Necroptosis Mediates Muscle Protein Degradation in a Cachexia Model of Weanling Pig with Lipopolysaccharide Challenge
Abstract
:1. Introduction
2. Results
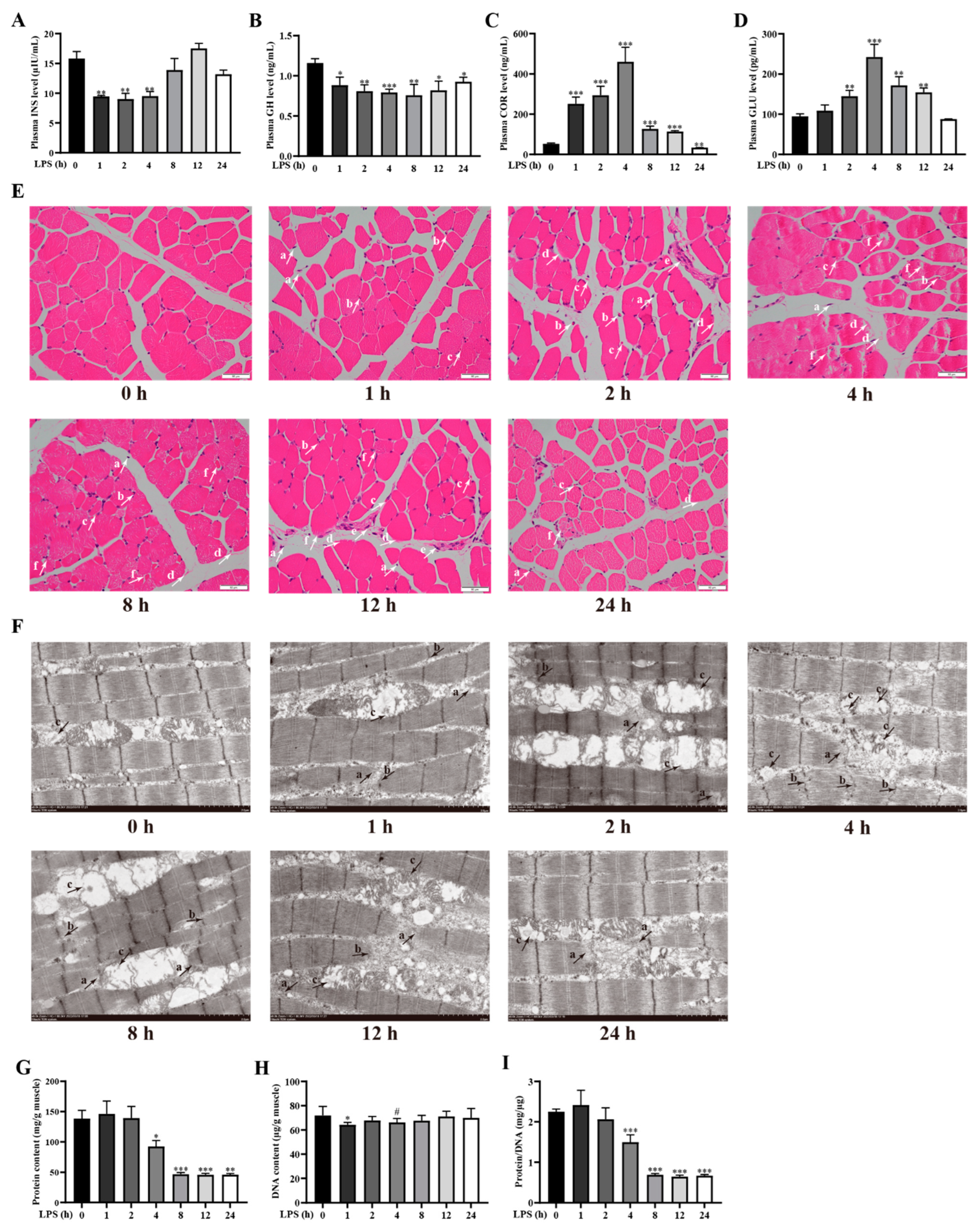
2.1. LPS Challenge Dynamically Changes Physiological and Biochemical Indexes for Diagnosis of Cachexia in Weanling Pigs
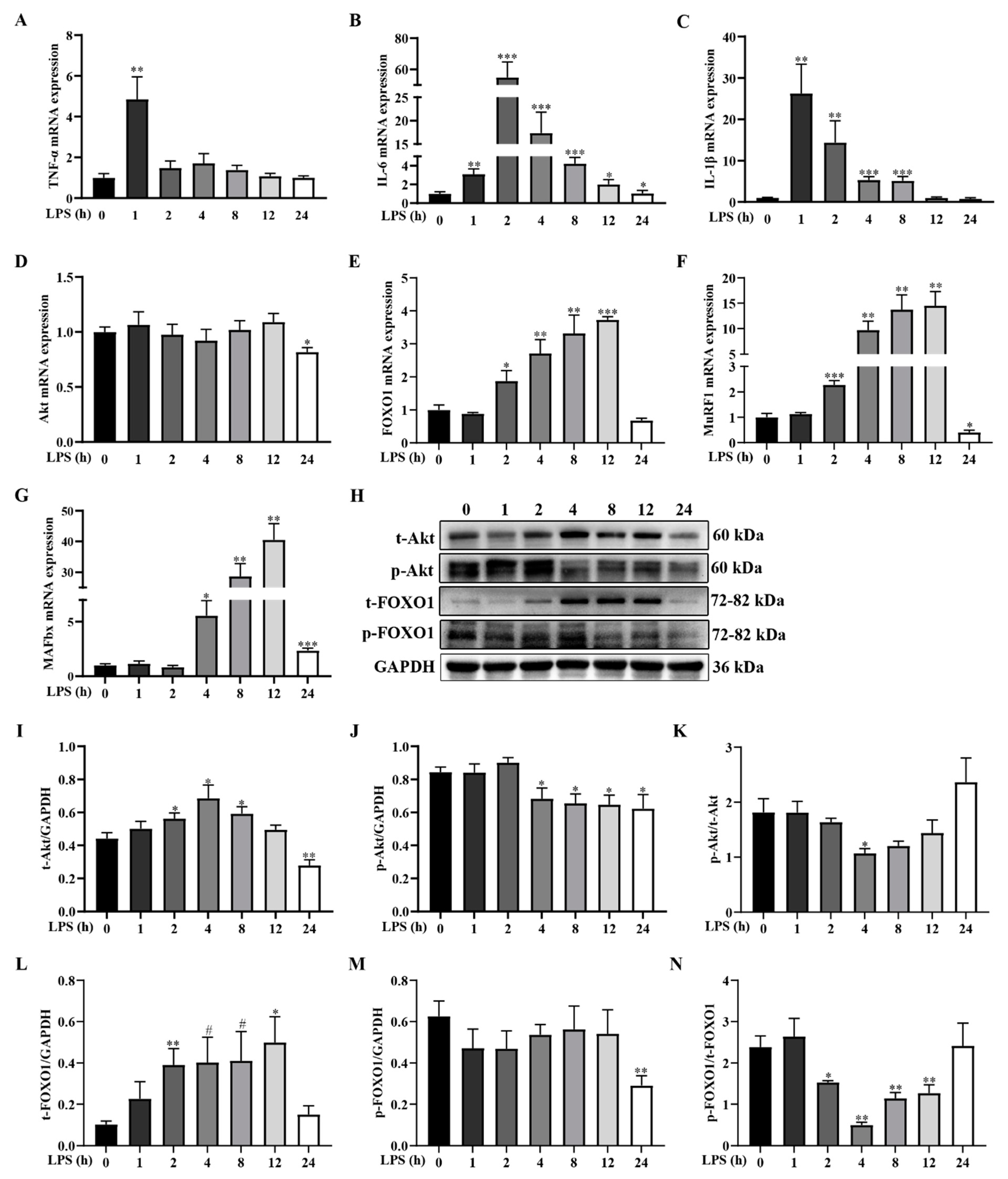
2.2. LPS Challenge Dynamically Induces Inflammatory Response and Activates Muscle Protein Degradation Signaling Pathway in Weanling Pigs
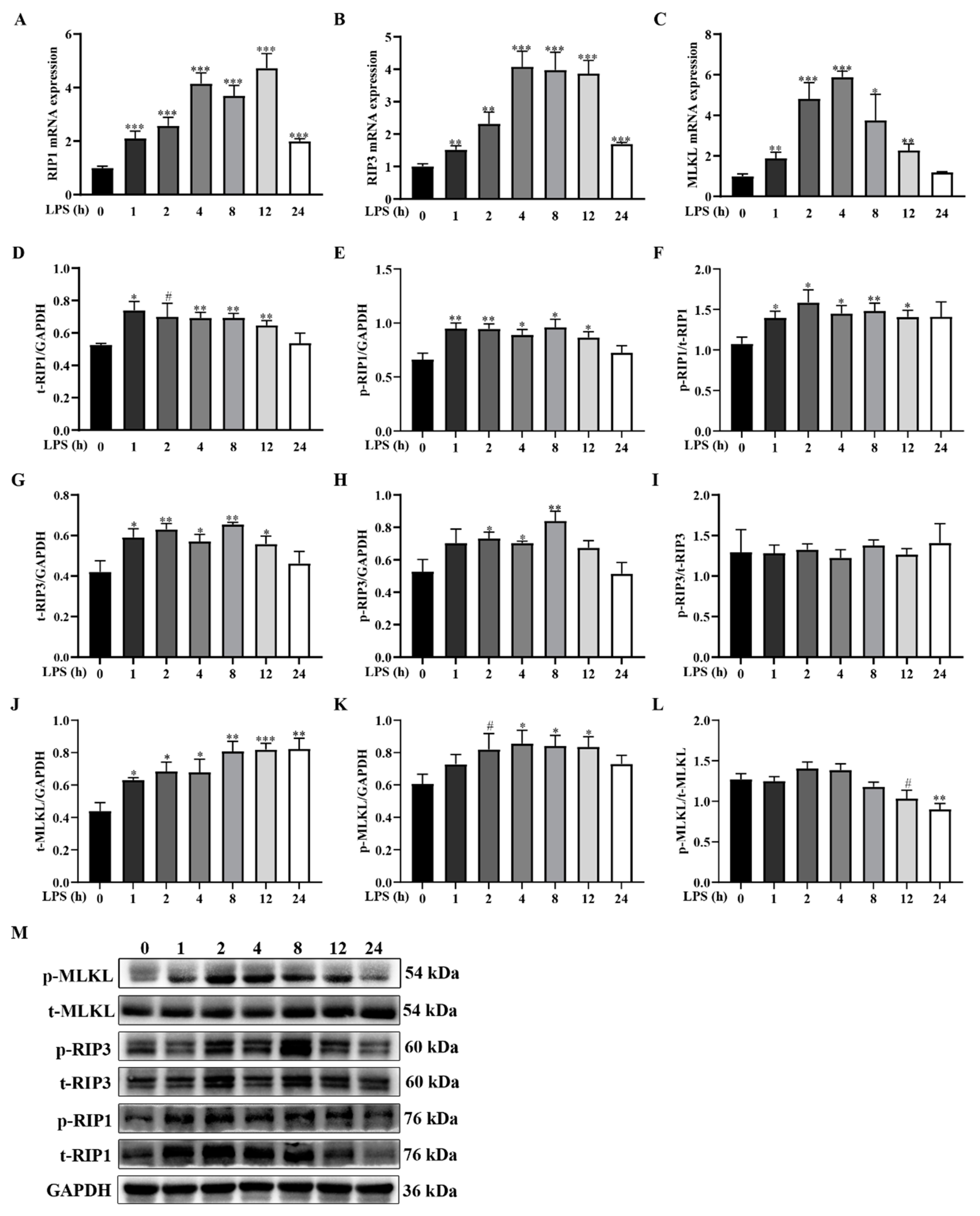
2.3. LPS Challenge Dynamically Induces Muscle Necroptosis in Weanling Pigs
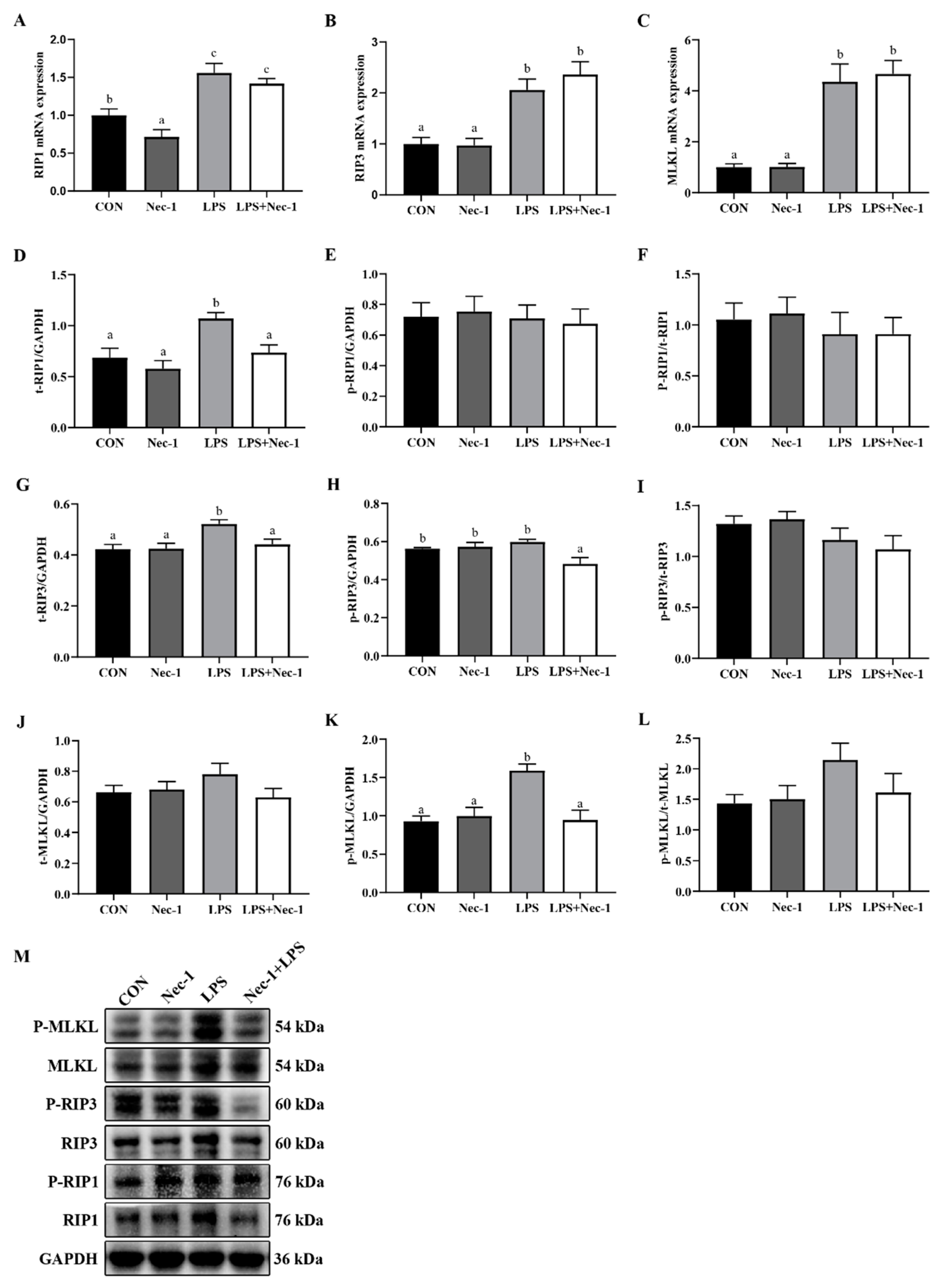
2.4. Nec-1 Inhibits Muscle Necroptosis Induced by LPS in Weanling Pigs
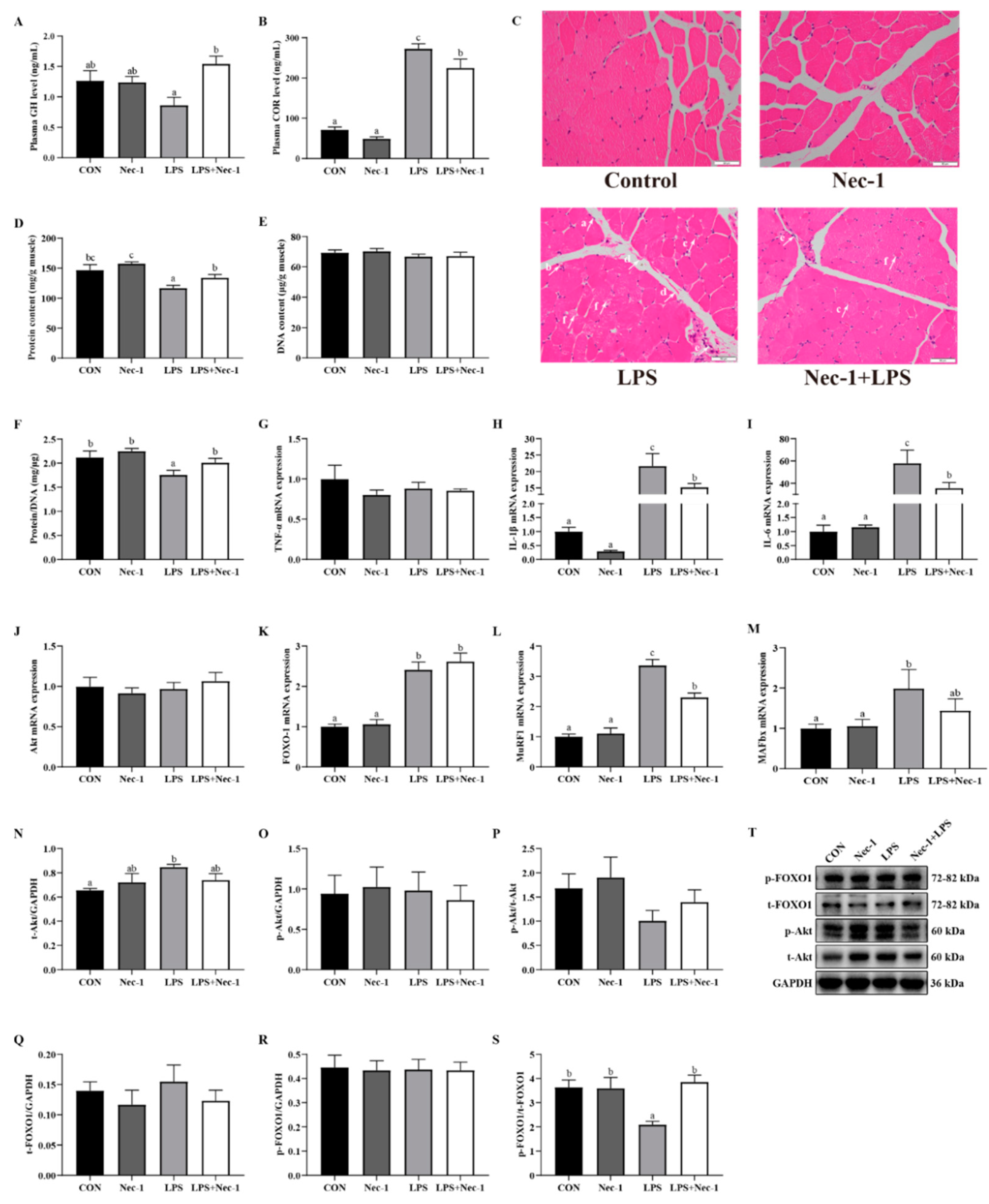
2.5. Inhibition of Necroptosis by Nec-1 Relieves Muscle Structure Injury, Inflammation, and Protein Degradation Induced by LPS in Weanling Pigs
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Blood and Muscle Sample Collection
4.3. Plasma Hormone Measurement
4.4. Muscle Morphologic Analysis
4.5. Muscle Ultrastructural Observation
4.6. Muscle Protein and DNA Concentration Determination
4.7. mRNA Abundance Analysis
4.8. Protein Expression Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishikawa, H.; Goto, M.; Fukunishi, S.; Asai, A.; Nishiguchi, S.; Higuchi, K. Cancer cachexia: Its mechanism and clinical significance. Int. J. Mol. Sci. 2021, 22, 8491. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Anker, S.D.; Argiles, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.H.; Glass, D.J.; Guttridge, D.C. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petruzzelli, M.; Wagner, E.F. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev. 2016, 30, 489–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Cho, J.; Song, E.J. Ubiquitin-proteasome system (UPS) as a target for anticancer treatment. Arch. Pharm. Res. 2020, 43, 1144–1161. [Google Scholar] [CrossRef]
- Kodani, N.; Nakae, J. Tissue-specific metabolic regulation of FOXO-binding protein: FOXO does not act alone. Cells 2020, 9, 702. [Google Scholar] [CrossRef] [Green Version]
- Rudar, M.; Fiorotto, M.L.; Davis, T.A. Regulation of muscle growth in early postnatal life in a swine model. Annu. Rev. Anim. Biosci. 2019, 7, 309–335. [Google Scholar] [CrossRef]
- Yin, L.; Li, N.; Jia, W.H.; Wang, N.Q.; Liang, M.D.; Yang, X.Y.; Du, G.H. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef]
- Crossland, H.; Constantin-Teodosiu, D.; Gardiner, S.M.; Constantin, D.; Greenhaff, P.L. A potential role for Akt/FOXO signalling in both protein loss and the impairment of muscle carbohydrate oxidation during sepsis in rodent skeletal muscle. J. Physiol. Sci. 2008, 586, 5589–5600. [Google Scholar] [CrossRef]
- Lala-Tabbert, N.; Lejmi-Mrad, R.; Timusk, K.; Fukano, M.; Holbrook, J.; St-Jean, M.; LaCasse, E.C.; Korneluk, R.G. Targeted ablation of the cellular inhibitor of apoptosis 1 (cIAP1) attenuates denervation-induced skeletal muscle atrophy. Skelet. Muscle 2019, 9, 13. [Google Scholar] [CrossRef]
- Dinulovic, I.; Furrer, R.; Di Fulvio, S.; Ferry, A.; Beer, M.; Handschin, C. PGC-1 alpha modulates necrosis, inflammatory response, and fibrotic tissue formation in injured skeletal muscle. Skelet. Muscle 2016, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Ogihara, K.; Aoki, K.; Zubkov, A.Y.; Zhang, J.H. Oxyhemoglobin produces apoptosis and necrosis in cultured smooth muscle cells. Brain Res. 2001, 889, 89–97. [Google Scholar] [CrossRef]
- Yuan, J.; Amin, P.; Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 2019, 20, 19–33. [Google Scholar] [CrossRef]
- Yan, J.; Wan, P.; Choksi, S.; Liu, Z.G. Necroptosis and tumor progression. Trends Cancer 2022, 8, 21–27. [Google Scholar] [CrossRef]
- Shan, B.; Pan, H.L.; Najafov, A.; Yuan, J.Y. Necroptosis in development and diseases. Genes Dev. 2018, 32, 327–340. [Google Scholar] [CrossRef]
- Zhou, W.; Yuan, J.Y. Necroptosis in health and diseases. Semin. Cell Dev. Biol. 2014, 35, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Kim, C. The modulation of necroptosis and its therapeutic potentials. Mol. Cell Toxicol. 2021, 17, 93–97. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, Y.; Shi, L.; Li, W.; Chen, K.; Li, M.; Chen, X.; Zhang, H.; Li, T.; Matsuzawa-Ishimoto, Y.; et al. Gut epithelial TSC1/mTOR controls RIPK3-dependent necroptosis in intestinal inflammation and cancer. J. Clin. Investig. 2020, 130, 2111–2128. [Google Scholar] [CrossRef] [Green Version]
- Majdi, A.; Aoudjehane, L.; Ratziu, V.; Islam, T.; Afonso, M.B.; Conti, F.; Mestiri, T.; Lagouge, M.; Foufelle, F.; Ballenghien, F.; et al. Inhibition of receptor-interacting protein kinase 1 improves experimental non-alcoholic fatty liver disease. J. Hepatol. 2020, 72, 627–635. [Google Scholar] [CrossRef]
- Wang, H.H.; Chen, Y.; Liu, X.D.; Zhang, R.; Wang, X.T.; Zhang, Q.H.; Wei, Y.T.; Fang, F.; Yuan, Y.; Zhou, Q.Q.; et al. TNF-alpha derived from arsenite-induced microglia activation mediated neuronal necroptosis. Ecotoxicol. Environ. Saf. 2022, 236, 13. [Google Scholar] [CrossRef]
- Odle, J.; Lin, X.; Jacobi, S.K.; Kim, S.W.; Stahl, C.H. The suckling piglet as an agrimedical model for the study of pediatric nutrition and metabolism. Annu. Rev. Anim. Biosci. 2014, 2, 419–444. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.P.; Grossberg, A.J.; Krasnow, S.M.; Levasseur, P.R.; Szumowski, M.; Zhu, X.X.; Maxson, J.E.; Knoll, J.G.; Barnes, A.P.; Marks, D.L. Cancer- and endotoxin-induced cachexia require intact glucocorticoid signaling in skeletal muscle. FASEB J. 2013, 27, 3572–3582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, E.Q.; Wang, Y.; Wang, C.X.; Zhang, R.Y.; Zhao, Y.M.; Hong, J. Necrostatin-1 attenuates lipopolysaccharide-induced acute lung injury in mice. Exp. Lung Res. 2017, 43, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Aniort, J.; Stella, A.; Philipponnet, C.; Poyet, A.; Polge, C.; Claustre, A.; Combaret, L.; Béchet, D.; Attaix, D.; Boisgard, S.; et al. Muscle wasting in patients with end-stage renal disease or early-stage lung cancer: Common mechanisms at work. J. Cachexia Sarcopenia Muscle 2019, 10, 323–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argiles, J.M.; Lopez-Soriano, F.J.; Busquets, S. Mediators of cachexia in cancer patients. Nutrition 2019, 66, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Sharma, R. The syndrome of cardiac cachexia. Int. J. Cardiol. 2002, 85, 51–66. [Google Scholar] [CrossRef]
- Biazi, G.R.; Frasson, I.G.; Miksza, D.R.; de Morais, H.; Silva, F.D.; Bertolini, G.L.; de Souza, H.M. Decreased hepatic response to glucagon, adrenergic agonists, and cAMP in glycogenolysis, gluconeogenesis, and glycolysis in tumor-bearing rats. J. Cell Biochem. 2018, 119, 7300–7309. [Google Scholar] [CrossRef]
- Trobec, K.; von Haehling, S.; Anker, S.D.; Lainscak, M. Growth hormone, insulin-like growth factor 1, and insulin signaling-a pharmacological target in body wasting and cachexia. J. Cachexia Sarcopenia Muscle 2011, 2, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Chiappalupi, S.; Sorci, G.; Vukasinovic, A.; Salvadori, L.; Sagheddu, R.; Coletti, D.; Renga, G.; Romani, L.; Donato, R.; Riuzzi, F. Targeting RAGE prevents muscle wasting and prolongs survival in cancer cachexia. J. Cachexia Sarcopenia Muscle 2020, 11, 929–946. [Google Scholar] [CrossRef] [Green Version]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Porporato, P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Jaffer, T.; Eguchi, S.; Wang, Z.; Linkermann, A.; Ma, D. Role of necroptosis in the pathogenesis of solid organ injury. Cell Death Dis. 2015, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Lv, X.; Hu, B.; Shao, Z.; Wang, B.; Ma, K.; Lin, H.; Cui, M. RIPK1/RIPK3/MLKL-mediated necroptosis contributes to compression-induced rat nucleus pulposus cells death. Apoptosis 2017, 22, 626–638. [Google Scholar] [CrossRef]
- Jog, N.R.; Caricchio, R. The role of necrotic cell death in the pathogenesis of immune mediated nephropathies. Clin. Immunol. 2014, 153, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Kang, P.; Zhang, L.; Hou, Y.; Ding, B.; Yi, D.; Wang, L.; Zhu, H.; Liu, Y.; Yin, Y.; Wu, G. Effects of L-proline on the growth performance, and blood parameters in weaned lipopolysaccharide (LPS)-challenged pigs. Asian-Australas J. Anim. Sci. 2014, 27, 1150–1156. [Google Scholar] [CrossRef]
- Landgraf, M.A.; Silva, R.C.; Corrêa-Costa, M.; Hiyane, M.I.; Carvalho, M.H.; Landgraf, R.G.; Câmara, N.O. Leptin downregulates LPS-induced lung injury: Role of corticosterone and insulin. Cell Physiol. Biochem. 2014, 33, 835–846. [Google Scholar] [CrossRef] [Green Version]
- Nordgreen, J.; Munsterhjelm, C.; Aae, F.; Popova, A.; Boysen, P.; Ranheim, B.; Heinonen, M.; Raszplewicz, J.; Piepponen, P.; Lervik, A.; et al. The effect of lipopolysaccharide (LPS) on inflammatory markers in blood and brain and on behavior in individually-housed pigs. Physiol. Behav. 2018, 195, 98–111. [Google Scholar] [CrossRef]
- Xiong, X.Y.; Liang, J.; Xu, Y.Q.; Liu, Y. The Tilapia collagen peptide mixture TY001 protects against LPS-induced inflammation, disruption of glucose metabolism, and aberrant expression of circadian clock genes in mice. Chronobiol. Int. 2019, 36, 1013–1023. [Google Scholar] [CrossRef]
- Schmidt, S.F.; Rohm, M.; Herzig, S.; Berriel Diaz, M. Cancer cachexia: More than skeletal muscle wasting. Trends Cancer 2018, 4, 849–860. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, F.; Odle, J.; Lin, X.; Zhu, H.; Shi, H.; Hou, Y.; Yin, J. Fish oil increases muscle protein mass and modulates Akt/FOXO, TLR4, and NOD signaling in weanling piglets after lipopolysaccharide challenge. J. Nutr. 2013, 143, 1331–1339. [Google Scholar] [CrossRef] [Green Version]
- Valentine, R.J.; Jefferson, M.A.; Kohut, M.L.; Eo, H. Imoxin attenuates LPS-induced inflammation and MuRF1 expression in mouse skeletal muscle. Physiol. Rep. 2018, 6, e13941. [Google Scholar] [CrossRef] [PubMed]
- Bivona, J.J., 3rd; Crymble, H.M.; Guigni, B.A.; Stapleton, R.D.; Files, D.C.; Toth, M.J.; Poynter, M.E.; Suratt, B.T. Macrophages augment the skeletal muscle proinflammatory response through TNFα following LPS-induced acute lung injury. FASEB J. 2021, 35, e21462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chi, M.; Chen, L.; Sun, X.; Wan, L.; Yang, Q.; Guo, C. Linalool prevents cisplatin induced muscle atrophy by regulating IGF-1/Akt/FOXO pathway. Front. Pharmacol. 2020, 11, 598166. [Google Scholar] [CrossRef] [PubMed]
- Goldbraikh, D.; Neufeld, D.; Eid-Mutlak, Y.; Lasry, I.; Gilda, J.E.; Parnis, A.; Cohen, S. USP1 deubiquitinates Akt to inhibit PI3K-Akt-FoxO signaling in muscle during prolonged starvation. EMBO Rep. 2020, 21, e48791. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Leng, W.; Pi, D.; Tu, Z.; Zhu, H.; Shi, H.; Li, S.; Hou, Y.; Hu, C.A. Aspartate inhibits LPS-induced MAFbx and MuRF1 expression in skeletal muscle in weaned pigs by regulating Akt, AMPKα and FOXO1. Innate Immun. 2017, 23, 34–43. [Google Scholar] [CrossRef]
- Lecker, S.H.; Jagoe, R.T.; Gilbert, A.; Gomes, M.; Baracos, V.; Bailey, J.; Price, S.R.; Mitch, W.E.; Goldberg, A.L. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004, 18, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Glass, D.J. Skeletal muscle hypertrophy and atrophy signaling pathways. Int. J. Biochem. Cell Biol. 2005, 37, 1974–1984. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the dependency of cellular protein levels on mRNA abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef] [Green Version]
- Morgan, J.E.; Prola, A.; Mariot, V.; Pini, V.; Meng, J.; Hourde, C.; Dumonceaux, J.; Conti, F.; Relaix, F.; Authier, F.J.; et al. Necroptosis mediates myofibre death in dystrophin-deficient mice. Nat. Commun. 2018, 9, 3655. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, H.; Dai, T.; Gu, Y.; Qiu, X.; Hu, C.; Liu, Y.; Wei, K.; Li, D. Necrostatin-1 promotes ectopic periodontal tissue like structure regeneration in LPS-treated PDLSCs. PLoS ONE 2018, 13, e0207760. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhu, C.J.; Zhu, F.; Dai, B.B.; Song, S.J.; Wang, Z.Q.; Feng, Y.B.; Ge, J.F.; Zhou, R.P.; Chen, F.H. Necrostatin-1 ameliorates adjuvant arthritis rat articular chondrocyte injury via inhibiting ASIC1a-mediated necroptosis. Biochem. Biophys. Res. Commun. 2018, 504, 843–850. [Google Scholar] [CrossRef]
- Han, X.A.; Jie, H.Y.; Wang, J.H.; Zhang, X.M.; Wang, J.; Yu, C.X.; Zhang, J.L.; He, J.; Chen, J.Q.; Lai, K.F.; et al. Necrostatin-1 ameliorates neutrophilic inflammation in asthma by suppressing MLKL phosphorylation to inhibiting NETs release. Front. Immunol. 2020, 11, 666. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.T.; Xue, X.D.; Zhao, Z.D.; Lu, J.Y.; Xie, P.L. Necrostatin-1, RIP1/RIP3 inhibitor, relieves transforming growth factor β-induced wound-healing process in formation of hypertrophic scars. J. Cosmet. Dermatol. 2021, 20, 2612–2618. [Google Scholar] [CrossRef]
- Kamiya, M.; Mizoguchi, F.; Kawahata, K.; Wang, D.; Nishibori, M.; Day, J.; Louis, C.; Wicks, I.P.; Kohsaka, H.; Yasuda, S. Targeting necroptosis in muscle fibers ameliorates inflammatory myopathies. Nat. Commun. 2022, 13, 166. [Google Scholar] [CrossRef]
- Johnson, L.R.; Chandler, A.M. RNA and DNA of gastric and duodenal mucosa in antrectomized and gastrin-treated rats. Am. J. Physiol. Renal. Physiol. 1973, 224, 937–940. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Gene | Primer Sequence | Amplification Length | SERIAL NUMBER |
|---|---|---|---|
| TNF-α | F: TCCAATGGCAGAGTGGGTATG | 67 | NM_214022.1 |
| R: AGCTGGTTGTCTTTCAGCTTCAC | |||
| IL-1β | F: GCTAACTACGGTGACAACAATAATG | 186 | NM_214055.1 |
| R: CTTCTCCACTGCCACGATGA | |||
| IL-6 | F: AAGGTGATGCCACCTCAGAC | 151 | JQ839263.1 |
| R: TCTGCCAGTACCTCCTTGCT | |||
| Akt | F: GAAGAAGGAGGTCATCGT | 178 | NM_001159776.1 |
| R: GGACAGGTGGAAGAAGAG | |||
| FOXO-1 | F: TTCACCAGGCACCATCAT | 236 | NM_214014.2 |
| R: GAGGAGAGTCGGAAGTAAGT | |||
| MuFR1 | F: ATGGAGAACCTGGAGAAGCA | 219 | FJ905227.1 |
| R: ACGGTCCATGATCACCTCAT | |||
| MAFbx | F: TCACAGCTCACATCCCTGAG | 167 | NM_001044588.1 |
| R: GACTTGCCGACTCTCTGGAC | |||
| RIP1 | F: ACATCCTGTACGGCAACTCT | 175 | XM_005665538.2 |
| R: CGGGTCCAGGTGTTTATCC | |||
| RIP3 | F: CTTGTTGTCTGTCCGTGAGC | 238 | XM_001927424.3 |
| R: GAGGAGGTTGGGCTGTTGA | |||
| MLKL | F: TCTCGCTGCTGCTTCA | 105 | XM_013998184.1 |
| R: CTCGCTTGTCTTCCTCTG | |||
| GAPDH | F: CGTCCCTGAGACACGATGGT | 194 | AF017079.1 |
| R: GCCTTGACTGTGCCGTGGAAT |
| Antibodies (Host) | Catalog Number | Manufacturers | Dilution Ratio |
|---|---|---|---|
| RIP1 (rabbit) | LS-B8214 | LifeSpan BioSciences (Lynnwood, WA, USA) | 1:1000 |
| p-RIP1 (rabbit) | 91702S | Cell Signaling Technology (Danvers, MA, USA) | 1:1000 |
| RIP3 (rabbit) | SC-135170 | Santa Cruz Biotechnology (Dallas, TX, USA) | 1:1000 |
| p-RIP1 (rabbit) | 91702S | Cell Signaling Technology | 1:1000 |
| MLKL (rabbit) | 37705S | Cell Signaling Technology | 1:1000 |
| p-MLKL (rabbit) | 62233S | Cell Signaling Technology | 1:1000 |
| Akt (rabbit) | 9272S | Cell Signaling Technology | 1:1000 |
| p-Akt (rabbit) | 9271S | Cell Signaling Technology | 1:1000 |
| FOXO1 (rabbit) | 9454S | Cell Signaling Technology | 1:1000 |
| p-FOXO1 (rabbit) | 9464S | Cell Signaling Technology | 1:1000 |
| GAPDH (mouse) | FD0063 | Hangzhou Fude Biological Technology (Hangzhou, China) | 1:5000 |
| IgG-HRP (goat anti-rabbit) | ANT020 | Wuhan Antejie Biotechnology(Wuhan, China) | 1:5000 |
| IgG-HRP (goat anti-mouse) | ANT019 | Wuhan Antejie Biotechnology | 1:5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Qin, X.; Wang, Y.; Li, X.; Wang, X.; Zhu, H.; Chen, S.; Zhao, J.; Xiao, K.; Liu, Y. Necroptosis Mediates Muscle Protein Degradation in a Cachexia Model of Weanling Pig with Lipopolysaccharide Challenge. Int. J. Mol. Sci. 2023, 24, 10923. https://doi.org/10.3390/ijms241310923
Guo J, Qin X, Wang Y, Li X, Wang X, Zhu H, Chen S, Zhao J, Xiao K, Liu Y. Necroptosis Mediates Muscle Protein Degradation in a Cachexia Model of Weanling Pig with Lipopolysaccharide Challenge. International Journal of Molecular Sciences. 2023; 24(13):10923. https://doi.org/10.3390/ijms241310923
Chicago/Turabian StyleGuo, Junjie, Xu Qin, Yang Wang, Xiangen Li, Xiuying Wang, Huiling Zhu, Shaokui Chen, Jiangchao Zhao, Kan Xiao, and Yulan Liu. 2023. "Necroptosis Mediates Muscle Protein Degradation in a Cachexia Model of Weanling Pig with Lipopolysaccharide Challenge" International Journal of Molecular Sciences 24, no. 13: 10923. https://doi.org/10.3390/ijms241310923





