Current Understanding on Why Ovarian Cancer Is Resistant to Immune Checkpoint Inhibitors
Abstract
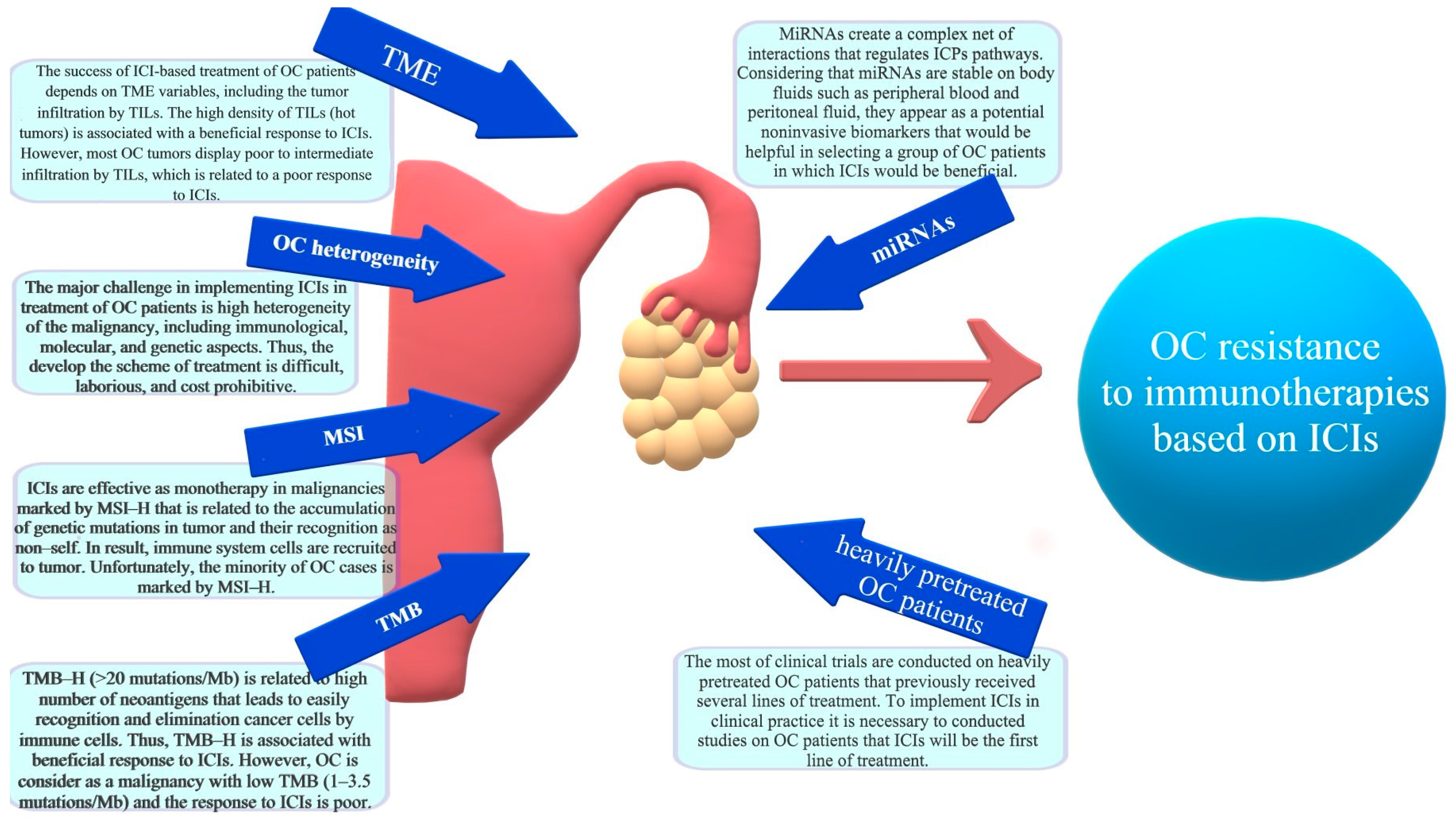
:1. Heterogeneity and Prognosis of Ovarian Cancer (OC)
2. Treatment of Ovarian Cancer
3. Clinical Trials in Ovarian Cancer
4. Mechanisms of Immunotherapy Resistance in Ovarian Cancer
4.1. Significance of Tumor Infiltrating Lymphocytes (TILs)
4.2. Dual Role of Tumor-Associated Macrophages (TAMs)
4.3. Significance of Microsatellite Instability (MSI)
4.4. Significance of Tumor Mutation Burden
4.5. The Regulation of ICPs by microRNA Net
5. Hyperprogression
6. Pseudoprogression
7. Future Directions
7.1. Double and Triple ICP Blockade
7.2. Vaccines
7.3. Machine Learning as a Hope for Ovarian Cancer Patients
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3′-UTR | 3′-untranslated region |
| ABCB1 | adenosine 5′-triphosphate–binding cassette subfamily B member 1 |
| AFP | alpha-fetoprotein |
| AI | artificial intelligence |
| ALPK2 | alpha kinase 2 |
| ALT | alanine aminotransferase |
| Ang-2 | angiopoietin 2 |
| APCs | antigen-presenting cells |
| ARID1A | AT-rich interactive domain-containing protein 1A |
| ASA | acetylsalicylic acid |
| AST | aspartate aminotransferase |
| BRAF | B-Raf proto-oncogene, serine/threonine kinase |
| BRCA | breast cancer gene |
| BTC | biliary tract cancers |
| BUB1 | budding uninhibited by benzimidazoles 1 |
| BUB1B | mitotic checkpoint serine/threonine-protein kinase BUB1 beta |
| CA-125 | cancer antigen 125 |
| CA19-9 | carbohydrate antigen 19-9 |
| CA72-4 | carbohydrate antigen 72-4 |
| CAMK1G | calcium/calmodulin-dependent protein kinase 1G |
| CCC | clear cell carcinomas |
| CCL2 | chemokine (C-C motif) ligand 2 |
| CCL22 | C-C motif chemokine 22 |
| CD | cluster of differentiation |
| CEA | carcinoembryonic antigen |
| cfDNA | cell-free DNA |
| cHL | classical Hodgkin Lymphoma |
| CRC | colorectal cancer |
| cSCC | cutaneous squamous cell carcinoma |
| CSF-1 | colony stimulating factor 1 |
| CT | computer tomography |
| CTLA-4 | cytotoxic T-lymphocyte-associated antigen 4 |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| DCNN | deep convolutional neural network |
| DCs | dendritic cells |
| DEG | differentially expressed gene |
| dMMR | deficient mismatch repair |
| DNAM-1 | DNAX accessory molecule-1 |
| DPYSL2 | dihydropyrimidinase like 2 |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| ER | estrogen receptor |
| ERK | extracellular signal-regulated kinase |
| ESMO | European Society for Medical Oncology |
| FcAb | antigen-binding Fc fragment |
| FDA | Food and Drug Administration |
| FIGO | International Federation of Gynecology and Obstetrics |
| FOLR1 | folate receptor alpha |
| FOXM1 | forkhead box M1 |
| FRα | folate receptor alpha |
| GBM | Gradient Boosting Machines |
| GEO | Gene Expression Omnibus |
| GTEx | The Genotype-Tissue Expression |
| HCC | hepatocellular carcinoma |
| HE4 | human epididymis protein 4 |
| HGF | hepatocyte growth factor |
| HGSOC | high-grade serous ovarian carcinoma |
| HLA | human leukocyte antigen |
| HLA-DOB | histocompatibility antigen, DO beta chain |
| HNSCC | head and neck squamous cell carcinoma |
| HOXA13 | homeobox A13 |
| HPD | hyperprogressive disease |
| ICI | immune checkpoint inhibitor |
| ICP | immune checkpoint |
| IDH1 | isocitrate dehydrogenase 1 |
| IFN-γ | interferon γ |
| IL | interleukin |
| INAVA | innate immunity activator |
| irAEs | immune-related adverse events |
| IRF9 | interferon regulatory factor 9 |
| ISG20 | interferon-stimulated gene of 20 kDa protein |
| JAK-STAT | Janus kinase/signal transducers and activators of transcription |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| LAG-3 | lymphocyte activation gene 3 |
| LDH | lactate dehydrogenase |
| LGBM | light gradient boosting machine |
| mAbs | monoclonal antibodies |
| MAP | mitogen-activated protein |
| MAPK | mitogen-activated protein kinase |
| Mb | megabase |
| MCC | Merkel cell carcinoma |
| MDM2 | mouse double minute 2 |
| MDM4 | mouse double minute 4 |
| MDSC | myeloid-derived suppressor cells |
| MGMT | O6-methylguanine-DNA methyltransferase methylated |
| miRNA | microRNA |
| MLH1 | MutL homolog 1 |
| MMR | mismatch repair |
| MO/MA | monocytes/macrophages |
| MSH2 | MutS homolog 2 |
| MSH6 | MutS homolog 6 |
| MSI | microsatellite instability |
| MSI-H | microsatellite instability-high |
| MSI-H | high microsatellite instability |
| NCCN | National Comprehensive Cancer Network |
| ncRNAs | non-coding RNAs |
| NK cell | natural killer cell |
| NSCLC | non-small-cell lung cancer |
| OC | ovarian cancer |
| OCDC | whole-tumor lysate-pulsed dendritic cell vaccine |
| OS | overall survival |
| PARPi | poly(ADP-ribose) polymerase inhibitor |
| PD-1 | Programmed cell death receptor 1 |
| PDCD1 | Programmed Cell Death 1 |
| PDCD4 | programmed cell death 4 |
| PDE8B | phosphodiesterase 8B |
| PD-L1 | Programmed death-ligand 1 |
| PD-L2 | Programmed death-ligand 2 |
| PFS | progression-free survival |
| PI3K/Akt | phosphoinositide 3-kinase/protein kinase B |
| PIK3CA | phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α |
| PLD | pegylated liposomal doxorubicin |
| PMLBCL | primary mediastinal large B-cell lymphoma |
| PMS2 | PMS1 homolog 2 |
| PR | progesterone receptor |
| PSAT1 | phosphoserine aminotransferase 1 |
| PTEN | phosphatase and tensin homolog deleted on chromosome ten |
| RCC | renal cell carcinoma |
| RF | random forest |
| rRNA | ribosomal RNA |
| SCLC | small cell lung cancer |
| SFRP1 | secreted frizzled-related protein 1 |
| SHG | second-harmonic generation |
| TAM | Tumor-associated macrophage |
| TCGA | The Cancer Genome Atlas |
| TCR | T cell receptor |
| TGF-β | transforming growth 276 factor β |
| TIDE | Tumor Immune Dysfunction and Exclusion algorithm |
| TIGIT | T cell immunoglobulin and ITIM domain |
| TIIClnc | tumor-infiltrating immune cell-associated long noncoding ribonucleic acids |
| TIL | tumor-infiltrating lymphocytes |
| TIM-3 | T cell immunoglobulin, mucin domain-containing protein 3 |
| TMB | tumor mutational burden |
| TMB-H | high tumor mutational burden |
| TME | tumor microenvironment |
| TMEM139 | transmembrane protein 139 |
| TNF-α | tumor necrosis factor α |
| TP53 | Tumor protein P53 |
| TPIV200 | a multi-epitope anti-folate receptor vaccine |
| TREM2 | triggering receptor expressed on myeloid cells 2 |
| tRNA | transfer RNA |
| UBR5 | ubiquitin 288 protein ligase E3 component n-recognin 5 |
| USP51 | ubiquitin specific peptidase |
| VEGF | vascular endothelial growth factor |
| VEGFi | vascular endothelial growth factor inhibitor |
| WHO | World Health Organization |
| XRCC1 | X-ray repair cross-complementing gene |
References
- Cancer (IARC), T.I.A. for R. on Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 12 February 2022).
- Bonifácio, V.D.B. Ovarian Cancer Biomarkers: Moving Forward in Early Detection. In Tumor Microenvironment: The Main Driver of Metabolic Adaptation; Serpa, J., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; pp. 355–363. ISBN 978-3-030-34025-4. [Google Scholar]
- Nebgen, D.R.; Lu, K.H.; Bast, R.C. Novel Approaches to Ovarian Cancer Screening. Curr. Oncol. Rep. 2019, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Terp, S.K.; Stoico, M.P.; Dybkær, K.; Pedersen, I.S. Early Diagnosis of Ovarian Cancer Based on Methylation Profiles in Peripheral Blood Cell-Free DNA: A Systematic Review. Clin. Epigenetics 2023, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, A.; Skiba, W.; Suszczyk, D.; Kuryło, W.; Jakubowicz-Gil, J.; Paduch, R.; Wertel, I. The Dual Blockade of the TIGIT and PD-1/PD-L1 Pathway as a New Hope for Ovarian Cancer Patients. Cancers 2022, 14, 5757. [Google Scholar] [CrossRef] [PubMed]
- Kossaï, M.; Leary, A.; Scoazec, J.-Y.; Genestie, C. Ovarian Cancer: A Heterogeneous Disease. Pathobiology 2018, 85, 41–49. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S.; Young, R.H. WHO Classification of Tumours of Female Reproductive Organs; IARC Press: Lyon, France, 2014; ISBN 978-92-832-2435-8. [Google Scholar]
- Awada, A.; Ahmad, S.; McKenzie, N.D.; Holloway, R.W. Immunotherapy in the Treatment of Platinum-Resistant Ovarian Cancer: Current Perspectives. Onco Targets Ther. 2022, 15, 853–866. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.-M. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am. J. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef] [Green Version]
- Shih, I.-M.; Kurman, R.J. Ovarian Tumorigenesis: A Proposed Model Based on Morphological and Molecular Genetic Analysis. Am. J. Pathol. 2004, 164, 1511–1518. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Q.; Xu, M.; Liu, D.; Yao, S.; Chen, M. Current Advances in PD-1/PD-L1 Blockade in Recurrent Epithelial Ovarian Cancer. Front. Immunol. 2022, 13, 901772. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, X.; Li, S.; Wang, S.; Sun, H.; Yang, M. Identification of Novel Immunologic Checkpoint Gene Prognostic Markers for Ovarian Cancer. J. Oncol. 2022, 2022, 8570882. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Konishi, I. Immune Checkpoint Inhibition in Ovarian Cancer. Int. Immunol. 2016, 28, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Terlikowska, K.M.; Dobrzycka, B.; Terlikowski, S.J. Chimeric Antigen Receptor Design and Efficacy in Ovarian Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 3495. [Google Scholar] [CrossRef] [PubMed]
- Commissioner of the U.S. Food and Drug Administration. Available online: https://www.fda.gov/home (accessed on 4 November 2022).
- Rutten, M.J.; Leeflang, M.M.G.; Kenter, G.G.; Mol, B.W.J.; Buist, M. Laparoscopy for Diagnosing Resectability of Disease in Patients with Advanced Ovarian Cancer. Cochrane Database Syst. Rev. 2014, 2014, CD009786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westergaard, M.C.W.; Milne, K.; Pedersen, M.; Hasselager, T.; Olsen, L.R.; Anglesio, M.S.; Borch, T.H.; Kennedy, M.; Briggs, G.; Ledoux, S.; et al. Changes in the Tumor Immune Microenvironment during Disease Progression in Patients with Ovarian Cancer. Cancers 2020, 12, 3828. [Google Scholar] [CrossRef]
- Yang, C.; Xia, B.-R.; Zhang, Z.-C.; Zhang, Y.-J.; Lou, G.; Jin, W.-L. Immunotherapy for Ovarian Cancer: Adjuvant, Combination, and Neoadjuvant. Front. Immunol. 2020, 11, 2595. [Google Scholar] [CrossRef]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial Ovarian Cancer: Evolution of Management in the Era of Precision Medicine. CA A Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef] [Green Version]
- Świderska, J.; Kozłowski, M.; Kwiatkowski, S.; Cymbaluk-Płoska, A. Immunotherapy of Ovarian Cancer with Particular Emphasis on the PD-1/PDL-1 as Target Points. Cancers 2021, 13, 6063. [Google Scholar] [CrossRef]
- Liu, X.; Hou, M.; Liu, Y. TIGIT, A Novel Therapeutic Target for Tumor Immunotherapy. Immunol. Investig. 2017, 46, 172–182. [Google Scholar] [CrossRef]
- Pawłowska, A.; Suszczyk, D.; Okła, K.; Barczyński, B.; Kotarski, J.; Wertel, I. Immunotherapies Based on PD-1/PD-L1 Pathway Inhibitors in Ovarian Cancer Treatment. Clin. Exp. Immunol. 2019, 195, 334–344. [Google Scholar] [CrossRef] [Green Version]
- Maiorano, B.A.; Maiorano, M.F.P.; Lorusso, D.; Maiello, E. Ovarian Cancer in the Era of Immune Checkpoint Inhibitors: State of the Art. and Future Perspectives. Cancers 2021, 13, 4438. [Google Scholar] [CrossRef]
- Doo, D.W.; Norian, L.A.; Arend, R.C. Checkpoint Inhibitors in Ovarian Cancer: A Review of Preclinical Data. Gynecol. Oncol. Rep. 2019, 29, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Chardin, L.; Leary, A. Immunotherapy in Ovarian Cancer: Thinking Beyond PD-1/PD-L1. Front. Oncol. 2021, 11, 795547. [Google Scholar] [CrossRef] [PubMed]
- Agyemang, A.F.; Lele, S. The Use of Immunotherapy for Treatment of Gynecologic Malignancies. In Ovarian Cancer; Lele, S., Ed.; Exon Publications: Brisbane, Australia, 2022; ISBN 978-0-645-33208-7. [Google Scholar]
- Ning, F.; Cole, C.B.; Annunziata, C.M. Driving Immune Responses in the Ovarian Tumor Microenvironment. Front. Oncol. 2020, 10, 604084. [Google Scholar] [CrossRef]
- Cassar, E.; Kartikasari, A.E.R.; Plebanski, M. Regulatory T Cells in Ovarian Carcinogenesis and Future Therapeutic Opportunities. Cancers 2022, 14, 5488. [Google Scholar] [CrossRef] [PubMed]
- Majidpoor, J.; Mortezaee, K. The Efficacy of PD-1/PD-L1 Blockade in Cold Cancers and Future Perspectives. Clin. Immunol. 2021, 226, 108707. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Lee, S.; Cho, H.-W.; Min, K.-J.; Hong, J.-H.; Song, J.-Y.; Lee, J.-K.; Lee, N.-W. Application of Immune Checkpoint Inhibitors in Gynecological Cancers: What Do Gynecologists Need to Know before Using Immune Checkpoint Inhibitors? Int. J. Mol. Sci. 2023, 24, 974. [Google Scholar] [CrossRef]
- Revythis, A.; Limbu, A.; Mikropoulos, C.; Ghose, A.; Sanchez, E.; Sheriff, M.; Boussios, S. Recent Insights into PARP and Immuno-Checkpoint Inhibitors in Epithelial Ovarian Cancer. Int. J. Environ. Res. Public Health 2022, 19, 8577. [Google Scholar] [CrossRef]
- Xu, S.; Tao, Z.; Hai, B.; Liang, H.; Shi, Y.; Wang, T.; Song, W.; Chen, Y.; OuYang, J.; Chen, J.; et al. MiR-424(322) Reverses Chemoresistance via T-Cell Immune Response Activation by Blocking the PD-L1 Immune Checkpoint. Nat. Commun. 2016, 7, 11406. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, J.; Dong, K.; Lin, F.; Long, M.; Ouyang, Y.; Wei, J.; Chen, X.; Weng, Y.; He, T.; et al. Tumor Suppressor MiR-34a Targets PD-L1 and Functions as a Potential Immunotherapeutic Target in Acute Myeloid Leukemia. Cell. Signal. 2015, 27, 443–452. [Google Scholar] [CrossRef]
- Chen, J.; Kang, S.; Wu, J.; Zhao, J.; Si, W.; Sun, H.; Li, Y. CTLA-4 Polymorphism Contributes to the Genetic Susceptibility of Epithelial Ovarian Cancer. J. Obstet. Gynaecol. Res. 2022, 48, 1240–1247. [Google Scholar] [CrossRef]
- Siminiak, N.; Czepczyński, R.; Zaborowski, M.P.; Iżycki, D. Immunotherapy in Ovarian Cancer. Arch. Immunol. Ther. Exp. 2022, 70, 19. [Google Scholar] [CrossRef] [PubMed]
- Frelaut, M.; du Rusquec, P.; de Moura, A.; Le Tourneau, C.; Borcoman, E. Pseudoprogression and Hyperprogression as New Forms of Response to Immunotherapy. BioDrugs 2020, 34, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [Green Version]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [Green Version]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Cascio, S.; Chandler, C.; Zhang, L.; Sinno, S.; Gao, B.; Onkar, S.; Bruno, T.C.; Vignali, D.A.A.; Mahdi, H.; Osmanbeyoglu, H.U.; et al. Cancer-Associated MSC Drive Tumor Immune Exclusion and Resistance to Immunotherapy, Which Can Be Overcome by Hedgehog Inhibition. Sci. Adv. 2021, 7, eabi5790. [Google Scholar] [CrossRef]
- Luyckx, M.; Squifflet, J.L.; Bruger, A.M.; Baurain, J.F. Recurrent High Grade Serous Ovarian Cancer Management. In Ovarian Cancer; Lele, S., Ed.; Exon Publications: Brisbane, Australia, 2022; Chapter 6; pp. 87–105. [Google Scholar] [CrossRef]
- Hudry, D.; Le Guellec, S.; Meignan, S.; Bécourt, S.; Pasquesoone, C.; El Hajj, H.; Martínez-Gómez, C.; Leblanc, É.; Narducci, F.; Ladoire, S. Tumor-Infiltrating Lymphocytes (TILs) in Epithelial Ovarian Cancer: Heterogeneity, Prognostic Impact, and Relationship with Immune Checkpoints. Cancers 2022, 14, 5332. [Google Scholar] [CrossRef]
- Shen, J.; Liu, T.; Bei, Q.; Xu, S. Comprehensive Landscape of Ovarian Cancer Immune Microenvironment Based on Integrated Multi-Omics Analysis. Front. Oncol. 2021, 11, 2180. [Google Scholar] [CrossRef] [PubMed]
- McHann, M.C.; Blanton, H.L.; Guindon, J. Role of sex hormones in modulating breast and ovarian cancer associated pain. Mol. Cell. Endocrinol. 2021, 533, 111320. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Wang, Y.; Zhao, X.; Qi, X. Hormone Therapy for Ovarian Cancer: Emphasis on Mechanisms and Applications (Review). Oncol. Rep. 2021, 46, 223. [Google Scholar] [CrossRef] [PubMed]
- Anbarasu, S.; Anbarasu, A. Cancer-Biomarkers Associated with Sex Hormone Receptors and Recent Therapeutic Advancements: A Comprehensive Review. Med. Oncol. 2023, 40, 171. [Google Scholar] [CrossRef]
- Langdon, S.P.; Herrington, C.S.; Hollis, R.L.; Gourley, C. Estrogen Signaling and Its Potential as a Target for Therapy in Ovarian Cancer. Cancers 2020, 12, 1647. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Andersen, C.L.; Sikora, M.J.; Boisen, M.M.; Ma, T.; Christie, A.; Tseng, G.; Park, Y.; Luthra, S.; Chandran, U.; Haluska, P.; et al. Active Estrogen Receptor-Alpha Signaling in Ovarian Cancer Models and Clinical Specimens. Clin. Cancer Res. 2017, 23, 3802–3812. [Google Scholar] [CrossRef] [Green Version]
- Gjorgoska, M.; Rižner, T.L. Estrogens and the Schrödinger’s Cat in the Ovarian Tumor Microenvironment. Cancers 2021, 13, 5011. [Google Scholar] [CrossRef]
- KEYTRUDA® (Pembrolizumab)—Official Site. Available online: https://www.keytruda.com/ (accessed on 17 April 2023).
- OPDIVO® (Nivolumab). Available online: https://www.opdivo.com/ (accessed on 17 April 2023).
- LIBTAYO® (Cemiplimab-Rwlc): Official Patient Website. Available online: https://www.libtayo.com/ (accessed on 24 April 2023).
- BAVENCIO® (Avelumab)|For Healthcare Professionals. Available online: https://www.bavencio.com/en_US/hcp.html (accessed on 17 April 2023).
- TECENTRIQ® (Atezolizumab) HCP|Efficacy, Safety, PI & MOA. Available online: https://www.tecentriq-hcp.com/ (accessed on 17 April 2023).
- Immunotherapy for BTC, UHCC, NSCLC & ES-SCLC—IMFINZI® (Durvalumab). Available online: https://www.imfinzi.com/ (accessed on 17 April 2023).
- EMA Imjudo. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/imjudo (accessed on 17 April 2023).
- YERVOY®(Ipilimumab)|Consumer|Gateway. Available online: https://www.yervoy.com/ (accessed on 17 April 2023).
- Kähler, K.C.; Hassel, J.C.; Heinzerling, L.; Loquai, C.; Thoms, K.-M.; Ugurel, S.; Zimmer, L.; Gutzmer, R.; for the committee on “Cutaneous Adverse Events“ of the German Working Group for Dermatological Oncology (Arbeitsgemeinschaft Dermatologische Onkologie, ADO). Side Effect Management during Immune Checkpoint Blockade Using CTLA-4 and PD-1 Antibodies for Metastatic Melanoma—An Update. J. Der Dtsch. Dermatol. Ges. 2020, 18, 582–609. [Google Scholar] [CrossRef] [PubMed]
- Hassel, J.C.; Heinzerling, L.; Aberle, J.; Bähr, O.; Eigentler, T.K.; Grimm, M.-O.; Grünwald, V.; Leipe, J.; Reinmuth, N.; Tietze, J.K.; et al. Combined Immune Checkpoint Blockade (Anti-PD-1/Anti-CTLA-4): Evaluation and Management of Adverse Drug Reactions. Cancer Treat. Rev. 2017, 57, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.J.; Sundar, R.; Lim, J.S.J. Immune Checkpoint Inhibitor Combinations—Current and Emerging Strategies. Br. J. Cancer 2023, 128, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Xu, Y.; Chen, X.; Zheng, L. Advances in the Application of Immune Checkpoint Inhibitors in Gynecological Tumors. Int. Immunopharmacol. 2023, 117, 109774. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO Consensus Conference Recommendations on Ovarian Cancer: Pathology and Molecular Biology, Early and Advanced Stages, Borderline Tumours and Recurrent Disease†. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [Green Version]
- Guidelines Detail. Available online: https://www.nccn.org/guidelines/guidelines-detail (accessed on 17 April 2023).
- Heo, Y.-A. Mirvetuximab Soravtansine: First Approval. Drugs 2023, 83, 265–273. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration: FDA Grants Accelerated Approval to Mirvetuximab Soravtansine-Gynx for FRα Positive, Platinum-Resistant Epithelial Ovarian, Fallopian Tube, or Peritoneal Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-elahere-mirvetuximab-soravtansine-gynx-fra-positive-platinum (accessed on 4 June 2023).
- Home—ClinicalTrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/home (accessed on 25 February 2022).
- Grupo Español de Investigación en Cáncer de Ovario A Phase III Randomized, Double-Blinded Trial of Platinum-Based Chemotherapy with or without Atezolizumab Followed by Niraparib Maintenance with or without Atezolizumab in Patients with Recurrent Ovarian, Tubal or Peritoneal Cancer and Platinum Treatment-Free. Interval (TFIp) >6 Months. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03598270 (accessed on 3 June 2023).
- Clovis Oncology, Inc. ATHENA (A Multicenter, Randomized, Double-Blind, Placebo- Controlled Phase 3 Study in Ovarian Cancer Patients Evaluating Rucaparib and Nivolumab as Maintenance Treatment Following Response to Front-Line Platinum-Based Chemotherapy). 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT03522246 (accessed on 3 June 2023).
- Merck Sharp & Dohme LLC. A Randomized Phase 3, Double-Blind Study of Chemotherapy with or without Pembrolizumab Followed by Maintenance with Olaparib or Placebo for the First-Line Treatment of BRCA Non-Mutated Advanced Epithelial Ovarian Cancer (EOC) (KEYLYNK-001/ENGOT-Ov43/GOG-3036). 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT03740165 (accessed on 3 June 2023).
- Tesaro, Inc. A Randomized, Double-Blind, Phase 3 Comparison of Platinum-Based Therapy with TSR-042 and Niraparib Versus Standard of Care Platinum-Based Therapy as First-Line Treatment of Stage III or IV Nonmucinous Epithelial Ovarian Cancer. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT03602859 (accessed on 3 June 2023).
- Merck Sharp & Dohme LLC. A Phase 3, Randomized, Double-Blind Study of Pembrolizumab Versus Placebo in Combination With Paclitaxel With or Without Bevacizumab for the Treatment of Platinum-Resistant Recurrent Ovarian Cancer (KEYNOTE-B96/ENGOT-Ov65). 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05116189 (accessed on 3 June 2023).
- AGO Research GmbH. Atezolizumab in Combination with Bevacizumab and Chemotherapy Versus Bevacizumab and Chemotherapy in Recurrent Ovarian Cancer—A Randomized Phase III Trial. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03353831 (accessed on 3 June 2023).
- Pfizer. A Phase 3, Multicenter, Randomized, Open-Label Study of Avelumab (MSB0010718C) Alone or in Combination with Pegylated Liposomal Doxorubicin Versus Pegylated Liposomal Doxorubicin alone in Patients with Platinum-Resistant/Refractory Ovarian Cancer. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02580058 (accessed on 3 June 2023).
- Pfizer. A Randomized, Open-Label, Multicenter, Phase 3 Study to Evaluate the Efficacy and Safety of Avelumab in Combination with Chemotherapy Followed by Maintenance Therapy of Avelumab in Combination with the Poly (Adenosine Diphosphate [ADP]-Ribose) Polymerase (PARP) Inhibitor Talazoparib in Patients with Previously Untreated Advanced Ovarian Cancer (Javelin Ovarian PARP100). 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT03642132 (accessed on 3 June 2023).
- Pfizer. A Randomized, Open-Label, Multicenter, Phase 3 Study to Evaluate the Efficacy and Safety of Avelumab (MSB0010718C) in Combination with and/or Following Chemotherapy in Patients with Previously Untreated Epithelial Ovarian Cancer Javelin Ovarian 100. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT02718417 (accessed on 3 June 2023).
- Hoffmann-La Roche. A Phase III, Multicenter, Randomized, Study of Atezolizumab Versus Placebo Administered in Combination with Paclitaxel, Carboplatin, and Bevacizumab to Patients with Newly-Diagnosed Stage III or Stage IV Ovarian, Fallopian Tube, or Primary Peritoneal Cancer. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT03038100 (accessed on 3 June 2023).
- ARCAGY/GINECO Group. A Randomized, Double-Blinded, Phase III Study of Atezolizumab Versus Placebo in Patients with Late Relapse of Epithelial Ovarian, Fallopian Tube, or Peritoneal Cancer Treated by Platinum-Based Chemotherapy and Bevacizumab. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT02891824 (accessed on 3 June 2023).
- National Cancer Institute (NCI). A Randomized, Phase II/III Study of Pegylated Liposomal Doxorubicin and CTEP-Supplied Atezolizumab Versus Pegylated Liposomal Doxorubicin, CTEP-Supplied Bevacizumab and CTEP-Supplied Atezolizumab Versus Pegylated Liposomal Doxorubicin and CTEP-Supplied Bevacizumab in Platinum Resistant Ovarian Cancer. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT02839707 (accessed on 3 June 2023).
- Second Affiliated Hospital of Guangzhou Medical University. A Phase II/III Trial of Comparison of Benefit of Administration of Checkpoint Inhibitors Plus Chemodrug Via Artery or Fine Needle to Tumor Versus Vein for Immunotherapy of Advanced Solid Tumors. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT03755739 (accessed on 3 June 2023).
- ARCAGY/GINECO Group. A Multicentric Randomized Phase II/III Evaluating TSR-042 (Anti-PD-1 MAb) in Combination with Niraparib (Parpi) Versus Niraparib Alone Compared to Chemotherapy in the Treatment of Metastatic or Recurrent Endometrial or Ovarian Carcinosarcoma after at Least One Line of Chemotherapy. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT03651206 (accessed on 3 June 2023).
- Fondazione Policlinico Universitario Agostino Gemelli IRCCS. Randomized Phase III Trial on NIraparib-TSR-042 (Dostarlimab) vs Physician’s Choice CHEmotherapy in Recurrent, Ovarian, Fallopian Tube or Primary Peritoneal Cancer Patients Not Candidate for Platinum Retreatment: NItCHE Trial (MITO 33). 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04679064 (accessed on 4 June 2023).
- Xencor, Inc. A Phase 1 Multiple-Dose Study to Evaluate the Safety and Tolerability of XmAb®22841 Monotherapy and in Combination with Pembrolizumab in Subjects with Selected Advanced Solid Tumors (DUET-4). 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT03849469 (accessed on 4 June 2023).
- MacroGenics. A Phase 1, First-in-Human, Open-Label, Dose Escalation Study of MGD013, A Bispecific DART® Protein Binding PD-1 and LAG-3 in Patients with Unresectable or Metastatic Neoplasms. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT03219268 (accessed on 4 June 2023).
- Svane, I.M. T-Cell Therapy in Combination with Nivolumab, Relatlimab and Ipilimumab for Patients with Advanced Ovarian-, Fallopian Tube- and Primary Peritoneal Cancer. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04611126 (accessed on 4 June 2023).
- Incyte Biosciences International Sàrl. A Phase 1 Open-Label, Dose-Escalation, Safety and Tolerability Study of INCAGN02385 in Participants with Select Advanced Malignancies. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03538028 (accessed on 4 June 2023).
- Compugen Ltd. A Phase 1 Study of The Safety and Tolerability of COM902 in Subjects with Advanced Malignancies. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04354246 (accessed on 4 June 2023).
- M.D. Anderson Cancer Center. EON: A Single-Arm Phase II Study of Etigilimab (OMP-313M32) in Combination with Checkpoint Inhibition (Nivolumab) in Patients with Platinum-Resistant, Recurrent Epithelial Ovarian Cancer. 2023. Available online: https://www.clinicaltrials.gov/ct2/show/NCT05715216/ (accessed on 4 June 2023).
- Search Results|Beta ClinicalTrials.Gov. Available online: https://beta.clinicaltrials.gov/search?cond=Ovarian%20Cancer&term=immune%20checkpoint (accessed on 1 June 2023).
- Drakes, M.L.; Mehrotra, S.; Aldulescu, M.; Potkul, R.K.; Liu, Y.; Grisoli, A.; Joyce, C.; O’Brien, T.E.; Stack, M.S.; Stiff, P.J. Stratification of Ovarian Tumor Pathology by Expression of Programmed Cell Death-1 (PD-1) and PD-Ligand- 1 (PD-L1) in Ovarian Cancer. J. Ovarian Res. 2018, 11, 43. [Google Scholar] [CrossRef]
- Drakes, M.L.; Czerlanis, C.M.; Stiff, P.J. Immune Checkpoint Blockade in Gynecologic Cancers: State of Affairs. Cancers 2020, 12, 3301. [Google Scholar] [CrossRef]
- Pirš, B.; Škof, E.; Smrkolj, V.; Smrkolj, Š. Overview of Immune Checkpoint Inhibitors in Gynecological Cancer Treatment. Cancers 2022, 14, 631. [Google Scholar] [CrossRef]
- Santoiemma, P.P.; Powell, D.J. Tumor Infiltrating Lymphocytes in Ovarian Cancer. Cancer Biol. Ther. 2015, 16, 807–820. [Google Scholar] [CrossRef]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, W.-T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic Significance of Tumor-Infiltrating T Cells in Ovarian Cancer: A Meta-Analysis. Gynecol. Oncol. 2012, 124, 192–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ Tumor-Infiltrating Lymphocytes and a High CD8+/Regulatory T Cell Ratio Are Associated with Favorable Prognosis in Ovarian Cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [Green Version]
- Bronger, H. Immunology and Immune Checkpoint Inhibition in Ovarian Cancer—Current Aspects. Geburtshilfe Frauenheilkd 2021, 81, 1128–1144. [Google Scholar] [CrossRef]
- Tumor Derived UBR5 Promotes Ovarian Cancer Growth and Metastasis through Inducing Immunosuppressive Macrophages|Nature Communications. Available online: https://www.nature.com/articles/s41467-020-20140-0 (accessed on 12 April 2023).
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Yeku, O.O.; Rafiq, S.; Purdon, T.; Dong, X.; Zhu, L.; Zhang, T.; Wang, H.; Yu, Z.; Mai, J.; et al. Tumor Derived UBR5 Promotes Ovarian Cancer Growth and Metastasis through Inducing Immunosuppressive Macrophages. Nat. Commun. 2020, 11, 6298. [Google Scholar] [CrossRef] [PubMed]
- Hensler, M.; Kasikova, L.; Fiser, K.; Rakova, J.; Skapa, P.; Laco, J.; Lanickova, T.; Pecen, L.; Truxova, I.; Vosahlikova, S.; et al. M2-like Macrophages Dictate Clinically Relevant Immunosuppression in Metastatic Ovarian Cancer. J. Immunother. Cancer 2020, 8, e000979. [Google Scholar] [CrossRef]
- Yin, M.; Li, X.; Tan, S.; Zhou, H.J.; Ji, W.; Bellone, S.; Xu, X.; Zhang, H.; Santin, A.D.; Lou, G.; et al. Tumor-Associated Macrophages Drive Spheroid Formation during Early Transcoelomic Metastasis of Ovarian Cancer. J. Clin. Investig. 2016, 126, 4157–4173. [Google Scholar] [CrossRef] [Green Version]
- Binnewies, M.; Pollack, J.L.; Rudolph, J.; Dash, S.; Abushawish, M.; Lee, T.; Jahchan, N.S.; Canaday, P.; Lu, E.; Norng, M.; et al. Targeting TREM2 on Tumor-Associated Macrophages Enhances Immunotherapy. Cell. Rep. 2021, 37, 109844. [Google Scholar] [CrossRef]
- Ardighieri, L.; Missale, F.; Bugatti, M.; Gatta, L.B.; Pezzali, I.; Monti, M.; Gottardi, S.; Zanotti, L.; Bignotti, E.; Ravaggi, A.; et al. Infiltration by CXCL10 Secreting Macrophages Is Associated With Antitumor Immunity and Response to Therapy in Ovarian Cancer Subtypes. Front. Immunol. 2021, 12, 690201. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, X.; Wang, L.; Liu, J.; Qu, Z.; Zhang, H.; Li, L.; Chen, J.; Zhou, Q. Angiogenesis and Immune Checkpoint Dual Blockade in Combination with Radiotherapy for Treatment of Solid Cancers: Opportunities and Challenges. Oncogenesis 2021, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing Cancer Immunotherapy Using Antiangiogenics: Opportunities and Challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, M.; Jiao, D.; Qin, S.; Chu, Q.; Wu, K.; Li, A. Synergistic Effect of Immune Checkpoint Blockade and Anti-Angiogenesis in Cancer Treatment. Mol. Cancer 2019, 18, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evrard, C.; Alexandre, J. Predictive and Prognostic Value of Microsatellite Instability in Gynecologic Cancer (Endometrial and Ovarian). Cancers 2021, 13, 2434. [Google Scholar] [CrossRef]
- Deshpande, M.; Romanski, P.A.; Rosenwaks, Z.; Gerhardt, J. Gynecological Cancers Caused by Deficient Mismatch Repair and Microsatellite Instability. Cancers 2020, 12, 3319. [Google Scholar] [CrossRef]
- Atwal, A.; Snowsill, T.; Dandy, M.C.; Krum, T.; Newton, C.; Evans, D.G.; Crosbie, E.J.; Ryan, N.A.J. The Prevalence of Mismatch Repair Deficiency in Ovarian Cancer: A Systematic Review and Meta-Analysis. Int. J. Cancer 2022, 151, 1626–1639. [Google Scholar] [CrossRef]
- Nonomura, Y.; Nakayama, K.; Nakamura, K.; Razia, S.; Yamashita, H.; Ishibashi, T.; Ishikawa, M.; Sato, S.; Nakayama, S.; Otsuki, Y.; et al. Ovarian Endometrioid and Clear Cell Carcinomas with Low Prevalence of Microsatellite Instability: A Unique Subset of Ovarian Carcinomas Could Benefit from Combination Therapy with Immune Checkpoint Inhibitors and Other Anticancer Agents. Healthcare 2022, 10, 694. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Sui, Q.; Zhang, X.; Chen, C.; Tang, J.; Yu, J.; Li, W.; Han, K.; Jiang, W.; Liao, L.; Kong, L.; et al. Inflammation Promotes Resistance to Immune Checkpoint Inhibitors in High Microsatellite Instability Colorectal Cancer. Nat. Commun. 2022, 13, 7316. [Google Scholar] [CrossRef]
- Shen, J.; Ju, Z.; Zhao, W.; Wang, L.; Peng, Y.; Ge, Z.; Nagel, Z.D.; Zou, J.; Wang, C.; Kapoor, P.; et al. ARID1A Deficiency Promotes Mutability and Potentiates Therapeutic Antitumor Immunity Unleashed by Immune Checkpoint Blockade. Nat. Med. 2018, 24, 556–562. [Google Scholar] [CrossRef]
- Yamashita, H.; Nakayama, K.; Ishikawa, M.; Ishibashi, T.; Nakamura, K.; Sawada, K.; Yoshimura, Y.; Tatsumi, N.; Kurose, S.; Minamoto, T.; et al. Relationship between Microsatellite Instability, Immune Cells Infiltration, and Expression of Immune Checkpoint Molecules in Ovarian Carcinoma: Immunotherapeutic Strategies for the Future. Int. J. Mol. Sci. 2019, 20, 5129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Liu, J.; Yang, J.; Wang, Z.; Zhang, Z.; Peng, J.; Wang, Y.; Hong, L. A Novel Tumor Mutational Burden-Based Risk Model Predicts Prognosis and Correlates with Immune Infiltration in Ovarian Cancer. Front. Immunol. 2022, 13, 943389. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.-L.; Xu, M.; Liu, C.; Wang, R.-S. Interactions between Tumor Mutation Burden and Immune Infiltration in Ovarian Cancer. Int. J. Clin. Exp. Pathol. 2020, 13, 2513–2523. [Google Scholar] [PubMed]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S. Development of Tumor Mutation Burden as an Immunotherapy Biomarker: Utility for the Oncology Clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Sha, D.; Jin, Z.; Budzcies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor Mutational Burden (TMB) as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef]
- Riviere, P.; Goodman, A.M.; Okamura, R.; Barkauskas, D.A.; Whitchurch, T.J.; Lee, S.; Khalid, N.; Collier, R.; Mareboina, M.; Frampton, G.M.; et al. High Tumor Mutational Burden Correlates with Longer Survival in Immunotherapy-Naïve Patients with Diverse Cancers. Mol. Cancer Ther. 2020, 19, 2139–2145. [Google Scholar] [CrossRef]
- Fan, S.; Gao, X.; Qin, Q.; Li, H.; Yuan, Z.; Zhao, S. Association between Tumor Mutation Burden and Immune Infiltration in Ovarian Cancer. Int. Immunopharmacol. 2020, 89, 107126. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, Y.; Li, B. CD8+ T Cell Exhaustion and Cancer Immunotherapy. Cancer Lett. 2023, 559, 216043. [Google Scholar] [CrossRef]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High Tumor Mutation Burden Fails to Predict Immune Checkpoint Blockade Response across All Cancer Types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef]
- Ukai, M.; Yokoi, A.; Yoshida, K.; Suzuki, S.; Shibata, K.; Kikkawa, F.; Nakatsura, T.; Kajiyama, H. Extracellular MiRNAs as Predictive Biomarkers for Glypican-3-Derived Peptide Vaccine Therapy Response in Ovarian Clear Cell Carcinoma. Cancers 2021, 13, 550. [Google Scholar] [CrossRef]
- Png, K.J.; Halberg, N.; Yoshida, M.; Tavazoie, S.F. A MicroRNA Regulon That Mediates Endothelial Recruitment and Metastasis by Cancer Cells. Nature 2011, 481, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.I.; Katsura, A.; Matsuyama, H.; Miyazono, K. MicroRNA Regulons in Tumor Microenvironment. Oncogene 2015, 34, 3085–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuyama, H.; Suzuki, H.I.; Nishimori, H.; Noguchi, M.; Yao, T.; Komatsu, N.; Mano, H.; Sugimoto, K.; Miyazono, K. MiR-135b Mediates NPM-ALK-Driven Oncogenicity and Renders IL-17-Producing Immunophenotype to Anaplastic Large Cell Lymphoma. Blood 2011, 118, 6881–6892. [Google Scholar] [CrossRef] [PubMed]
- Au Yeung, C.L.; Co, N.-N.; Tsuruga, T.; Yeung, T.-L.; Kwan, S.-Y.; Leung, C.S.; Li, Y.; Lu, E.S.; Kwan, K.; Wong, K.-K.; et al. Exosomal Transfer of Stroma-Derived MiR21 Confers Paclitaxel Resistance in Ovarian Cancer Cells through Targeting APAF1. Nat. Commun. 2016, 7, 11150. [Google Scholar] [CrossRef] [Green Version]
- Nanbakhsh, A.; Malarkannan, S. The Role of MicroRNAs in NK Cell Development and Function. Cells 2021, 10, 2020. [Google Scholar] [CrossRef]
- Weiss, C.N.; Ito, K. A Macro View of MicroRNAs: The Discovery of MicroRNAs and Their Role in Hematopoiesis and Hematologic Disease. Int. Rev. Cell. Mol. Biol. 2017, 334, 99–175. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Lin, W.; Tang, X.; Li, S.; Guo, L.; Lin, Y.; Kwok, H.F. The Roles of MicroRNAs in Regulating the Expression of PD-1/PD-L1 Immune Checkpoint. Int. J. Mol. Sci. 2017, 18, 2540. [Google Scholar] [CrossRef] [Green Version]
- Kousar, K.; Ahmad, T.; Abduh, M.S.; Kanwal, B.; Shah, S.S.; Naseer, F.; Anjum, S. MiRNAs in Regulation of Tumor Microenvironment, Chemotherapy Resistance, Immunotherapy Modulation and MiRNA Therapeutics in Cancer. Int. J. Mol. Sci. 2022, 23, 13822. [Google Scholar] [CrossRef]
- Sohel, M.H. Extracellular/Circulating MicroRNAs: Release Mechanisms, Functions and Challenges. Achiev. Life Sci. 2016, 10, 175–186. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Zhao, Z.; Cai, Q.; Zhang, Y.; Zhang, P.; Shi, S.; Xie, H.; Peng, X.; Yin, W.; Tao, Y.; et al. MiRNA-Based Biomarkers, Therapies, and Resistance in Cancer. Int. J. Biol. Sci. 2020, 16, 2628–2647. [Google Scholar] [CrossRef]
- Li, Q.; Johnston, N.; Zheng, X.; Wang, H.; Zhang, X.; Gao, D.; Min, W. MiR-28 Modulates Exhaustive Differentiation of T Cells through Silencing Programmed Cell Death-1 and Regulating Cytokine Secretion. Oncotarget 2016, 7, 53735–53750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Daly, S.M.; Bayraktar, R.; Anfossi, S.; Calin, G.A. The Interplay between MicroRNAs and the Components of the Tumor Microenvironment in B-Cell Malignancies. Int. J. Mol. Sci. 2020, 21, 3387. [Google Scholar] [CrossRef] [PubMed]
- Diener, C.; Keller, A.; Meese, E. Emerging Concepts of MiRNA Therapeutics: From Cells to Clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Ferragut Cardoso, A.P.; Banerjee, M.; Nail, A.N.; Lykoudi, A.; States, J.C. MiRNA Dysregulation Is an Emerging Modulator of Genomic Instability. Semin. Cancer Biol. 2021, 76, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yin, H.; Zhang, Y.; Feng, Y.; Yan, Z.; Jiang, X.; Bukhari, I.; Iqbal, F.; Cooke, H.J.; Shi, Q. MiR-214-Mediated Downregulation of RNF8 Induces Chromosomal Instability in Ovarian Cancer Cells. Cell Cycle 2014, 13, 3519–3528. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.; Tran, N. Global MiRNA to MiRNA Interactions: Impacts for MiR-21. Trends Cell Biol. 2021, 31, 3–5. [Google Scholar] [CrossRef]
- Terkelsen, T.; Russo, F.; Gromov, P.; Haakensen, V.D.; Brunak, S.; Gromova, I.; Krogh, A.; Papaleo, E. Secreted Breast Tumor Interstitial Fluid MicroRNAs and Their Target Genes Are Associated with Triple-Negative Breast Cancer, Tumor Grade, and Immune Infiltration. Breast Cancer Res. 2020, 22, 73. [Google Scholar] [CrossRef]
- Felekkis, K.; Touvana, E.; Stefanou, C.; Deltas, C. MicroRNAs: A Newly Described Class of Encoded Molecules That Play a Role in Health and Disease. Hippokratia 2010, 14, 236–240. [Google Scholar]
- Gong, A.-Y.; Zhou, R.; Hu, G.; Li, X.; Splinter, P.L.; O’Hara, S.P.; LaRusso, N.F.; Soukup, G.A.; Dong, H.; Chen, X.-M. MicroRNA-513 Regulates B7-H1 Translation and Is Involved in IFN-γ-Induced B7-H1 Expression in Cholangiocytes. J. Immunol. 2009, 182, 1325–1333. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Gibbons, D.L.; Goswami, S.; Cortez, M.A.; Ahn, Y.-H.; Byers, L.A.; Zhang, X.; Yi, X.; Dwyer, D.; Lin, W.; et al. Metastasis Is Regulated via MicroRNA-200/ZEB1 Axis Control of Tumour Cell PD-L1 Expression and Intratumoral Immunosuppression. Nat. Commun. 2014, 5, 5241. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.T.; Phung, C.D.; Tran, T.H.; Pham, T.T.; Pham, L.M.; Nguyen, T.T.; Jeong, J.-H.; Choi, H.-G.; Ku, S.K.; Yong, C.S.; et al. Manipulating Immune System Using Nanoparticles for an Effective Cancer Treatment: Combination of Targeted Therapy and Checkpoint Blockage MiRNA. J. Control. Release 2021, 329, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Huang, Q.; Wang, L.; Ma, X.; Deng, Q.; Kumar, M.; Zhou, Z.; Li, L.; Zeng, Z.; Young, K.H.; et al. MiR-21 Depletion in Macrophages Promotes Tumoricidal Polarization and Enhances PD-1 Immunotherapy. Oncogene 2018, 37, 3151–3165. [Google Scholar] [CrossRef] [PubMed]
- Schmid, G.; Notaro, S.; Reimer, D.; Abdel-Azim, S.; Duggan-Peer, M.; Holly, J.; Fiegl, H.; Rössler, J.; Wiedemair, A.; Concin, N.; et al. Expression and Promotor Hypermethylation of MiR-34a in the Various Histological Subtypes of Ovarian Cancer. BMC Cancer 2016, 16, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyon, N.; Garnier, D.; Briand, J.; Nadaradjane, A.; Bougras-Cartron, G.; Raimbourg, J.; Campone, M.; Heymann, D.; Vallette, F.M.; Frenel, J.-S.; et al. Anti-PD1 Therapy Induces Lymphocyte-Derived Exosomal MiRNA-4315 Release Inhibiting Bim-Mediated Apoptosis of Tumor Cells. Cell. Death Dis. 2020, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, C.C.; Jin, L.; Zhang, X.D. Regulation of PD-L1: A Novel Role of pro-Survival Signalling in Cancer. Ann. Oncol. 2016, 27, 409–416. [Google Scholar] [CrossRef]
- Pathania, A.S.; Prathipati, P.; Olwenyi, O.A.; Chava, S.; Smith, O.V.; Gupta, S.C.; Chaturvedi, N.K.; Byrareddy, S.N.; Coulter, D.W.; Challagundla, K.B. MiR-15a and MiR-15b Modulate Natural Killer and CD8+T-Cell Activation and Anti-Tumor Immune Response by Targeting PD-L1 in Neuroblastoma. Mol. Ther.-Oncolytics 2022, 25, 308–329. [Google Scholar] [CrossRef]
- Ji, X.; Wang, E.; Tian, F. MicroRNA-140 Suppresses Osteosarcoma Tumor Growth by Enhancing Anti-Tumor Immune Response and Blocking MTOR Signaling. Biochem. Biophys. Res. Commun. 2018, 495, 1342–1348. [Google Scholar] [CrossRef]
- Di Martino, M.T.; Riillo, C.; Scionti, F.; Grillone, K.; Polerà, N.; Caracciolo, D.; Arbitrio, M.; Tagliaferri, P.; Tassone, P. MiRNAs and LncRNAs as Novel Therapeutic Targets to Improve Cancer Immunotherapy. Cancers 2021, 13, 1587. [Google Scholar] [CrossRef]
- Yokoi, A.; Matsuzaki, J.; Yamamoto, Y.; Yoneoka, Y.; Takahashi, K.; Shimizu, H.; Uehara, T.; Ishikawa, M.; Ikeda, S.; Sonoda, T.; et al. Integrated Extracellular MicroRNA Profiling for Ovarian Cancer Screening. Nat. Commun. 2018, 9, 4319. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Sawada, K.; Yoshimura, A.; Kinose, Y.; Nakatsuka, E.; Kimura, T. Clinical Relevance of Circulating Cell-Free MicroRNAs in Ovarian Cancer. Mol. Cancer 2016, 15, 48. [Google Scholar] [CrossRef] [Green Version]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating MicroRNA in Body Fluid: A New Potential Biomarker for Cancer Diagnosis and Prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Rapado-González, Ó.; Álvarez-Castro, A.; López-López, R.; Iglesias-Canle, J.; Suárez-Cunqueiro, M.M.; Muinelo-Romay, L. Circulating MicroRNAs as Promising Biomarkers in Colorectal Cancer. Cancers 2019, 11, 898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quandt, D.; Dieter Zucht, H.; Amann, A.; Wulf-Goldenberg, A.; Borrebaeck, C.; Cannarile, M.; Lambrechts, D.; Oberacher, H.; Garrett, J.; Nayak, T.; et al. Implementing Liquid Biopsies into Clinical Decision Making for Cancer Immunotherapy. Oncotarget 2017, 8, 48507–48520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mari, R.; Mamessier, E.; Lambaudie, E.; Provansal, M.; Birnbaum, D.; Bertucci, F.; Sabatier, R. Liquid Biopsies for Ovarian Carcinoma: How Blood Tests May Improve the Clinical Management of a Deadly Disease. Cancers 2019, 11, 774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Failing, J.J.; Dudek, O.A.; Marin Acevedo, J.A.; Chirila, R.M.; Dong, H.; Markovic, S.N.; Dronca, R.S. Biomarkers of Hyperprogression and Pseudoprogression with Immune Checkpoint Inhibitor Therapy. Future Oncol. 2019, 15, 2645–2656. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, J.; Wu, X. Pseudoprogression and Hyperprogression after Checkpoint Blockade. Int. Immunopharmacol. 2018, 58, 125–135. [Google Scholar] [CrossRef]
- Chubachi, S.; Yasuda, H.; Irie, H.; Fukunaga, K.; Naoki, K.; Soejima, K.; Betsuyaku, T. A Case of Non-Small Cell Lung Cancer with Possible “Disease Flare” on Nivolumab Treatment. Case Rep. Oncol. Med. 2016, 2016, e1075641. [Google Scholar] [CrossRef]
- Matos, I.; Martin-Liberal, J.; Hierro, C.; Ochoa De Olza, M.; Viaplana, C.; Costa, M.; Felip-Falg’s, E.; Mur-Bonet, G.; Vieito, M.; Brana, I.; et al. Incidence and Clinical Implications of a New Definition of Hyperprogression (HPD) with Immune Checkpoint Inhibitors (ICIs) in Patients Treated in Phase 1 (Ph1) Trials. J. Clin. Oncol. 2018, 36, 3032. [Google Scholar] [CrossRef]
- Kim, C.G.; Kim, K.H.; Pyo, K.-H.; Xin, C.-F.; Hong, M.H.; Ahn, B.-C.; Kim, Y.; Choi, S.J.; Yoon, H.I.; Lee, J.G.; et al. Hyperprogressive Disease during PD-1/PD-L1 Blockade in Patients with Non-Small-Cell Lung Cancer. Ann. Oncol. 2019, 30, 1104–1113. [Google Scholar] [CrossRef]
- Sasaki, A.; Nakamura, Y.; Mishima, S.; Kawazoe, A.; Kuboki, Y.; Bando, H.; Kojima, T.; Doi, T.; Ohtsu, A.; Yoshino, T.; et al. Predictive Factors for Hyperprogressive Disease during Nivolumab as Anti-PD1 Treatment in Patients with Advanced Gastric Cancer. Gastric Cancer 2019, 22, 793–802. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, R.; Mezquita, L.; Texier, M.; Lahmar, J.; Audigier-Valette, C.; Tessonnier, L.; Mazieres, J.; Zalcman, G.; Brosseau, S.; Le Moulec, S.; et al. Hyperprogressive Disease in Patients with Advanced Non–Small Cell Lung Cancer Treated with PD-1/PD-L1 Inhibitors or with Single-Agent Chemotherapy. JAMA Oncol. 2018, 4, 1543–1552. [Google Scholar] [CrossRef]
- Kato, S.; Goodman, A.; Walavalkar, V.; Barkauskas, D.A.; Sharabi, A.; Kurzrock, R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin. Cancer Res. 2017, 23, 4242–4250. [Google Scholar] [CrossRef] [Green Version]
- Frelaut, M.; Le Tourneau, C.; Borcoman, E. Hyperprogression under Immunotherapy. Int. J. Mol. Sci. 2019, 20, 2674. [Google Scholar] [CrossRef] [Green Version]
- Saâda-Bouzid, E.; Defaucheux, C.; Karabajakian, A.; Coloma, V.P.; Servois, V.; Paoletti, X.; Even, C.; Fayette, J.; Guigay, J.; Loirat, D.; et al. Hyperprogression during Anti-PD-1/PD-L1 Therapy in Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma. Ann. Oncol. 2017, 28, 1605–1611. [Google Scholar] [CrossRef]
- Boland, J.L.; Zhou, Q.; Martin, M.; Callahan, M.K.; Konner, J.; O’Cearbhaill, R.E.; Friedman, C.F.; Tew, W.; Makker, V.; Grisham, R.N.; et al. Early Disease Progression and Treatment Discontinuation in Patients with Advanced Ovarian Cancer Receiving Immune Checkpoint Blockade. Gynecol. Oncol. 2019, 152, 251–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arasanz, H.; Zuazo, M.; Bocanegra, A.; Gato, M.; Martínez-Aguillo, M.; Morilla, I.; Fernández, G.; Hernández, B.; López, P.; Alberdi, N.; et al. Early Detection of Hyperprogressive Disease in Non-Small Cell Lung Cancer by Monitoring of Systemic T Cell Dynamics. Cancers 2020, 12, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Russo, G.; Moro, M.; Sommariva, M.; Cancila, V.; Boeri, M.; Centonze, G.; Ferro, S.; Ganzinelli, M.; Gasparini, P.; Huber, V.; et al. Antibody–Fc/FcR Interaction on Macrophages as a Mechanism for Hyperprogressive Disease in Non–Small Cell Lung Cancer Subsequent to PD-1/PD-L1 Blockade. Clin. Cancer Res. 2019, 25, 989–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin, I.; Zhang, S.; Navaraj, A.; Zhou, L.; Dizon, D.; Safran, H.; El-Deiry, W.S. AMG-232 Sensitizes High MDM2-Expressing Tumor Cells to T-Cell-Mediated Killing. Cell Death Discov. 2020, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.; Marabelle, A.; Soria, J.-C.; et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [CrossRef] [Green Version]
- Kanjanapan, Y.; Day, D.; Wang, L.; Al-Sawaihey, H.; Abbas, E.; Namini, A.; Siu, L.L.; Hansen, A.; Razak, A.A.; Spreafico, A.; et al. Hyperprogressive Disease in Early-Phase Immunotherapy Trials: Clinical Predictors and Association with Immune-Related Toxicities. Cancer 2019, 125, 1341–1349. [Google Scholar] [CrossRef]
- Di Giacomo, A.M.; Danielli, R.; Guidoboni, M.; Calabrò, L.; Carlucci, D.; Miracco, C.; Volterrani, L.; Mazzei, M.A.; Biagioli, M.; Altomonte, M.; et al. Therapeutic Efficacy of Ipilimumab, an Anti-CTLA-4 Monoclonal Antibody, in Patients with Metastatic Melanoma Unresponsive to Prior Systemic Treatments: Clinical and Immunological Evidence from Three Patient Cases. Cancer Immunol. Immunother. 2009, 58, 1297–1306. [Google Scholar] [CrossRef]
- Chiou, V.L.; Burotto, M. Pseudoprogression and Immune-Related Response in Solid Tumors. J. Clin. Oncol. 2015, 33, 3541–3543. [Google Scholar] [CrossRef] [Green Version]
- Queirolo, P.; Spagnolo, F. Atypical Responses in Patients with Advanced Melanoma, Lung Cancer, Renal-Cell Carcinoma and Other Solid Tumors Treated with Anti-PD-1 Drugs: A Systematic Review. Cancer Treat. Rev. 2017, 59, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, X.; Zhang, D.; Wang, G.; Cheng, X.; Xu, C.; Yao, B.; Pang, L.; Chen, J. Early Onset Immune-Related Adverse Event to Identify Pseudo-Progression in a Patient With Ovarian Cancer Treated With Nivolumab: A Case Report and Review of the Literature. Front. Med. 2020, 7, 366. [Google Scholar] [CrossRef] [PubMed]
- Passler, M.; Taube, E.T.; Sehouli, J.; Pietzner, K. Pseudo- or Real Progression? An Ovarian Cancer Patient under Nivolumab: A Case Report. World J. Clin. Oncol. 2019, 10, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Q.; Dong, Q.; Zhan, L.; Zhang, J. How to Differentiate Pseudoprogression from True Progression in Cancer Patients Treated with Immunotherapy. Am. J. Cancer Res. 2019, 9, 1546–1553. [Google Scholar] [PubMed]
- Nero, C.; Ciccarone, F.; Pietragalla, A.; Duranti, S.; Daniele, G.; Salutari, V.; Carbone, M.V.; Scambia, G.; Lorusso, D. Ovarian Cancer Treatments Strategy: Focus on PARP Inhibitors and Immune Check Point Inhibitors. Cancers 2021, 13, 1298. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Ohashi, P.S. Clinical Blockade of PD1 and LAG3--Potential Mechanisms of Action. Nat. Rev. Immunol. 2015, 15, 45–56. [Google Scholar] [CrossRef]
- Sanchez-Correa, B.; Valhondo, I.; Hassouneh, F.; Lopez-Sejas, N.; Pera, A.; Bergua, J.M.; Arcos, M.J.; Bañas, H.; Casas-Avilés, I.; Durán, E.; et al. DNAM-1 and the TIGIT/PVRIG/TACTILE Axis: Novel Immune Checkpoints for Natural Killer Cell-Based Cancer Immunotherapy. Cancers 2019, 11, 877. [Google Scholar] [CrossRef] [Green Version]
- Chauvin, J.-M.; Zarour, H.M. TIGIT in Cancer Immunotherapy. J. Immunother. Cancer 2020, 8, e000957. [Google Scholar] [CrossRef]
- Ge, Z.; Peppelenbosch, M.P.; Sprengers, D.; Kwekkeboom, J. TIGIT, the Next Step Towards Successful Combination Immune Checkpoint Therapy in Cancer. Front. Immunol. 2021, 12, 699895. [Google Scholar] [CrossRef]
- Mariniello, A.; Novello, S.; Scagliotti, G.V.; Ramalingam, S.S. Double Immune Checkpoint Blockade in Advanced NSCLC. Crit. Rev. Oncol./Hematol. 2020, 152, 102980. [Google Scholar] [CrossRef]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 Combination Blockade Expands Infiltrating T Cells and Reduces Regulatory T and Myeloid Cells within B16 Melanoma Tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef] [Green Version]
- Baumeister, S.H.; Freeman, G.J.; Dranoff, G.; Sharpe, A.H. Coinhibitory Pathways in Immunotherapy for Cancer. Annu. Rev. Immunol. 2016, 34, 539–573. [Google Scholar] [CrossRef] [PubMed]
- Boutros, C.; Tarhini, A.; Routier, E.; Lambotte, O.; Ladurie, F.L.; Carbonnel, F.; Izzeddine, H.; Marabelle, A.; Champiat, S.; Berdelou, A.; et al. Safety Profiles of Anti-CTLA-4 and Anti-PD-1 Antibodies Alone and in Combination. Nat. Rev. Clin. Oncol. 2016, 13, 473–486. [Google Scholar] [CrossRef]
- Simpson, T.R.; Li, F.; Montalvo-Ortiz, W.; Sepulveda, M.A.; Bergerhoff, K.; Arce, F.; Roddie, C.; Henry, J.Y.; Yagita, H.; Wolchok, H.D.; et al. Fc-Dependent Depletion of Tumor-Infiltrating Regulatory t Cells Co-Defines the Efficacy of Anti-CTLA-4 Therapy against Melanoma. J. Exp. Med. 2013, 210, 1695–1710. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Harden, K.; C Gonzalez, L.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The Surface Protein TIGIT Suppresses T Cell Activation by Promoting the Generation of Mature Immunoregulatory Dendritic Cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Whelan, S.; Ophir, E.; Kotturi, M.F.; Levy, O.; Ganguly, S.; Leung, L.; Vaknin, I.; Kumar, S.; Dassa, L.; Hansen, K.; et al. PVRIG and PVRL2 Are Induced in Cancer and Inhibit CD8+ T-Cell Function. Cancer Immunol. Res. 2019, 7, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Solomon, B.L.; Garrido-Laguna, I. TIGIT: A Novel Immunotherapy Target Moving from Bench to Bedside. Cancer Immunol. Immunother. 2018, 67, 1659–1667. [Google Scholar] [CrossRef]
- Manieri, N.A.; Chiang, E.Y.; Grogan, J.L. TIGIT: A Key Inhibitor of the Cancer Immunity Cycle. Trends Immunol. 2017, 38, 20–28. [Google Scholar] [CrossRef]
- Banta, K.L.; Xu, X.; Chitre, A.S.; Au-Yeung, A.; Takahashi, C.; O’Gorman, W.E.; Wu, T.D.; Mittman, S.; Cubas, R.; Comps-Agrar, L.; et al. Mechanistic Convergence of the TIGIT and PD-1 Inhibitory Pathways Necessitates Co-Blockade to Optimize Anti-Tumor CD8+ T Cell Responses. Immunity 2022, 55, 512–526.e9. [Google Scholar] [CrossRef]
- Chen, F.; Xu, Y.; Chen, Y.; Shan, S. TIGIT Enhances CD4+ Regulatory T-Cell Response and Mediates Immune Suppression in a Murine Ovarian Cancer Model. Cancer Med. 2020, 9, 3584–3591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Chen, X.; Lou, J.; Zhang, S.; Zhang, X.; Huang, L.; Sun, R.; Huang, P.; Pan, S.; Wang, F. Changes in Regulatory T Cells in Patients with Ovarian Cancer Undergoing Surgery: Preliminary Results. Int. Immunopharmacol. 2017, 47, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Kurtulus, S.; Sakuishi, K.; Ngiow, S.-F.; Joller, N.; Tan, D.J.; Teng, M.W.L.; Smyth, M.J.; Kuchroo, V.K.; Anderson, A.C. TIGIT Predominantly Regulates the Immune Response via Regulatory T Cells. Available online: https://www.jci.org/articles/view/81187/pdf (accessed on 17 August 2022).
- Hoogstad-van Evert, J.S.; Maas, R.J.; van der Meer, J.; Cany, J.; van der Steen, S.; Jansen, J.H.; Miller, J.S.; Bekkers, R.; Hobo, W.; Massuger, L.; et al. Peritoneal NK Cells Are Responsive to IL-15 and Percentages Are Correlated with Outcome in Advanced Ovarian Cancer Patients. Oncotarget 2018, 9, 34810–34820. [Google Scholar] [CrossRef] [Green Version]
- Second Affiliated Hospital of Guangzhou Medical University. Triplex CTLA4/PD1/PDL1 Checkpoint Inhibitors Combination Therapy for Advanced Solid. Tumors. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05187338 (accessed on 4 June 2023).
- Anderson, K.; Su, Y.; Burnett, M.; Bates, B.; Suarez, M.R.; Ruskin, S.; Vakil, A.; Voillet, V.; Gottardo, R.; Greenberg, P. 561 Triple Checkpoint Blockade, but Not Anti-PD1 Alone, Enhances the Efficacy of Engineered Adoptive T Cell Therapy in Advanced Ovarian Cancer. J. Immunother. Cancer 2021, 9, A590. [Google Scholar] [CrossRef]
- Archilla-Ortega, A.; Domuro, C.; Martin-Liberal, J.; Muñoz, P. Blockade of Novel Immune Checkpoints and New Therapeutic Combinations to Boost Antitumor Immunity. J. Exp. Clin. Cancer Res. 2022, 41, 62. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-Y.; Francois, A.; McGray, A.R.; Miliotto, A.; Odunsi, K. Compensatory Upregulation of PD-1, LAG-3, and CTLA-4 Limits the Efficacy of Single-Agent Checkpoint Blockade in Metastatic Ovarian Cancer. Oncoimmunology 2017, 6, e1249561. [Google Scholar] [CrossRef] [Green Version]
- Vetter, V.; Denizer, G.; Friedland, L.R.; Krishnan, J.; Shapiro, M. Understanding Modern-Day Vaccines: What You Need to Know. Ann. Med. 2018, 50, 110–120. [Google Scholar] [CrossRef]
- Kimiz-Gebologlu, I.; Gulce-Iz, S.; Biray-Avci, C. Monoclonal Antibodies in Cancer Immunotherapy. Mol. Biol. Rep. 2018, 45, 2935–2940. [Google Scholar] [CrossRef]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic Cancer Vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Chiang, C.L.-L.; Kandalaft, L.E.; Tanyi, J.; Hagemann, A.R.; Motz, G.T.; Svoronos, N.; Montone, K.; Mantia-Smaldone, G.M.; Smith, L.; Nisenbaum, H.L.; et al. A Dendritic Cell Vaccine Pulsed with Autologous Hypochlorous Acid-Oxidized Ovarian Cancer Lysate Primes Effective Broad Antitumor Immunity: From Bench to Bedside. Clin. Cancer Res. 2013, 19, 4801–4815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanyi, J.L.; Bobisse, S.; Ophir, E.; Tuyaerts, S.; Roberti, A.; Genolet, R.; Baumgartner, P.; Stevenson, B.J.; Iseli, C.; Dangaj, D.; et al. Personalized Cancer Vaccine Effectively Mobilizes Antitumor T Cell Immunity in Ovarian Cancer. Sci. Transl. Med. 2018, 10, eaao5931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanyi, J.L.; Chiang, C.L.-L.; Chiffelle, J.; Thierry, A.-C.; Baumgartener, P.; Huber, F.; Goepfert, C.; Tarussio, D.; Tissot, S.; Torigian, D.A.; et al. Personalized Cancer Vaccine Strategy Elicits Polyfunctional T Cells and Demonstrates Clinical Benefits in Ovarian Cancer. NPJ Vaccines 2021, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, T.; Li, Y.; Chen, L.; Liu, H.; Wu, Y.; Guo, H. Dendritic Cell Vaccines in Ovarian Cancer. Front. Immunol. 2021, 11, 613773. [Google Scholar] [CrossRef]
- Martin-Lluesma, S.; Graciotti, M.; Grimm, A.J.; Boudousquié, C.; Chiang, C.L.; Kandalaft, L.E. Are Dendritic Cells the Most Appropriate Therapeutic Vaccine for Patients with Ovarian Cancer? Curr. Opin. Biotechnol. 2020, 65, 190–196. [Google Scholar] [CrossRef]
- Brentville, V.A.; Metheringham, R.L.; Daniels, I.; Atabani, S.; Symonds, P.; Cook, K.W.; Vankemmelbeke, M.; Choudhury, R.; Vaghela, P.; Gijon, M.; et al. Combination Vaccine Based on Citrullinated Vimentin and Enolase Peptides Induces Potent CD4-Mediated Anti-Tumor Responses. J. Immunother. Cancer 2020, 8, e000560. [Google Scholar] [CrossRef]
- Zamarin, D.; Walderich, S.; Holland, A.; Zhou, Q.; Iasonos, A.E.; Torrisi, J.M.; Merghoub, T.; Chesebrough, L.F.; Mcdonnell, A.S.; Gallagher, J.M.; et al. Safety, Immunogenicity, and Clinical Efficacy of Durvalumab in Combination with Folate Receptor Alpha Vaccine TPIV200 in Patients with Advanced Ovarian Cancer: A Phase II Trial. J. Immunother. Cancer 2020, 8, e000829. [Google Scholar] [CrossRef]
- Edgar, T.W.; Manz, D.O. Chapter 6—Machine Learning. In Research Methods for Cyber Security; Edgar, T.W., Manz, D.O., Eds.; Syngress: Burlington, MA, USA, 2017; pp. 153–173. ISBN 978-0-12-805349-2. [Google Scholar]
- Schneider, P.; Xhafa, F. Chapter 8—Machine Learning: ML for EHealth Systems. In Anomaly Detection and Complex Event Processing Over IoT Data Streams; Schneider, P., Xhafa, F., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 149–191. ISBN 978-0-12-823818-9. [Google Scholar]
- Deo, R.C. Machine Learning in Medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zeng, S.; Xu, X.; Li, H.; Yao, S.; Song, K.; Li, X.; Chen, L.; Tang, J.; Xing, H.; et al. Deep Learning-Enabled Pelvic Ultrasound Images for Accurate Diagnosis of Ovarian Cancer in China: A Retrospective, Multicentre, Diagnostic Study. Lancet Digit. Health 2022, 4, e179–e187. [Google Scholar] [CrossRef]
- Ahamad, M.M.; Aktar, S.; Uddin, M.J.; Rahman, T.; Alyami, S.A.; Al-Ashhab, S.; Akhdar, H.F.; Azad, A.K.M.; Moni, M.A. Early-Stage Detection of Ovarian Cancer Based on Clinical Data Using Machine Learning Approaches. J. Pers. Med. 2022, 12, 1211. [Google Scholar] [CrossRef]
- Wang, G.; Sun, Y.; Jiang, S.; Wu, G.; Liao, W.; Chen, Y.; Lin, Z.; Liu, Z.; Zhuo, S. Machine Learning-Based Rapid Diagnosis of Human Borderline Ovarian Cancer on Second-Harmonic Generation Images. Biomed. Opt. Express 2021, 12, 5658–5669. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Antwi, P.A.; Luo, Y.; Liang, F. Identification and Validation of the Diagnostic Characteristic Genes of Ovarian Cancer by Bioinformatics and Machine Learning. Front. Genet. 2022, 13, 858466. [Google Scholar] [CrossRef]
- Johannet, P.; Coudray, N.; Donnelly, D.M.; Jour, G.; Bochaca, I.I.; Xia, Y.; Johnson, D.B.; Wheless, L.; Patrinely, J.R.; Nomikou, S.; et al. Using Machine Learning Algorithms to Predict Immunotherapy Response in Patients with Advanced Melanoma. Clin. Cancer Res. 2021, 27, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Ha, D.; Lee, J.; Kim, I.; Park, M.; Im, S.-H.; Shin, K.; Kim, S. Network-Based Machine Learning Approach to Predict Immunotherapy Response in Cancer Patients. Nat. Commun. 2022, 13, 3703. [Google Scholar] [CrossRef] [PubMed]
- Harder, N.; Schönmeyer, R.; Nekolla, K.; Meier, A.; Brieu, N.; Vanegas, C.; Madonna, G.; Capone, M.; Botti, G.; Ascierto, P.A.; et al. Automatic Discovery of Image-Based Signatures for Ipilimumab Response Prediction in Malignant Melanoma. Sci. Rep. 2019, 9, 7449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhang, N.; Wu, W.; Zhou, R.; Li, S.; Wang, Z.; Dai, Z.; Zhang, L.; Liu, Z.; Zhang, J.; et al. Machine Learning-Based Tumor-Infiltrating Immune Cell-Associated LncRNAs for Predicting Prognosis and Immunotherapy Response in Patients with Glioblastoma. Brief. Bioinform. 2022, 23, bbac386. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Yang, T.; Xing, H.; Wang, Y.; Gao, L.; Guo, X.; Xing, B.; Wang, Y.; Ma, W. Machine Learning Revealed Stemness Features and a Novel Stemness-Based Classification with Appealing Implications in Discriminating the Prognosis, Immunotherapy and Temozolomide Responses of 906 Glioblastoma Patients. Brief. Bioinform. 2021, 22, bbab032. [Google Scholar] [CrossRef]
- Chen, D.; Liu, J.; Zang, L.; Xiao, T.; Zhang, X.; Li, Z.; Zhu, H.; Gao, W.; Yu, X. Integrated Machine Learning and Bioinformatic Analyses Constructed a Novel Stemness-Related Classifier to Predict Prognosis and Immunotherapy Responses for Hepatocellular Carcinoma Patients. Int. J. Biol. Sci. 2022, 18, 360–373. [Google Scholar] [CrossRef]


| NCT Number | Acronym | Condition | mAbs Anti-ICPs | Additional Drugs | Participants | Phase | Company | Ref. |
|---|---|---|---|---|---|---|---|---|
| NCT03598270 | ANITA | Recurrent ovarian carcinoma | Atezolizumab | placebo carboplatin paclitaxel niraparib gemcitabine PLD | 414 | 3 | Grupo Español de Investigación en Cáncer de Ovario | [74] |
| NCT03522246 | ATHENA | Epithelial ovarian cancer | Nivolumab | rucaparib placebo oral tablet placebo IV infusion | 1000 | 3 | Clovis Oncology, Inc. | [75] |
| NCT03740165 | - | Epithelial ovarian cancer | Pembrolizumab | placebo for pembrolizumab carboplatin paclitaxel olaparib placebo for olaparib bevacizumab docetaxel | 1367 | 3 | Merck Sharp & Dohme LLC | [76] |
| NCT03602859 | FIRST | First-line treatment of stage III/IV non-mucinous epithelial OC | Dostarlimab (TSR-042) | niraparib standard care dostarlimab-placebo niraparib-placebo | 1403 | 3 | Tesaro, Inc. | [77] |
| NCT05116189 | - | Platinum-resistant recurrent ovarian cancer | Pembrolizumab | paclitaxel bevacizumab placebo for pembrolizumab docetaxel | 616 | 3 | Merck Sharp & Dohme LLC | [78] |
| NCT03353831 | - | early relapse ovarian cancer | Atezolizumab | bevacizumab chemotherapy placebos | 550 | 3 | AGO Research GmbH | [79] |
| NCT02580058 | JAVELIN OVARIAN 200 | Platinum resistant/ refractory ovarian cancer | Avelumab | PLD | 566 | 3 | Pfizer | [80] |
| NCT03642132 | JAVELIN OVARIAN PARP - | Untreated advanced ovarian cancer | Avelumab | chemotherapy + avelumab followed by avelumab + talazoparib chemotherapy + bevacizumab followed by bevacizumab chemotherapy, followed by talazoparib maintenance | 79 | 3 | Pfizer | [81] |
| NCT02718417 | JAVELIN OVARIAN 100 | Previously untreated patients with epithelial ovarian cancer | Avelumab | carboplatin paclitaxel | 998 | 3 | Pfizer | [82] |
| NCT03038100 | IMagyn050 | Newly-diagnosed stage III or stage IV ovarian cancer | Atezolizumab | paclitaxel carboplatin bevacizumab atezolizumab placebo | 1301 | 3 | Hoffmann-La Roche | [83] |
| NCT02891824 | ARCAGY/GINECO GROUP | Late relapse ovarian cancer | Atezolizumab | atezolizumab + avastin + platinum-based chemotherapy placebo + avastin + platinum-based chemotherapy | 614 | 3 | ARCAGY/GINECO GROUP | [84] |
| NCT02839707 | - | Recurrent ovarian cancer | Atezolizumab | bevacizumab computed tomography PLD hydrochloride quality-of-life assessment | 444 | 2/3 | National Cancer Institute (NCI) | [85] |
| NCT03755739 | - | Ovarian cancer | Pembrolizumab, ipilimumab | immune checkpoint inhibitors such as pembrolizumab, ipilimumab plus chemotherapy | 200 | 2/3 | Second Affiliated Hospital of Guangzhou Medical University | [86] |
| NCT03651206 | ROCSAN | Recurrent ovarian carcinosarcoma | Dostarlimab | niraparib niraparib + dostarlimab chemotherapy drugs | 196 | 2/3 | ARCAGY/GINECO GROUP | [87] |
| NCT04679064 | NItCHE-MITO33 | Recurrent ovarian cancer patients not a candidate for platinum retreatment | Dostarlimab | niraparib pegylated liposomal doxorubicin paclitaxel gemcitabine topotecan bevacizumab | 427 | 3 | Fondazione Policlinico Universitario Agostino Gemelli IRCCS | [88] |
| NCT Number | Acronym | Condition | mAbs Anti-ICPs | Additional Drugs | Participants | Phase | Company | Ref. |
|---|---|---|---|---|---|---|---|---|
| NCT04611126 | - | Metastatic ovarian cancer | Ipilimumab Nivolumab Relatlimab | cyclophosphamid fludarabine phosphate tumor-infiltrating lymphocytes infusion | 18 | 1/2 | Inge Marie Svane | [91] |
| NCT03219268 | - | Ovarian cancer | Tebotelimab Margetuximab | - | 353 | 1 | MacroGenics | [90] |
| NCT03538028 | - | Advanced ovarian cancer | INCAGN02385 | - | 22 | 1 | Incyte Biosciences International Sàrl | [92] |
| NCT03849469 | DUET-4 | Advanced ovarian cancer | Xmab®22841 Pembrolizumab | - | 78 | 1 | Xencor, Inc. | [89] |
| NCT04354246 | - | Advanced ovarian cancer | COM902 COM701 (antiCD112R) pembrolizumab. | - | 110 | 1 | Compugen Ltd. | [93] |
| NCT05026606 | - | Recurrent ovarian clear cell adenocarcinoma Recurrent platinum-resistant ovarian carcinoma | Etigilimab nivolumab | - | 20 | 2 | M.D. Anderson Cancer Center | [94] |
| mAbs Anti-ICPs | Additional Drugs | NCT Number |
|---|---|---|
| Pembrolizumab | - (monotherapy) | NCT05368207 NCT04575961 NCT03732950 NCT04602377 NCT03430700 NCT04375956 NCT02644369 NCT03012620 |
| chemotherapy | NCT03734692 NCT05467670 NCT04387227 NCT02766582 NCT03410784 NCT03755739 NCT02520154 NCT03126812 | |
| VEGFi + chemotherapy | NCT03596281 NCT03275506 NCT05116189 | |
| VEGFi + PARPi + chemotherapy | NCT03740165 NCT05158062 | |
| PARPi | NCT04417192 | |
| VEGFi + PARPi | NCT04361370 | |
| PY314 | NCT04691375 | |
| KVA12123 | NCT05708950 | |
| Anti-CTLA4 | NCT04140526 | |
| Modified vaccinia virus Ankara vaccine expressing p53 | NCT03113487 | |
| Nivolumab | PARPi (Rucaparib) | NCT03522246 |
| PARPi + VEGFi | NCT02873962 | |
| Chemotherapy + PARPi | NCT03245892 | |
| Etigilimab | NCT05715216 | |
| NY-ESO-1 peptide vaccine | NCT05479045 | |
| Atezolizumab | Chemotherapy + PARPi | NCT03598270 |
| Chemotherapy + VEGFi | NCT03353831 NCT02891824 NCT02839707 | |
| VEGFi | NCT04510584 | |
| Durvalumab | Olaparib + Bevacizumab | NCT04015739 |
| Durvalumab + Tremelimumab | (ICIs combination) | NCT03026062 |
| Tremelimumab | PARPi | NCT04034927 |
| Nivolumab + Ipilimumab | (ICIs combination) | NCT03355976 NCT03508570 NCT02498600 |
| Ipilimumab +Pembrolizumab +Durvalumab | (ICIs combination) | NCT05187338 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawłowska, A.; Rekowska, A.; Kuryło, W.; Pańczyszyn, A.; Kotarski, J.; Wertel, I. Current Understanding on Why Ovarian Cancer Is Resistant to Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2023, 24, 10859. https://doi.org/10.3390/ijms241310859
Pawłowska A, Rekowska A, Kuryło W, Pańczyszyn A, Kotarski J, Wertel I. Current Understanding on Why Ovarian Cancer Is Resistant to Immune Checkpoint Inhibitors. International Journal of Molecular Sciences. 2023; 24(13):10859. https://doi.org/10.3390/ijms241310859
Chicago/Turabian StylePawłowska, Anna, Anna Rekowska, Weronika Kuryło, Anna Pańczyszyn, Jan Kotarski, and Iwona Wertel. 2023. "Current Understanding on Why Ovarian Cancer Is Resistant to Immune Checkpoint Inhibitors" International Journal of Molecular Sciences 24, no. 13: 10859. https://doi.org/10.3390/ijms241310859
APA StylePawłowska, A., Rekowska, A., Kuryło, W., Pańczyszyn, A., Kotarski, J., & Wertel, I. (2023). Current Understanding on Why Ovarian Cancer Is Resistant to Immune Checkpoint Inhibitors. International Journal of Molecular Sciences, 24(13), 10859. https://doi.org/10.3390/ijms241310859






