An Inferred Ancestral CotA Laccase with Improved Expression and Kinetic Efficiency
Abstract
1. Introduction
2. Results
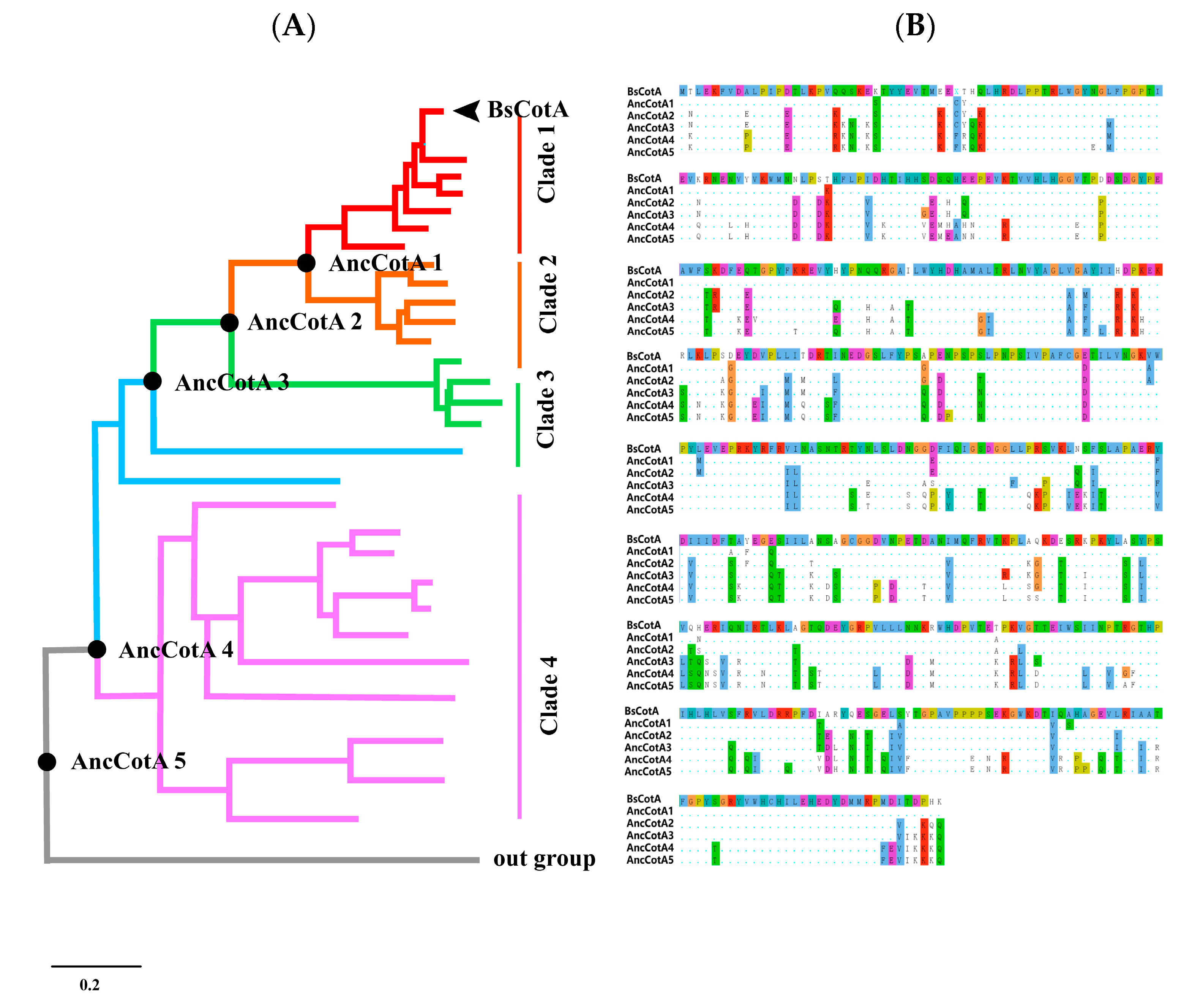
2.1. Inferring BsCotA Laccase Ancestor
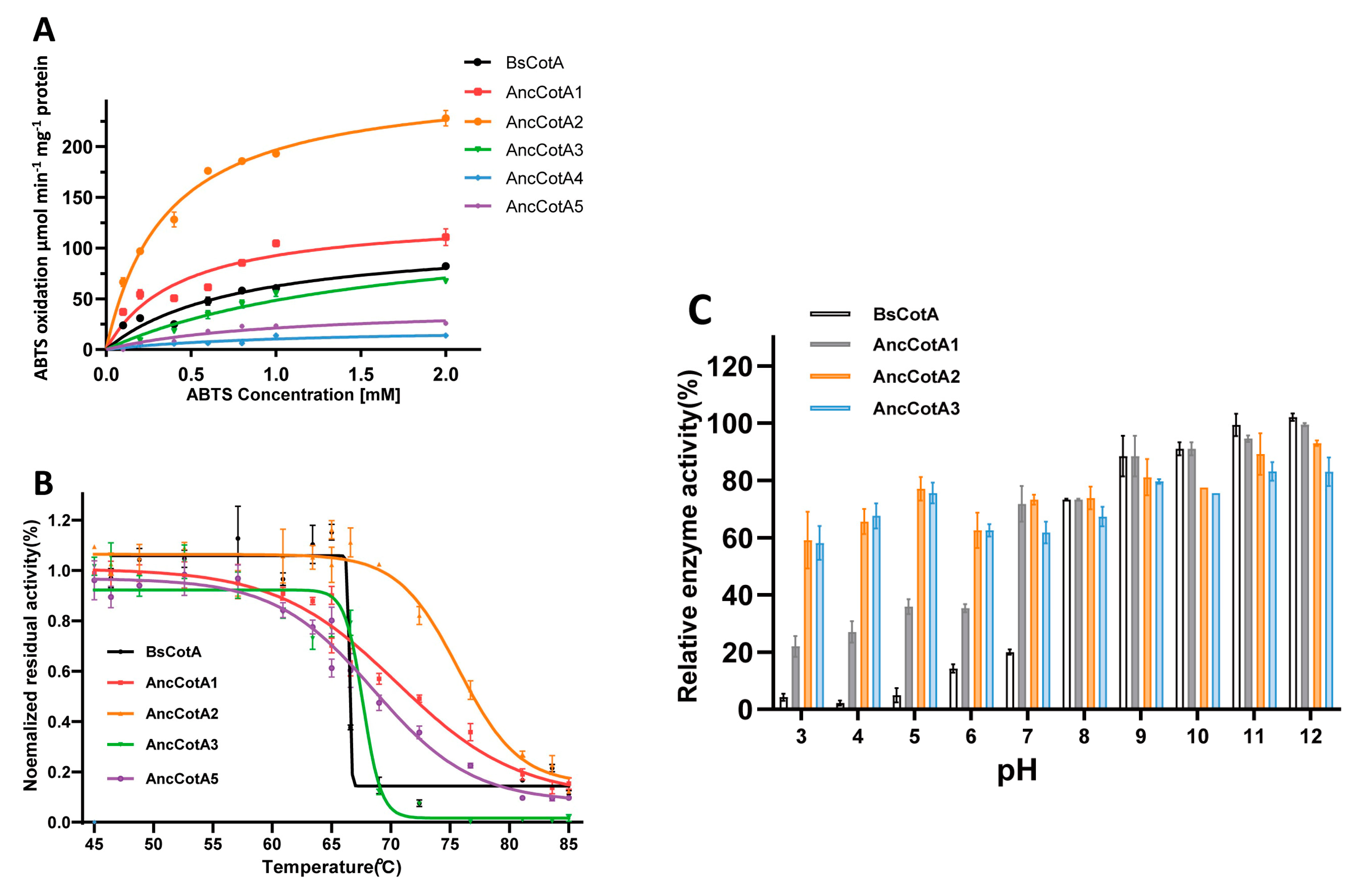
2.2. Ancestral Inferring Improved Catalytic Efficiency and Stability
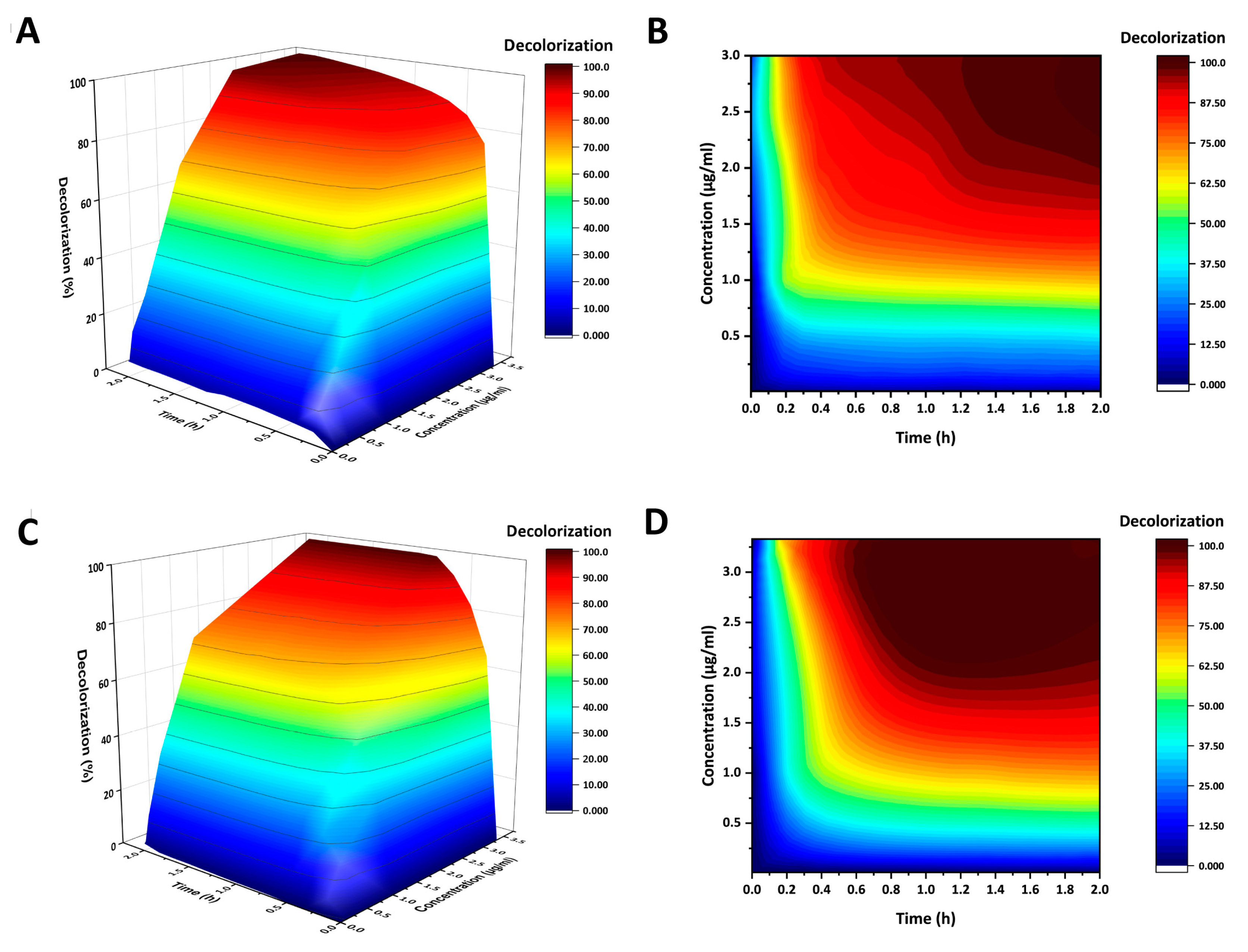
2.3. AncCotA2 Showed Higher Application Potential
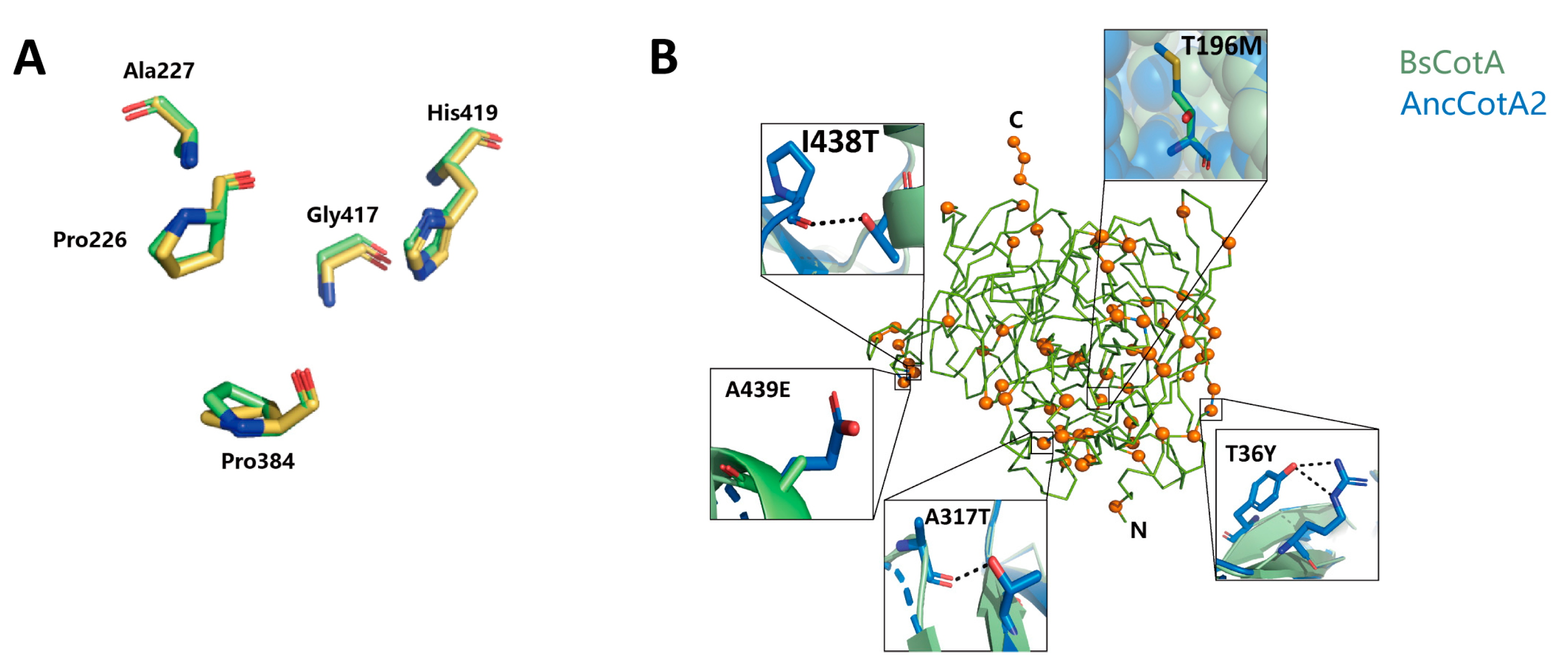
2.4. Plausible Structural Explanation for the Effect of Residues on the Stability of AncCotA2
3. Discussion
4. Materials and Methods
4.1. Ancestral Sequence Reconstruction
4.2. Protein Expression and Purification
4.3. Enzyme Assay
4.4. Thermostability and pH Stability
4.5. Decolorization of Indigo Carmine (IC)
4.6. Structural Modeling
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshida, H. LXIII.—Chemistry of lacquer (Urushi). Part I. Communication from the Chemical Society of Tokio. J. Chem. Soc. Trans. 1883, 43, 472–486. [Google Scholar] [CrossRef]
- Bertrand, G. Sur la laccase et sur le pouvoir oxydant de cette diastase. CR Acad. Sci. 1985, 120, 266–269. [Google Scholar]
- Agrawal, K.; Chaturvedi, V.; Verma, P. Fungal laccase discovered but yet undiscovered. Bioresour. Bioprocess. 2018, 5, 4. [Google Scholar] [CrossRef]
- Khatami, S.H.; Vakili, O.; Movahedpour, A.; Ghesmati, Z.; Ghasemi, H.; Taheri-Anganeh, M. Laccase: Various types and applications. Biotechnol. Appl. Biochem. 2022, 69, 2658–2672. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef] [PubMed]
- Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzym. Res. 2011, 2011, 217861. [Google Scholar]
- Si, J.; Ma, H.; Cao, Y.; Cui, B.; Dai, Y. Introducing a thermo-alkali-stable, metallic ion-tolerant laccase purified from white rot fungus Trametes hirsuta. Front. Microbiol. 2021, 12, 670163. [Google Scholar] [CrossRef]
- Zheng, F.; An, Q.; Meng, G.; Wu, X.J.; Dai, Y.C.; Si, J.; Cui, B.K. A novel laccase from white rot fungus Trametes orientalis: Purification, characterization, and application. Int. J. Biol. Macromol. 2017, 102, 758–770. [Google Scholar] [CrossRef]
- Si, J.; Wu, Y.; Ma, H.-F.; Cao, Y.-J.; Sun, Y.-F.; Cui, B.-K. Selection of a pH-and temperature-stable laccase from Ganoderma australe and its application for bioremediation of textile dyes. J. Environ. Manag. 2021, 299, 113619. [Google Scholar] [CrossRef]
- Martins, L.O.; Soares, C.M.; Pereira, M.M.; Teixeira, M.; Costa, T.; Jones, G.H.; Henriques, A.O. Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J. Biol. Chem. 2002, 277, 18849–18859. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, K.K.; Bhardwaj, K.N.; Chakraborty, S.; Kuhad, R.C. Multiple Genes in a Single Host: Cost-Effective Production of Bacterial Laccase (cotA), Pectate Lyase (pel), and Endoxylanase (xyl) by Simultaneous Expression and Cloning in Single Vector in E. coli. PLoS ONE 2015, 10, e0144379. [Google Scholar] [CrossRef] [PubMed]
- Ihssen, J.; Reiss, R.; Luchsinger, R.; Thöny-Meyer, L.; Richter, M. Biochemical properties and yields of diverse bacterial laccase-like multicopper oxidases expressed in Escherichia coli. Sci. Rep. 2015, 5, 10465. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhao, M.; Wang, Y. Expression of CotA laccase in Pichia pastoris and its electrocatalytic sensing application for hydrogen peroxide. Appl. Microbiol. Biotechnol. 2015, 99, 9483–9493. [Google Scholar] [CrossRef] [PubMed]
- Spence, M.A.; Kaczmarski, J.A.; Saunders, J.W.; Jackson, C.J. Ancestral sequence reconstruction for protein engineers. Curr. Opin. Struct. Biol. 2021, 69, 131–141. [Google Scholar] [CrossRef]
- Furukawa, R.; Toma, W.; Yamazaki, K.; Akanuma, S. Ancestral sequence reconstruction produces thermally stable enzymes with mesophilic enzyme-like catalytic properties. Sci. Rep. 2020, 10, 15493. [Google Scholar] [CrossRef]
- Moshe, A.; Pupko, T. Ancestral sequence reconstruction: Accounting for structural information by averaging over replacement matrices. Bioinformatics 2019, 35, 2562–2568. [Google Scholar] [CrossRef]
- Trudeau, D.L.; Kaltenbach, M.; Tawfik, D.S. On the Potential Origins of the High Stability of Reconstructed Ancestral Proteins. Mol. Biol. Evol. 2016, 33, 2633–2641. [Google Scholar] [CrossRef]
- Risso, V.A.; Gavira, J.A.; Mejia-Carmona, D.F.; Gaucher, E.A.; Sanchez-Ruiz, J.M. Hyperstability and substrate promiscuity in laboratory resurrections of Precambrian β-lactamases. J. Am. Chem. Soc. 2013, 135, 2899–2902, Correction in J. Am. Chem. Soc. 2013, 135, 10580. [Google Scholar] [CrossRef]
- Enguita, F.J.; Martins, L.O.; Henriques, A.O.; Carrondo, M.A. Crystal structure of a bacterial endospore coat component. A laccase with enhanced thermostability properties. J. Biol. Chem. 2003, 278, 19416–19425. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazy, H.; Penn, O.; Doron-Faigenboim, A.; Cohen, O.; Cannarozzi, G.; Zomer, O.; Pupko, T. FastML: A web server for probabilistic reconstruction of ancestral sequences. Nucleic Acids Res. 2012, 40, W580–W584. [Google Scholar] [CrossRef] [PubMed]
- Khlifi, R.; Belbahri, L.; Woodward, S.; Ellouz, M.; Dhouib, A.; Sayadi, S.; Mechichi, T. Decolourization and detoxification of textile industry wastewater by the laccase-mediator system. J. Hazard. Mater. 2010, 175, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.R.; Chao, Y.P.; Zhang, G.Q.; Xue, Z.Q.; Qian, S. Laccase-mediator system in the decolorization of different types of recalcitrant dyes. J. Ind. Microbiol. Biotechnol. 2009, 36, 45–51. [Google Scholar] [CrossRef]
- Du, Z.; Su, H.; Wang, W.; Ye, L.; Wei, H.; Peng, Z.; Anishchenko, I.; Baker, D.; Yang, J. The trRosetta server for fast and accurate protein structure prediction. Nat. Protoc. 2021, 16, 5634–5651. [Google Scholar] [CrossRef]
- Yang, J.; Anishchenko, I.; Park, H.; Peng, Z.; Ovchinnikov, S.; Baker, D. Improved protein structure prediction using predicted interresidue orientations. Proc. Natl. Acad. Sci. USA 2020, 117, 1496–1503. [Google Scholar] [CrossRef]
- Pace, C.N.; Fu, H.; Fryar, K.L.; Landua, J.; Trevino, S.R.; Schell, D.; Thurlkill, R.L.; Imura, S.; Scholtz, J.M.; Gajiwala, K.S.; et al. Contribution of hydrogen bonds to protein stability. Protein Sci. 2014, 23, 652–661. [Google Scholar] [CrossRef]
- Tych, K.M.; Batchelor, M.; Hoffmann, T.; Wilson, M.C.; Hughes, M.L.; Paci, E.; Brockwell, D.J.; Dougan, L. Differential Effects of Hydrophobic Core Packing Residues for Thermodynamic and Mechanical Stability of a Hyperthermophilic Protein. Langmuir 2016, 32, 7392–7402. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Phillips, K.J.; Liu, D.R. Supercharging Proteins Can Impart Unusual Resilience. J. Am. Chem. Soc. 2007, 129, 10110–10112. [Google Scholar] [CrossRef]
- Barber-Zucker, S.; Mateljak, I.; Goldsmith, M.; Kupervaser, M.; Alcalde, M.; Fleishman, S.J. Designed High-Redox Potential Laccases Exhibit High Functional Diversity. ACS Catal. 2022, 12, 13164–13173. [Google Scholar] [CrossRef]
- Lu, L.; Wang, T.-N.; Xu, T.-F.; Wang, J.-Y.; Wang, C.-L.; Zhao, M. Cloning and expression of thermo-alkali-stable laccase of Bacillus licheniformis in Pichia pastoris and its characterization. Bioresour. Technol. 2013, 134, 81–86. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, K.K.; Rani, S.; Bhardwaj, K.N.; Goel, M.; Kuhad, R.C. In-Vitro Refolding and Characterization of Recombinant Laccase (CotA) from Bacillus pumilus MK001 and Its Potential for Phenolics Degradation. Mol. Biotechnol. 2016, 58, 789–800. [Google Scholar] [CrossRef]
- Reiss, R.; Ihssen, J.; Thöny-Meyer, L. Bacillus pumilus laccase: A heat stable enzyme with a wide substrate spectrum. BMC Biotechnol. 2011, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Rasekh, B.; Khajeh, K.; Ranjbar, B.; Mollania, N.; Almasinia, B.; Tirandaz, H. Protein engineering of laccase to enhance its activity and stability in the presence of organic solvents. Eng. Life Sci. 2014, 14, 442–448. [Google Scholar] [CrossRef]
- Mollania, N.; Khajeh, K.; Ranjbar, B.; Hosseinkhani, S. Enhancement of a bacterial laccase thermostability through directed mutagenesis of a surface loop. Enzym. Microb. Technol. 2011, 49, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, D.; Yang, E.; Niu, J.; Chen, Y.; Chagan, I. Purification and characterization of a thermotolerant laccase isoform in Trametes trogii strain and its potential in dye decolorization. Int. Biodeterior. Biodegradation 2014, 93, 186–194. [Google Scholar] [CrossRef]
- Durão, P.; Chen, Z.; Fernandes, A.T.; Hildebrandt, P.; Murgida, D.H.; Todorovic, S.; Pereira, M.M.; Melo, E.P.; Martins, L.O. Copper incorporation into recombinant CotA laccase from Bacillus subtilis: Characterization of fully copper loaded enzymes. JBIC J. Biol. Inorg. Chem. 2008, 13, 183–193. [Google Scholar] [CrossRef]
- Gomez-Fernandez, B.J.; Risso, V.A.; Rueda, A.; Sanchez-Ruiz, J.M.; Alcalde, M. Ancestral Resurrection and Directed Evolution of Fungal Mesozoic Laccases. Appl. Environ. Microbiol. 2020, 86, e00778-20. [Google Scholar] [CrossRef] [PubMed]
- Hamuro, Y.; Tajima, K.; Matsumoto-Akanuma, A.; Sakamoto, S.; Furukawa, R.; Yamagishi, A.; Ohno, N.; Akanuma, S. Characterization of a thermostable mutant of Agaricus brasiliensis laccase created by phylogeny-based design. J. Biosci. Bioeng. 2017, 124, 623–629. [Google Scholar] [CrossRef]
- Wheeler, L.C.; A Lim, S.; Marqusee, S.; Harms, M.J. The thermostability and specificity of ancient proteins. Curr. Opin. Struct. Biol. 2016, 38, 37–43. [Google Scholar] [CrossRef]
- Tawfik, D.S. Enzyme promiscuity and evolution in light of cellular metabolism. FEBS J. 2020, 287, 1260–1261. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, P.; Kobus, S.; Gertzen, C.G.W.; Hoeppner, A.; Holzscheck, N.; Strunk, C.H.; Huber, H.; Jaeger, K.-E.; Gohlke, H.; Kovacic, F.; et al. A promiscuous ancestral enzyme´s structure unveils protein variable regions of the highly diverse metallo-β-lactamase family. Commun. Biol. 2021, 4, 132. [Google Scholar] [CrossRef] [PubMed]
- Akanuma, S.; Nakajima, Y.; Yokobori, S.-I.; Kimura, M.; Nemoto, N.; Mase, T.; Miyazono, K.-I.; Tanokura, M.; Yamagishi, A. Experimental evidence for the thermophilicity of ancestral life. Proc. Natl. Acad. Sci. USA 2013, 110, 11067–11072. [Google Scholar] [CrossRef]
- Hobbs, J.; Shepherd, C.; Saul, D.J.; Demetras, N.J.; Haaning, S.; Monk, C.R.; Daniel, R.M.; Arcus, V.L. On the origin and evolution of thermophily: Reconstruction of functional precambrian enzymes from ancestors of Bacillus. Mol. Biol. Evol. 2012, 29, 825–835. [Google Scholar] [CrossRef]
- Coria-Oriundo, L.L.; Battaglini, F.; Wirth, S.A. Efficient decolorization of recalcitrant dyes at neutral/alkaline pH by a new bacterial laccase-mediator system. Ecotoxicol. Environ. Saf. 2021, 217, 112237. [Google Scholar] [CrossRef]
- Yao, C.; Xia, W.; Dou, M.; Du, Y.; Wu, J. Oxidative degradation of UV-irradiated polyethylene by laccase-mediator system. J. Hazard. Mater. 2022, 440, 129709. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Xiang, S.; Raleigh, D.P. On the relationship between protein stability and folding kinetics: A comparative study of the N-terminal domains of RNase HI, E. coli and Bacillus stearothermophilus L9. J. Mol. Biol. 2001, 312, 569–577. [Google Scholar] [CrossRef]
- Glyakina, A.V.; Galzitskaya, O.V. How Quickly Do Proteins Fold and Unfold, and What Structural Parameters Correlate with These Values? Biomolecules 2020, 10, 197. [Google Scholar] [CrossRef]
- Gamiz-Arco, G.; Risso, V.A.; Gaucher, E.A.; Gavira, J.A.; Naganathan, A.N.; Ibarra-Molero, B.; Sanchez-Ruiz, J.M. Combining Ancestral Reconstruction with Folding-Landscape Simulations to Engineer Heterologous Protein Expression. J. Mol. Biol. 2021, 433, 167321. [Google Scholar] [CrossRef]
- Goldsmith, M.; Tawfik, D.S. Enzyme engineering by targeted libraries. Methods Enzymol. 2013, 523, 257–283. [Google Scholar]
- Pey, A.L.; Rodriguez-Larrea, D.; Bomke, S.; Dammers, S.; Godoy-Ruiz, R.; Garcia-Mira, M.M.; Sanchez-Ruiz, J.M. Engineering proteins with tunable thermodynamic and kinetic stabilities. Proteins 2008, 71, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.H.; Linke, K.; Graf, P.; Lilie, H.; Jakob, U. Identification of a redox-regulated chaperone network. EMBO J. 2004, 23, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Alcolombri, U.; Elias, M.; Tawfik, D.S. Directed Evolution of Sulfotransferases and Paraoxonases by Ancestral Libraries. J. Mol. Biol. 2011, 411, 837–853. [Google Scholar] [CrossRef] [PubMed]
- Arenas, M.; Bastolla, U. ProtASR2: Ancestral reconstruction of protein sequences accounting for folding stability. Methods Ecol. Evol. 2020, 11, 248–257. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Randall, R.N.; Radford, C.E.; Roof, K.A.; Natarajan, D.K.; Gaucher, E.A. An experimental phylogeny to benchmark ancestral sequence reconstruction. Nat. Commun. 2016, 7, 12847. [Google Scholar] [CrossRef]
- Hoover, D.M.; Lubkowski, J. DNAWorks: An automated method for designing oligonucleotides for PCR-based gene synthesis. Nucleic Acids Res. 2002, 30, e43. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol. Biol. 1994, 32, 5–8. [Google Scholar] [CrossRef]
- Wong, K.-S.; Cheung, M.-K.; Au, C.-H.; Kwan, H.-S. A Novel Lentinula edodes Laccase and Its Comparative Enzymology Suggest Guaiacol-Based Laccase Engineering for Bioremediation. PLoS ONE 2013, 8, e66426. [Google Scholar] [CrossRef]
- Despotović, D.; Aharon, E.; Dubovetskyi, A.; Leader, H.; Ashani, Y.; Tawfik, D.S. A mixture of three engineered phosphotriesterases enables rapid detoxification of the entire spectrum of known threat nerve agents. Protein Eng. Des. Sel. PEDS 2019, 32, 169–174. [Google Scholar] [CrossRef]
| CotA Variants | Mut a | Normalized Activity (μmol min−1 mg Enzyme−1) | Yield (mg/L) | Thermostability (T50) | ABTS Oxidation | ||
|---|---|---|---|---|---|---|---|
| KM (mM) | kcat (S−1) | kcat/KM (M−1·s−1) | |||||
| BsCotA | 0 | 82.54 | 0.801 | 66 ± 4 °C | 0.9 ± 0.2 | 127 ± 15 | 1.4 × 105 |
| AncCotA1 | 19 | 107.48 | 3.133 | 71 ± 0.1 °C | 0.5 ± 0.1 | 155 ± 28 | 3.0 × 105 |
| AncCotA2 | 69 | 198.43 | 4.705 | 76 ± 0.1 °C | 0.4 ± 0.04 | 306 ± 12 | 7.5 × 105 |
| AncCotA3 | 101 | 68.67 | 3.083 | 64 ± 0.3 °C | 1.9 ± 0.5 | 152 ± 23 | 0.8 × 105 |
| AncCotA4 | 152 | 13.65 | 2.317 | NA | 1 ± 0.4 | 21 ± 4 | 0.2 × 105 |
| AncCotA5 | 159 | 25.12 | 2.932 | 69 ± 0.10 °C | 1 ± 0.4 | 48 ± 9 | 0.4 × 105 |
| Name | Expression Host | Specific Activity (U/mg) | Optimal Temperature | Optimal pH | KM (mM) | kcat (s−1) | kcat/KM (M−1·s−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Trametes trogii | native form | 352.1 | 45 °C | 3 | 0.069 | 7985 | 1.15 × 108 | [36] |
| Bacillus subtilis | native form | 82.54 | 75 °C | 3 | 0.106 | 16.8 ± 0.8 | 1.52 × 105 | [10] |
| Basidiomycete PM1 laccase OB-1 mutant | S. cerevisiae | 400 | 60 °C | 4 | 0.0044 | 110 ± 3 | 2.47 × 108 | [30] |
| Bacillus pumilus CotA | E. coli | 117 | 70 °C | 4 | 0.080 | 291 ± 2.7 | 3.64 × 106 | [33] |
| BsCotA WT | E. coli | 82.54 | 70 °C | 4 | 0.79 ± 0.23 | 120.7 | 1.44 × 105 | This study |
| BsCotA AncCotA2 | E. coli | 198.43 | 70 °C | 4 | 0.35 ± 0.03 | 288.3 | 7.52 × 105 | This study |
| Trametes orientalis | native form | 20.667 | 80 °C | 4 | 0.33 | 21.81 | ND | [8] |
| Trametes hirsuta. | native form | 22.111 | 50 °C | 6 | 0.087 | 1.479 | 1.48 × 106 | [7] |
| Ganoderma australe Galacc-F | native form | 22.214 | 55 °C | 6 | 0.164 | ND | 1.66 × 106 | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, L.; Zhao, L.; Hou, Y.; Yue, C.; Liu, P.; Zheng, Y.; Peng, W.; Yang, J. An Inferred Ancestral CotA Laccase with Improved Expression and Kinetic Efficiency. Int. J. Mol. Sci. 2023, 24, 10901. https://doi.org/10.3390/ijms241310901
Lei L, Zhao L, Hou Y, Yue C, Liu P, Zheng Y, Peng W, Yang J. An Inferred Ancestral CotA Laccase with Improved Expression and Kinetic Efficiency. International Journal of Molecular Sciences. 2023; 24(13):10901. https://doi.org/10.3390/ijms241310901
Chicago/Turabian StyleLei, Lei, Lijun Zhao, Yiqia Hou, Chen Yue, Pulin Liu, Yanli Zheng, Wenfang Peng, and Jiangke Yang. 2023. "An Inferred Ancestral CotA Laccase with Improved Expression and Kinetic Efficiency" International Journal of Molecular Sciences 24, no. 13: 10901. https://doi.org/10.3390/ijms241310901
APA StyleLei, L., Zhao, L., Hou, Y., Yue, C., Liu, P., Zheng, Y., Peng, W., & Yang, J. (2023). An Inferred Ancestral CotA Laccase with Improved Expression and Kinetic Efficiency. International Journal of Molecular Sciences, 24(13), 10901. https://doi.org/10.3390/ijms241310901






