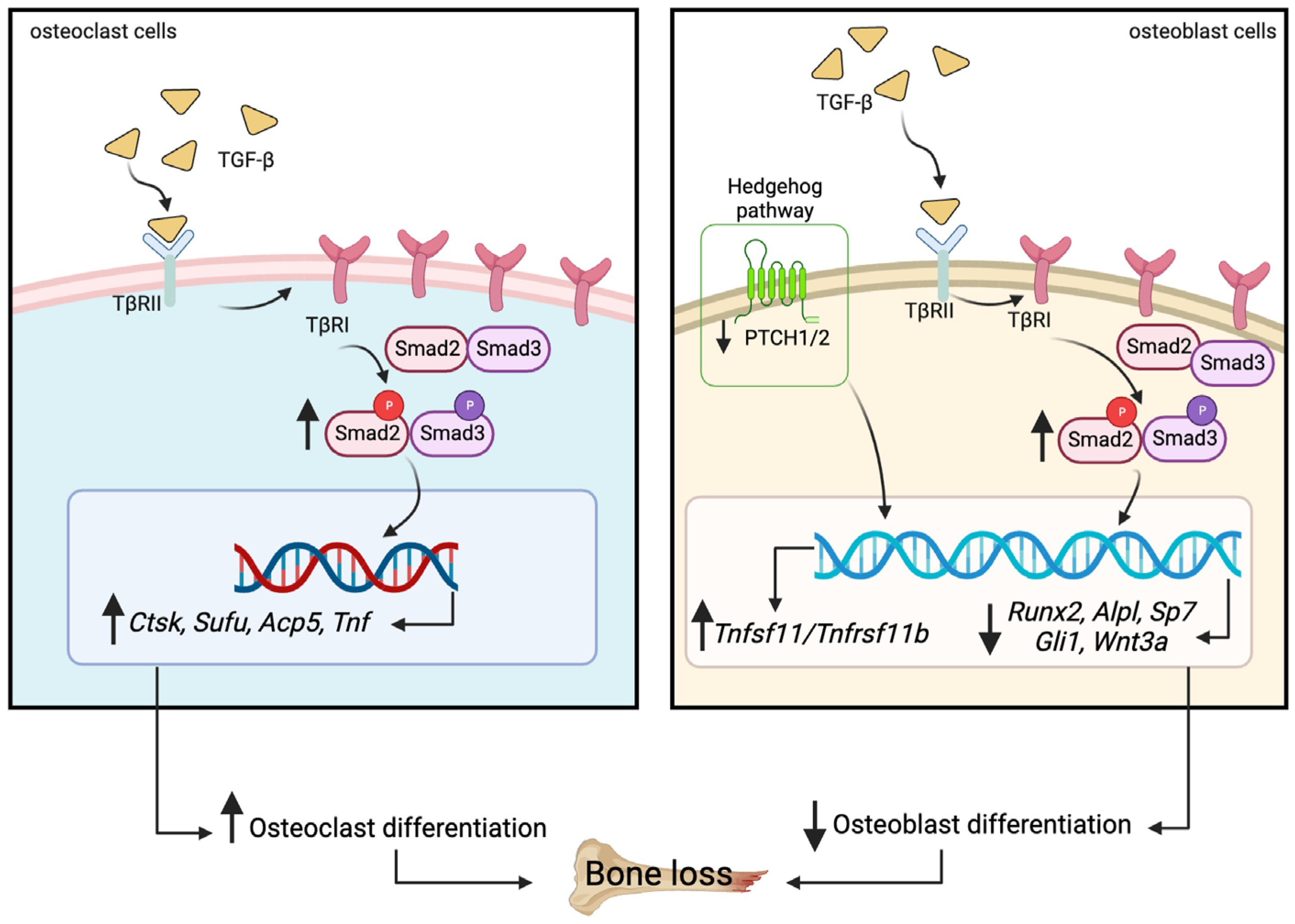
3. Discussion
4. Materials and Methods
4.1. Mice
- pRCA: 5′-TTG TGA ACA GAA GTT AAG GC-3′
- pPA: 5′-AGA AAG AAC AAT CAA GGG TCC-3′
- pHPRT-f: 5′-GAG GGA GAA AAA TGC GGA GTG-3′
- pHPRT-r: 5′-CTC CGG AAA GCA GTG AGG TAA G-3′
- Mx1-Cre-f: 5′-GTG AGT TTC GTT TCT GAG CTC C-3′
- Mx1-Cre-r: 5′-CGG TTA TTC AAC TTG CAC CA-3′
- Internal control-f: 5′-GAC AAA ATG GTG AAG GTC GG-3′
- Internal control-r: 5′-CAA AGG CGG AGT TAC CAG AG-3′
4.2. µCT Analysis
4.3. qPCR Analysis
4.4. Osteoclast Culture
4.5. Osteoblast Culture
4.6. Histomorphometry
4.7. Western Blot Analysis
4.8. Microindentation Analysis
4.9. Measurement of Bone Length
4.10. Serum Chemistry
4.11. Statistically Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janssens, K.; ten Dijke, P.; Janssens, S.; Van Hul, W. Transforming Growth Factor-β1 to the Bone. Endocr. Rev. 2005, 26, 743–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaidya, A.; Kale, V.P. TGF-β signaling and its role in the regulation of hematopoietic stem cells. Syst. Synth. Biol. 2015, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bonewald, L.F.; Dallas, S.L. Role of active and latent transforming growth factor β in bone formation. J. Cell. Biochem. 1994, 55, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.R.; Nyman, J.S.; Lwin, S.T.; Moore, M.M.; Esparza, J.; O’Quinn, E.C.; Hart, A.J.; Biswas, S.; Patil, C.A.; Lonning, S.; et al. Inhibition of TGF-β signaling by 1D11 antibody treatment increases bone mass and quality in vivo. J. Bone Min. Res. 2010, 25, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Karst, M.; Gorny, G.; Galvin, R.J.; Oursler, M.J. Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-beta regulation of osteoclast differentiation. J. Cell. Physiol. 2004, 200, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009, 15, 757–765. [Google Scholar] [CrossRef] [Green Version]
- Jiao, K.; Zhang, M.; Niu, L.; Yu, S.; Zhen, G.; Xian, L.; Yu, B.; Yang, K.; Liu, P.; Cao, X.; et al. Overexpressed TGF-β in subchondral bone leads to mandibular condyle degradation. J. Dent. Res. 2014, 93, 140–147. [Google Scholar] [CrossRef]
- Zhen, G.; Wen, C.; Jia, X.; Li, Y.; Crane, J.L.; Mears, S.C.; Askin, F.B.; Frassica, F.J.; Chang, W.; Yao, J.; et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013, 19, 704–712. [Google Scholar] [CrossRef] [Green Version]
- Longobardi, L.; Li, T.; Myers, T.J.; O’Rear, L.; Ozkan, H.; Li, Y.; Contaldo, C.; Spagnoli, A. TGF-β type II receptor/MCP-5 axis: At the crossroad between joint and growth plate development. Dev. Cell 2012, 23, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Serra, R.; Johnson, M.; Filvaroff, E.H.; LaBorde, J.; Sheehan, D.M.; Derynck, R.; Moses, H.L. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J. Cell Biol. 1997, 139, 541–552. [Google Scholar] [CrossRef]
- Sanford, L.P.; Ormsby, I.; Gittenberger-de Groot, A.C.; Sariola, H.; Friedman, R.; Boivin, G.P.; Cardell, E.L.; Doetschman, T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 1997, 124, 2659–2670. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Alliston, T. Regulation of postnatal bone homeostasis by TGFβ. BoneKEy Rep. 2013, 2, 255. [Google Scholar] [CrossRef] [Green Version]
- Kühn, R.; Schwenk, F.; Aguet, M.; Rajewsky, K. Inducible gene targeting in mice. Science 1995, 269, 1427–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliprantis, A.; Ueki, Y.; Sulyanto, R.; Park, A.; Sigrist, K.; Sharma, S.; Ostrowski, M.; Olsen, B.; Glimcher, L. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J. Clin. Investig. 2008, 118, 3775–3789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Sells Galvin, R.J.; Gatlin, C.L.; Horn, J.W.; Fuson, T.R. TGF-beta enhances osteoclast differentiation in hematopoietic cell cultures stimulated with RANKL and M-CSF. Biochem. Biophys. Res. Commun. 1999, 265, 233–239. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Yamato, H.; Fukagawa, M. TGF-Beta Signaling in Bone with Chronic Kidney Disease. Int. J. Mol. Sci. 2018, 19, 2352. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Partridge, N.C. Parathyroid hormone signaling in bone and kidney. Curr. Opin. Nephrol. Hypertens. 2009, 18, 298–302. [Google Scholar] [CrossRef]
- Pfeilschifter, J.; Laukhuf, F.; Müller-Beckmann, B.; Blum, W.F.; Pfister, T.; Ziegler, R. Parathyroid hormone increases the concentration of insulin-like growth factor-I and transforming growth factor beta 1 in rat bone. J. Clin. Investig. 1995, 96, 767–774. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Kumar, R. Parathyroid Hormone Regulates Transforming Growth Factor β1 and β2 Synthesis in Osteoblasts Via Divergent Signaling Pathways. J. Bone Miner. Res. 2000, 15, 879–884. [Google Scholar] [CrossRef]
- Parker, W.L.; Finnson, K.W.; Soe-Lin, H.; Knaus, P.; Philip, A. Expression and function of TβRII-B, a variant of the type II TGF-β receptor, in human chondrocytes. Osteoarthr. Cartil. 2007, 15, 442–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erlebacher, A.; Derynck, R. Increased expression of TGF-beta 2 in osteoblasts results in an osteoporosis-like phenotype. J. Cell Biol. 1996, 132, 195–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, K.S.; Chen, C.G.; Balooch, G.; Stebbins, E.; McKenna, C.R.; Davis, H.; Niewolna, M.; Peng, X.H.; Nguyen, D.H.N.; Ionova-Martin, S.S.; et al. Pharmacologic Inhibition of the TGF-β Type I Receptor Kinase Has Anabolic and Anti-Catabolic Effects on Bone. PLoS ONE 2009, 4, e5275. [Google Scholar] [CrossRef] [Green Version]
- Lian, N.; Lin, T.; Liu, W.; Wang, W.; Li, L.; Sun, S.; Nyman, J.S.; Yang, X. Transforming growth factor β suppresses osteoblast differentiation via the vimentin activating transcription factor 4 (ATF4) axis. J. Biol. Chem. 2012, 287, 35975–35984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [Green Version]
- Dieudonné, S.C.; Foo, P.; Van Zoelen, E.J.J.; Burger, E.H. Inhibiting and stimulating effects of TGF-β1 on osteoclastic bone resorption in fetal mouse bone organ cultures. J. Bone Miner. Res. 1991, 6, 479–487. [Google Scholar] [CrossRef]
- Kale, V.P.; Vaidya, A.A. Molecular mechanisms behind the dose-dependent differential activation of MAPK pathways induced by transforming growth factor-beta1 in hematopoietic cells. Stem Cells Dev. 2004, 13, 536–547. [Google Scholar] [CrossRef]
- Yasui, T.; Kadono, Y.; Nakamura, M.; Oshima, Y.; Matsumoto, T.; Masuda, H.; Hirose, J.; Omata, Y.; Yasuda, H.; Imamura, T.; et al. Regulation of RANKL-induced osteoclastogenesis by TGF-β through molecular interaction between Smad3 and Traf6. J. Bone Min. Res. 2011, 26, 1447–1456. [Google Scholar] [CrossRef]
- Matsunobu, T.; Torigoe, K.; Ishikawa, M.; de Vega, S.; Kulkarni, A.B.; Iwamoto, Y.; Yamada, Y. Critical roles of the TGF-beta type I receptor ALK5 in perichondrial formation and function, cartilage integrity, and osteoblast differentiation during growth plate development. Dev. Biol. 2009, 332, 325–338. [Google Scholar] [CrossRef] [Green Version]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Ehnert, S.; Baur, J.; Schmitt, A.; Neumaier, M.; Lucke, M.; Dooley, S.; Vester, H.; Wildemann, B.; Stöckle, U.; Nussler, A.K. TGF-β1 As Possible Link between Loss of Bone Mineral Density and Chronic Inflammation. PLoS ONE 2010, 5, e14073. [Google Scholar] [CrossRef] [PubMed]
- Jann, J.; Gascon, S.; Roux, S.; Faucheux, N. Influence of the TGF-beta Superfamily on Osteoclasts/Osteoblasts Balance in Physiological and Pathological Bone Conditions. Int. J. Mol. Sci. 2020, 21, 7597. [Google Scholar] [CrossRef]
- Dai, R.; Wu, Z.; Chu, H.Y.; Lu, J.; Lyu, A.; Liu, J.; Zhang, G. Cathepsin K: The Action in and Beyond Bone. Front. Cell Dev. Biol. 2020, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Balkan, W.; Martinez, A.F.; Fernandez, I.; Rodriguez, M.A.; Pang, M.; Troen, B.R. Identification of NFAT binding sites that mediate stimulation of cathepsin K promoter activity by RANK ligand. Gene 2009, 446, 90–98. [Google Scholar] [CrossRef]
- Li, J.; Cui, Y.; Xu, J.; Wang, Q.; Yang, X.; Li, Y.; Zhang, X.; Qiu, M.; Zhang, Z.; Zhang, Z. Suppressor of Fused restraint of Hedgehog activity level is critical for osteogenic proliferation and differentiation during calvarial bone development. J. Biol. Chem. 2017, 292, 15814–15825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.C.; Liu, Z.G.; Yu, B.; Zhang, X.R. MicroRNA-874 targeting SUFU involves in osteoblast proliferation and differentiation in osteoporosis rats through the Hedgehog signaling pathway. Biochem. Biophys. Res. Commun. 2018, 506, 194–203. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Y.; Liao, Z.; Liu, Q.; Lei, X.; Li, M.; Saijilafu; Zhang, Z.; Hong, D.; Zhu, M.; et al. Genetic and pharmacological activation of Hedgehog signaling inhibits osteoclastogenesis and attenuates titanium particle-induced osteolysis partly through suppressing the JNK/c-Fos-NFATc1 cascade. Theranostics 2020, 10, 6638–6660. [Google Scholar] [CrossRef]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [Green Version]
- Sowa, H.; Kaji, H.; Yamaguchi, T.; Sugimoto, T.; Chihara, K. Activations of ERK1/2 and JNK by transforming growth factor beta negatively regulate Smad3-induced alkaline phosphatase activity and mineralization in mouse osteoblastic cells. J. Biol. Chem. 2002, 277, 36024–36031. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Andre, P.; Ye, L.; Yang, Y.Z. The Hedgehog signalling pathway in bone formation. Int. J. Oral. Sci. 2015, 7, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, Y.; Hojo, H.; Komiyama, Y.; Takato, T.; Chung, U.-i.; Ohba, S. Gli1 Haploinsufficiency Leads to Decreased Bone Mass with an Uncoupling of Bone Metabolism in Adult Mice. PLoS ONE 2014, 9, e109597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grafe, I.; Yang, T.; Alexander, S.; Homan, E.P.; Lietman, C.; Jiang, M.M.; Bertin, T.; Munivez, E.; Chen, Y.; Dawson, B.; et al. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat. Med. 2014, 20, 670–675. [Google Scholar] [CrossRef] [Green Version]
- Zou, M.L.; Chen, Z.H.; Teng, Y.Y.; Liu, S.Y.; Jia, Y.; Zhang, K.W.; Sun, Z.L.; Wu, J.J.; Yuan, Z.D.; Feng, Y.; et al. The Smad Dependent TGF-β and BMP Signaling Pathway in Bone Remodeling and Therapies. Front. Mol. Biosci. 2021, 8, 593310. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, L.; Wang, S.; Huang, B.; Jing, Y.; Su, J. Bone Marrow Mesenchymal Stromal Cells: Identification, Classification, and Differentiation. Front. Cell Dev. Biol. 2021, 9, 787118. [Google Scholar] [CrossRef]
- Park, D.; Spencer, J.A.; Koh, B.I.; Kobayashi, T.; Fujisaki, J.; Clemens, T.L.; Lin, C.P.; Kronenberg, H.M.; Scadden, D.T. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 2012, 10, 259–272. [Google Scholar] [CrossRef] [Green Version]
- Ortinau, L.C.; Wang, H.; Lei, K.; Deveza, L.; Jeong, Y.; Hara, Y.; Grafe, I.; Rosenfeld, S.B.; Lee, D.; Lee, B.; et al. Identification of Functionally Distinct Mx1+αSMA+ Periosteal Skeletal Stem Cells. Cell Stem Cell 2019, 25, 784–796.e5. [Google Scholar] [CrossRef]
- LeBoff, M.S.; Greenspan, S.L.; Insogna, K.L.; Lewiecki, E.M.; Saag, K.G.; Singer, A.J.; Siris, E.S. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2022, 33, 2049–2102. [Google Scholar] [CrossRef]
- Ciardiello, D.; Elez, E.; Tabernero, J.; Seoane, J. Clinical development of therapies targeting TGFβ: Current knowledge and future perspectives. Ann. Oncol. 2020, 31, 1336–1349. [Google Scholar] [CrossRef]
- Vincent, D.F.; Kaniewski, B.; Powers, S.E.; Havenar-Daughton, C.; Marie, J.C.; Wotton, D.; Bartholin, L. A rapid strategy to detect the recombined allele in LSL-TβRICA transgenic mice. Genesis 2010, 48, 559–562. [Google Scholar] [CrossRef] [Green Version]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Min. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, C.; Çolakoğlu, M.; Brun, J.; Thouverey, C.; Bonnet, N.; Ferrari, S.; Trajkovski, M. Primary mouse osteoblast and osteoclast culturing and analysis. STAR Protoc. 2021, 2, 100452. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brylka, L.J.; Köppert, S.; Babler, A.; Kratz, B.; Denecke, B.; Yorgan, T.A.; Etich, J.; Costa, I.G.; Brachvogel, B.; Boor, P.; et al. Post-weaning epiphysiolysis causes distal femur dysplasia and foreshortened hindlimbs in fetuin-A-deficient mice. PLoS ONE 2017, 12, e0187030. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, F.; Perpiñán, D. Measurement Error in Morphometric Studies: Comparison between Manual and Computerized Methods. Ann. Zool. Fenn. 2010, 47, 46–56. [Google Scholar] [CrossRef]





| Parameters | Tibia | Mandible | ||
|---|---|---|---|---|
| WT (n = 8) | Mx1;TβRICA (n = 7) | WT (n = 6) | Mx1;TβRICA (n = 6) | |
| BV/TV (%) | 5.95 ± 0.62 | 2.44 ± 0.43 * | 58.73 ± 3.10 | 33.93 ± 3.07 * |
| Tb.Th (μm) | 35.41 ± 2.04 | 28.92 ± 1.67 * | 66.74 ± 4.89 | 48.45 ± 3.72 * |
| Tb.N (/mm) | 1.69 ± 0.18 | 0.84 ± 0.14 * | 9.04 ± 0.84 | 7.01 ± 0.31 * |
| Tb.Sp (μm) | 613.02 ± 82.29 | 1350.27 ± 209.88 * | 47.77 ± 5.85 | 95.81 ± 7.77 * |
| Ob.S/BS (%) | 21.38 ± 2.02 | 13.59 ± 1.48 * | 6.59 ± 0.79 | 4.40 ± 0.39 * |
| N.Ob/T.Ar (/mm2) | 69.42 ± 7.57 | 22.64 ± 4.61 * | 120.11 ± 7.83 | 73.27 ± 4.32 * |
| N.Ob/B.Pm (/mm) | 20.75 ± 1.96 | 13.25 ± 1.51 * | 6.80 ± 0.52 | 5.26 ± 0.34 * |
| Oc.S/BS (%) | 0.37 ± 0.05 | 1.63 ± 0.30 * | 0.10 ± 0.02 | 0.60 ± 0.03 * |
| N.Oc/T.Ar (/mm2) | 1.12 ± 0.17 | 2.39 ± 0.58 * | 1.54 ± 0.37 | 5.68 ± 0.50 * |
| N.Oc/B.Pm (/mm) | 0.33 ± 0.03 | 1.49 ± 0.28 * | 0.08 ± 0.02 | 0.41 ± 0.04 * |
| ES/BS (%) | 0.73 ± 0.08 | 2.55 ± 0.39 * | 0.18 ± 0.06 | 0.98 ± 0.06 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toejing, P.; Sakunrangsit, N.; Pho-on, P.; Phetkong, C.; Leelahavanichkul, A.; Sridurongrit, S.; Greenblatt, M.B.; Lotinun, S. Accelerated Bone Loss in Transgenic Mice Expressing Constitutively Active TGF-β Receptor Type I. Int. J. Mol. Sci. 2023, 24, 10797. https://doi.org/10.3390/ijms241310797
Toejing P, Sakunrangsit N, Pho-on P, Phetkong C, Leelahavanichkul A, Sridurongrit S, Greenblatt MB, Lotinun S. Accelerated Bone Loss in Transgenic Mice Expressing Constitutively Active TGF-β Receptor Type I. International Journal of Molecular Sciences. 2023; 24(13):10797. https://doi.org/10.3390/ijms241310797
Chicago/Turabian StyleToejing, Parichart, Nithidol Sakunrangsit, Pinyada Pho-on, Chinnatam Phetkong, Asada Leelahavanichkul, Somyoth Sridurongrit, Matthew B. Greenblatt, and Sutada Lotinun. 2023. "Accelerated Bone Loss in Transgenic Mice Expressing Constitutively Active TGF-β Receptor Type I" International Journal of Molecular Sciences 24, no. 13: 10797. https://doi.org/10.3390/ijms241310797






