Whole-Genome Resequencing Reveals the Diversity of Patchouli Germplasm
Abstract
1. Introduction
2. Results
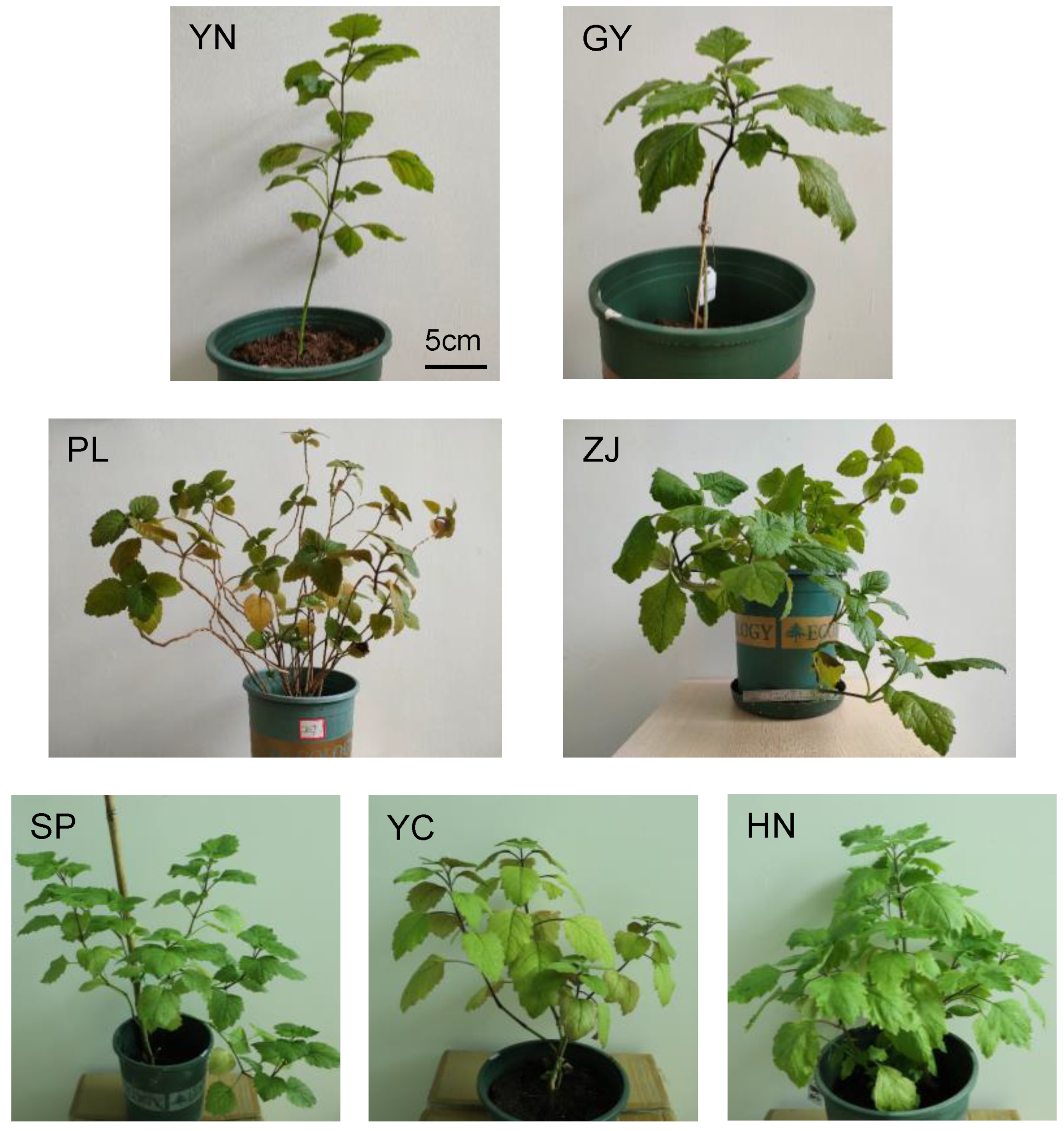
2.1. Whole-Genome Resequencing of Seven Representative Patchouli Accessions
2.2. Massive Genetic Variants Exist among Patchouli Accessions
2.3. Patchouli Accessions Can Be Divided into Three Groups
2.4. Detection and Verification of Molecular Markers for the Genuine Patchouli SP
2.5. Genetic Variations Alter the Protein Structure of Sesquiterpene Synthesis Gene
2.6. Genetic Variants in PatNES Cause the Loss of Nerolidol Synthetase Activity in Patchouli Accessions from Southeast Asia and SP
3. Discussion
4. Materials and Methods
4.1. Sample Preparation, DNA Extraction, and Whole-Genome Resequencing
4.2. Sequence Processing and Alignment to the Reference Genome
4.3. Genetic Variation Detection and Annotation
4.4. Population Structure Analysis
4.5. The Detection and Verification of Molecular Markers for Patchouli Accession SP
4.6. The Prediction of Three-Dimensional Structure and Binding Pockets of Protein
4.7. RNA Extraction and Amplification of cDNA
4.8. Cloning of the Full-Length CDS of PatNES
4.9. In Vitro Assays
4.10. Metabolite Analysis with GC-MS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Jeong, J.B.; Shin, Y.K.; Lee, S.H. Anti-inflammatory activity of patchouli alcohol in RAW264.7 and HT-29 cells. Food Chem. Toxicol. 2013, 55, 229–233. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Lian, D.W.; Xu, Y.F.; Ren, W.K.; Fu, L.J.; Chen, F.J.; Tang, L.Y.; Zhuang, H.L.; Cao, H.Y.; Huang, P. Unraveling the Novel Protective Effect of Patchouli Alcohol Against Helicobacter pylori-Induced Gastritis: Insights Into the Molecular Mechanism in vitro and in vivo. Front. Pharmacol. 2018, 9, 1347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, M.; Song, M.; Wang, J.; Cai, J.; Lin, C.; Li, Y.; Jin, X.; Shen, C.; Chen, Z.; et al. Patchouli alcohol activates PXR and suppresses the NF-κB-mediated intestinal inflammatory. J. Ethnopharmacol. 2020, 248, 112302. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gan, Y.; Li, M.; Chen, L.; Liang, J.; Zhuo, J.; Luo, H.; Xu, N.; Wu, X.; Wu, Q.; et al. Patchouli alcohol attenuates 5-fluorouracil-induced intestinal mucositis via TLR2/MyD88/NF-kB pathway and regulation of microbiota. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 124, 109883. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.L.; Chen, Y.; Yu, Q.Y.; Wang, Y.H.; Liu, G. Patchouli alcohol protects against ischemia/reperfusion-induced brain injury via inhibiting neuroinflammation in normal and obese mice. Brain Res. 2018, 1682, 61–70. [Google Scholar] [CrossRef]
- Bhatia, S.P.; Letizia, C.S.; Api, A.M. Fragrance material review on patchouli alcohol. Food Chem. Toxicol. 2008, 46 (Suppl. S11), S255–S256. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R. A Comprehensive Review on the Phytochemical Constituents and Pharmacological Activities of Pogostemon cablin Benth.: An Aromatic Medicinal Plant of Industrial Importance. Molecules 2015, 20, 8521–8547. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R. Patchouli (Pogostemon cablin Benth.): Botany, agrotechnology and biotechnological aspects. Ind. Crop. Prod. 2016, 87, 161–176. [Google Scholar] [CrossRef]
- Wu, Y.-G.; Guo, Q.-S.; Zheng, H.-Q. Textual research on history of introduction corrected and herbal medicine of Pogostemon cablin. China J. Chin. Mater. Med. 2007, 32, 2114–2117, 2181. [Google Scholar]
- Ouyang, P.; Liu, Y.; Wang, Y.; Mo, X.; Zeng, S. Aging and/or tissue-specific regulation of patchoulol and pogostone in two Pogostemon cablin (Blanco) Benth. cultivars. Physiol. Plant. 2016, 158, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.F.; Li, S.P.; Cao, H.; Liu, J.J.; Gao, J.L.; Yang, F.Q.; Wang, Y.T. GC-MS fingerprint of Pogostemon cablin in China. J. Pharm. Biomed. Anal. 2006, 42, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pan, C.; Xu, H.; Liu, X. The observation and comparison of Pogostemon cablin from different habitats. J. Chin. Med. Mater. 2002, 25, 463–465. [Google Scholar]
- Li, W.; Pan, C.; Song, L.; Liu, X.; Xu, L.; Xu, H. Observation and comparison of the flowers of Pogostemon cablin from different habitats. Zhong Yao Cai 2003, 26, 79–82. [Google Scholar]
- Pan, C.-M.; Li, W.; He, H.; Deng, W.-Q.; Li, T.-H.; Xu, H.-H. Study on intraspecific genetic diversity in different plant populations of Pogostemon cabli. China J. Chin. Mater. Med. 2006, 31, 723–726. [Google Scholar]
- Wu, Y.G.; Guo, Q.S.; He, J.C.; Lin, Y.F.; Luo, L.J.; Liu, G.D. Genetic diversity analysis among and within populations of Pogostemon cablin from China with ISSR and SRAP markers. Biochem. Syst. Ecol. 2010, 38, 63–72. [Google Scholar] [CrossRef]
- Huang, H.R.; Wu, W.; Zhang, J.X.; Wang, L.J.; Yuan, Y.M.; Ge, X.J. A genetic delineation of Patchouli (Pogostemon cablin) revealed by specific-locus amplified fragment sequencing. J. Syst. Evol. 2016, 54, 491–501. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, L.; Zou, X.; Gong, L.; Zhuang, J.; Zhang, D.; Zheng, H.; Wang, X.; Wu, D.; Zhan, R.; et al. GC-MS and UHPLC-QTOFMS-assisted identification of the differential metabolites and metabolic pathways in key tissues of Pogostemon cablin. Front. Plant Sci. 2023, 14, 1098280. [Google Scholar] [CrossRef]
- Newman, J.D.; Chappell, J. Isoprenoid biosynthesis in plants: Carbon partitioning within the cytoplasmic pathway. Crit. Rev. Biochem. Mol. Biol. 1999, 34, 95–106. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Schwender, J.; Disch, A.; Rohmer, M. Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett. 1997, 400, 271–274. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Deng, W.J.; Lu, C.H.; He, M.L.; Yan, H.J. SMRT sequencing of full-length transcriptome and gene expression analysis in two chemical types of Pogostemon cablin (Blanco) Benth. PeerJ 2022, 10, e12940. [Google Scholar] [CrossRef]
- Yan, W.P.; Ye, Z.C.; Cao, S.J.; Yao, G.L.; Yu, J.; Yang, D.M.; Chen, P.; Zhang, J.F.; Wu, Y.G. Transcriptome analysis of two Pogostemon cablin chemotypes reveals genes related to patchouli alcohol biosynthesis. PeerJ 2021, 9, e12025. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, L.; Guo, S.; An, F.; Du, D. Fine Mapping and Whole-Genome Resequencing Identify the Seed Coat Color Gene in Brassica rapa. PLoS ONE 2016, 11, e0166464. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, W.; Zeng, Y.; Li, Z.; Chen, Y.; Zhang, J.; Zhao, H.; Feng, L.; Ma, D.; Mo, X.; et al. Chromosome-level and haplotype-resolved genome provides insight into the tetraploid hybrid origin of patchouli. Nat. Commun. 2022, 13, 3511. [Google Scholar] [CrossRef]
- He, S.; Wang, X.H.; Yin, M.; Ye, J.P.; Meng, J.Z.; Zhou, L.Q. Molecular identification of Lingyun Baihao wild and cultivated tea through genome-wide sequencing. Genet. Resour. Crop. Evol. 2022, 70, 1407–1417. [Google Scholar] [CrossRef]
- Xiang, X.J.; Li, C.; Li, L.; Bian, Y.B.; Kwan, H.S.; Nong, W.Y.; Cheung, M.K.; Xiao, Y. Genetic diversity and population structure of Chinese Lentinula edodes revealed by InDel and SSR markers. Mycol. Prog. 2016, 15, 37. [Google Scholar] [CrossRef]
- Luo, J.P.; Liu, Y.P.; Feng, Y.F.; Guo, X.L.; Cao, H. Two chemotypes of Pogostemon cablin and influence of region of cultivation and harvesting time on volatile oil composition. Acta Pharm. Sin. 2003, 38, 307–310. [Google Scholar]
- Lopez-Gallego, F.; Agger, S.A.; Abate-Pella, D.; Distefano, M.D.; Schmidt-Dannert, C. Sesquiterpene synthases Cop4 and Cop6 from Coprinus cinereus: Catalytic promiscuity and cyclization of farnesyl pyrophosphate geometric isomers. Chembiochem 2010, 11, 1093–1106. [Google Scholar] [CrossRef]
- Little, D.B.; Croteau, R.B. Alteration of product formation by directed mutagenesis and truncation of the multiple-product sesquiterpene synthases delta-selinene synthase and gamma-humulene synthase. Arch. Biochem. Biophys. 2002, 402, 120–135. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zhu, H.L.; Zhou, J.H.; Jiang, S.J.; Wang, Y.; Kuang, J.; Ji, Q.; Peng, J.; Wang, J.; Gao, L.; et al. Resequencing of 296 cultivated and wild lotus accessions unravels its evolution and breeding history. Plant J. 2020, 104, 1673–1684. [Google Scholar] [CrossRef]
- Wang, Y. A draft genome, resequencing, and metabolomes reveal the genetic background and molecular basis of the nutritional and medicinal properties of loquat (Eriobotrya japonica (Thunb.) Lindl). Hortic. Res. 2021, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Rustgi, S.; Mir, R.R. Array-based high-throughput DNA markers for crop improvement. Heredity 2008, 101, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.; Sharma, P.C. Development and use of molecular markers: Past and present. Crit. Rev. Biotechnol. 2016, 36, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Russell, R.R. The natural history of protein domains. Annu. Rev. Biophys. Biomol. Struct. 2002, 31, 45–71. [Google Scholar] [CrossRef]
- Murugan, R.; Mallavarapu, G.R.; Padmashree, K.V.; Rao, R.R.; Livingstone, C. Volatile oil composition of Pogostemon heyneanus and comparison of its composition with patchouli oil. Nat. Prod. Commun. 2010, 5, 1961–1964. [Google Scholar] [CrossRef]
- Chan, W.K.; Tan, L.T.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- Lee, S.; Badieyan, S.; Bevan, D.R.; Herde, M.; Gatz, C.; Tholl, D. Herbivore-induced and floral homoterpene volatiles are biosynthesized by a single P450 enzyme (CYP82G1) in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 21205–21210. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Bittencourt, S.A. FastQC: A Quality Control Tool for High Throughput Sequence Data; ScienceOpen, Inc.: Berlin, Germany, 2010. [Google Scholar]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Retief, J.D. Phylogenetic analysis using PHYLIP. Methods Mol. Biol. 2000, 132, 243–258. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Tang, H.; Peng, J.; Wang, P.; Risch, N.J. Estimation of individual admixture: Analytical and study design considerations. Genet. Epidemiol. 2005, 28, 289–301. [Google Scholar] [CrossRef]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef]
- Konieczny, A.; Ausubel, F.M. A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 1993, 4, 403–410. [Google Scholar] [CrossRef]
- Neff, M.M.; Neff, J.D.; Chory, J.; Pepper, A.E. dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: Experimental applications in Arabidopsis thaliana genetics. Plant J. 1998, 14, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Peng, C.; Wang, J.; Xu, Z.; Chen, K.; Shi, J.; Zhu, W. D3Pockets: A Method and Web Server for Systematic Analysis of Protein Pocket Dynamics. J. Chem. Inf. Model. 2019, 59, 3353–3358. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, X.; Zhang, S.; Zhu, J.; Tang, B.; Wang, A.; Dong, L.; Zhang, Z.; Yu, C.; Sun, Y.; et al. The Genome Sequence Archive Family: Toward Explosive Data Growth and Diverse Data Types. Genom. Proteom. Bioinform. 2021, 19, 578–583. [Google Scholar] [CrossRef] [PubMed]
- CNCB-NGDC Members and Partners. Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2022. Nucleic Acids Res. 2022, 50, D27–D38. [Google Scholar] [CrossRef] [PubMed]




| ID | Raw Reads Number | Clean Reads Number | Duplication Rate (%) | Mean Depth (×) | Mean Coverage (%) | Mapped Ratio (%) | SNP Number | INDEL Number |
|---|---|---|---|---|---|---|---|---|
| YC | 284,919,332 | 281,852,310 | 15.99 | 21.00 | 99.58 | 97.51 | 110,944 | 33,837 |
| ZJ | 286,562,660 | 284,505,424 | 14.94 | 21.80 | 99.55 | 99.87 | 95,858 | 29,126 |
| HN | 312,327,368 | 310,953,452 | 19.28 | 22.48 | 99.59 | 94.56 | 104,200 | 32,370 |
| GY | 275,765,722 | 273,674,814 | 16.21 | 20.67 | 99.53 | 98.41 | 103,142 | 30,697 |
| PL | 296,466,716 | 293,764,344 | 14.41 | 22.52 | 99.46 | 99.84 | 250,663 | 73,501 |
| YN | 272,246,368 | 270,794,548 | 16.51 | 19.69 | 99.54 | 95.05 | 248,053 | 66,545 |
| SP | 303,353,674 | 300,234,292 | 15.84 | 22.32 | 99.54 | 96.96 | 249,746 | 64,447 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Chen, Y.; Li, Y.; Zeng, Y.; Li, W.; Ma, X.; Huang, L.; Shen, Y. Whole-Genome Resequencing Reveals the Diversity of Patchouli Germplasm. Int. J. Mol. Sci. 2023, 24, 10970. https://doi.org/10.3390/ijms241310970
Li Z, Chen Y, Li Y, Zeng Y, Li W, Ma X, Huang L, Shen Y. Whole-Genome Resequencing Reveals the Diversity of Patchouli Germplasm. International Journal of Molecular Sciences. 2023; 24(13):10970. https://doi.org/10.3390/ijms241310970
Chicago/Turabian StyleLi, Zhipeng, Yiqiong Chen, Yangyan Li, Ying Zeng, Wanying Li, Xiaona Ma, Lili Huang, and Yanting Shen. 2023. "Whole-Genome Resequencing Reveals the Diversity of Patchouli Germplasm" International Journal of Molecular Sciences 24, no. 13: 10970. https://doi.org/10.3390/ijms241310970
APA StyleLi, Z., Chen, Y., Li, Y., Zeng, Y., Li, W., Ma, X., Huang, L., & Shen, Y. (2023). Whole-Genome Resequencing Reveals the Diversity of Patchouli Germplasm. International Journal of Molecular Sciences, 24(13), 10970. https://doi.org/10.3390/ijms241310970






