The Bacterial Spore as a Mucosal Vaccine Delivery System
Abstract
:1. Introduction
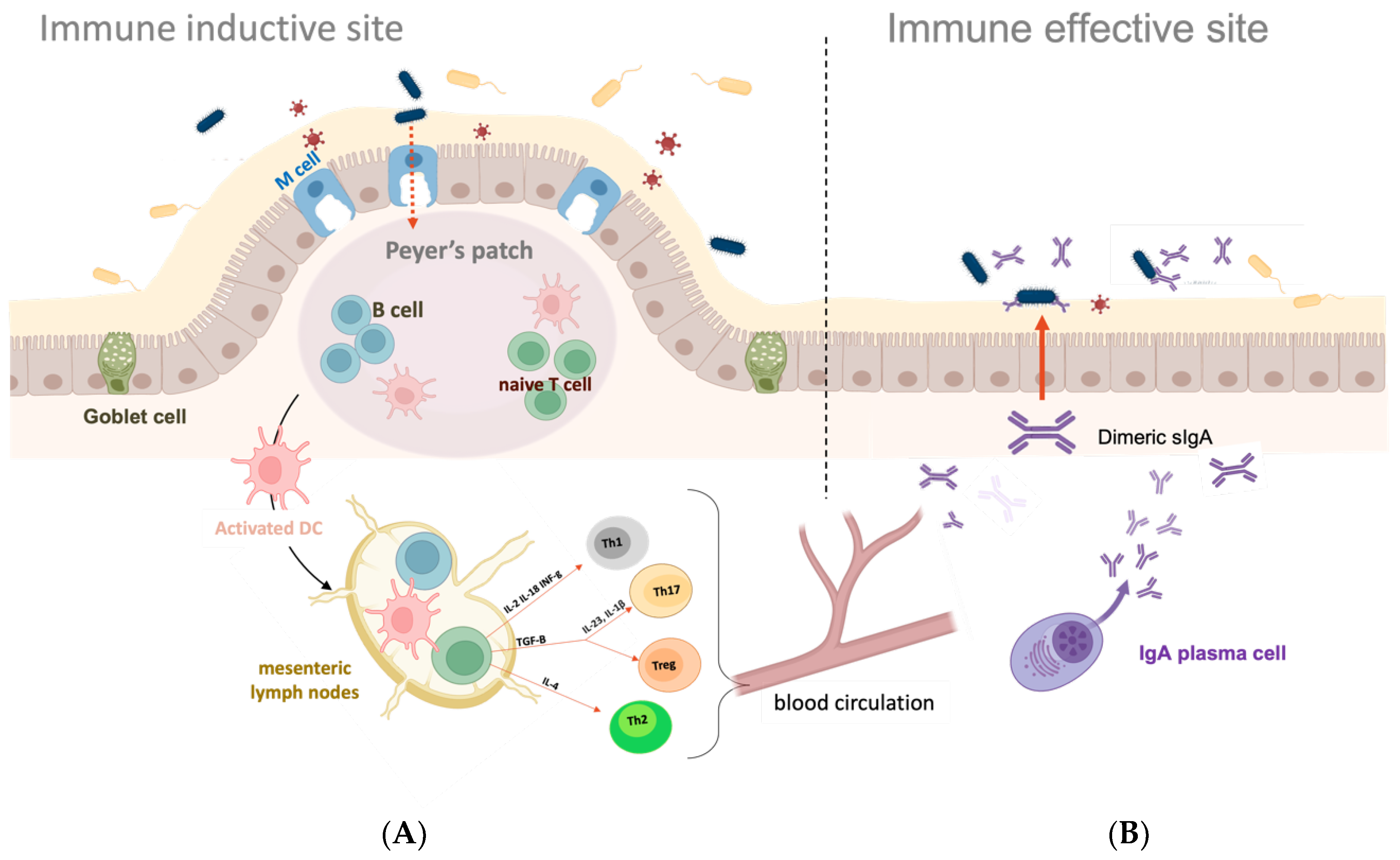
2. Mucosal Surfaces and Mucosal Immune System
3. Mucosal Adjuvants and Delivery Systems
3.1. Mucosal Adjuvants
3.2. Mucosal Delivery Systems
4. The Bacillus Spore as Mucosal Vaccine Vehicles
4.1. The Bacillus Spore
4.2. The Spore Delivery Systems: Recombinant Approach
4.3. The Spore Delivery Systems: Non-Recombinant Approach
4.4. Recombinant vs. Non-Recombinant Spore Display
5. Mucosal Immunizations with Recombinant and Non-Recombinant Spores
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pashine, A.; Valiante, N.M.; Ulmer, J.B. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 2005, 11, S63–S68. [Google Scholar] [CrossRef]
- Correa, V.A.; Portilho, A.I.; De Gaspari, E. Vaccines, adjuvants and key factors for mucosal immune response. Immunology 2022, 167, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, W.; Wang, S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev. Vaccines 2015, 14, 1509–1523. [Google Scholar] [CrossRef] [Green Version]
- Lycke, N.; Bemark, M. Mucosal adjuvants and long-term memory development with special focus on CTA1-DD and other ADP-ribosylating toxins. Mucosal Immunol. 2010, 3, 556–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasetti, M.F.; Simon, J.K.; Sztein, M.B.; Levine, M.M. Immunology of gut mucosal vaccines. Immunol. Rev. 2011, 239, 125–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Criscuolo, E.; Caputo, V.; Diotti, R.A.; Sautto, G.A.; Kirchenbaum, G.A.; Clementi, N. Alternative methods of vaccine delivery: An overview of edible and intradermal vaccines. J. Immunol. Res. 2019, 2019, 8303648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nature Rev. Immunol. 2022, 22, 237–250. [Google Scholar] [CrossRef]
- Wilson, H.L.; Gerdts, V.; Babiul, L.A. Mucosal vaccine development for veterinary and aquatic diseases. In Mucosal Vaccines, 2nd ed.; Kiyono, H., Pascual, D.W., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 811–829. [Google Scholar]
- Huang, M.; Zhang, M.; Zhu, H.; Du, X.; Wnag, J. Mucosal vaccine delivery: A focus on the breakthrough of specific barriers. Acta Pharm. Sin. B 2022, 12, 3456–3474. [Google Scholar] [CrossRef]
- Traxinger, B.R.; Richert-Spuhler, L.E.; Lund, J.M. Mucosal tissue regulatory T cells are integral in balancing immunity and tolerance at portals of antigen entry. Mucosal Immunol. 2021, 15, 398–407. [Google Scholar] [CrossRef]
- Mörbe, U.M.; Jørgensen, P.B.; Fenton, T.M.; Von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef]
- Tordesillas, L.; Berin, M.C. Mechanisms of oral tolerance. Clin. Rev. Allergy Immunol. 2018, 55, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Lycke, N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef]
- Isticato, R.; Sirec, T.; Treppiccione, L.; Maurano, F.; De Felice, M.; Rossi, M.; Ricca, E. Non-recombinant display of the B subunit of the heat labile toxin of Escherichia coli on wild type and mutant spores of Bacillus subtilis. Microb. Cell Factories 2013, 29, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Splutter, M.; Van Hoffen, E.; Floris-Vollenbroek, E.G.; Timmerman, H.; Lucas van de Bos, E.; Mejier, B.; Ulfman, L.H.; Witteman, B.; Wells, J.M.; Brugman, S.; et al. Oral cholera vaccination promotes homing of IgA+ memory B cells to the large intestine and the respiratory tract. Mucosal Immunol. 2018, 11, 1254–1264. [Google Scholar] [CrossRef] [Green Version]
- Ou, B.; Yang, Y.; Lin, X.; Zhang, M. Current progress and challenges in the study of adjuvants for oral vaccines. BioDrugs 2023, 37, 143–180. [Google Scholar] [CrossRef] [PubMed]
- Lycke, N.; Lebrero-Fernandez, C. ADP-ribosylating enterotoxins as vaccine adjuvants. Curr. Opin. Pharmacol. 2018, 41, 42–51. [Google Scholar] [CrossRef]
- Innocentin, S.; Guimares, V.; Miyoshi, A.; Azevedo, V.; Langella, P.; Chatel, J.-M. Lactococcus lactis expressing either Staphylococcus aureus fibronectin-binding protein A or Listeria monocytogenes internalin A can efficiently internalize and deliver DNA in human epithelial cells. Appl. Environ. Microbiol. 2009, 75, 4870–4878. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Seo, K.-W.; Kim, J.; Lee, K.-Y.; Jang, Y.-S. The M cell-targeting ligand promotes antigen delivery and induces antigenspecific immune responses in mucosal vaccination. J. Immunol. 2010, 185, 5787–5795. [Google Scholar] [CrossRef] [Green Version]
- Owen, J.L.; Sahay, B.; Mohamadzadeh, M. New generation of oral mucosal vaccines targeting dendritic cells. Curr. Opin. Chem. Biol. 2013, 17, 918–924. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Zeng, R.; Bai, Y.; Roopenian, D.C.; Zhu, X. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat. Biotechnol. 2011, 29, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Manohar, M.M.; Campbell, B.E.; Walduck, A.K.; Moore, R.J. Enhancement of live vaccines by co-delivery of immune modulating proteins. Vaccine 2022, 40, 5769–5780. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.D.S.; Maubrigades, L.R.; Gonsalves, V.S.; Franz, H.C.; Rodrigues, P.R.C.; Cunha, R.C.; Leite, F.P.L. Bacillus toyonensis BCT-7112 spores as parenteral adjuvant of BoHV-5 vaccine in a murine model. Probiot. Antimicrob. Prot. 2021, 13, 655–663. [Google Scholar] [CrossRef]
- Santos, F.D.S.; Mazzoli, A.; Maia, A.R.; Saggese, A.; Isticato, R.; Leite, F.; Iossa, S.; Ricca, E.; Baccigalupi, L. A probiotic treatment increases the immune response induced by the nasal delivery of spore-adsorbed TTFC. Microb. Cell Factories 2020, 19, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Zhu, Y.; Zhang, L.; Zhang, Z. Recent advances in delivery systems for genetic and other novel vaccines. Adv. Mater. 2022, 34, 2107946. [Google Scholar] [CrossRef]
- Benhar, I. Biotechnological applications of phage and cell display. Biotechnol. Adv. 2001, 19, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Isticato, R.; Ricca, E. Spore surface display. Microbiol. Spect. 2014, 2, TBS-0011-2012. [Google Scholar] [CrossRef]
- Wu, J.Y.; Newton, S.; Judd, A.; Stocker, B.; Robinson, W.S. Expression of immunogenic epitopes of hepatitis B surface antigen with hybrid flagellin proteins by a vaccine strain of Salmonella. Proc. Natl. Acad. Sci. USA 1989, 86, 4726–4730. [Google Scholar] [CrossRef] [Green Version]
- Newton, S.M.; Jacob, C.O.; Stocker, B.A. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science 1989, 244, 70–72. [Google Scholar] [CrossRef] [Green Version]
- Schorr, J.; Knapp, B.; Hundt, E.; Kupper, H.A.; Amann, E. Surface expression of malarial antigens in Salmonella typhimurium: Induction of serum antibody response upon oral vaccination of mice. Vaccine 1991, 9, 675–681. [Google Scholar] [CrossRef]
- Fischetti, V.A.; Medaglini, D.; Pozzi, G. Gram-positive commensal bacteria for mucosal vaccine delivery. Curr. Opin. Biotechnol. 1996, 7, 659–666. [Google Scholar] [CrossRef]
- Lieberman, L.A. Outer membrane vesicles: A bacterial-derived vaccination system. Front. Microbiol. 2022, 13, 1029146. [Google Scholar] [CrossRef] [PubMed]
- Van der Ley, P.A.; Zariri, A.; Van Riet, E.; Oosterhoff, D.; Kruiswijk, C.P. An intranasal OMV-based vaccine induces high mucosal and systemic protecting immunity against a SARS-CoV-2 infection. Front. Immunol. 2021, 12, 5303. [Google Scholar] [CrossRef] [PubMed]
- Haddad, H.F.; Roe, E.F.; Collier, J.H. Expanding opportunities to engineer mucosal vaccination with biomaterials. Biomater. Sci. 2023, 11, 1625–1647. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, V.; Norling, K.; Bally, M.; Hook, F.; Lycke, N.Y. Mucosal vaccine development based on liposome technology. J. Immunol. Res. 2016, 2016, 5482087. [Google Scholar] [CrossRef]
- Wu, F.; Qin, M.; Wang, H.; Sun, X. Nanovaccines to combat virus-related diseases. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1857. [Google Scholar] [CrossRef]
- He, J.; Yu, L.; Lin, X.; Liu, X.; Zhang, Y.; Yang, F.; Deng, W. Virus-like particles as nanocarriers for intracellular delivery of biomolecules and compounds. Viruses 2022, 14, 1905. [Google Scholar] [CrossRef]
- Garcia-Sastre, A. Mucosal delivery of RNA vaccines by Newcastle disease virus vectors. Curr. Res. Immunol. 2022, 3, 234–238. [Google Scholar] [CrossRef]
- Chen, H.; Ji, H.; Kong, X.; Lei, P.; Yang, Q.; Wu, W.; Jin, L.; Sun, D. Bacterial ghosts-based vaccine and drug delivery. Pharmaceutics 2021, 13, 1892. [Google Scholar] [CrossRef]
- Isticato, R.; Cangiano, G.; Tran, T.-H.; Ciabattini, A.; Medaglini, D.; Oggioni, M.R.; De Felice, M.; Pozzi, G.; Ricca, E. Surface display of recombinant proteins on Bacillus subtilis spores. J. Bacteriol. 2001, 183, 6294–6301. [Google Scholar] [CrossRef] [Green Version]
- Duc, L.H.; Huynh, H.A.; Fairweather, N.; Ricca, E.; Cutting, S.M. Bacterial Spores as Vaccine Vehicles. Infect. Immun. 2003, 71, 2810–2818. [Google Scholar] [CrossRef] [Green Version]
- Ricca, E.; Baccigalupi, L.; Cangiano, G.; De Felice, M.; Isticato, R. Mucosal vaccine delivery by non-recombinant spores of Bacillus subtilis. Microb. Cell Factories 2014, 13, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenney, P.T.; Driks, A.; Eichenberger, P. The Bacillus subtilis endospore: Assembly and function of the multilayered coat. Nat. Rev. Microbiol. 2013, 11, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, A.; Trauth, S.; Ziesack, M.; Nagler, K.; Bergeest, J.P.; Rohr, K.; Becker, N.; Höfer, T.; Bischofs, I.B. Phenotypic memory in Bacillus subtilis links dormancy entry and exit by a spore quantity-quality tradeoff. Nat. Commun. 2018, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paredes-Sabja, D.; Cid-Rojas, F.; Pizarro-Guajardo, M. Assembly of the exosporium layer in Clostridioides difficile spores. Curr. Opin. Microbiol. 2022, 67, 102137. [Google Scholar] [CrossRef] [PubMed]
- Cangiano, G.; Sirec, T.; Panarella, C.; Isticato, R.; Baccigalupi, L.; De Felice, M.; Ricca, E. The sps gene products affect germination, hydrophobicity and protein adsorption of Bacillus subtilis spores. Appl. Environ. Microbiol. 2014, 80, 7293–7302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois, T.; Krzewinski, F.; Yamakawa, N.; Lemy, C.; Hamiot, A.; Brunet, L.; Lacoste, A.S.; Knirel, Y.; Guerardel, Y.; Faille, C. The sps genes encode an original legionaminic acid pathway required for crust assembly in Bacillus subtilis. mBio 2020, 11, e01153-20. [Google Scholar] [CrossRef]
- Pesce, G.; Rusciano, G.; Sirec, T.; Isticato, R.; Sasso, A.; Ricca, E. Surface charge and hydrodynamic coefficient measurements of Bacillus subtilis spore by optical tweezers. Colloids Surf. B Biointerfaces 2014, 116, 568–575. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Krajcikova, D.; Zhu, R.; Ebner, A.; Cutting, S.; Gruber, H.J.; Barak, I.; Hinterdorfer, P. Atomic force microscopy imaging and single molecule recognition force spectroscopy of coat proteins on the surface of Bacillus subtilis spore. J. Mol. Recognit. 2007, 20, 483–489. [Google Scholar] [CrossRef]
- Ramamurthi, K.S.; Losick, R. ATP-driven self-assembly of a morphogenetic protein in Bacillus subtilis. Mol. Cell 2008, 31, 406–414. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Wan, Q.; Krajcikova, D.; Tang, J.; Tzokov, S.B.; Barak, I.; Bullogh, P.A. Diverse supramolecular structures formed by self-assembling proteins of the Bacillus subtilis spore coat. Molec. Microbiol. 2015, 97, 347–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janganan, T.K.; Mullin, N.; Dafis-Sagarmendi, A.; Brunt, J.; Tzokov, S.B.; Stringer, S.; Moir, A.; Chaudhuri, R.R.; Fagan, R.P.; Hobbs, J.K.; et al. Architecture and self-assembly of Clostridium sporogenes and Clostridium botulinum spore surfaces illustrate a general protective strategy across spore formers. mSphere 2020, 5, e00424-20. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, S.; Sorrentini, I.; Ricca, E.; De Felice, M.; Baccigalupi, L. Characterization of spore forming Bacilli isolated from the human gastrointestinal tract. J. Appl. Microbiol. 2008, 105, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.A.; To, E.; Fakhry, S.; Baccigalupi, L.; Ricca, E.; Cutting, S.M. Defining the natural habitat of Bacillus spore-formers. Res. Microbiol. 2009, 160, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Casula, G.; Cutting, S.M. Bacillus probiotics: Spore germination in the gastrointestinal tract. Appl. Environ. Microbiol. 2002, 68, 2344–2352. [Google Scholar] [CrossRef] [Green Version]
- Duc, L.H.; Hong, A.H.; Nguyen, Q.U.; Cutting, S.M. Intracellular fate and immunogenicity of B. subtilis spores. Vaccine 2004, 22, 1873–1885. [Google Scholar] [CrossRef]
- Cutting, S.M.; Hong, H.A.; Baccigalupi, L.; Ricca, E. Oral vaccine delivery by recombinant spore probiotics. Int. Rev. Immunol. 2009, 28, 487–505. [Google Scholar] [CrossRef] [PubMed]
- Rhee, K.-J.; Sethupathi, P.; Driks, A.; Lanning, D.K.; Knight, K.L. Role of commensal bacteria in development of gut-associated lymphoid tissue and preimmune antibody repertoire. J. Immunol. 2004, 172, 1118–1124. [Google Scholar] [CrossRef] [Green Version]
- D’Arienzo, R.; Maurano, F.; Mazzarella, G.; Luongo, D.; Stefanile, R.; Ricca, E.; Rossi, M. Bacillus subtilis spores reduce susceptibility to Citrobacter rodentium-mediated enteropathy in a mouse model. Res. Microbiol. 2006, 157, 891–897. [Google Scholar] [CrossRef]
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef]
- Saggese, A.; Baccigalupi, L.; Ricca, E. Spore formers as beneficial microbes for humans and animals. Appl. Microbiol. 2021, 1, 498–509. [Google Scholar] [CrossRef]
- Mauriello, E.M.F.; Duc, L.H.; Isticato, R.; Cangiano, G.; Hong, H.A.; De Felice, M.; Ricca, E.; Cutting, S.M. Display of heterologous antigens on the Bacillus subtilis spore coat using CotC as a fusion partner. Vaccine 2004, 22, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Negri, A.; Potocki, W.; Iwanicki, A.; Obuchowski, M.; Hinc, K. Expression and display of Clostridium difficile protein FliD on the surface of Bacillus subtilis spores. J. Med. Microbiol. 2013, 62, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Piekarska, A.; Pełka, P.; Peszyńska-Sularz, G.; Negri, A.; Hinc, K.; Obuchowski, M.; Iwanicki, A. The choice of the anchoring protein influences the interaction of recombinant Bacillus spores with the immune system. Acta Biochim. Pol. 2017, 64, 239–244. [Google Scholar] [CrossRef]
- Duc, L.H.; Hong, H.A.; Atkins, H.S.; Flick-Smith, H.C.; Durrani, Z.; Rijpkema, S.; Titball, R.W.; Cutting, S.M. Immunization against anthrax using Bacillus subtilis spores expressing the anthrax protective antigen. Vaccine 2007, 25, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Hinc, K.; Isticato, R.; Dembek, M.; Karczewska, J.; Iwanicki, A.; Peszyńska-Sularz, G.; De Felice, M.; Obuchowski, M.; Ricca, E. Expression and display of UreA of Helicobacter acinonychis on the surface of Bacillus subtilis spores. Microb. Cell Factories 2010, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Permpoonpattana, P.; Hong, H.A.; Phetcharaburanin, J.; Huang, J.M.; Cook, J.; Fairweather, N.; Cutting, S.M. Immunization with Bacillus spores expressing toxin A peptide repeats protects against infection with Clostridium difficile. Infect. Immun. 2011, 79, 2295–2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang, T.H.; Hong, H.A.; Clark, G.C.; Titball, R.W.; Cutting, S.M. Recombinant Bacillus subtilis expressing the Clostridium perfringens alpha toxoid is a candidate orally delivered vaccine against necrotic enteritis. Infect. Immun. 2008, 76, 5257–5265. [Google Scholar] [CrossRef] [Green Version]
- Ning, D.; Leng, X.; Li, Q.; Xu, W. Surface-displayed VP28 on Bacillus subtilis spores induces protection against white spot syndrome virus in crayfish by oral administration. J. Appl. Microbiol. 2011, 111, 1327–1336. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Pham, C.K.; Pham, H.T.; Pham, H.L.; Nguyen, A.H.; Dang, L.T.; Huynh, H.A.; Cutting, S.M.; Phan, T.N. Bacillus subtilis spores expressing the VP28 antigen: A potential oral treatment to protect Litopenaeus vannamei against white spot syndrome. FEMS Microbiol. Lett. 2014, 358, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Miao, Y.; Guo, Y.; Qiu, H.; Sun, S.; Kou, Z.; Yu, H.; Li, J.; Chen, Y.; Jiang, S.; et al. Development of a heat-stable and orally delivered recombinant M2e-expressing B. subtilis spore-based influenza vaccine. Hum. Vaccines Immunother. 2014, 10, 3649–3658. [Google Scholar] [CrossRef] [Green Version]
- Batista, M.T.; Souza, R.D.; Paccez, J.D.; Luiz, W.B.; Ferreira, E.L.; Cavalcante, R.C.; Ferreira, R.C.; Ferreira, L.C. Gut adhesive Bacillus subtilis spores as a platform for mucosal delivery of antigens. Infect. Immun. 2014, 82, 1414–1423. [Google Scholar] [CrossRef] [Green Version]
- Sibley, L.; Reljic, R.; Radford, D.S.; Huang, J.M.; Hong, H.A.; Cranenburgh, R.M. Recombinant spores expressing MPT64 evaluated as a vaccine against tuberculosis in the murine model. FEMS Microbiol. Lett. 2014, 358, 170–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maia, A.R.; Reyes-Ramírez, R.; Pizarro-Guajardo, M.; Saggese, A.; Ricca, E.; Baccigalupi, L.; Paredes-Sabja, D. Nasal Immunization with the C-Terminal Domain of Bcla3 Induced Specific IgG Production and Attenuated Disease Symptoms in Mice Infected with Clostridioides difficile Spores. Int. J. Mol. Sci. 2020, 13, 6696. [Google Scholar] [CrossRef]
- Cao, Y.-G.; Li, Z.-H.; Yue, Y.-Y.; Song, N.-N.; Peng, L.; Wang, L.-X. Construction and evaluation of a novel spores-based enterovirus 71 vaccine. J. Appl. Biomed. 2013, 11, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Hen, C.; Li, Y.L.; Lv, F.L.; Xu, L.D.; Huang, Y.W. Surface Display of Peptides Corresponding to the Heptad Repeat 2 Domain of the Feline Enteric Coronavirus Spike Protein on Bacillus subtilis Spores Elicits Protective Immune Responses Against Homologous Infection in a Feline Aminopeptidase-N-Transduced Mouse Model. Front. Immunol. 2022, 28, 925922. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, M.; Chen, H.; Wei, Y.; Ning, D. Germination-Arrest Bacillus subtilis Spores as An Oral Delivery Vehicle of Grass Carp Reovirus (GCRV) Vp7 Antigen Augment Protective Immunity in Grass Carp (Ctenopharyngodon idella). Genes 2020, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, J.; Dai, X.; Liu, M.; Khalique, A.; Wang, Z.; Zeng, Y.; Zhang, D.; Ni, X.; Zeng, D.; et al. Surface Display of Porcine circovirus type 2 antigen protein cap on the spores of Bacillus subtilis 168: An effective mucosal vaccine candidate. Front. Immunol. 2022, 13, 1007202. [Google Scholar] [CrossRef]
- Katsande, P.M.; Fernández-Bastit, L.; Ferreira, W.T.; Vergara-Alert, J.; Hess, M.; Lloyd-Jones, K.; Hong, H.A.; Segales, J.; Cutting, S.M. Heterologous systemic prime–intranasal boosting using a spore SARS-CoV-2 vaccine confers mucosal immunity and cross-reactive antibodies in mice as well as protection in hamsters. Vaccines 2022, 10, 1900. [Google Scholar] [CrossRef]
- Zhou, Z.; Dong, H.; Huang, Y.; Yao, S.; Liang, B.; Xie, Y.; Long, Y.; Mai, J.; Gong, S. Recombinant Bacillus subtilis spores expressing cholera toxin B subunit and Helicobacter pylori urease B confer protection against H. pylori in mice. J. Med. Microbiol. 2017, 66, 83–89. [Google Scholar] [CrossRef]
- Zhou, Z.; Xia, H.; Hu, X.; Huang, Y.; Li, Y.; Li, L.; Ma, C.; Chen, X.; Hu, F.; Xu, J.; et al. Oral administration of a Bacillus subtilis spore-based vaccine expressing Clonorchis sinensis tegumental protein 22.3 kDa confers protection against Clonorchis sinensis. Vaccine 2008, 28, 1817–1825. [Google Scholar] [CrossRef]
- Lin, Z.; Sun, H.; Ma, Y.; Zhou, X.; Jiang, H.; Wang, X.; Song, J.; Tang, Z.; Bian, Q.; Zhang, Z.; et al. Evaluation of immune response to Bacillus subtilis spores expressing Clonorchis sinensis serpin3. Parasitology 2020, 147, 1080–1087. [Google Scholar] [CrossRef]
- D’Apice, L.; Sartorius, R.; Caivano, A.; Mascolo, D.; Del Pozzo, G.; Di Mase, D.S.; Ricca, E.; Pira, G.L.; Manca, F.; Malanga, D.; et al. Comparative analysis of new innovative vaccine formulations based on the use of procaryotic display systems. Vaccine 2007, 25, 1993–2000. [Google Scholar] [CrossRef]
- Li, L.; Hu, X.; Wu, Z.; Xiong, S.; Zhou, Z.; Wang, X.; Xu, J.; Lu, F.; Yu, X. Immunogenicity of self-adjuvanticity oral vaccinecandidate based on use of Bacillus subtilis spore displaying Schistosoma japonicum 26 KDa GST protein. Parasitol. Res. 2009, 105, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tang, Q.; Chen, H.; Yao, Q.; Ning, D.; Chen, K. Display of Bombyx mori nucleopolyhedrovirus GP64 on the Bacillus subtilis spore coat. Curr. Microbiol. 2011, 62, 1368–1373. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.; Tian, Y.; Mao, Q.; Lv, X.; Shang, M.; Li, X.; Yu, X.; Huang, Y. Surface display of Clonorchis sinensis enolase on Bacillus subtilis spores potentializes an oral vaccine candidate. Vaccine 2014, 10, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lin, Z.; Zhao, L.; Chen, T.; Shang, M.; Jiang, H.; Tang, Z.; Zhou, X.; Shi, M.; Zhou, L.; et al. Bacillus subtilis spore with surface display of paramyosin from Clonorchis sinensis potentializes a promising oral vaccine candidate. Parasit. Vectors 2018, 7, 156. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Liu, M.; Pan, K.; Yang, J. Surface display of OmpC of Salmonella serovar Pullorum on Bacillus subtilis spores. PLoS ONE 2018, 25, e0191627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Bian, Q.; Zeng, W.; Ren, P.; Sun, H.; Lin, Z.; Tang, Z.; Zhou, X.; Wang, Q.; Wang, Y.; et al. Oral delivery of Bacillus subtilis spores expressing grass carp reovirus VP4 protein produces protection against grass carp reovirus infection. Fish Shellfish Immunol. 2019, 84, 768–780. [Google Scholar] [CrossRef]
- Gao, Y.; Huo, X.; Wang, Z.; Yuan, G.; Liu, X.; Ai, T.; Su, J. Oral Administration of Bacillus subtilis Subunit Vaccine Significantly Enhances the Immune Protection of Grass Carp against GCRV-II Infection. Viruses 2021, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Valdez, A.; Yepiz-Plascencia, G.; Ricca, E.; Olmos, J. First Litopenaeus vannamei WSSV 100% oral vaccination protection using CotC::Vp26 fusion protein displayed on Bacillus subtilis spores surface. J. Appl. Microbiol. 2014, 117, 347–357. [Google Scholar] [CrossRef]
- Mai, W.; Yan, B.; Xin, J. Oral immunizations with Bacillus subtilis spores expressing MCP protein provide protection against red-spotted grouper nervous necrosis virus (RGNNV) infection in juvenile grouper, Epinephelus coioides. Aquaculture 2022, 552, 738008. [Google Scholar] [CrossRef]
- Gonçalves, G.; Santos, R.A.; Coutinho, F.; Pedrosa, N.; Curado, M.; Machado, M.; Costas, B.; Bonneville, L.; Serrano, M.; Carvalho, A.P.; et al. Oral vaccination of fish against vibriosis using spore-display technology. Front. Immunol. 2022, 13, 1012301. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.Y.; Chen, D.D.; Cui, Z.W.; Zhang, X.Y.; Zhou, Y.Y.; Guo, X.; Li, A.H.; Zhang, Y.A. Oral vaccination of tilapia against Streptococcus agalactiae using Bacillus subtilis spores expressing Sip. Fish Shellfish Immunol. 2019, 86, 999–1008. [Google Scholar]
- Vetráková, A.; Chovanová, R.K.; Rechtoríková, R.; Krajčíková, D.; Barák, I. Bacillus. subtilis spores displaying RBD domain of SARS-CoV-2 spike protein. Comput. Struct. Biotechnol. J. 2023, 21, 1550–1556. [Google Scholar] [CrossRef]
- Iwanicki, A.; Piątek, I.; Stasiłojć, M.; Grela, A.; Lęga, T.; Obuchowski, M.; Hinc, K. A system of vectors for Bacillus subtilis spore surface display. Microb. Cell Factories 2014, 13, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, C.; Chan, W.C.; McKeithan, W.; Nickerson, K.W. Surface display of recombinant proteins on Bacillus thuringiensis spores. Appl. Environ. Microbiol. 2005, 71, 3337–3341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, T.J.; Choi, S.-K.; Jung, H.-C.; Lee, S.Y.; Pan, J.-G. Spore display using Bacillus thuringiensis exosporium protein InhA. J. Microbiol. Biotechnol. 2009, 19, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.E.; Kye, Y.C.; Park, S.M.; Shim, B.S.; Yoo, S.; Hwang, E.; Kim, H.; Kim, S.J.; Han, S.H.; Park, T.S.; et al. Bacillus subtilis spores as adjuvants against avian influenza H9n2 induce antigen-specific antibody and T cell responses in white leghorn chickens. Vet. Res. 2020, 51, 68. [Google Scholar] [CrossRef]
- Pan, J.G.; Choim, S.K.; Jung, H.C.; Kim, E.J. Display of native proteins on Bacillus. subtilis spores. FEMS Microbiol. Lett. 2014, 358, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Oh, Y.; Kim, J.A.; Kim, C.-H.; Choi, S.-K.; Pan, J.-G. Bacillus subtilis spore vaccines displaying protective antigen induce functional antibodies and protective potency. BMC Veter. Res. 2020, 16, 259. [Google Scholar] [CrossRef]
- Huang, J.M.; Hong, H.A.; Tong, H.V.; Hoang, T.H.; Brisson, A.; Cutting, S.M. Mucosal delivery of antigens using adsorption to bacterial spores. Vaccine 2010, 28, 1021–1030. [Google Scholar] [CrossRef]
- Ricca, E.; Baccigalupi, L.; Isticato, R. Spore-adsorption: Mechanism and applications of a non-recombinant display system. Biotechnol. Adv. 2021, 47, 107693. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.L.; Narayan, K.; Castaing, J.-P.; Tian, F.; Subramaniam, S.; Ramamurthi, K.S. A versatile nano display platform from bacterial spore coat proteins. Nat. Commun. 2015, 6, 6777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, C.M.; Schraner, E.M.; Aguilar, C.; Eichwald, C. Heterologous expression of antigenic peptides in Bacillus subtilis biofilm. Microb. Cell Factories 2016, 15, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, C.M.; Armua-Fernandez, M.T.; Tobler, K.; Hilbe, M.; Ackermann, M.; Deplazes, P.; Aguilar, C.; Eichwald, C. Oral application of recombinant Bacillus subtilis spores to dogs results in a humoral response against specific Echinococcus granulosus paramyosin and tropomyosin antigens. Infect. Immun. 2018, 86, e00495-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maia, A.R.; Reyes-Ramírez, R.; Pizarro-Guajardo, M.; Saggese, A.; Castro-Córdova, P.; Isticato, R.; Ricca, E.; Paredes-Sabja, D.; Baccigalupi, L. Induction of a Specific Humoral Immune Response by Nasal Delivery of Bcla2ctd of Clostridioides difficile. Int. J. Mol. Sci. 2020, 21, 1277. [Google Scholar] [CrossRef] [Green Version]
- Donadio, G.; Lanzilli, M.; Sirec, T.; Ricca, E.; Isticato, R. Localization of a red fluorescence protein adsorbed on wild type and mutant spores of Bacillus subtilis. Microb. Cell Factories 2016, 15, 153. [Google Scholar] [CrossRef] [Green Version]
- Lanzilli, M.; Donadio, G.; Addevico, R.; Saggese, A.; Cangiano, G.; Baccigalupi, L.; Christie, G.; Ricca, E.; Isticato, R. The Exosporium of Bacillus megaterium QM B1551 Is Permeable to the Red Fluorescence Protein of the Coral Discosoma sp. Front. Microbiol. 2016, 7, 1752. [Google Scholar] [CrossRef] [Green Version]
- Setlow, P. Germination of spores of Bacillus species: What we know and do not know. J. Bacteriol. 2014, 196, 1297–1305. [Google Scholar] [CrossRef] [Green Version]
- Saggese, A.; Di Gregorio Barletta, G.; Vittoria, M.; Donadio, G.; Isticato, R.; Baccigalupi, L.; Ricca, E. CotG Mediates Spore Surface Permeability in Bacillus subtilis. mbio 2022, 13, e0276022. [Google Scholar] [CrossRef]
- Sirec, T.; Strazzulli, A.; Isticato, R.; De Felice, M.; Moracci, M.; Ricca, E. Adsorption of β-galactosidase of Alicyclobacillus acidocaldarius on wild type and mutants spores of Bacillus subtilis. Microb. Cell Factories 2012, 11, 100. [Google Scholar] [CrossRef] [Green Version]
- Sirec, T.; Cangiano, G.; Baccigalupi, L.; Ricca, E.; Isticato, R. The spore surface of intestinal isolates of Bacillus subtilis. FEMS Microbiol. Lett. 2014, 358, 194–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrillo, C.; Castaldi, S.; Lanzilli, M.; Saggese, A.; Donadio, G.; Baccigalupi, L.; Ricca, E.; Isticato, R. The temperature of growth and sporulation modulates the efficiency of spore-display in Bacillus subtilis. Microb. Cell Factories 2020, 19, 185. [Google Scholar] [CrossRef]
- Song, M.; Hong, H.A.; Huang, J.M.; Colenutt, C.; Khang, D.D.; Nguyen, T.V.; Park, S.M.; Shim, B.S.; Song, H.H.; Cheon, I.S.; et al. Killed Bacillus subtilis spores as a mucosal adjuvant for an H5N1 vaccine. Vaccine 2012, 30, 3266–3277. [Google Scholar] [CrossRef]
- Reljic, R.; Sibley, L.; Huang, J.M.; Pepponi, I.; Hoppe, A.; Hong, H.A.; Cutting, S.M. Mucosal vaccination against tuberculosis using inert bioparticles. Infect. Immun. 2013, 81, 4071–4080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-M.; Park, S.-M.; Kim, J.-A.; Park, J.-A.; Yi, M.-H.; Kim, N.-S.; Bae, J.-L.; Park, G.S.; Jang, J.-S.; Yang, M.-S.; et al. Functional pentameric formation via coexpression of the Escherichia coli heat-labile enterotoxin B subunit and its fusion protein subunit with a Neutralizing Epitope of ApxIIA Exotoxin improves the mucosal immunogenicity and protection against challenge by Actinobacillus pleuropneumoniae. Clin. Vaccine Immunol. 2011, 18, 2168–2177. [Google Scholar] [CrossRef] [Green Version]
- Ricci, S.; Medaglini, D.; Rush, C.M.; Marcello, A.; Peppoloni, S.; Manganelli, R.; Palú, G.; Pozzi, G. Immunogenicity of the B monomer of Escherichia coli heatlabile toxin expressed on the surface of Streptococcus gordonii. Infect. Immun. 2000, 68, 760–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.-M.; La Ragione, R.; Nunez, A.; Cutting, S.M. Immunostimulatory activity of Bacillus spores. FEMS Immunol. Med. Microbiol. 2008, 53, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Schauer, D.B.; Falkow, S. The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect. Immun. 1993, 61, 4654–4661. [Google Scholar] [CrossRef] [Green Version]
- La Ragione, R.M.; Woodward, M.J. Competitive exclusion by Bacillus subtilis spores of Salmonella enterica serotype Enteritidis and Clostridium perfringens in young chickens. Vet. Microbiol. 2003, 94, 245–256. [Google Scholar] [CrossRef]
- La Ragione, R.M.; Casula, G.; Cutting, S.M.; Woodward, M.J. Bacillus subtilis spores competitively exclude Escherichia coli O78:K80 in poultry. Vet. Microbiol. 2001, 79, 113–142. [Google Scholar] [CrossRef]
- Ciabattini, A.; Parigi, R.; Isticato, R.; Oggioni, M.; Pozzi, G. Oral priming of mice by recombinant spores of Bacillus subtilis. Vaccine 2004, 22, 4139–4143. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, E.M.; Cangiano, G.; Maurano, F.; Saggese, V.; De Felice, M.; Rossi, M.; Ricca, E. Germination-independent induction of cellular immune response by Bacillus subtilis spores displaying the C fragment of the tetanus toxin. Vaccine 2007, 25, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Potocki, W.; Negri, A.; Peszyńska-Sularz, G.; Hinc, K.; Obuchowski, M.; Iwanicki, A. The combination of recombinant and non-recombinant Bacillus subtilis spore display technology for presentation of antigen and adjuvant on single spore. Microb. Cell Factories 2017, 16, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.A.; Huynh, H.A.; Hoang, T.V.; Ninh, N.T.; Pham, A.T.; Nguyen, H.A.; Phan, T.N.; Cutting, S.M. Killed Bacillus subtilis spores expressing streptavidin: A novel carrier of drugs to target cancer cells. J. Drug Target 2013, 21, 528–541. [Google Scholar] [CrossRef]



| Pathogen | Trade Name | Composition | Route, Dose | Immunological Mechanism | Efficacy |
|---|---|---|---|---|---|
| Rotavirus | Rotarix; RotaTeq | Live attenuated | Oral, 3 doses | sIgA and systemic neutralizing IgG | Over 70–90% |
| Poliovirus | Orimune; OPV; Poliomyelitis vaccine | Live attenuated | Oral, 3 doses | sIgA and systemic IgG | Over 90% |
| Salmonella typhi | Vivotif; Ty21A | Live attenuated | Oral, 3–4 doses | sIgA, systemic IgG and CTL responses | Variable, but more than 50% |
| Vibrio cholera | Dukoral; ORC-Vax; Shanchol | Inactivated V. cholera | Oral, 2–3 doses | Antibacterial, toxin-specific and LPS-specific IgA | Over 85% |
| Influenza Virus A | FluMist Quadrivalent® | Antigens incorporated into live attenuated, cold adapted influenza vector | Nasal, 1 dose | Mucosal IgA and systemic IgG | Over 90% |
| Influenza Virus A and B | Fluenz Tetra® | Antigens into live attenuated, cold-adapted influenza vector | Nasal, 1 dose | Mucosal IgA, systemic IgG and CTL responses | Variable, but more than 50% |
| Carriers | Antigens | References |
|---|---|---|
| CotB | TTFC of Clostridium tetani | [40] |
| LTB of Escherichia coli | [62] | |
| FliD of Clostridium difficile | [63,64] | |
| PA of Bacillus anthracis | [65] | |
| UreA of Helicobacter acinonychis | [66] | |
| TcdA-TcdB of Clostridium difficile | [67] | |
| Cpa of Clostridium perfringens | [68] | |
| VP28 of White Spot Syndrome Virus | [69,70] | |
| M2 protein of influenza virus | [71] | |
| SlpA of Lactobacillus brevis | [72] | |
| InvA of Yersinia pseudotuberculosis | [72] | |
| MPT64 of Mycobacterium tuberculosis | [73] | |
| BclA3 of Clostridium difficile | [74] | |
| VP1 capsid protein of Enterovirus 71 | [75] | |
| HR2P of SARS-CoV-2 spike | [76] | |
| PCV2 Cap protein of Porcine circovirus | [77] | |
| Vp7 of grass carp reovirus | [78] | |
| RBD of SARS-CoV-2 spike | [79] | |
| CotC | TTFC of Clostridium tetani | [40] |
| LTB of Escherichia coli | [62] | |
| FliD of Clostridium difficile | [63,64] | |
| PA of Bacillus anthracis | [65] | |
| UreA of Helicobacter acinonychis | [66] | |
| TcdA-TcdB of Clostridium difficile | [67] | |
| UreB of Helicobacter pylori | [80] | |
| TP22.3 of Clonorchis sinensis | [81] | |
| CsSerpin3 of Clonorchis sinensis | [82] | |
| Pep23 of HIV | [83] | |
| GST of Schistosoma japonicum | [84] | |
| GP64 of Bombyx mori | [85] | |
| Enolase of Clonorchis sinensis | [86] | |
| Paramyosin of Clonorchis sinensis | [87] | |
| OmpC of Salmonella serovar Pullorum | [88] | |
| VP4 of Grass carp reovirus | [89] | |
| VP56 of Grass carp reovirus | [90] | |
| Vp26 of White spot syndrome virus | [69,91] | |
| Vp7 of grass carp reovirus | [78] | |
| MCP of Nervous necrosis virus (RGNNV) | [92] | |
| HR1HR2 of SARS-CoV-2 spike | [79] | |
| Sip of Streptococcus agalactiae | [93] | |
| CotG | UreA of Helicobacter acinonychis | [66] |
| FliD of Clostridium difficile | [63,64] | |
| CotY | OmpK of Vibrio vulnificus | [94] |
| RBD of SARS-CoV-2 spike | [95] | |
| CotZ | FliD of Clostridium difficile | [63,64] |
| UreA of Helicobacter acinonychis | [66] | |
| RBD of SARS-CoV-2 spike | [95] | |
| CgeA | CagA of Helicobacter pylori | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saggese, A.; Baccigalupi, L.; Donadio, G.; Ricca, E.; Isticato, R. The Bacterial Spore as a Mucosal Vaccine Delivery System. Int. J. Mol. Sci. 2023, 24, 10880. https://doi.org/10.3390/ijms241310880
Saggese A, Baccigalupi L, Donadio G, Ricca E, Isticato R. The Bacterial Spore as a Mucosal Vaccine Delivery System. International Journal of Molecular Sciences. 2023; 24(13):10880. https://doi.org/10.3390/ijms241310880
Chicago/Turabian StyleSaggese, Anella, Loredana Baccigalupi, Giuliana Donadio, Ezio Ricca, and Rachele Isticato. 2023. "The Bacterial Spore as a Mucosal Vaccine Delivery System" International Journal of Molecular Sciences 24, no. 13: 10880. https://doi.org/10.3390/ijms241310880
APA StyleSaggese, A., Baccigalupi, L., Donadio, G., Ricca, E., & Isticato, R. (2023). The Bacterial Spore as a Mucosal Vaccine Delivery System. International Journal of Molecular Sciences, 24(13), 10880. https://doi.org/10.3390/ijms241310880








