Accumulation of Renal Fibrosis in Hyperuricemia Rats Is Attributed to the Recruitment of Mast Cells, Activation of the TGF-β1/Smad2/3 Pathway, and Aggravation of Oxidative Stress
Abstract
1. Introduction
2. Results
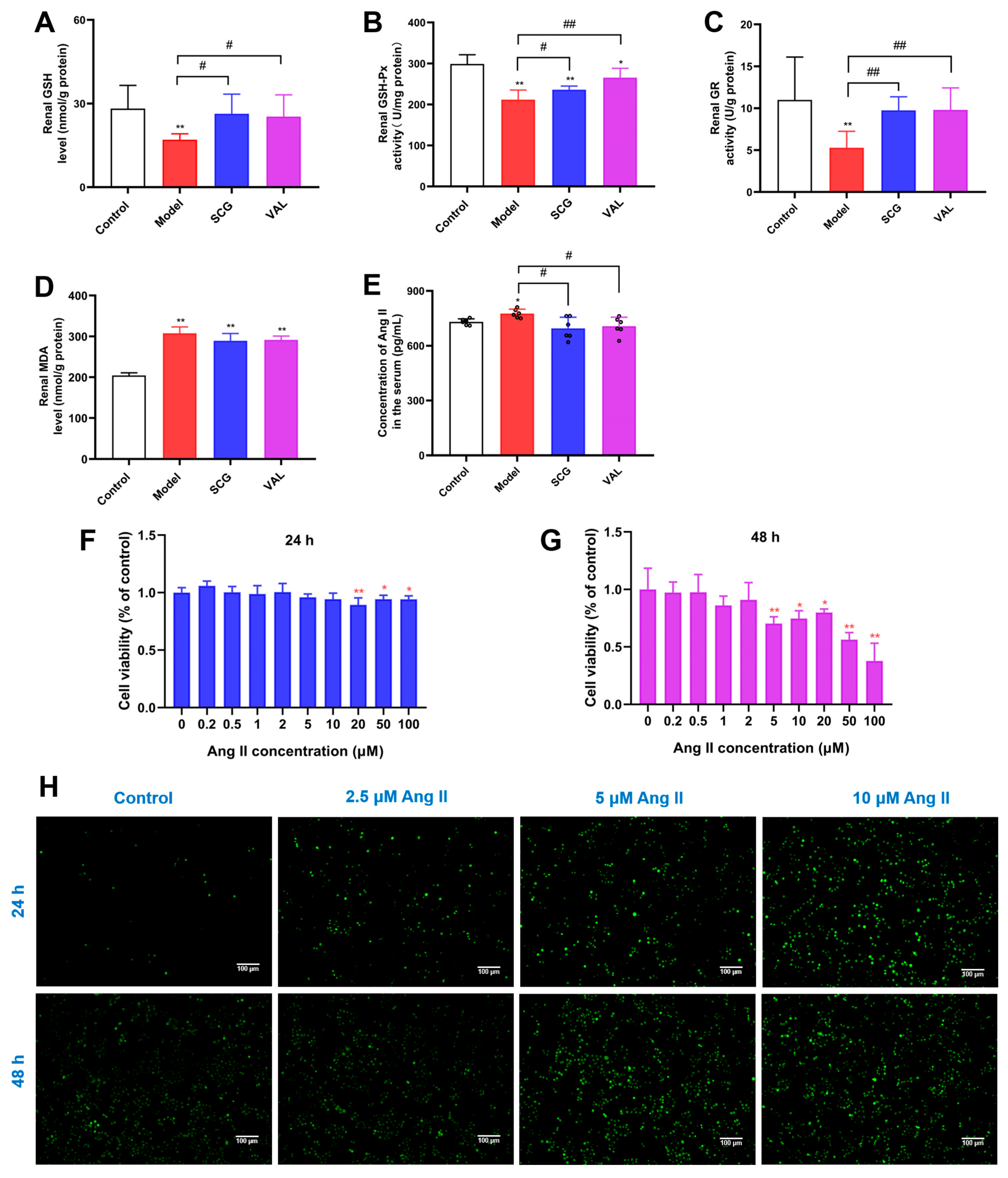
2.1. Effect of Administration of Uric Acid on the Release of Ang II from RBL-2H3 Cells
2.2. Effects of SCG or VAL on Renal Function in Hyperuricemic Rats
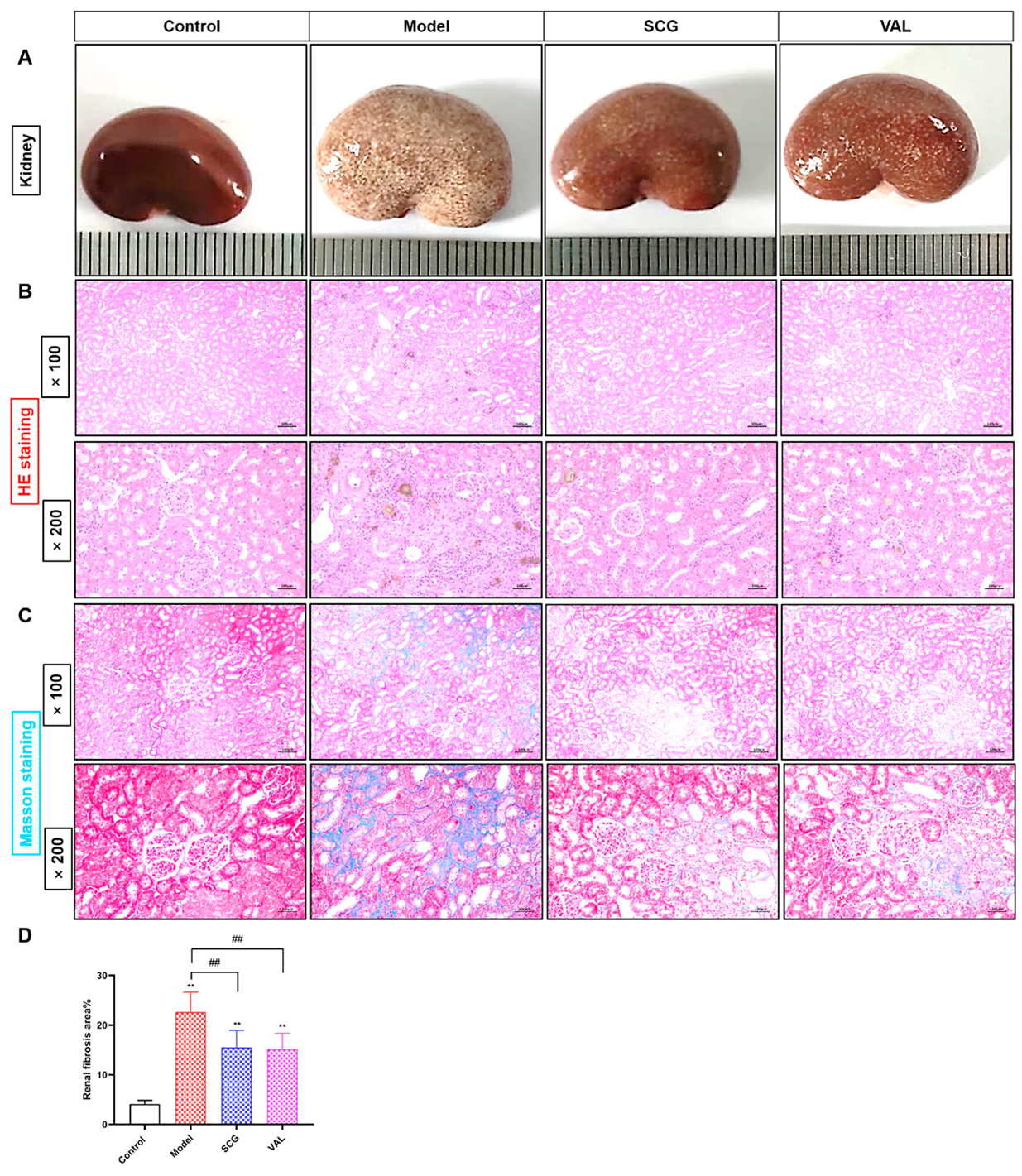
2.3. Effects of SCG or VAL on Renal Histopathology in Hyperuricemic Rats
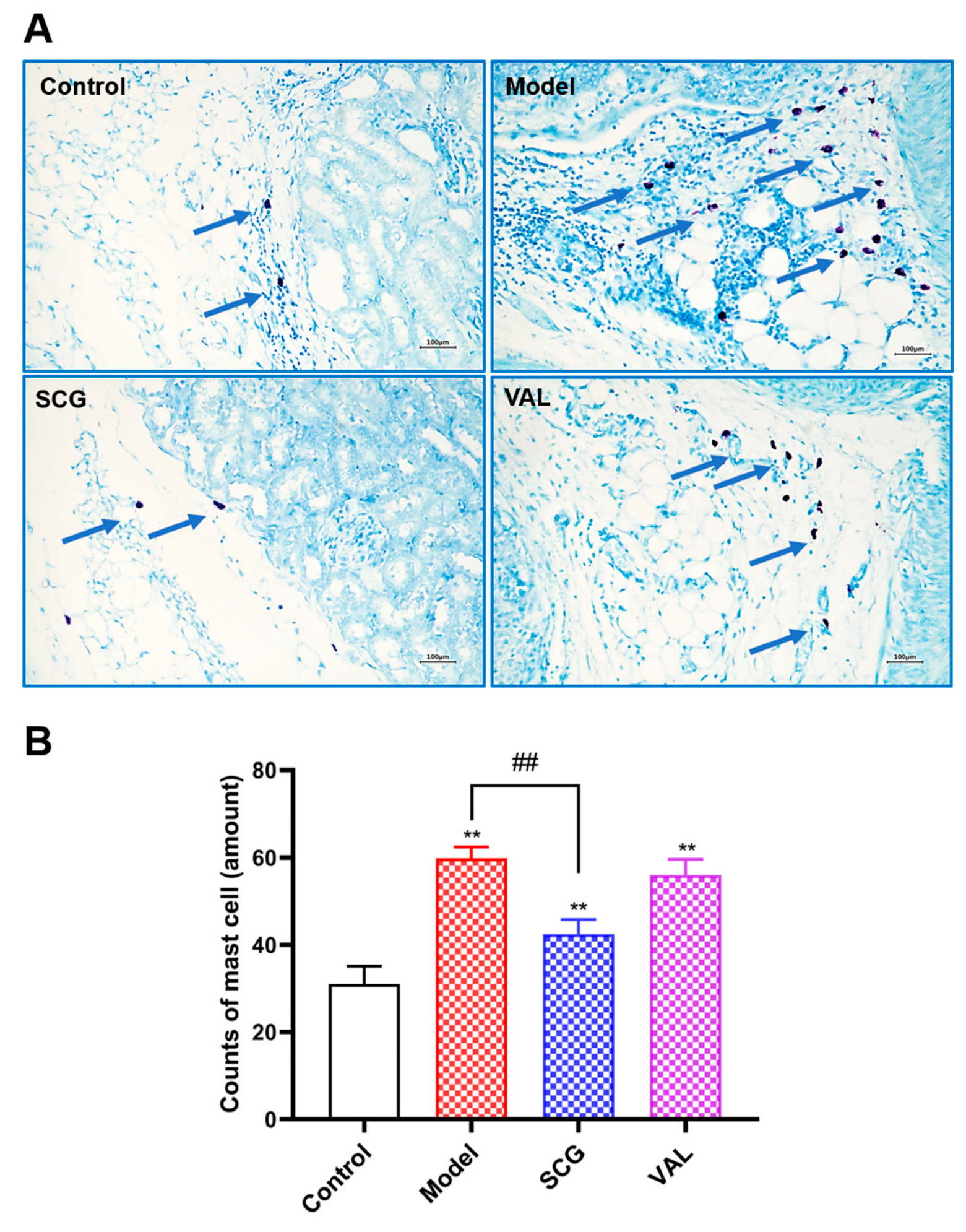
2.4. MCs Recruitment in the Kidney of Hyperuricemic Rats
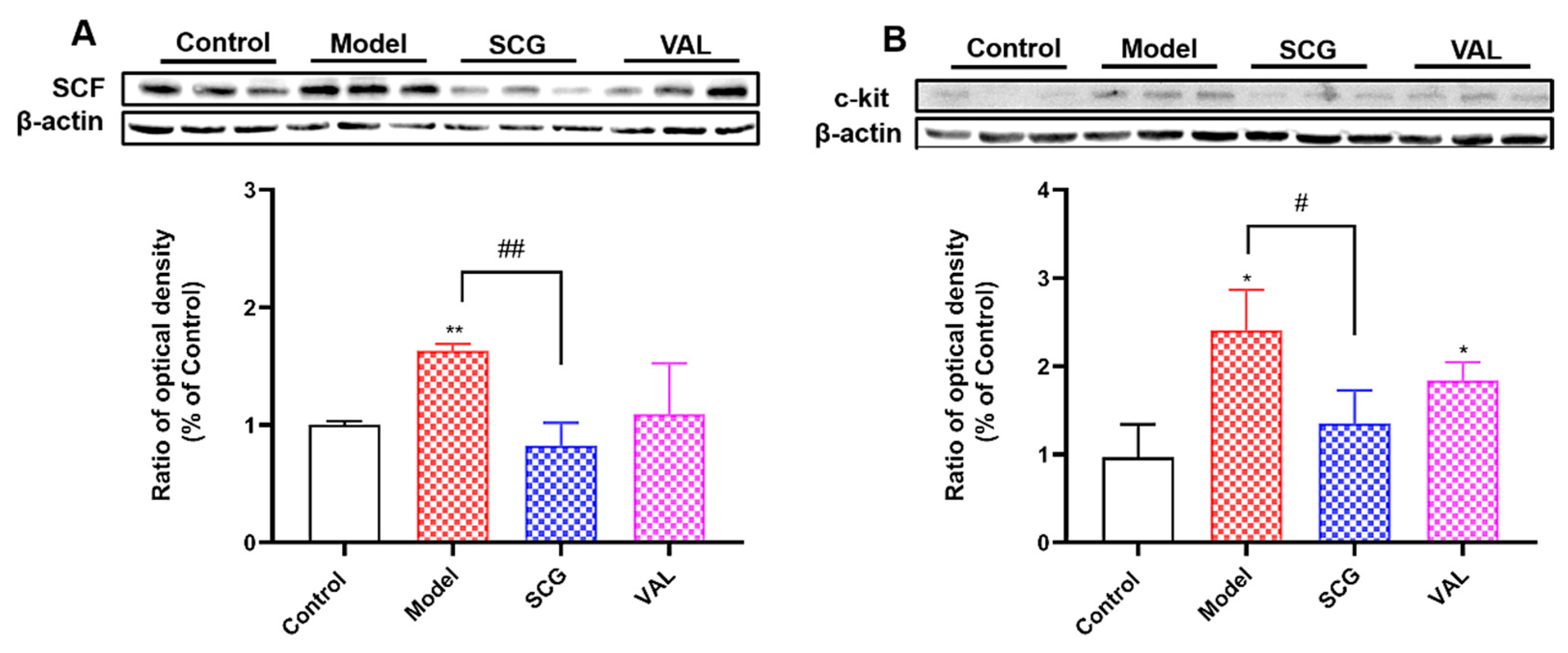
2.5. Effects of SCG or VAL on the Expression of Renal SCF/C-Kit in Hyperuricemic Rats
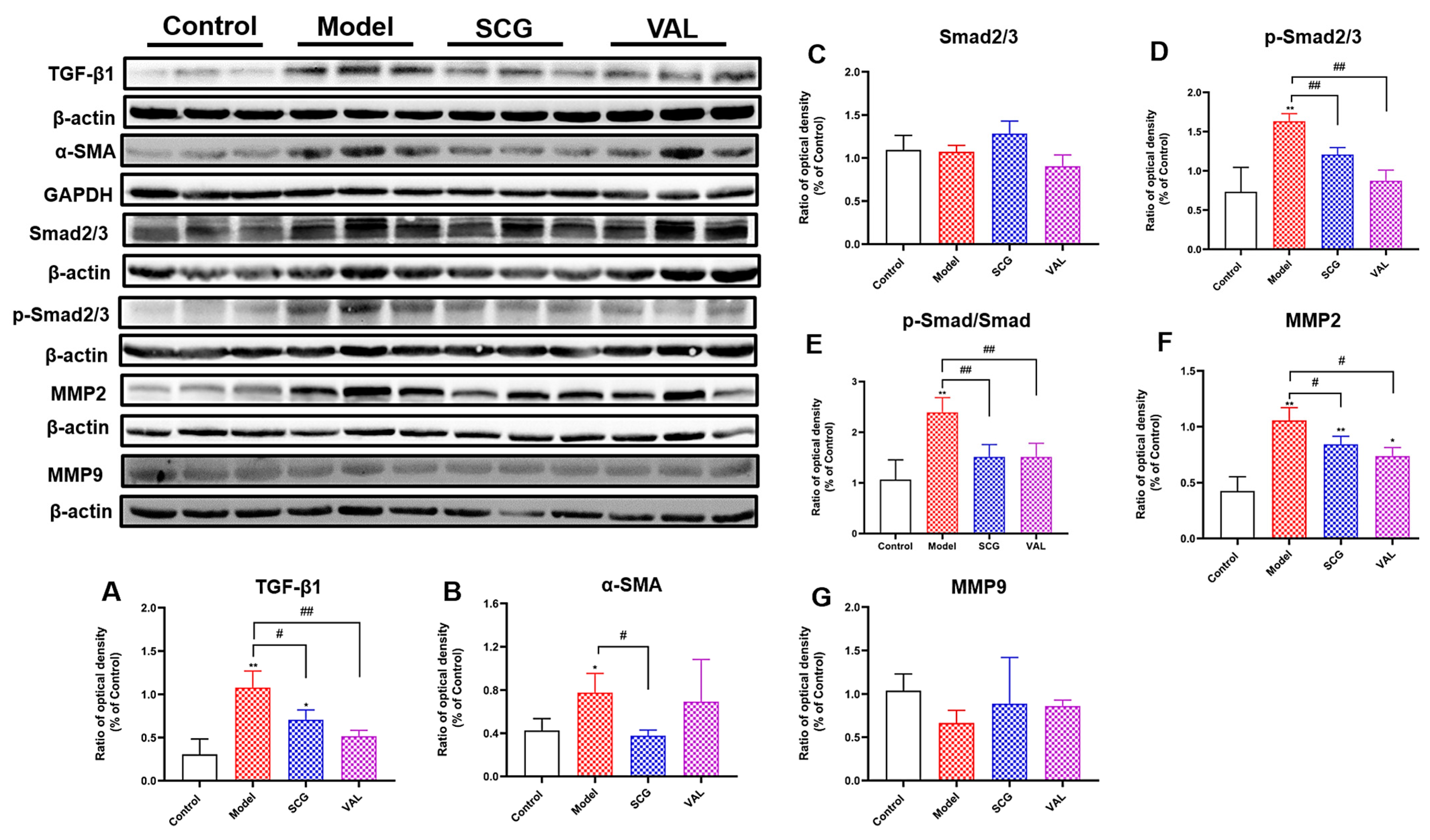
2.6. Effects of SCG or VAL on Renal Fibrosis in Hyperuricemic Rats
2.7. Effects of SCG or VAL on Mitochondria of Renal Tubular Epithelial Cells in Hyperuricemic Rats
2.8. Effects of SCG or VAL on Renal Oxidative Stress in Hyperuricemic Rats
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Biochemical Analysis
4.4. Observation of MCs
4.5. Western Blotting Analysis
4.6. Histopathological Evaluation
4.7. Observation of Mitochondrial Structure
4.8. Oxidative Stress Evaluation
4.9. Cell Culture and Cell Viability Assay
4.10. Detection of Ang II Release from Rat Serum and RBL-2H3 Cells
4.11. Detection of Reactive Oxygen Species
4.12. Statistical Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, H.-Y.; Yang, C.; Liang, D.; Liu, H.-F. Research Advances in the Mechanisms of Hyperuricemia-Induced Renal Injury. BioMed Res. Int. 2020, 2020, 5817348. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, X.; Lee, M.H.; Li, H. Vitamin C alleviates hyperuricemia nephropathy by reducing inflammation and fibrosis. J. Food Sci. 2021, 86, 3265–3276. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9221. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011, 63, 3136–3141. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, L.; Xu, T.; Liu, J.; Han, S. Association of Hyperuricemia with 10-Year Atherosclerotic Cardiovascular Disease Risk among Chinese Adults and Elders. Int. J. Environ. Res. Public Health 2022, 19, 6713. [Google Scholar] [CrossRef]
- Long, T.; Liu, L. Research Progress on the Relationship between Dietary Patterns and Hyperuricemia. Appl. Bionics Biomech. 2022, 2022, 1263–1270. [Google Scholar] [CrossRef]
- Pan, J.; Shi, M.; Li, L.; Liu, J.; Guo, F.; Feng, Y.; Ma, L.; Fu, P. Pterostilbene, a bioactive component of blueberries, alleviates renal fibrosis in a severe mouse model of hyperuricemic nephropathy. Biomed. Pharmacother. 2019, 109, 1802–1808. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol. Asp. Med. 2019, 65, 2–15. [Google Scholar] [CrossRef]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhang, P.; Wang, A.Y.; Wang, X.; Wang, L.; Li, G.; Hong, D. Hyperuricemia and its related histopathological features on renal biopsy. BMC Nephrol. 2019, 20, 95. [Google Scholar] [CrossRef]
- Dobrian, A.D.; Schriver, S.D.; Prewitt, R.L. Role of angiotensin II and free radicals in blood pressure regulation in a rat model of renal hypertension. Hypertension 2001, 38, 361–366. [Google Scholar] [CrossRef]
- Pan, X.X.; Wu, F.; Chen, X.H.; Chen, D.R.; Chen, H.J.; Kong, L.R.; Ruan, C.C.; Gao, P.J. T-cell senescence accelerates angiotensin II-induced target organ damage. Cardiovasc. Res. 2021, 117, 271–283. [Google Scholar] [CrossRef]
- Shen, Y.; Miao, N.J.; Xu, J.L.; Gan, X.X.; Xu, D.; Zhou, L.; Xue, H.; Zhang, W.; Lu, L.M. N-acetylcysteine alleviates angiotensin II-mediated renal fibrosis in mouse obstructed kidneys. Acta Pharmacol. Sin. 2016, 37, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, S.; Wang, J.; Huang, S.; Zhang, A.; Zhang, Y.; Gu, W.; Yu, X.; Jia, Z. Cilomilast Ameliorates Renal Tubulointerstitial Fibrosis by Inhibiting the TGF-beta1-Smad2/3 Signaling Pathway. Front. Med. 2020, 7, 626140. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ning, X.; Li, R.; Yang, Z.; Yang, X.; Sun, S.; Qian, Q. Signalling pathways involved in hypoxia-induced renal fibrosis. J. Cell. Mol. Med. 2017, 21, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xiao, Y.; Zhu, H.; Guo, H.; Zhou, Y.; Shentu, Y.; Zheng, C.; Chen, C.; Bai, Y. Inhibition of proliferation-linked signaling cascades with atractylenolide I reduces myofibroblastic phenotype and renal fibrosis. Biochem. Pharmacol. 2021, 183, 114344. [Google Scholar] [CrossRef]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef]
- El-Agamy, D.S. Targeting c-kit in the therapy of mast cell disorders: Current update. Eur. J. Pharmacol. 2012, 690, 1–3. [Google Scholar] [CrossRef]
- Levick, S.; Widiapradja, A. Mast Cells: Key Contributors to Cardiac Fibrosis. Int. J. Mol. Sci. 2018, 19, 231. [Google Scholar] [CrossRef]
- Yin, D.D.; Luo, J.H.; Zhao, Z.Y.; Liao, Y.J.; Li, Y. Tranilast prevents renal interstitial fibrosis by blocking mast cell infiltration in a rat model of diabetic kidney disease. Mol. Med. Rep. 2018, 17, 7356–7364. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Zhang, M.; Liu, Z. Roles of mast cells and monocyte chemoattractant protein-1 in the renal injury of obesity-related glomerulopathy. Clin. Investig. 2013, 346, 295–301. [Google Scholar] [CrossRef]
- Stanchev, S.; Landzhov, B.; Kotov, G.; Stamenov, N.; Dikov, T.; Iliev, A. The potential role of mast cells and fibroblast growth factor-2 in the development of hypertension-induced renal damage. Acta Histochem. 2020, 122, 151599. [Google Scholar] [CrossRef]
- Veerappan, A.; Reid, A.C.; O’Connor, N.; Mora, R.; Brazin, J.A.; Estephan, R.; Kameue, T.; Chen, J.; Felsen, D.; Seshan, S.V.; et al. Mast cells are required for the development of renal fibrosis in the rodent unilateral ureteral obstruction model. Am. J. Physiol. Ren. Physiol. 2012, 302, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wei, M.; Zhang, M.; Zhang, J.; Tang, F.; Wu, X. Adefovir accumulation in the renal interstitium triggers mast cell degranulation and promotes renal interstitial fibrosis. Toxicol. Lett. 2022, 359, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.A.; Gan, P.Y.; Dewage, L.; Ma, F.T.; Ooi, J.D.; O’Sullivan, K.M.; Nikolic-Paterson, D.J.; Kitching, A.R.; Holdsworth, S.R. Mast cell activation and degranulation promotes renal fibrosis in experimental unilateral ureteric obstruction. Kidney Int. 2012, 82, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.M.; Hung, Y.L.; Wang, S.J.; Tsai, Y.J.; Wu, N.L.; Liang, C.W.; Chang, D.C.; Hung, C.F. Anti-Allergic and Anti-Inflammatory Effects of Neferine on RBL-2H3 Cells. Int. J. Mol. Sci. 2021, 22, 10994. [Google Scholar] [CrossRef]
- Zeng, H.R.; Wang, B.; Zhao, Z.; Zhang, Q.; Liang, M.Y.; Yao, Y.Q.; Bian, K.; Zhang, W.R. Effects of Viola yedoensis Makino anti-itching compound on degranulation and cytokine generation in RBL-2H3 mast cells. J. Ethnopharmacol. 2016, 189, 132–138. [Google Scholar] [CrossRef]
- Ribatti, D. The Staining of Mast Cells: A Historical Overview. Int. Arch. Allergy Immunol. 2018, 176, 55–60. [Google Scholar] [CrossRef]
- Draber, P.; Halova, I.; Polakovicova, I.; Kawakami, T. Signal transduction and chemotaxis in mast cells. Eur. J. Pharmacol. 2016, 778, 11–23. [Google Scholar] [CrossRef]
- Ma, Y.; Kuang, Y.; Bo, W.; Liang, Q.; Zhu, W.; Cai, M.; Tian, Z. Exercise Training Alleviates Cardiac Fibrosis through Increasing Fibroblast Growth Factor 21 and Regulating TGF-beta1-Smad2/3-MMP2/9 Signaling in Mice with Myocardial Infarction. Int. J. Mol. Sci. 2021, 22, 12341. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, W.; Li, X.; Tang, S.; Meng, D.; Xia, W.; Wang, H.; Wu, Y.; Zhou, X.; Zhang, J. Capsaicin ameliorates renal fibrosis by inhibiting TGF-beta1-Smad2/3 signaling. Phytomedicine 2022, 100, 154067. [Google Scholar] [CrossRef] [PubMed]
- Duann, P.; Lin, P.-H. Mitochondria Damage and Kidney Disease. Adv. Exp. Med. Biol. 2017, 982, 529–551. [Google Scholar]
- Feng, J.; Chen, Z.; Liang, W.; Wei, Z.; Ding, G. Roles of Mitochondrial DNA Damage in Kidney Diseases: A New Biomarker. Int. J. Mol. Sci. 2022, 23, 15166. [Google Scholar] [CrossRef] [PubMed]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Chang, C.; Worley, B.L.; Phaeton, R.; Hempel, N. Extracellular Glutathione Peroxidase GPx3 and Its Role in Cancer. Cancers 2020, 12, 2197. [Google Scholar] [CrossRef]
- Tsikas, D. Malondialdehyde-Induced Post-Translational Modification of Human Hemoglobin. J. Proteome Res. 2023, 22, 2141–2143. [Google Scholar] [CrossRef]
- Meng, X.; Mao, Z.; Li, X.; Yang, B.; Zhou, H.; Zhong, D.; Li, M.; Jia, Y.; Wei, J. Baicalein decreases uric acid and prevents hyperuricemic nephropathy in mice. Oncotarget 2017, 8, 40305–40317. [Google Scholar] [CrossRef]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free radicals, antioxidant defense systems, and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Okami, N.; Yamada, T.; Azushima, K.; Yamaji, T.; Kinguchi, S.; Uneda, K.; Kanaoka, T.; Wakui, H.; Tamura, K. Prevention of kidney function decline using uric acid-lowering therapy in chronic kidney disease patients: A systematic review and network meta-analysis. Clin. Rheumatol. 2022, 41, 911–919. [Google Scholar] [CrossRef]
- Peng, Y.L.; Tain, Y.L.; Lee, C.T.; Yang, Y.H.; Huang, Y.B.; Wen, Y.H.; Hsu, C.N. Comparison of uric acid reduction and renal outcomes of febuxostat vs allopurinol in patients with chronic kidney disease. Sci. Rep. 2020, 10, 10734. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Meurer, S.K.; Liedtke, C.; Huber, M. Mast Cells in Liver Fibrogenesis. Cells 2019, 8, 1429. [Google Scholar] [CrossRef]
- Zheng, J.M.; Yao, G.H.; Cheng, Z.; Wang, R.; Liu, Z.H. Pathogenic role of mast cells in the development of diabetic nephropathy: A study of patients at different stages of the disease. Diabetologia 2012, 55, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Silvaa, G.E.B.; Costaa, R.S.; Ravinala, R.C.; Reisb, M.A.d.; Dantasc, M.; Coimbra, T.M. Mast cells, TGF-beta1 and alpha-SMA expression in IgA nephropathy. Dis. Mrk. 2008, 24, 181–190. [Google Scholar]
- Welker, P.; Kramer, S.; Groneberg, D.A.; Neumayer, H.H.; Bachmann, S.; Amann, K.; Peters, H. Increased mast cell number in human hypertensive nephropathy. Am. J. Physiol. Ren. Physiol. 2008, 295, F1103–F1109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Gomez, G.; Yu, S.-H.; Ryan, J.J.; Schwartz, L.B. TGF-β1 Attenuates Mediator Release and De Novo Kit Expression by Human Skin Mast Cells through a Smad-Dependent Pathway. J. Immunol. 2008, 181, 7263–7272. [Google Scholar] [CrossRef]
- Rasky, A.; Habiel, D.M.; Morris, S.; Schaller, M.; Moore, B.B.; Phan, S.; Kunkel, S.L.; Phillips, M.; Hogaboam, C.; Lukacs, N.W. Inhibition of the stem cell factor 248 isoform attenuates the development of pulmonary remodeling disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, 200–211. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, D.; Cui, S.; Yin, Z.; Huang, Z.; Zhang, Y.; Cai, G.; Chen, X.; Sun, X. Effect of youthful blood environment and its key factor SCF on renal interstitial fibrosis in elderly mice. Gerontology 2023, 69, 628–640. [Google Scholar] [CrossRef]
- Pan, J.; Shi, M.; Guo, F.; Ma, L.; Fu, P. Pharmacologic inhibiting STAT3 delays the progression of kidney fibrosis in hyperuricemia-induced chronic kidney disease. Life Sci. 2021, 285, 119946. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, W.; Peng, X.; Zhu, L.; Wang, Z.; Shen, H.; Chen, C.; Xiao, L.; Li, S.; Zhao, Y.; et al. Parthenolide alleviates peritoneal fibrosis by inhibiting inflammation via the NF-κB/ TGF-β/Smad signaling axis. Lab. Investig. 2022, 102, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wan, C.; Song, A.; Qiu, Y.; Xiong, W.; Zhang, C. Oxidative Stress and Renal Fibrosis: Mechanisms and Therapies. Adv. Exp. Med. Biol. 2019, 1165, 585–604. [Google Scholar] [PubMed]
- Cristobal-Garcia, M.; Garcia-Arroyo, F.E.; Tapia, E.; Osorio, H.; Arellano-Buendia, A.S.; Madero, M.; Rodriguez-Iturbe, B.; Pedraza-Chaverri, J.; Correa, F.; Zazueta, C.; et al. Renal oxidative stress induced by long-term hyperuricemia alters mitochondrial function and maintains systemic hypertension. Oxidative Med. Cell. Longev. 2015, 2015, 535686. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Roumeliotis, A.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Dietary Antioxidant Supplements and Uric Acid in Chronic Kidney Disease: A Review. Nutrients 2019, 11, 1911. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef] [PubMed]
- Giam, B.; Kaye, D.M.; Rajapakse, N.W. Role of Renal Oxidative Stress in the Pathogenesis of the Cardiorenal Syndrome. Heart Lung Circ. 2016, 25, 874–880. [Google Scholar] [CrossRef]
- He, P.; Li, Z.; Yue, Z.; Gao, H.; Feng, G.; Wang, P.; Huang, Y.; Luo, W.; Hong, H.; Liang, L.; et al. SIRT3 prevents angiotensin II-induced renal tubular epithelial-mesenchymal transition by ameliorating oxidative stress and mitochondrial dysfunction. Mol. Cell. Endocrinol. 2018, 460, 1–13. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Lou, A.; Wang, G.; Hu, Y.; Zhang, Y.; Huang, W.; Wang, J.; Li, Y.; Zhu, X.; et al. Alamandine attenuates hepatic fibrosis by regulating autophagy induced by NOX4-dependent ROS. Clin. Sci. 2020, 134, 853–869. [Google Scholar] [CrossRef]
- Huang, C.; Gao, J.; Wei, T.; Shen, W. Angiotensin II-Induced Erythrocyte Senescence Contributes to Oxidative Stress. Rejuvenation Res. 2022, 25, 30–38. [Google Scholar] [CrossRef]
- Xu, D.F.; Liu, Y.J.; Mao, Y.F.; Wang, Y.; Xu, C.F.; Zhu, X.Y.; Jiang, L. Elevated angiotensin II induces platelet apoptosis through promoting oxidative stress in an AT1R-dependent manner during sepsis. J. Cell. Mol. Med. 2021, 25, 4124–4135. [Google Scholar] [CrossRef]
- Zhan, H.; Huang, F.; Niu, Q.; Jiao, M.; Han, X.; Zhang, K.; Ma, W.; Mi, S.; Guo, S.; Zhao, Z. Downregulation of miR-128 Ameliorates Ang II-Induced Cardiac Remodeling via SIRT1/PIK3R1 Multiple Targets. Oxidative Med. Cell. Longev. 2021, 2021, 8889195. [Google Scholar] [CrossRef] [PubMed]
- Wakayama, K.; Shimamura, M.; Suzuki, J.; Watanabe, R.; Koriyama, H.; Akazawa, H.; Nakagami, H.; Mochizuki, H.; Isobe, M.; Morishita, R. Angiotensin II Peptide Vaccine Protects Ischemic Brain Through Reducing Oxidative Stress. Stroke 2017, 48, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Calvo, B.; Cassina, A.; Rios, N.; Peluffo, G.; Boggia, J.; Radi, R.; Rubbo, H.; Trostchansky, A. Nitro-Arachidonic Acid Prevents Angiotensin II-Induced Mitochondrial Dysfunction in a Cell Line of Kidney Proximal Tubular Cells. PLoS ONE 2016, 11, e0150459. [Google Scholar] [CrossRef] [PubMed]
- Milanesi, S.; Verzola, D.; Cappadona, F.; Bonino, B.; Murugavel, A.; Pontremoli, R.; Garibotto, G.; Viazzi, F. Uric acid and angiotensin II additively promote inflammation and oxidative stress in human proximal tubule cells by activation of toll-like receptor 4. J. Cell. Physiol. 2019, 234, 10868–10876. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tao, Y.; Xie, H.; Liu, C.; Liu, P. Fuzheng Huayu recipe, a traditional Chinese compound herbal medicine, attenuates renal interstitial fibrosis via targeting the miR-21/PTEN/AKT axis. J. Integr. Med. 2020, 18, 505–513. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, J.; Zhang, T.; He, L.; Ma, S.; Zuo, Q.; Zhang, G.; Wang, Y.; Guo, Y. Canagliflozin ameliorates epithelial-mesenchymal transition in high-salt diet-induced hypertensive renal injury through restoration of sirtuin 3 expression and the reduction of oxidative stress. Biochem. Biophys. Res. Commun. 2023, 653, 53–61. [Google Scholar] [CrossRef]
- Veerappan, A.; Reid, A.C.; Estephan, R.; O’Connor, N.; Thadani-Mulero, M.; Salazar-Rodriguez, M.; Levi, R.; Silver, R.B. Mast cell renin and a local renin-angiotensin system in the airway role in bronchoconstriction. Proc. Natl. Acad. Sci. USA 2008, 105, 1315–1320. [Google Scholar] [CrossRef]
- Zhang, X.; Agborbesong, E.; Li, X. The Role of Mitochondria in Acute Kidney Injury and Chronic Kidney Disease and Its Therapeutic Potential. Int. J. Mol. Sci. 2021, 22, 11253. [Google Scholar] [CrossRef]

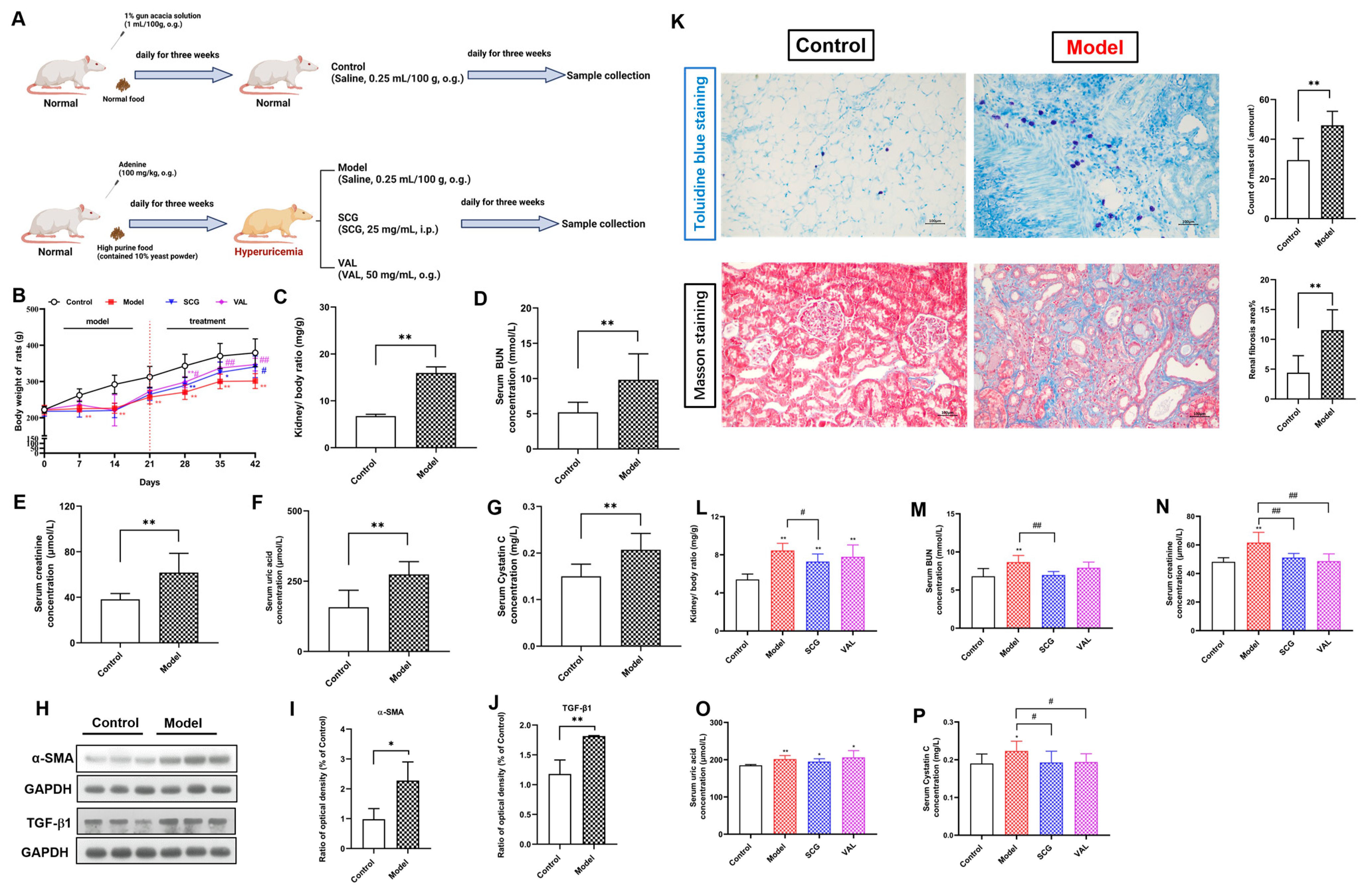

| Protocol One | Protocol Two | ||||
|---|---|---|---|---|---|
| Groups | Treatment (daily for three weeks) | Treatment (daily for three weeks) | |||
| Food | Oral gavage (o.g.) | Groups | Intraperitoneal injection (i.p.) | Oral gavage (o.g.) | |
| Control | Normal food | 1% gum acacia solution (1 mL/100 g) | Control | saline (0.25 mL/100 g) | saline (0.25 mL/100 g) |
| Model | High purine food (contained 10% yeast powder) | Adenine (100 mg/kg) | Model SCG | saline (0.25 mL/100 g) SCG (25 mg/kg) | saline (0.25 mL/100 g) saline (0.25 mL/100 g) |
| VAL | saline (0.25 mL/100 g) | VAL (50 mg/kg) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Cui, R.; Zhou, Y.; Ma, Y.; Jin, Y.; Wang, L.; Kou, W.; Wu, X. Accumulation of Renal Fibrosis in Hyperuricemia Rats Is Attributed to the Recruitment of Mast Cells, Activation of the TGF-β1/Smad2/3 Pathway, and Aggravation of Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 10839. https://doi.org/10.3390/ijms241310839
Zhang M, Cui R, Zhou Y, Ma Y, Jin Y, Wang L, Kou W, Wu X. Accumulation of Renal Fibrosis in Hyperuricemia Rats Is Attributed to the Recruitment of Mast Cells, Activation of the TGF-β1/Smad2/3 Pathway, and Aggravation of Oxidative Stress. International Journal of Molecular Sciences. 2023; 24(13):10839. https://doi.org/10.3390/ijms241310839
Chicago/Turabian StyleZhang, Mingkang, Ruirui Cui, Yan Zhou, Yanrong Ma, Yongwen Jin, Lina Wang, Wen Kou, and Xin’an Wu. 2023. "Accumulation of Renal Fibrosis in Hyperuricemia Rats Is Attributed to the Recruitment of Mast Cells, Activation of the TGF-β1/Smad2/3 Pathway, and Aggravation of Oxidative Stress" International Journal of Molecular Sciences 24, no. 13: 10839. https://doi.org/10.3390/ijms241310839
APA StyleZhang, M., Cui, R., Zhou, Y., Ma, Y., Jin, Y., Wang, L., Kou, W., & Wu, X. (2023). Accumulation of Renal Fibrosis in Hyperuricemia Rats Is Attributed to the Recruitment of Mast Cells, Activation of the TGF-β1/Smad2/3 Pathway, and Aggravation of Oxidative Stress. International Journal of Molecular Sciences, 24(13), 10839. https://doi.org/10.3390/ijms241310839






