Zeolite Nanoparticles Loaded with 2-Methoxystradiol as a Novel Drug Delivery System for the Prostate Cancer Therapy
Abstract
:1. Introduction
2. Results and Discussion
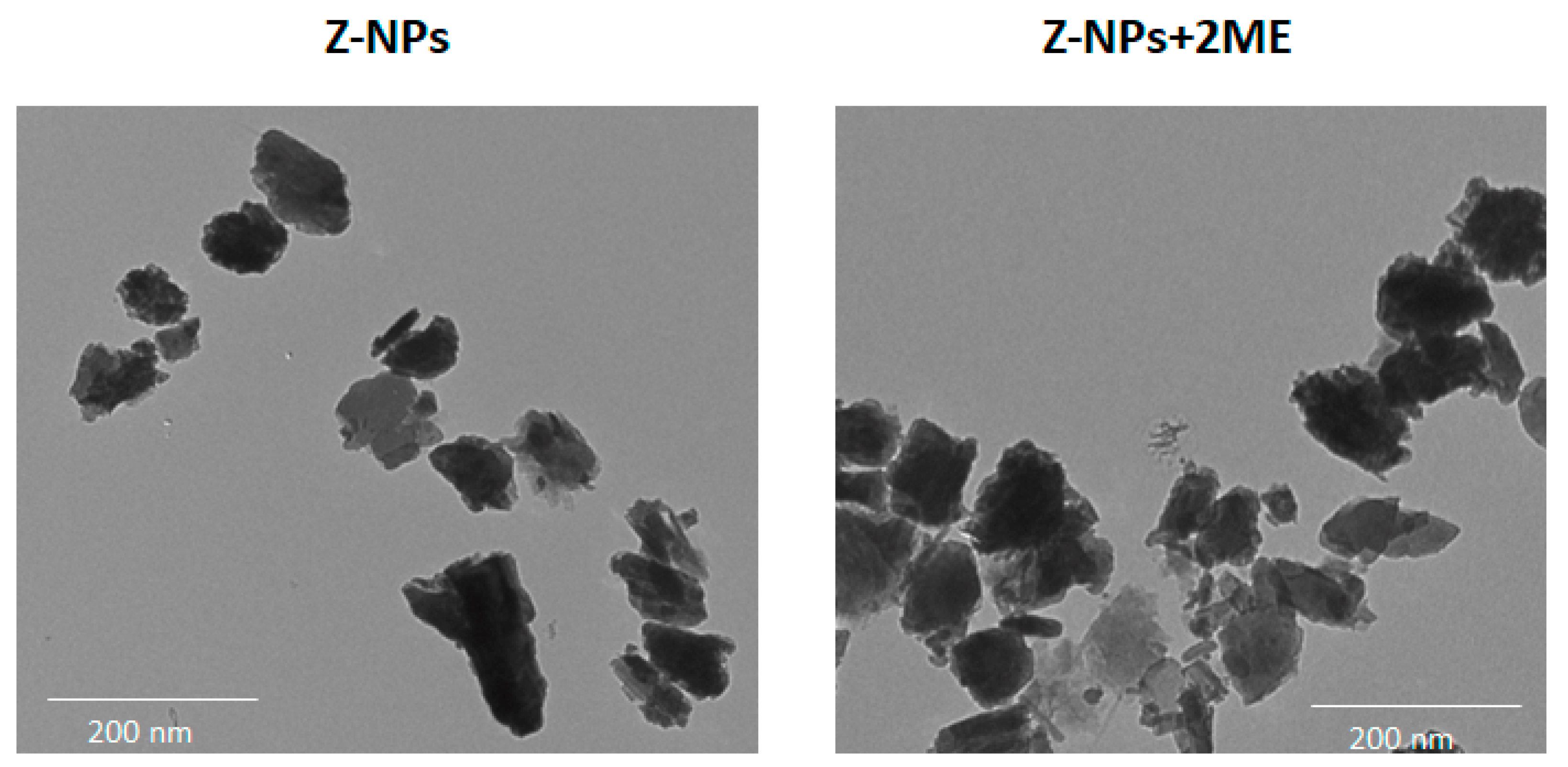
2.1. Characterization of the 2ME-Loaded Zeolite Nanoparticles
2.2. Interaction of 2ME with Zeolite Nanoparticles
2.3. Adsorption of 2ME
2.4. Release Profile of 2ME
2.5. Analysis of the 2ME Releasing Kinetics
2.6. Viability of LNCaP Cells Treated with 2ME-Loaded Zeolite Nanoparticles
2.7. 2ME-Loaded Zeolite Nanoparticles Increased the Expression of the mRNA for SPON1
2.8. Viability of Primary Cell Culture from Mouse Prostate Cancer Treated with 2ME-Loade Zeolite Nanoparticles
3. Conclusions
4. Materials and Methods
4.1. Separation of Zeolite Nanoparticles
4.2. Zeolite Nanoparticles Loaded with 2ME
4.3. Characterization Techniques
4.3.1. Dynamic Light Scattering
4.3.2. Transmission Electron Microscopy
4.3.3. Ultraviolet Visible (UV-Vis) Spectroscopy
4.3.4. Attenuated Total Reflectance Infrared Fourier-Transform Spectroscopy
4.4. Loading Efficiency of 2ME
4.5. Release Efficiency of 2ME
4.6. 2ME Releasing Kinetics
4.7. LNCaP Cells Culture
4.8. Mouse Prostate Cancer Primary Cultures
4.8.1. Prostate Tumor Induction
4.8.2. Primary Cultures
4.9. Measurement of Cell Viability
4.10. Determination of SPON1 Transcripts by Real-Time PCR
4.11. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, A.J.; Autio, K.A.; Roach, M., III; Scher, H.I. High-risk prostate cancer-classification and therapy. Nat. Rev. Clin. Oncol. 2014, 11, 308–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowes, L.E.; Lock, M.; Rodrigues, G.; D’Souza, D.; Bauman, G.; Ahmad, B.; Venkatesan, V.; Allan, A.L.; Sexton, T. The significance of circulating tumor cells in prostate cancer patients undergoing adjuvant or salvage radiation therapy. Prostate Cancer Prostatic Dis. 2015, 18, 358–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravichandiran, P.; Subramaniyan, S.A.; Kim, S.Y.; Kim, J.S.; Park, B.H.; Shim, K.S.; Yoo, D.J. Synthesis and anticancer evaluation of 1,4-naphthoquinone derivates containing a phenyaminosulfanyl moiety. Chem. Med. Chem. 2019, 14, 532–544. [Google Scholar] [CrossRef] [Green Version]
- Ravichandiran, P.; Jegan, A.; Premnath, D.; Periasamy, V.S.; Muthusubramanian, S.; Vasanthkumar, S. Syntheis, molecular docking and cytotoxicity evaluation of novel 2-(4-amino-benzosulfonyl)-5H-benzo[b]carbazole-6,11-dione derivates as histone deacetylase (HDAC8) inhibitors. Bioorg. Chem. 2014, 53, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Parada-Bustamante, A.; Valencia, C.; Reuquen, P.; Diaz, P.; Rincon-Rodriguez, R.; Orihuela, P.A. Role of 2-methoxyestradiol, an endogenous estrogen metabolite, in health and disease. Mini Rev. Med. Chem. 2015, 15, 427–438. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Sadarangani, A.; Lange, S.; Villalón, M.; Brañes, J.; Brosens, J.J.; Owen, G.I.; Cuello, M. The oestrogen metabolite 2-methoxyoestradiol alone or in combination with tumour necrosis factor-related apop-tosis-inducing ligand mediates apoptosis in cancerous but not healthy cells of the human endometrium. En-Docr Relat. Cancer 2007, 14, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Rodriguez, R.; Oróstica, M.L.; Diaz, P.; Reuquen, P.; Cárdenas, H.; Orihuela, P.A. Changes in the gene expression pattern induced by 2-methoxyestradiol in the mouse uterus. Endocrine 2013, 44, 773–783. [Google Scholar] [CrossRef]
- Rincón-Rodriguez, R.; Mena, D.; Mena, J.; Diaz-Saldivar, P.; Guajardo-Correa, E.; Godoy-Guzman, C.; Cardenas, H.; Orihuela, P.A. F-spondin is the signal by which 2-methoxyestradiol induces apoptosis in the endometrial cancer cell line Ishikawa. Int. J. Mol. Sci. 2019, 20, 3850. [Google Scholar] [CrossRef] [Green Version]
- Verenich, S.; Gerk, P.M. Therapeutic promises of 2-methoxyestradiol and its drug disposition challenges. Mol. Pharm. 2010, 7, 2030–2039. [Google Scholar] [CrossRef] [Green Version]
- León, A.; Reuquen, P.; Garin, C.; Segura, R.; Vargas, P.; Zapata, P.A.; Orihuela, P.A. FTIR and Raman characterization coated with polyethylene glycol as carrier for 2-methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef] [Green Version]
- Alfaro, A.; León, A.; Guajardo-Correa, E.; Reuquen, P.; Torres, F.; Mery, M.; Segura, R.; Zapata, P.A.; Orihuela, P.A. MgO nanoparticles coated with polyethylene glicol as Carrier for 2-methoxyestradiol anti-cancer drug. PLoS ONE 2019, 14, e0214900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavolaro, P.; Catalano, S.; Martino, G.; Tavolaro, A. Zeolite inorganic scaffolds for novel biomedical applications: Effect of physicochemical characteristic of zeolite membranes on cell adhesion and viability. Appl. Surf. Sci. 2016, 380, 135–140. [Google Scholar] [CrossRef]
- Myroslav, S. Solid-liquid-solid extraction of heavy metals (Cr, Cu, Cd, Ni and Pb) in aqueous system of zeolite-sewage sludge. J. Hazard. Mater. 2009, 161, 1377–1383. [Google Scholar]
- Chutia, P.; Kato, S.; Kojima, T.; Satokama, S. Adsorption of As. (V) on surfactant-modified natural zeo-lites. J. Hazard. Mater. 2009, 162, 204–211. [Google Scholar] [CrossRef]
- Calvo, B.; Canoira, L.; Morante, F.; Martinez-Bedia, J.M.; Vinagre, C.; Garcia-Gonzales, J.E.; Elsen, J.; Alcantara, R. Continuous elimination of Pb2+, Cu2+, Zn2+, H+ and NH4+ from acidic waters by ionic ex-change on natural zeolites. J. Hazard. Mater. 2009, 166, 619–627. [Google Scholar] [CrossRef]
- Serata-Nouri, H.; Jafari, A.; Roshangar, L.; Dadashpour, M.; Pilehvar-Soltanahmadi, Y.; Zarhami, N. Biomedical applications of zeolite-based materials: A review. Mater. Sci. Eng. C. 2020, 116, 111225. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Jafari, S.; Sarvari, S.; Barzegari, E.; Moakedl, F.; Ghorbani, M.; Shiri Varnam-Khasti, B.; Jaymand, M.; Izadi, Z.; Tayebi, L. Biomedical applications of zeolite nanoparticles, with an em-phasis on medical interventions. Inter. J. Nanomed. 2020, 15, 363–386. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Stavljenic, I.; Batu, Z.; Mravak-Stipetic, M.; Pavelic, K.; Ozer, F. Effects of zeolite as a drug delivery system on cancer therapy. A systemic review. Molecules 2021, 26, 6196. [Google Scholar] [CrossRef]
- Rahimi, M.; Ng, E.P.; Bakhtiari, K.; Vinciguerra, M.; Ali Ahmad, H.; Awala, H.; Mintova, S.; Daghiri, M.; Bakhshandeh, R.F.; de Vries, M.; et al. Zeolite nanoparticles for selective sorption of plasma proteins. Sci. Rep. 2015, 5, 17259. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elsatar, A.G.; Farag, M.M.; Youssef, H.F.; Salih, S.A.; Mounier, M.M.; El-Mellegy, E. Different zeolite systems for colon cancer therapy: Monitoring of ion release, cytotoxicity and drug release behavior. Prog. Biomater. 2018, 8, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Servatan, M.; Zarrintaj, P.; Mahomodi, G.; Kim, S.J.; Reza Ganjali, M.; Reza Saeb, M.; Mozafari, M. Zeolites in drug delivery: Progress, challenges and opportunities. Drug. Discov. Today 2020, 25, 642–656. [Google Scholar] [CrossRef]
- Mintova, S.; Gilson, J.P.; Valtchev, V. Advances in nanosized zeolites. Nanoscale 2013, 5, 6693–6703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Gu, J.; Zhang, L.; Chen, H.; Shi, J. Fabrication of uniform magnetic nanocomposite spheres with a magnetic core/mesoporous silica shell structure. J. Chem. Soc. 2005, 127, 8916–8917. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, S.; Ataei, F.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, S.; Khoras-ani, S.; Mozafar, R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.J.; Chen, C.; Zhao, Y.; Jia, L.; Wang, P.C. Biopharmaceutics and therapeutic potential of en-gineered nanomaterials. Curr. Drug. Metab. 2008, 9, 697–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Guo, R.; Cao, X.; Shen, M.; Shi, X. Encapsulation of 2-methoxyestradiol within multifunc-tional poly(amidoamine) dendrimers for targeted cancer therapy. Biomaterials 2011, 32, 3322–3329. [Google Scholar] [CrossRef]
- de Peña, Y.P.; Rondón, W. Linde type a zeolite and type Y faujasite as a solid-phase for lead, cadmium, nickel and cobalt preconcentration and determination using a flow injection system coupled to flame atomic absorption spectrometry. Am. J. Anal. Chem. 2013, 4, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Prasetyo, T.A.B.; Soegijono, B. Characterization of sonicated natural zeolite/ferric chloride hexahydrate by infrared spectroscopy. J. Phys. Conf. Ser. 2018, 985, 012022. [Google Scholar] [CrossRef]
- Katarzyna, M.; Jelen, P. The effect of heat treatment on the structure of zeolite A. Materials 2021, 14, 4642. [Google Scholar] [CrossRef]
- Lei, G.D.; Carvill, B.T.; Sachtler, W.M.H. Single file diffusion in mordenite channels: Neopentane conversion and H/D exchange as catalytic probes. Appl. Catal. A Gen. 1996, 142, 347–359. [Google Scholar] [CrossRef]
- Lozano-Castello, D.; Zhu, W.; Linares-Solano, A.; Kapteijn, F.; Moulijn, J.A. Micropore accessibility of large modenite crystals. Microporous Mesoporous Mater. 2006, 92, 145–153. [Google Scholar] [CrossRef]
- de Gennaro, B.; Catalanotti, L.; Cappelletti, P.; Langella, A.; Mercurio, M.; Serri, C.; Biondi, M.; Mayol, L. Surface modified natural zeolite as a Carrier for sustained diclofenac release: A preliminary feasibility study. Colloids Surf. B 2015, 130, 101–109. [Google Scholar] [CrossRef]
- Rimoli, M.G.; Rabaioli, M.R.; Melisi, D.; Curcio, A.; Mondello, S.; Mirabelli, R.; Abignente, E. Synthetic zeolites as a new tool for drug delivery. J. Biomed. Mater. Res. A 2007, 87, 156–164. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Soleimani, H.A.; Mohammadpour, F.; Hadizadeh, F. Synthetic zeolites as controlled-release delivery systems for anti-inflammatory drugs. Chem. Biol. Drug. Des. 2016, 87, 849–857. [Google Scholar] [CrossRef]
- Abadeh, Z.A.; Saviano, G.; Ballirano, P.; Santonicola, G. Curcumin-loaded zeolite as anticáncer drug Carrier: Effect of curcumin adsorption on zeolite structure. Pure Appl. Chem. 2020, 92, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.E.; Madler, L.; Velegol, D.; Xia, T.; Hock, E.M.; Somasundaran, P.; Klaesssig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Bajpai, P.K. Synthesis of mordenite type zeolite. Zeolites 1986, 6, 2–9. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z. Zeolite imidazole framework materials: Recent progress in synthesis and applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Danyuo, Y.; Ani, C.J.; Salifu, A.A.; Obayemi, J.D.; Dozie-Nwachukwu, S.; Obanawu, V.O.; Akpan, U.M.; Odusanya, O.S.; Abade-Abugre, M.; McBagonluri, F.; et al. Anomalous release kinetics of prodi-giosin from poly-N-isopropyl-acrylamid based hydrogels for the treatment of triple negative breast cancer. Sci. Rep. 2019, 9, 3862. [Google Scholar] [CrossRef] [Green Version]
- Moradi, M.R.; Salahinejad, E.; Sharifi, L.; Tayebi, L. Controlled drug deliveri from chitosan-coated hep-arin-loaded nanopores anodically grown on nitinol shape-memory alloy. Carbohydr. Polym. 2023, 314, 120961. [Google Scholar] [CrossRef]
- Ruiz-Garcia, A.; Tan, W.; Li, J.; Haughey, M.; Masters, J.; Hibma, J.; Lin, S. Pharmacokinetic models to characterize the absorption phase and the influence of a proton pump inhibitor on the overall exposure of dacomitinib. Pharmaceutics 2020, 12, 330. [Google Scholar] [CrossRef] [Green Version]
- Sawaftah, N.A.; Paul, V.; Awad, N.; Husseini, G.A. Modeling of anticancer drug release kinetics from liposomes and miscelles: A review. IEEE Trans. NanoBiosci. 2021, 20, 565–576. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Al-Rabia, M.W.; Asfour, H.Z.; Alshehri, S.A.; Alharbi, W.S.; Halawani, A.; Alamoudi, A.J.; Noor, A.O.; Bannan, D.F.; Fahmy, U.A.; et al. 2-methoxyestradiol loaded Alpha lipoic acid nano-particles augment cytotoxicity in MCF-7 breast cancer cells. DOSE-RESPONSE 2021, 19, 1–8. [Google Scholar] [CrossRef]
- Ali, H.; Kilic, G.; Vincent, K.; Motamedi, M.; Rytting, E. Nanomedicine for uterine leiomyoma therapy. Ther. Deliv. 2013, 4, 161–175. [Google Scholar] [CrossRef] [Green Version]
- Pyle-Chenault, R.A.; Stolk, J.A.; Molesh, D.A.; Boyle-Harlan, D.; McNeill, P.D. VSGP/F-Spondin: A New Ovarian Cancer Marker. Tumour Biol. 2005, 26, 245–257. [Google Scholar] [CrossRef]
- Cacciatore, A.; Albino, D.; Catapano, C.V.; Carbone, G.M. Preclinical models of neuroendocrine prostate cancer. Curr. Protoc. 2023, 3, e742. [Google Scholar] [CrossRef]
- Bosland, M.C.; Schlicht, M.J.; Acevedo, N.; Soto, A.M.; Prins, G. Effects of perinatal exposure to bi-sphenol A on induction of prostate cancer in Sprague Dawley rats by MNU and testosterone. Toxicology 2023, 484, 153394. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analyses: Advanced Course, 3rd ed.; Wisconsin-Madison: Madison, WI, USA, 1969; p. 894. [Google Scholar]
- Gutierrez, M.; Escudey, M.; Escrig, J.; Denardin, J.C.; Altbir, D.; Fabris, J.D.; Cavalcante, L.C.D.; García-Gonzales, M.T. Preparation and characterization of magnetic composites based on a natural zeolite. Clays Clay Miner. 2010, 58, 589–595. [Google Scholar] [CrossRef]
- Pizarro, C.; Escudey, M.; Bravo, C.; Gacitua, M.; Pavez, L. Sulfate kinetics and adsorption studies on a zeolite/polyammonium cation composite for environmental remediation. Minerals 2021, 11, 180. [Google Scholar] [CrossRef]
- Vilos, C.; Morales, F.A.; Solar, P.A.; Herrera, N.S.; Gonzalez-Nilo, F.D.; Aguayo, D.A.; Mendoza, H.L.; Comer, J.; Bravo, M.L.; Gonzalez, P.A.; et al. Paclitaxel-PHBV nanoparticles and their toxicity to endometrial and primary ovarian cancer cells. Biomaterials 2013, 345, 4098–4108. [Google Scholar] [CrossRef] [PubMed]
- Guajardo-Correa, E.; Mena-Silva, D.; Diaz, P.; Godoy-Guzmán, C.; Cardenas, H.; Orihuela, P.A. 2-Met-hoxyoestradiol impairs mouse embryo implantation via F-spondin. Reprod. Fertil. Dev. 2019, 31, 689–697. [Google Scholar] [CrossRef]
- Banudevi, S.; Elumalai, P.; Arunkumar, R.; Senthilkumar, K.; Gunadharini, D.N.; Sharmita, G.; Arunakaran, J. Chemoprevntive effects of zinc on prostate carcinogenesis induced by N-methyl-N-nitrosurea and testosterone in adult male Sprague-Dawley rats. J. Cancer Res. Clin. Oncol. 2011, 137, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Oróstica, M.L.; Reuquen, P.; Guajardo-Correa, E.; Parada-Bustamante, A.; Cardenas, H.; Orihuela, P.A. Sperm utilize tumor necrosis factor alpha to shut down a 2-methoxyestradiol nongenomic pathway that ac-celerates oviductal egg transport in the rat. Reproduction 2023, 165, 383–393. [Google Scholar] [CrossRef]








| Phase 1 (0–72 h) | Phase 2 (72–168 h) | |||||
|---|---|---|---|---|---|---|
| pH 4 | pH 5 | pH 7.4 | pH 4 | pH 5 | pH 7.4 | |
| n | 0.53544 | 0.50453 | 0.55881 | 0.27752 | 0.29661 | 0.35284 |
| R² | 0.98221 | 0.98786 | 0.98259 | 0.99423 | 0.99321 | 0.98612 |
| KKP | 0.00768 [1/0.53544] | 0.00715 [1/h^0.50453] | 0.00654 [1/^0.55881] | 0.00089 [1/h^0.27752] | 0.00101 [1/h^0.29661] | 0.00134 [1/h^0.35284] |
| Phase 1 (0–72 h) | Phase 2 (72–168 h) | |||||
|---|---|---|---|---|---|---|
| pH 4 | pH 5 | pH 7.4 | pH 4 | pH 5 | pH 7.4 | |
| R² | 0.97678 | 0.96969 | 0.97768 | 0.99636 | 0.99545 | 0.98514 |
| KH | 2.08708 [%/sqrt(h)] | 2.10728 [%/sqrt(h)] | 1.91517 [%/sqrt(h)] | 0.16405 [%/sqrt(h)] | 0.14557 [%/sqrt(h)] | 0.10289 [%/sqrt(h)] |
| Phase 1 (0–72 h) | Phase 2 (72–168 h) | |||||
|---|---|---|---|---|---|---|
| pH 4 | pH 5 | pH 7.4 | pH 4 | pH 5 | pH 7.4 | |
| R² | 0.97519 | 0.97982 | 0.97785 | 0.99818 | 0.99734 | 0.99754 |
| K1 | 0.04032 [1/h] | 0.04094 [1/h] | 0.03547 [1/h] | 0.00401 [1/h] | 0.00458 [1/h] | 0.00495 [1/h] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mena-Silva, D.; Alfaro, A.; León, A.; Guajardo-Correa, E.; Elgueta, E.; Diaz, P.; Vilos, C.; Cardenas, H.; Denardin, J.C.; Orihuela, P.A. Zeolite Nanoparticles Loaded with 2-Methoxystradiol as a Novel Drug Delivery System for the Prostate Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 10967. https://doi.org/10.3390/ijms241310967
Mena-Silva D, Alfaro A, León A, Guajardo-Correa E, Elgueta E, Diaz P, Vilos C, Cardenas H, Denardin JC, Orihuela PA. Zeolite Nanoparticles Loaded with 2-Methoxystradiol as a Novel Drug Delivery System for the Prostate Cancer Therapy. International Journal of Molecular Sciences. 2023; 24(13):10967. https://doi.org/10.3390/ijms241310967
Chicago/Turabian StyleMena-Silva, Denisse, Aline Alfaro, Andrea León, Emanuel Guajardo-Correa, Estefania Elgueta, Patricia Diaz, Cristian Vilos, Hugo Cardenas, Juliano C. Denardin, and Pedro A. Orihuela. 2023. "Zeolite Nanoparticles Loaded with 2-Methoxystradiol as a Novel Drug Delivery System for the Prostate Cancer Therapy" International Journal of Molecular Sciences 24, no. 13: 10967. https://doi.org/10.3390/ijms241310967
APA StyleMena-Silva, D., Alfaro, A., León, A., Guajardo-Correa, E., Elgueta, E., Diaz, P., Vilos, C., Cardenas, H., Denardin, J. C., & Orihuela, P. A. (2023). Zeolite Nanoparticles Loaded with 2-Methoxystradiol as a Novel Drug Delivery System for the Prostate Cancer Therapy. International Journal of Molecular Sciences, 24(13), 10967. https://doi.org/10.3390/ijms241310967






