Discovery and Mechanism of Novel 7-Aliphatic Amine Tryptanthrin Derivatives against Phytopathogenic Bacteria
Abstract
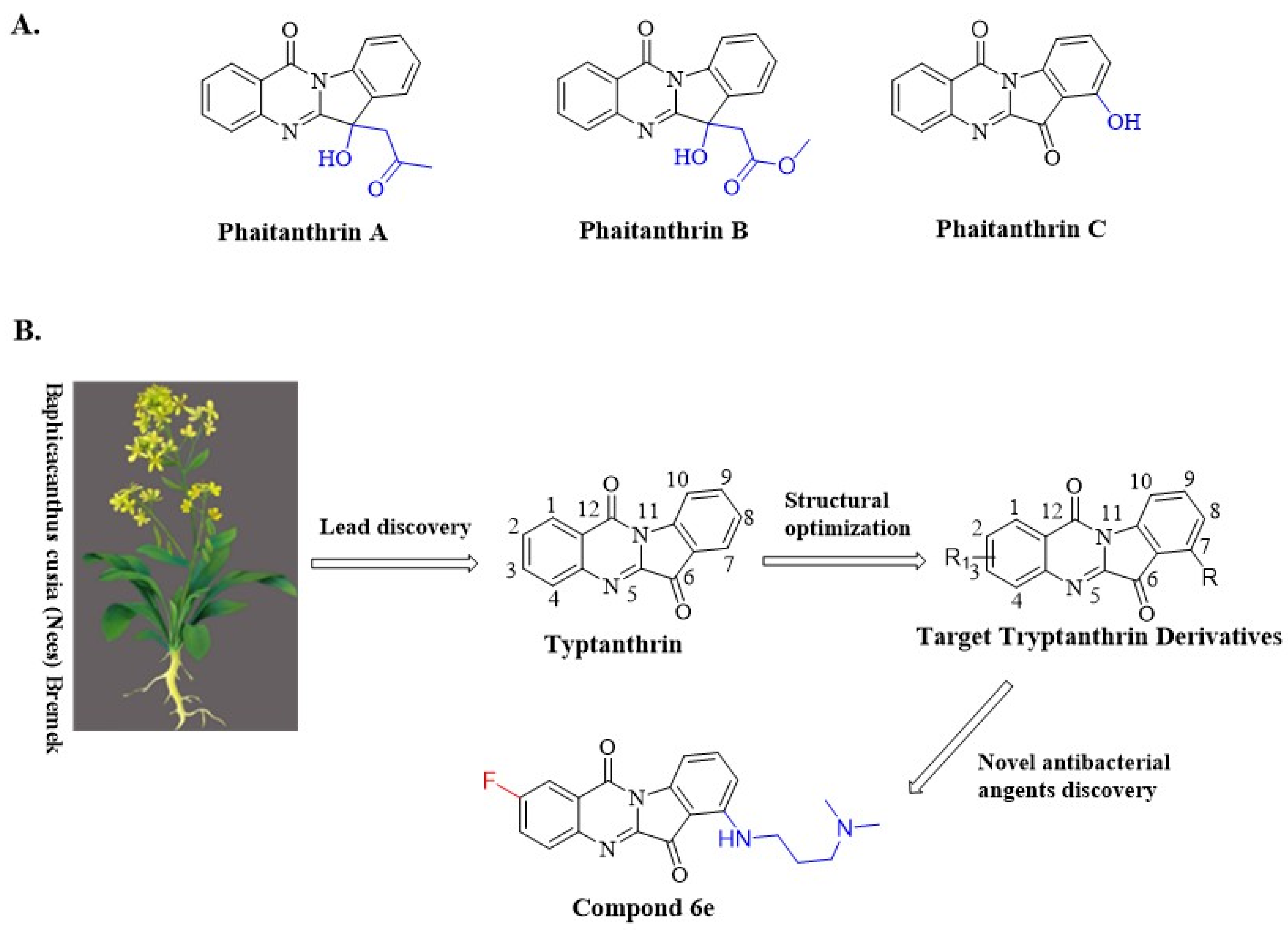
:1. Introduction
2. Results and Discussion
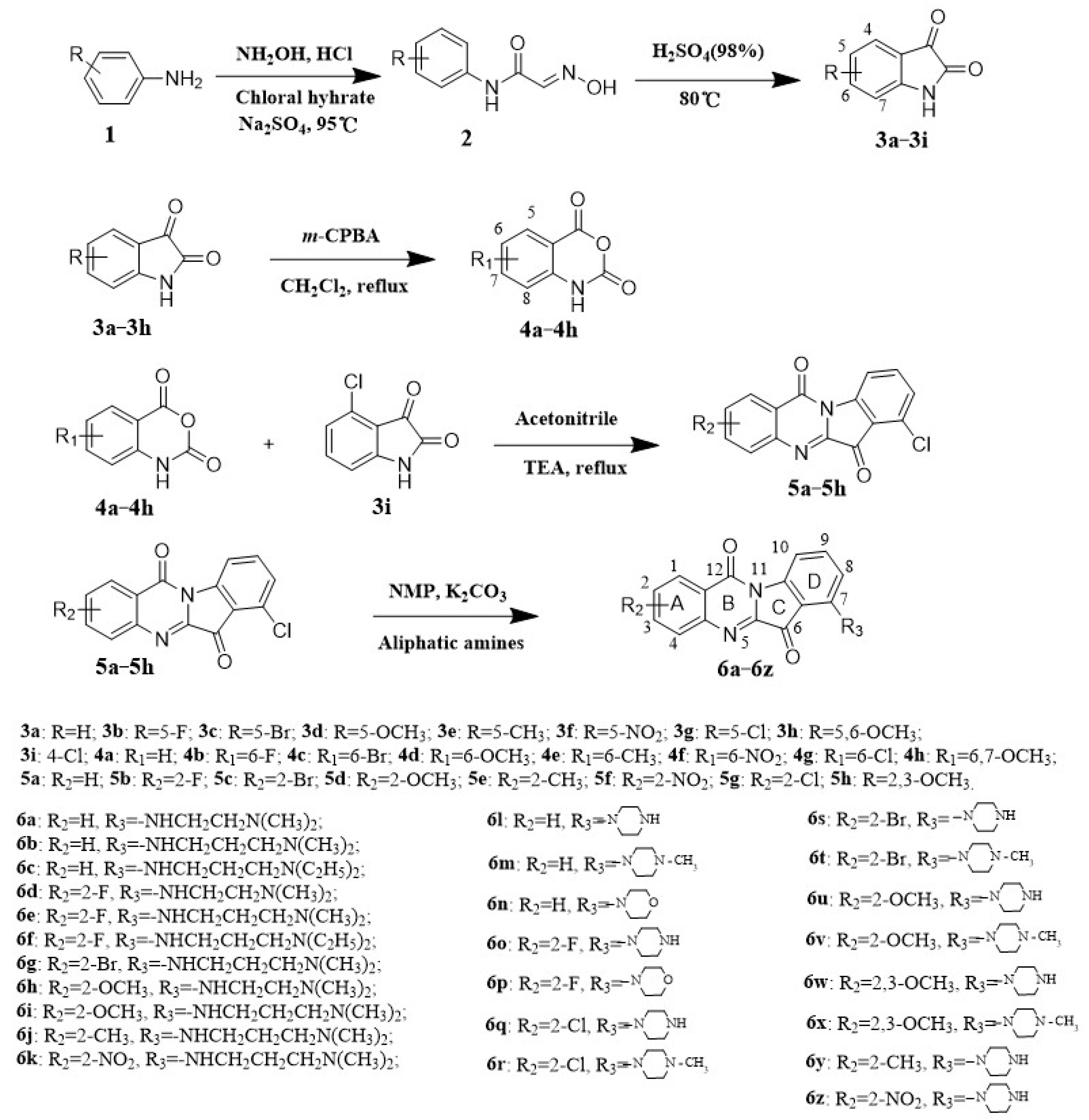
2.1. Chemistry
2.2. Biological Activity
2.2.1. Antibacterial Activity of Target Compounds 6a–6z
2.2.2. In Vivo Bioassay Results of Compound 6e against Rice Bacterial Leaf Blight
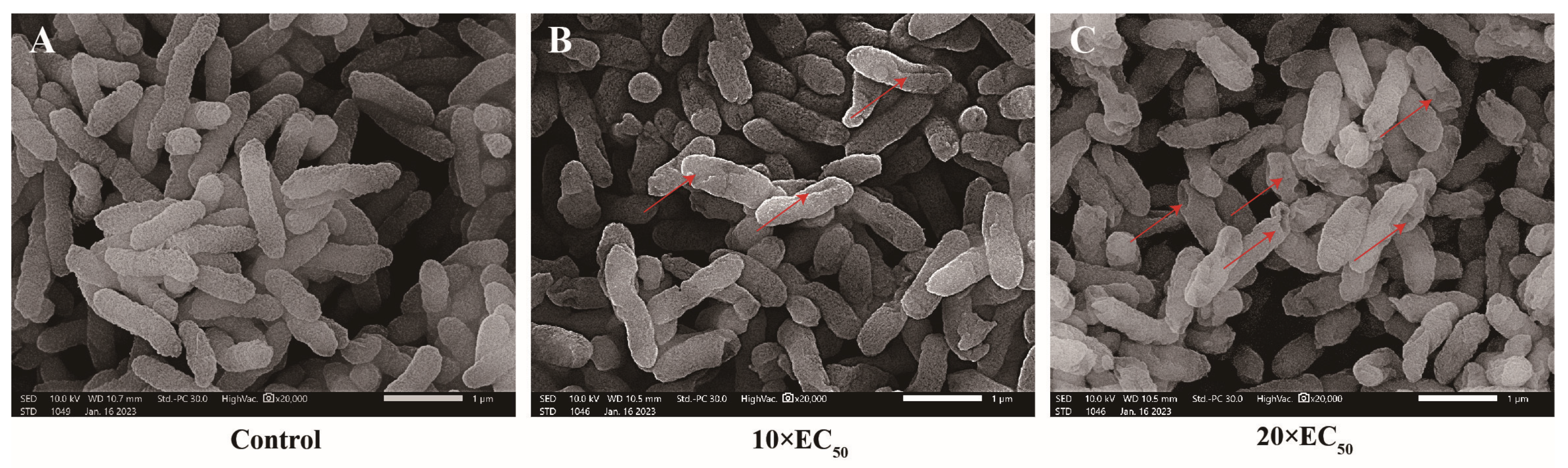
2.2.3. Effect of Compound 6e on the Morphology of Xoo Cells
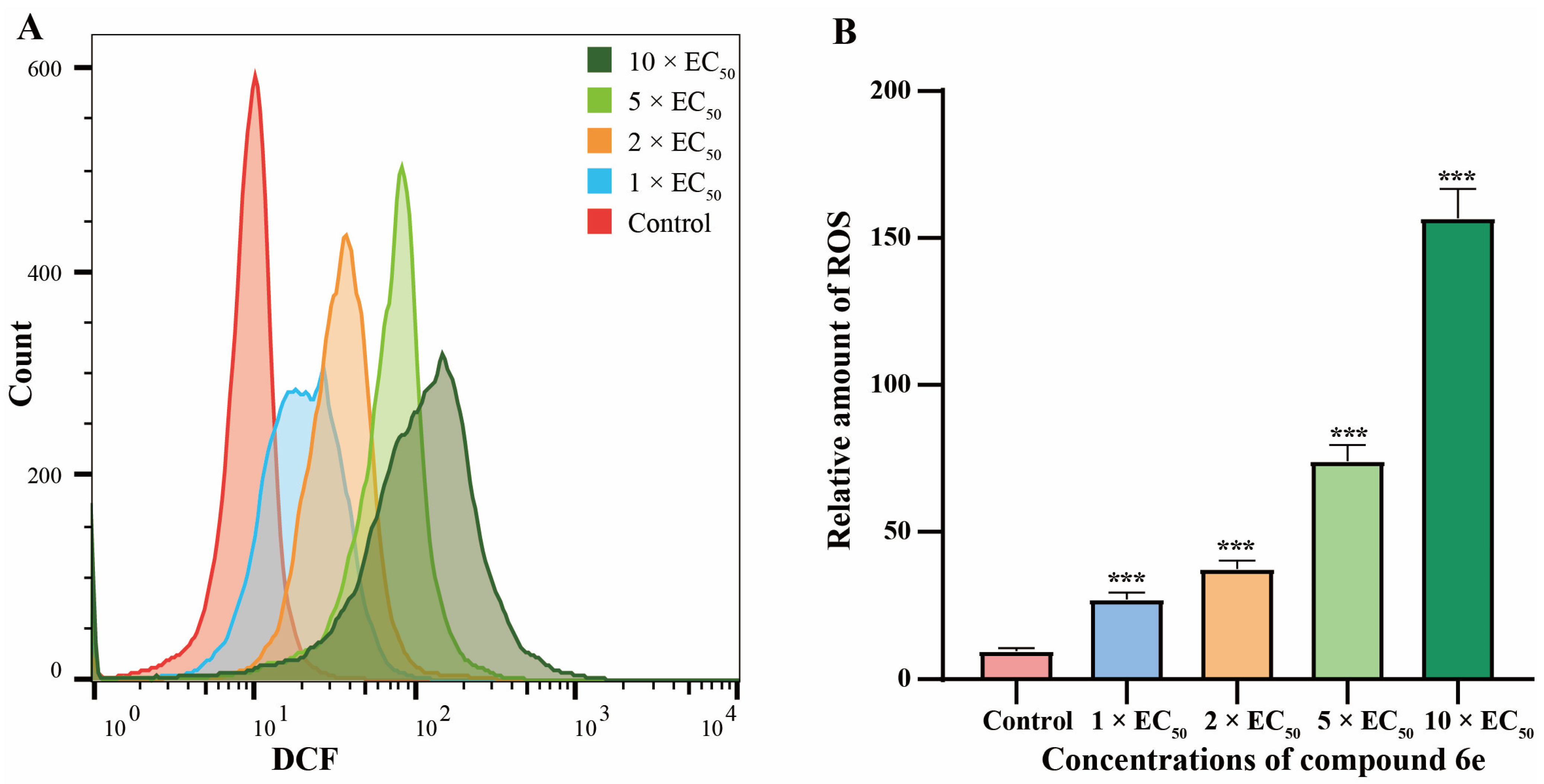
2.2.4. Compound 6e Induced ROS Accumulation
2.2.5. Compound 6e Induced Bacterial Cells’ Apoptosis
2.2.6. Compound 6e Inhibited the Formation of Bacterial Biofilms
3. Materials and Methods
3.1. Chemistry
3.1.1. General Synthesis Procedure for Intermediates 3a–3i and 4a–4h
3.1.2. General Synthesis Procedure for Intermediates 5a–5h
3.1.3. General Procedures for the Synthesis of Target Compounds 6a–6z
3.2. In Vitro Antibacterial Bioassay
3.3. In Vivo Assay against Rice Bacterial Blight
3.4. Morphological Investigations Using SEM
3.5. Detection of Reactive Oxygen Species
3.6. Induction of Apoptosis in Pathogenic Bacterial Cells
3.7. Bacterial Biofilm Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, N.; Yan, J.; Liang, Y.; Shi, Y.L.; He, Z.Z.; Wu, Y.T.; Zeng, Q.; Liu, X.L.; Peng, J.H. Resistance Genes and their Interactions with Bacterial Blight/Leaf Streak Pathogens (Xanthomonas oryzae) in Rice (Oryza sativa L.)-an Updated Review. Rice 2020, 13, 3. [Google Scholar] [CrossRef] [Green Version]
- Xiang, J.; Liu, D.Y.; Chen, J.X.; Hu, D.Y.; Song, B.A. Design and synthesis of novel 1,3,4-oxadiazole sulfone compounds containing 3,4-dichloroisothiazolylamide moiety and evaluation of rice bacterial activity. Pestic. Biochem. Physiol. 2020, 170, 104695. [Google Scholar] [CrossRef]
- Sanya, D.R.A.; Syed-Ab-Rahman, S.F.; Jia, A.; Onésime, D.; Kim, K.M.; Ahohuendo, B.C.; Rohr, J.R. A review of approaches to control bacterial leaf blight in rice. World J. Microbiol. Biotechnol. 2022, 38, 113. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.B.; Arul, L.; Ramalingam, J.; Uthandi, S. Advances in the Xoo-rice pathosystem interaction and its exploitation in disease management. J. Biosci. 2020, 45, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Wang, S.P. Rice versus Xanthomonas oryzae pv. oryzae: A unique pathosystem. Curr. Opin. Plant Biol. 2013, 16, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Shekhar, S.; Sinha, D.; Kumari, A. An Overview of Bacterial Leaf Blight Disease of Rice and Different Strategies for its Management. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 2250–2265. [Google Scholar] [CrossRef]
- Niño-Liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hu, D.Y.; Xie, D.D.; Chen, J.X.; Jin, L.H.; Song, B.A. Design, synthesis, and evaluation of new sulfone derivatives containing a 1,3,4-oxadiazole moiety as active antibacterial agents. J. Agric. Food Chem. 2018, 66, 3093–3100. [Google Scholar] [CrossRef]
- Wang, M.W.; Zhu, H.H.; Wang, P.Y.; Zeng, D.; Wu, Y.Y.; Liu, L.W.; Wu, Z.B.; Li, Z.; Yang, S. Synthesis of Thiazolium-Labeled 1,3,4-Oxadiazole Thioethers as Prospective Antimicrobials: In Vitro and in Vivo Bioactivity and Mechanism of Action. J. Agric. Food Chem. 2019, 67, 12696–12708. [Google Scholar] [CrossRef]
- Alexandrino, D.A.M.; Almeida, C.M.R.; Mucha, A.P.; Carvalho, M.F. Revisiting pesticide pollution: The case of fluorinated pesticides. Environ. Pollut. 2022, 292, 118315. [Google Scholar] [CrossRef]
- Zhang, J.H.; Wei, C.L.; Li, S.Y.; Hu, D.Y.; Song, B.A. Discovery of novel bis-sulfoxide derivatives bearing acylhydrazone and benzothiazole moieties as potential antibacterial agents. Pestic. Biochem. Physiol. 2020, 167, 104605. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Peng, F.; Zhu, Y.Y.; Cao, X.; Wang, Q.F.; Liu, F.; Liu, L.W.; Xue, W. Design, synthesis, biological activity evaluation and mechanism of action of myricetin derivatives containing thioether quinazolinone. Arab. J. Chem. 2022, 15, 104019. [Google Scholar] [CrossRef]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef]
- Chen, J.C.; Li, W.L.; Yao, H.Q.; Xu, J.Y. Insights into drug discovery from natural products through structural modification. Fitoterapia 2015, 103, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Hesse-Macabata, J.; Morgner, B.; Elsner, P.; Hipler, U.C.; Wiegand, C. Tryptanthrin promotes keratinocyte and fibroblast responses in vitro after infection with Trichophyton benhamiae DSM6916. Sci. Rep. 2020, 10, 1863. [Google Scholar] [CrossRef] [Green Version]
- Obafemi, C.A.; Adegbite, O.B.; Fadare, O.A.; Iwalewa, E.O.; Omisore, N.O.; Sanusi, K.; Yilmaz, Y.; Ceylan, Ü. Tryptanthrin from microwave-assisted reduction of isatin using solid-state-supported sodium borohydride: DFT calculations, molecular docking and evaluation of its analgesic and anti-inflammatory activity. Heliyon 2020, 7, e05756. [Google Scholar] [CrossRef]
- Bhattacharjee, A.K.; Skanchy, D.J.; Jennings, B.; Hudson, T.H.; Brendle, J.J.; Werbovetz, K.A. Analysis of stereoelectronic properties, mechanism of action and pharmacophore of synthetic indolo[2,1-b]quinazoline-6,12-dione derivatives in relation to antileishmanial activity using quantum chemical, cyclic voltammetry and 3-D-QSAR CATALYST procedures. Bioorg. Med. Chem. 2002, 10, 1979–1989. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Lee, C.L.; Yen, H.R.; Chang, Y.S.; Lin, Y.P.; Huang, S.H.; Lin, C.W. Antiviral Action of Tryptanthrin Isolated from Strobilanthes cusia Leaf against Human Coronavirus NL63. Biomolecules 2020, 10, 366. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, A.K.; Hartell, M.G.; Nichols, D.A.; Hicks, R.P.; Stanton, B.; Hamont, J.E.V.; Milhous, W.K. Structure-activity relationship study of antimalarial indolo [2,1-b]quinazoline-6,12-diones (tryptanthrins). Three dimensional pharmacophore modeling and identification of new antimalarial candidates. Eur. J. Med. Chem. 2004, 39, 59–67. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Sakuraba, H.; Kikuchi, H.; Numao, N.; Asari, T.; Hiraga, H.; Ding, J.; Matsumiya, T.; Seya, K.; Fukuda, S.; et al. Tryptanthrin suppresses double-stranded RNA-induced CXCL10 expression via inhibiting the phosphorylation of STAT1 in human umbilical vein endothelial cells. Mol. Immunol. 2021, 129, 32–38. [Google Scholar] [CrossRef]
- Leena, S.S.; Kaul, G.; Akhir, A.; Saxena, D.; Chopra, S.; Deepthi, A. Green synthesis and antibacterial evaluation of spiro fused tryptanthrin-thiopyrano[2,3-b]indole hybrids targeting drug-resistant S. aureus. Bioorg. Chem. 2022, 128, 106046. [Google Scholar] [CrossRef]
- Jao, C.W.; Lin, W.C.; Wu, Y.T.; Wu, P.L. Isolation, structure elucidation, and synthesis of cytotoxic tryptanthrin analogues from Phaius mishmensis. J. Nat. Prod. 2008, 71, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Pattarawarapan, M.; Wiriya, N.; Hongsibsong, S.; Phakhodee, W. Divergent Synthesis of Methylisatoid and Tryptanthrin Derivatives by Ph3P-I2-Mediated Reaction of Isatins with and without Alcohols. J. Org. Chem. 2020, 85, 15743–15751. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Varun; Sonam; Kakkar, R. Isatin and its derivatives: A survey of recent syntheses, reactions, and applications. Medchemcomm 2019, 10, 351–368. [Google Scholar] [CrossRef]
- Han, K.L.; Zhou, Y.; Liu, F.X.; Guo, Q.N.; Wang, P.F.; Yang, Y.; Song, B.B.; Liu, W.; Yao, Q.W.; Teng, Y.O.; et al. Design, synthesis and in vitro cytotoxicity evaluation of 5-(2-carboxyethenyl)isatin derivatives as anticancer agents. Bioorg. Med. Chem. Lett. 2014, 24, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.T.; Lee, W.C.; Liao, J.; Cheng, J.J.; Lin, L.C.; Chen, C.Y.; Song, J.S.; Wu, M.H.; Shia, K.S.; Li, W.T. Synthesis and evaluation of 3-ylideneoxindole acetamides as potent anticancer agents. Eur. J. Med. Chem. 2015, 98, 1–12. [Google Scholar] [CrossRef]
- Bogdanov, A.V.; Mironov, V.F. Synthesis of isatoic anhydride derivatives (microreview). Chem. Heterocycl. Compd. 2016, 52, 90–92. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, S.N.; Fang, X.; Guo, L.L.; Hu, N.; Guo, Z.L.; Li, X.S.; Yang, S.S.; He, J.C.; Kuang, C.X.; et al. N-Benzyl/Aryl Substituted Tryptanthrin as Dual Inhibitors of Indoleamine 2,3-Dioxygenase and Tryptophan 2,3-Dioxygenase. J. Med. Chem. 2019, 62, 9161–9174. [Google Scholar] [CrossRef]
- Wang, X.; Li, P.; Li, Z.N.; Yin, J.; He, M.; Xue, W.; Chen, Z.W.; Song, B.A. Synthesis and bioactivity evaluation of novel arylimines containing a 3-aminoethyl-2-[(p-trifluoromethoxy)-anilino]-4(3H)-quinazolinone moiety. J. Agric. Food Chem. 2013, 61, 9575–9582. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Huang, X.; Liu, L.W.; Wang, P.Y.; Long, Q.S.; Tao, Q.Q.; Li, Z.; Yang, S. Identification of racemic and chiral carbazole derivatives containing an isopropanolamine linker as prospective surrogates against plant pathogenic bacteria: In vitro and in vivo assays and quantitative proteomics. J. Agric. Food Chem. 2019, 67, 7512–7525. [Google Scholar] [CrossRef]
- Xiang, M.; Zhou, X.; Luo, T.R.; Wang, P.Y.; Liu, L.W.; Li, Z.; Wu, Z.B.; Yang, S. Design, synthesis, antibacterial evaluation, and induced apoptotic behaviors of epimeric and chiral 18β-glycyrrhetinic acid ester derivatives with an isopropanolamine bridge against phytopathogens. J. Agric. Food Chem. 2019, 67, 13212–13220. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.J.; Shi, J.; Luo, N.; Ding, M.H.; Bao, X.P. Synthesis, Crystal Structure, and Agricultural Antimicrobial Evaluation of Novel Quinazoline Thioether Derivatives Incorporating the 1,2,4-Triazolo[4,3-a]pyridine Moiety. J. Agric. Food Chem. 2019, 67, 11598–11606. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Y.; Wang, M.W.; Zeng, D.; Xiang, M.; Rao, J.R.; Liu, Q.Q.; Liu, L.W.; Wu, Z.B.; Li, Z.; Song, B.A.; et al. Rational Optimization and Action Mechanism of Novel Imidazole (or Imidazolium)-Labeled 1,3,4-Oxadiazole Thioethers as Promising Antibacterial Agents against Plant Bacterial Diseases. J. Agric. Food Chem. 2019, 67, 3535–3545. [Google Scholar] [CrossRef]
- Shi, J.; Ding, M.H.; Luo, N.; Wan, S.R.; Li, P.J.; Li, J.H.; Bao, X.P. Design, Synthesis, Crystal Structure, and Antimicrobial Evaluation of 6-Fluoroquinazolinylpiperidinyl-Containing 1,2,4-Triazole Mannich Base Derivatives against Phytopathogenic Bacteria and Fungi. J. Agric. Food Chem. 2020, 68, 9613–9623. [Google Scholar] [CrossRef] [PubMed]
- Li, A.P.; He, Y.H.; Zhang, S.Y.; Shi, Y.P. Antibacterial activity and action mechanism of flavonoids against phytopathogenic bacteria. Pestic. Biochem. Physiol. 2022, 188, 105221. [Google Scholar] [CrossRef]
- Zhang, G.L.; Li, C.P.; Li, Y.; Chen, D.P.; Li, Z.R.; Ouyang, G.P.; Wang, Z.C. Discovery and Mechanism of Azatryptanthrin Derivatives as Novel Anti-Phytopathogenic Bacterial Agents for Potent Bactericide Candidates. J. Agric. Food Chem. 2023, 71, 6288–6300. [Google Scholar] [CrossRef]
- Ahadi, H.; Shokrzadeh, M.; Hosseini-Khah, Z.; Barghi, N.G.; Ghasemian, M.; Emadi, E.; Zargari, M.; Razzaghi-Asl, N.; Emami, S. Synthesis and biological assessment of ciprofloxacin-derived 1,3,4-thiadiazoles as anticancer agents. Bioorg. Chem. 2020, 105, 104383. [Google Scholar] [CrossRef]
- Zhang, G.L.; Tang, Z.H.; Fan, S.L.; Li, C.P.; Li, Y.; Liu, W.Q.; Long, X.S.; Zhang, W.J.; Zhang, Y.; Li, Z.R.; et al. Synthesis and biological assessment of indole derivatives containing penta-heterocycles scaffold as novel anticancer agents towards A549 and K562 cells. J. Enzyme Inhib. Med. Chem. 2023, 38, 2163393. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Raorane, C.J.; Ryu, S.Y.; Shim, J.J.; Lee, J. The anti-biofilm and anti-virulence activities of trans-resveratrol and oxyresveratrol against uropathogenic Escherichia coli. Biofouling 2019, 35, 758–767. [Google Scholar] [CrossRef]
- Qi, P.Y.; Zhang, T.H.; Feng, Y.M.; Wang, M.W.; Shao, W.B.; Zeng, D.; Jin, L.H.; Wang, P.Y.; Zhou, X.; Yang, S. Exploring an Innovative Strategy for Suppressing Bacterial Plant Disease: Excavated Novel Isopropanolamine-Tailored Pterostilbene Derivatives as Potential Antibiofilm Agents. J. Agric. Food Chem. 2022, 70, 4899–4911. [Google Scholar] [CrossRef] [PubMed]



| Compd. | Xoo | Xac | Psa | |||
|---|---|---|---|---|---|---|
| Regression Equation | EC50 (μg/mL) | Regression Equation | EC50 (μg/mL) | Regression Equation | EC50 (μg/mL) | |
| 6a | y = 6.4546x − 0.7761 | 7.85 ± 1.26 | y = 2.4227x + 2.5164 | 10.6 ± 2.32 | y = 1.6022x + 2.4058 | 41.6 ± 0.78 |
| 6b | y = 3.0144x + 2.6913 | 5.83 ± 2.02 | y = 2.5298x + 2.3164 | 11.5 ± 1.37 | y = 1.4243x + 2.1996 | 92.5 ± 0.13 |
| 6c | y = 2.8801x + 2.9822 | 8.67 ± 1.29 | y = 2.0884x + 2.6988 | 12.6 ± 2.16 | y = 2.0566x + 0.8516 | 104 ± 0.09 |
| 6d | y = 0.9523x + 3.518 | 35.9 ± 0.83 | y = 1.8634x + 1.3966 | 85.9 ± 0.91 | y = 1.9052x + 0.6258 | 198 ± 0.55 |
| 6e | y = 2.8081x + 3.859 | 2.55 ± 0.63 | y = 1.8687x + 3.872 | 4.01 ± 2.13 | y = 1.9445x + 1.1506 | 65.4 ± 0.89 |
| 6f | y = 2.6196x + 3.2526 | 4.64 ± 0.86 | y = 1.4927x + 3.878 | 5.64 ± 1.70 | y = 1.4030x + 1.839 | 179 ± 1.04 |
| 6g | y = 2.9744x + 2.8651 | 5.22 ± 1.05 | y = 1.5277x + 3.2167 | 14.7 ± 0.48 | y = 1.4590x + 1.6785 | 189 ± 0.45 |
| 6h | y = 1.8963x + 3.1991 | 8.91 ± 2.11 | y = 1.3691x + 3.212 | 20.2 ± 1.57 | y = 2.5878x − 0.1844 | 101 ± 0.27 |
| 6i | y = 2.0305x + 3.6196 | 4.78 ± 0.54 | y = 1.6713x + 3.0283 | 15.1 ± 1.25 | y = 3.2277x − 1.338 | 92.0 ± 1.16 |
| 6j | y = 1.7663x + 2.9203 | 15.0 ± 0.77 | y = 1.9519x + 2.3878 | 21.8 ± 0.95 | y = 2.1527x + 0.499 | 123 ± 0.88 |
| 6k | y = 2.1311x + 2.055 | 24.1 ± 0.91 | y = 1.3754x + 2.6473 | 51.4 ± 1.23 | y = 1.3195x + 1.9708 | 198 ± 0.52 |
| 6l | y = 2.2259x + 3.5945 | 4.28 ± 2.32 | y = 1.2230x + 4.1502 | 4.95 ± 0.76 | y = 3.9030x - 0.7109 | 29.1 ± 1.58 |
| 6m | y = 1.0886x + 4.0373 | 7.66 ± 1.20 | y = 1.6773x + 3.3029 | 10.3 ± 0.91 | y = 2.5406x + 0.5878 | 54.5 ± 0.93 |
| 6n | / | >200 | / | >200 | y = 1.9399x + 1.0976 | 103 ± 1.17 |
| 6o | y = 2.5637x + 3.3706 | 4.32 ± 1.54 | y =1.2994x + 4.0043 | 5.84 ± 1.34 | y = 2.9462x + 0.8423 | 25.8 ± 0.64 |
| 6p | / | >200 | / | >200 | y = 1.5239x + 2.0559 | 85.5 ± 1.30 |
| 6q | y = 2.6239x + 3.1246 | 5.18 ± 0.96 | y = 1.4250x + 3.7055 | 8.10 ± 1.10 | y = 1.8106x + 2.1212 | 38.9 ± 0.54 |
| 6r | y = 2.4005x + 2.7039 | 9.05 ± 0.24 | y = 1.7637x + 3.1238 | 11.6 ± 1.47 | y = 1.6338x + 2.3123 | 44.2 ± 0.92 |
| 6s | y = 2.1919x + 3.2875 | 6.04 ± 1.18 | y = 2.0811x + 2.4628 | 16.6 ± 0.82 | y = 1.6229x + 2.1567 | 56.5 ± 1.38 |
| 6t | y = 2.0066x + 2.9113 | 10.9 ± 1.42 | y = 2.0753x + 2.2416 | 21.3 ± 1.03 | y = 1.9107x + 1.4848 | 69.1 ± 0.59 |
| 6u | y = 1.8610x + 3.4908 | 6.47 ± 1.27 | y = 2.7359x + 1.6863 | 16.3 ± 0.57 | y = 2.2314x + 0.8208 | 74.6 ± 1.11 |
| 6v | y = 1.9002x + 3.1388 | 9.54 ± 0.65 | y = 2.1932x + 2.1738 | 19.4 ± 1.43 | y = 1.7158x + 1.6172 | 93.7 ± 2.32 |
| 6w | y = 1.9052x + 3.1924 | 8.89 ± 0.81 | y = 2.2753x + 2.2518 | 16.1 ± 0.77 | y = 1.7448x + 2.018 | 51.2 ± 1.69 |
| 6x | y = 1.5924x + 3.1474 | 14.6 ± 1.76 | y = 1.7406x + 2.4935 | 27.5 ± 1.93 | y = 1.9229x + 0.9635 | 126 ± 1.34 |
| 6y | y = 1.7546x + 3.067 | 12.6 ± 1.29 | y = 1.7877x + 2.9907 | 13.3 ± 1.36 | y = 1.4929x + 2.3105 | 63.3 ± 1.25 |
| 6z | y = 1.1302x + 3.3554 | 28.5 ± 2.22 | y = 1.3206x + 2.9337 | 36.7 ± 1.92 | y = 0.7881x + 3.2194 | 182 ± 0.81 |
| Tryp. c | y = 1.9890x + 0.8834 | 117 ± 1.63 | y = 1.6262x + 1.5816 | 126 ± 0.56 | / | >200 |
| BT b | y = 2.6069x + 0.9742 | 35.0 ± 1.06 | y = 2.6498x + 0.4164 | 53.7 ± 0.63 | y = 2.2293x + 0.3741 | 119 ± 0.67 |
| TC b | y = 2.2754x + 0.6772 | 79.4 ± 0.94 | y = 2.6141x + 0.1945 | 68.9 ± 1.18 | y = 1.8074x + 1.5289 | 83.3 ± 1.25 |
| Chemicals | Protective Activity | Curative Activity | ||||
|---|---|---|---|---|---|---|
| Morbidity (%) | Disease Index (%) | Control Efficiency (%) b | Morbidity (%) | Disease Index (%) | Control Efficiency (%) b | |
| CK a | 100 | 78.15 | / | 100 | 82.96 | / |
| 6e | 100 | 42.96 | 45.02 | 100 | 46.67 | 43.75 |
| BT | 100 | 45.19 | 42.18 | 100 | 43.70 | 47.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, X.; Zhang, G.; Long, H.; Wang, Q.; Wang, C.; Zhu, M.; Wang, W.; Li, C.; Wang, Z.; Ouyang, G. Discovery and Mechanism of Novel 7-Aliphatic Amine Tryptanthrin Derivatives against Phytopathogenic Bacteria. Int. J. Mol. Sci. 2023, 24, 10900. https://doi.org/10.3390/ijms241310900
Long X, Zhang G, Long H, Wang Q, Wang C, Zhu M, Wang W, Li C, Wang Z, Ouyang G. Discovery and Mechanism of Novel 7-Aliphatic Amine Tryptanthrin Derivatives against Phytopathogenic Bacteria. International Journal of Molecular Sciences. 2023; 24(13):10900. https://doi.org/10.3390/ijms241310900
Chicago/Turabian StyleLong, Xuesha, Guanglong Zhang, Haitao Long, Qin Wang, Congyu Wang, Mei Zhu, Wenhang Wang, Chengpeng Li, Zhenchao Wang, and Guiping Ouyang. 2023. "Discovery and Mechanism of Novel 7-Aliphatic Amine Tryptanthrin Derivatives against Phytopathogenic Bacteria" International Journal of Molecular Sciences 24, no. 13: 10900. https://doi.org/10.3390/ijms241310900
APA StyleLong, X., Zhang, G., Long, H., Wang, Q., Wang, C., Zhu, M., Wang, W., Li, C., Wang, Z., & Ouyang, G. (2023). Discovery and Mechanism of Novel 7-Aliphatic Amine Tryptanthrin Derivatives against Phytopathogenic Bacteria. International Journal of Molecular Sciences, 24(13), 10900. https://doi.org/10.3390/ijms241310900





