Photons Induce Vesicular Exocytotic Release of Glutamate in a Power-Dependent Way
Abstract
:1. Introduction
2. Results
2.1. Synaptosomes Proved to Be a Purified Preparation of Nerve Terminals
2.2. Release of Glutamate from Synaptosomes: Effect of Laser Light
2.3. Power Evaluation and Temperature Measurement
3. Discussion
3.1. Photon-Evoked Glutamate Release from Glutamatergic Nerve Terminals in a Dose-Dependent Way
3.2. Photons Evoked Vesicular Exocytotic Release of Glutamate Release from the Nerve Terminals
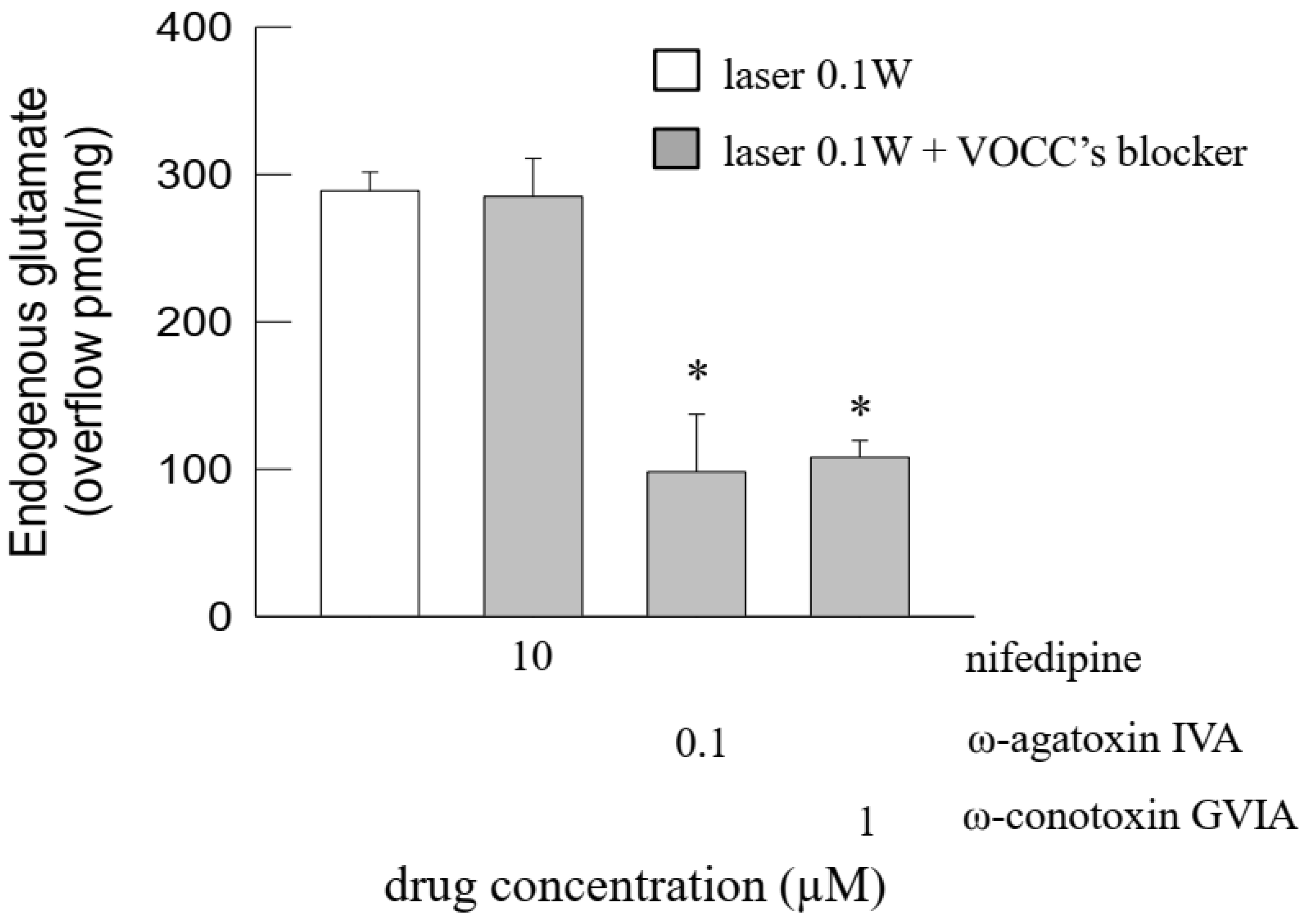
3.3. The Photon-Evoked Glutamate Release Involved Activation of Plasma Membrane Na+ Channels and Opening of Ca2+ Channels
4. Materials and Methods
4.1. Animals
4.2. Preparation of Purified Nerve Terminals and Perfusion
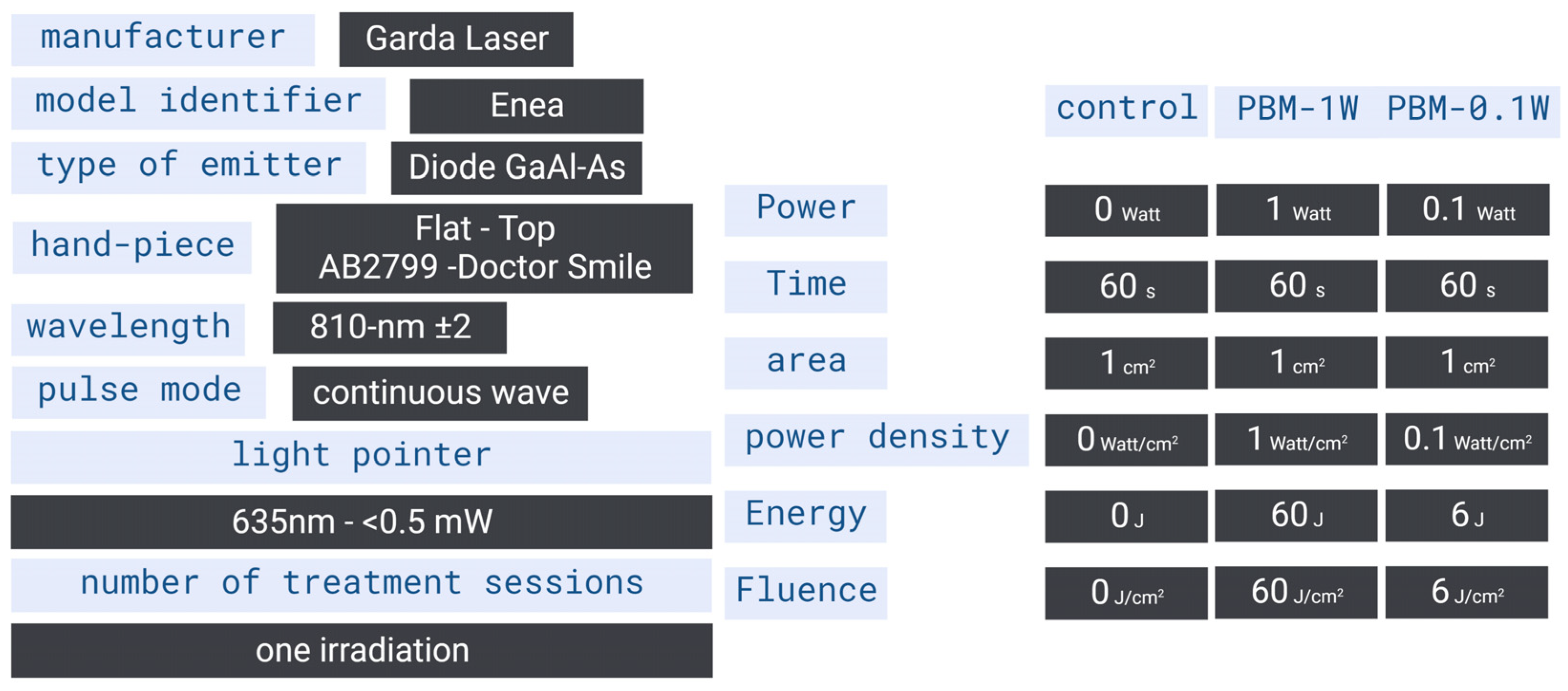
4.3. Delivering Device and Parameters Setting
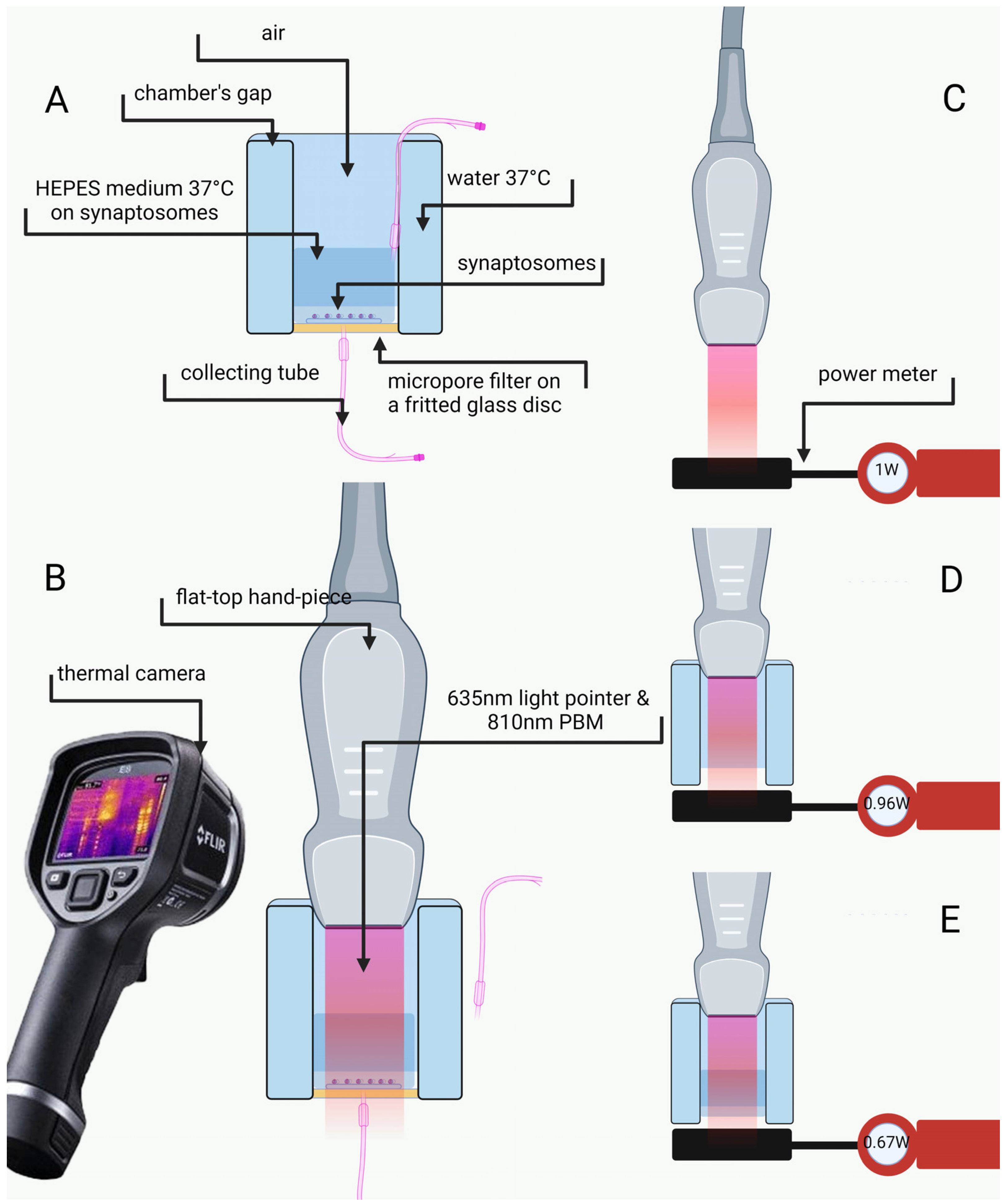
4.4. Irradiation Design, Laser Power Assessment and Temperature Monitoring
4.5. Immunofluorescent Confocal Laser Scanning Microscopy
4.6. Electron Microscopy
4.7. Determination of Endogenous Glutamate
4.8. Calculations and Statistical Analysis
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fitzgerald, M.; Hodgetts, S.; Van Den Heuvel, C.; Natoli, R.; Hart, N.S.; Valter, K.; Harvey, A.R.; Vink, R.; Provis, J.; Dunlop, S.A. Red/near-Infrared Irradiation Therapy for Treatment of Central Nervous System Injuries and Disorders. Rev. Neurosci. 2013, 24, 205–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamblin, M.R. Shining Light on the Head: Photobiomodulation for Brain Disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain Photobiomodulation Therapy: A Narrative Review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef]
- Salehpour, F.; Gholipour-Khalili, S.; Farajdokht, F.; Kamari, F.; Walski, T.; Hamblin, M.R.; DiDuro, J.O.; Cassano, P. Therapeutic Potential of Intranasal Photobiomodulation Therapy for Neurological and Neuropsychiatric Disorders: A Narrative Review. Rev. Neurosci. 2020, 31, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, R.; Hu, J.; Sun, L.; Zhao, X.; Zhao, Y.; Han, D.; Hu, S. Photobiomodulation Promotes Hippocampal CA1 NSC Differentiation Toward Neurons and Facilitates Cognitive Function Recovery Involving NLRP3 Inflammasome Mitigation Following Global Cerebral Ischemia. Front. Cell. Neurosci. 2021, 15, 731855. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Colombo, E.; Pasquale, C.; Benedicenti, S.; Solimei, L.; Signore, A.; Amaroli, A. Mitochondrial Bioenergetic, Photobiomodulation and Trigeminal Branches Nerve Damage, What’s the Connection? A Review. Int. J. Mol. Sci. 2021, 22, 4347. [Google Scholar] [CrossRef]
- Gerace, E.; Cialdai, F.; Sereni, E.; Lana, D.; Nosi, D.; Giovannini, M.G.; Monici, M.; Mannaioni, G. NIR Laser Photobiomodulation Induces Neuroprotection in an In Vitro Model of Cerebral Hypoxia/Ischemia. Mol. Neurobiol. 2021, 58, 5383–5395. [Google Scholar] [CrossRef]
- Bathini, M.; Raghushaker, C.R.; Mahato, K.K. The Molecular Mechanisms of Action of Photobiomodulation Against Neurodegenerative Diseases: A Systematic Review. Cell. Mol. Neurobiol. 2022, 42, 955–971. [Google Scholar] [CrossRef]
- De Taboada, L.; Yu, J.; El-Amouri, S.; Gattoni-Celli, S.; Richieri, S.; McCarthy, T.; Streeter, J.; Kindy, M.S. Transcranial Laser Therapy Attenuates Amyloid-β Peptide Neuropathology in Amyloid-β Protein Precursor Transgenic Mice. J. Alzheimers. Dis. 2011, 23, 521–535. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, W.; Luo, Y.; Wang, Z. Transcranial Photobiomodulation Therapy Ameliorates Perioperative Neurocognitive Disorder Through Modulation of Mitochondrial Function in Aged Mice. Neuroscience 2022, 490, 236–249. [Google Scholar] [CrossRef]
- Yang, L.; Youngblood, H.; Wu, C.; Zhang, Q. Mitochondria as a Target for Neuroprotection: Role of Methylene Blue and Photobiomodulation. Transl. Neurodegener. 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Signore, A.; Aicardi, S.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Amaroli, A. Experimental and Clinical Applications of Red and Near- Infrared Photobiomodulation on Endothelial Dysfunction: A Review. Biomedicines 2021, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Majdi, A.; Pazhuhi, M.; Ghasemi, F.; Khademi, M.; Pashazadeh, F.; Hamblin, M.R.; Cassano, P. Transcranial Photobiomodulation Improves Cognitive Performance in Young Healthy Adults: A Systematic Review and Meta-Analysis. Photobiomodul. Photomed. Laser Surg. 2019, 37, 635–643. [Google Scholar] [CrossRef]
- Cassano, P.; Petrie, S.R.; Hamblin, M.R.; Henderson, T.A.; Iosifescu, D.V. Review of Transcranial Photobiomodulation for Major Depressive Disorder: Targeting Brain Metabolism, Inflammation, Oxidative Stress, and Neurogenesis. Neurophotonics 2016, 3, 31404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassano, P.; Tran, A.P.; Katnani, H.; Bleier, B.S.; Hamblin, M.R.; Yuan, Y.; Fang, Q. Selective Photobiomodulation for Emotion Regulation: Model-Based Dosimetry Study. Neurophotonics 2019, 6, 15004. [Google Scholar] [CrossRef]
- Nizamutdinov, D.; Ezeudu, C.; Wu, E.; Huang, J.H.; Yi, S.S. Transcranial Near-Infrared Light in Treatment of Neurodegenerative Diseases. Front. Pharmacol. 2022, 13, 965788. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Zekiy, A.; Benedicenti, S.; Pasquale, C. A Narrative Review on Oral and Periodontal Bacteria Microbiota Photobiomodulation, through Visible and Near-Infrared Light: From the Origins to Modern Therapies. Int. J. Mol. Sci. 2022, 23, 1372. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, F.d.S.; Barrett, D.W.; Wade, Z.; Gomes da Silva, S.; Gonzalez-Lima, F. Photobiomodulation of Cytochrome c Oxidase by Chronic Transcranial Laser in Young and Aged Brains. Front. Neurosci. 2022, 16, 818005. [Google Scholar] [CrossRef]
- Amaroli, A.; Ferrando, S.; Benedicenti, S. Photobiomodulation Affects Key Cellular Pathways of All Life-Forms: Considerations on Old and New Laser Light Targets and the Calcium Issue. Photochem. Photobiol. 2019, 95, 455–459. [Google Scholar] [CrossRef] [Green Version]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE Lasers Electro.-Optics Soc. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebert, A.; Pang, V.; Bicknell, B.; McLachlan, C.; Mitrofanis, J.; Kiat, H. A Perspective on the Potential of Opsins as an Integral Mechanism of Photobiomodulation: It’s Not Just the Eyes. Photobiomodul. Photomed. Laser Surg. 2022, 40, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M. Mechanisms of Photobiomodulation in the Brain; Elsevier: Amsterdam, The Netherlands, 2019; pp. 97–110. ISBN 9780128153055. [Google Scholar]
- Arany, P.R. Photobiomodulation-Activated Latent Transforming Growth Factor-Β1: A Critical Clinical Therapeutic Pathway and an Endogenous Optogenetic Tool for Discovery. Photobiomodul. Photomed. Laser Surg. 2022, 40, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Glutamate and Neurotrophic Factors in Neuronal Plasticity and Disease. Ann. N. Y. Acad. Sci. 2008, 1144, 97–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Danbolt, N.C. Glutamate as a Neurotransmitter in the Healthy Brain. J. Neural Transm. 2014, 12, 799–817. [Google Scholar] [CrossRef] [Green Version]
- Amaroli, A.; Marcoli, M.; Venturini, A.; Passalacqua, M.; Agnati, L.F.; Signore, A.; Raffetto, M.; Maura, G.; Benedicenti, S.; Cervetto, C. Near-Infrared Laser Photons Induce Glutamate Release from Cerebrocortical Nerve Terminals. J. Biophotonics 2018, 11, e201800102. [Google Scholar] [CrossRef]
- Choi, S.W.; Gerencser, A.A.; Nicholls, D.G. Bioenergetic Analysis of Isolated Cerebrocortical Nerve Terminals on a Microgram Scale: Spare Respiratory Capacity and Stochastic Mitochondrial Failure. J. Neurochem. 2009, 109, 1179–1191. [Google Scholar] [CrossRef] [Green Version]
- Dresbach, T.; Qualmann, B.; Kessels, M.M.; Garner, C.C.; Gundelfinger, E.D. The Presynaptic Cytomatrix of Brain Synapses. Cell. Mol. Life Sci. 2001, 58, 94–116. [Google Scholar] [CrossRef]
- Zhai, R.G.; Bellen, H.J. The Architecture of the Active Zone in the Presynaptic Nerve Terminal. Physiology 2004, 19, 262–270. [Google Scholar] [CrossRef]
- Kaeser, P.S.; Deng, L.; Wang, Y.; Dulubova, I.; Liu, X.; Rizo, J.; Südhof, T.C. RIM Proteins Tether Ca2+ Channels to Presynaptic Active Zones via a Direct PDZ-Domain Interaction. Cell 2011, 144, 282–295. [Google Scholar] [CrossRef] [Green Version]
- Eroglu, C.; Barres, B.A. Regulation of Synaptic Connectivity by Glia. Nature 2010, 468, 223–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, S.; Davis, M.W.; Kusick, G.F.; Iwasa, J.; Jorgensen, E.M. SynapsEM: Computer-Assisted Synapse Morphometry. Front. Synaptic Neurosci. 2020, 12, 584549. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Maura, G.; Marcoli, M. Inhibition of Presynaptic Release-Facilitatory Kainate Autoreceptors by Extracellular Cyclic GMP. J. Pharmacol. Exp. Ther. 2010, 332, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Khorasani, M.; Gibson, I.; Ghasemi, A.H.; Hadavi, E.; Rolfe, B. Laser Subtractive and Laser Powder Bed Fusion of Metals: Review of Process and Production Features. Rapid Prototyp. J. 2023, 5, 935–958. [Google Scholar] [CrossRef]
- Tapia, R.; Sitges, M. Effect of 4-Aminopyridine on Transmitter Release in Synaptosomes. Brain Res. 1982, 250, 291–299. [Google Scholar] [CrossRef]
- Segovia, G.; Porras, A.; Mora, F. Effects of 4-Aminopyridine on Extracellular Concentrations of Glutamate in Striatum of the Freely Moving Rat. Neurochem. Res. 1997, 22, 1491–1497. [Google Scholar] [CrossRef]
- Luján, R.; Shigemoto, R.; López-Bendito, G. Glutamate and GABA Receptor Signalling in the Developing Brain. Neuroscience 2005, 130, 567–580. [Google Scholar] [CrossRef]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimers. Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef] [Green Version]
- Chase, T.N.; Oh, J.D. Striatal Dopamine- and Glutamate-Mediated Dysregulation in Experimental Parkinsonism. Trends Neurosci. 2000, 23, S86–S91. [Google Scholar] [CrossRef]
- Leung, C.C.-Y.; Gadelrab, R.; Ntephe, C.U.; McGuire, P.K.; Demjaha, A. Clinical Course, Neurobiology and Therapeutic Approaches to Treatment Resistant Schizophrenia. Toward an Integrated View. Front. Psychiatry 2019, 10, 601. [Google Scholar] [CrossRef]
- Roberts, R.C.; McCollum, L.A.; Schoonover, K.E.; Mabry, S.J.; Roche, J.K.; Lahti, A.C. Ultrastructural Evidence for Glutamatergic Dysregulation in Schizophrenia. Schizophr. Res. 2022, 249, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Sanacora, G.; Treccani, G.; Popoli, M. Towards a Glutamate Hypothesis of Depression: An Emerging Frontier of Neuropsychopharmacology for Mood Disorders. Neuropharmacology 2012, 62, 63–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javitt, D.C. Glutamate as a Therapeutic Target in Psychiatric Disorders. Mol. Psychiatry 2004, 9, 979, 984–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henter, I.D.; Park, L.T.; Zarate, C.A.J. Novel Glutamatergic Modulators for the Treatment of Mood Disorders: Current Status. CNS Drugs 2021, 35, 527–543. [Google Scholar] [CrossRef]
- Wang, S.; Tang, S.; Huang, J.; Chen, H. Rapid-Acting Antidepressants Targeting Modulation of the Glutamatergic System: Clinical and Preclinical Evidence and Mechanisms. Gen. Psychiatry 2022, 35, e100922. [Google Scholar] [CrossRef]
- Duman, R.S.; Shinohara, R.; Fogaça, M.V.; Hare, B. Neurobiology of Rapid-Acting Antidepressants: Convergent Effects on GluA1-Synaptic Function. Mol. Psychiatry 2019, 24, 1816–1832. [Google Scholar] [CrossRef]
- Sato, S.; Bunney, B.; Mendoza-Viveros, L.; Bunney, W.; Borrelli, E.; Sassone-Corsi, P.; Orozco-Solis, R. Rapid-Acting Antidepressants and the Circadian Clock. Neuropsychopharmacology 2022, 47, 805–816. [Google Scholar] [CrossRef]
- Brooks, M. FDA Approves “Rapid-Acting” Oral Drug for Major Depression. Available online: https://www.medscape.com/viewarticle/979568 (accessed on 14 March 2023).
- McIntyre, R.S. Rapid-Acting Antidepressants in Psychiatry: Psychedelics, Episodic Treatments, Innovation, and Clarion Call for Methodologic Rigor in Drug Development. Expert Opin. Drug Saf. 2022, 21, 715–716. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photobiomodulation for Alzheimer’s Disease: Has the Light Dawned? Photonics 2019, 6, 77. [Google Scholar] [CrossRef] [Green Version]
- McEntee, W.J.; Crook, T.H. Glutamate: Its Role in Learning, Memory, and the Aging Brain. Psychopharmacology 1993, 111, 391–401. [Google Scholar] [CrossRef]
- Cox, M.F.; Hascup, E.R.; Bartke, A.; Hascup, K.N. Friend or Foe? Defining the Role of Glutamate in Aging and Alzheimer’s Disease. Front. Aging 2022, 3, 65. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The Role of NMDA Receptors in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef] [Green Version]
- Bukke, V.N.; Archana, M.; Villani, R.; Romano, A.D.; Wawrzyniak, A.; Balawender, K.; Orkisz, S.; Beggiato, S.; Serviddio, G.; Cassano, T. The Dual Role of Glutamatergic Neurotransmission in Alzheimer’s Disease: From Pathophysiology to Pharmacotherapy. Int. J. Mol. Sci. 2020, 21, 7452. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, C.; Parker, E.; Li, Y.; Dong, Y.; Tucker, L.; Brann, D.W.; Lin, H.W.; Zhang, Q. Non-Invasive Photobiomodulation Treatment in an Alzheimer Disease-like Transgenic Rat Model. Theranostics 2022, 12, 2205–2231. [Google Scholar] [CrossRef] [PubMed]
- Cassano, T.; Serviddio, G.; Gaetani, S.; Romano, A.; Dipasquale, P.; Cianci, S.; Bellanti, F.; Laconca, L.; Romano, A.D.; Padalino, I.; et al. Glutamatergic Alterations and Mitochondrial Impairment in a Murine Model of Alzheimer Disease. Neurobiol. Aging 2012, 33, 1121.e1–1121.e12. [Google Scholar] [CrossRef] [PubMed]
- Rupsingh, R.; Borrie, M.; Smith, M.; Wells, J.L.; Bartha, R. Reduced Hippocampal Glutamate in Alzheimer Disease. Neurobiol. Aging 2011, 32, 802–810. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Y.; Qin, Z. Molecular Mechanisms of Excitotoxicity and Their Relevance to Pathogenesis of Neurodegenerative Diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Kostandy, B.B. The Role of Glutamate in Neuronal Ischemic Injury: The Role of Spark in Fire. Neurol. Sci. 2012, 33, 223–237. [Google Scholar] [CrossRef]
- Obrenovitch, T.P.; Urenjak, J. Is High Extracellular Glutamate the Key to Excitotoxicity in Traumatic Brain Injury? J. Neurotrauma 1997, 14, 677–698. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Nagata, K.; Tedford, C.E.; Hamblin, M.R. Low-Level Laser Therapy (810 Nm) Protects Primary Cortical Neurons against Excitotoxicity in Vitro. J. Biophotonics 2014, 7, 656–664. [Google Scholar] [CrossRef] [Green Version]
- Amaroli, A.; Chessa, M.G.; Bavestrello, G.; Bianco, B. Effects of an Extremely Low-Frequency Electromagnetic Field on Stress Factors: A Study in Dictyostelium Discoideum Cells. Eur. J. Protistol. 2013, 49, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. Effect of 808 Nm Diode Laser on Swimming Behavior, Food Vacuole Formation and Endogenous ATP Production of Paramecium primaurelia (Protozoa). Photochem. Photobiol. 2015, 91, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Benedicenti, A.; Ferrando, S.; Parker, S.; Selting, W.; Gallus, L.; Benedicenti, S. Photobiomodulation by Infrared Diode Laser: Effects on Intracellular Calcium Concentration and Nitric Oxide Production of Paramecium. Photochem. Photobiol. 2016, 92, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, S.; Agas, D.; Mirata, S.; Signore, A.; De Angelis, N.; Ravera, S.; Utyuzh, A.S.; Parker, S.; Sabbieti, M.G.; Benedicenti, S.; et al. The 808 nm and 980 nm Infrared Laser Irradiation Affects Spore Germination and Stored Calcium Homeostasis: A Comparative Study Using Delivery Hand-Pieces with Standard (Gaussian) or Flat-Top Profile. J. Photochem. Photobiol. B Biol. 2019, 199, 111627. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. 808-Nm Laser Therapy with a Flat-Top Handpiece Photobiomodulates Mitochondria Activities of Paramecium primaurelia (Protozoa). Lasers Med. Sci. 2016, 31, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Nelson, S.T.; Strahan, J.R. Photobiomodulation and Cancer: What Is the Truth? Photomed. Laser Surg. 2018, 36, 241–245. [Google Scholar] [CrossRef]
- Robijns, J.; Nair, R.G.; Lodewijckx, J.; Arany, P.; Barasch, A.; Bjordal, J.M.; Bossi, P.; Chilles, A.; Corby, P.M.; Epstein, J.B.; et al. Photobiomodulation Therapy in Management of Cancer Therapy-Induced Side Effects: WALT Position Paper 2022. Front. Oncol. 2022, 12, 7685. [Google Scholar] [CrossRef]
- Chaudhry, F.A.; Boulland, J.-L.; Jenstad, M.; Bredahl, M.K.L.; Edwards, R.H. Pharmacology of Neurotransmitter Transport into Secretory Vesicles. Handb. Exp. Pharmacol. 2008, 184, 77–106. [Google Scholar] [CrossRef]
- Pieribone, V.A.; Shupliakov, O.; Brodin, L.; Hilfiker-Rothenfluh, S.; Czernik, A.J.; Greengard, P. Distinct Pools of Synaptic Vesicles in Neurotransmitter Release. Nature 1995, 375, 493–497. [Google Scholar] [CrossRef]
- Südhof, T.C. The Synaptic Vesicle Cycle in the Nerve Terminal. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Siegel, G.J., Agranoff, B.W., Albers, R.W., Lippincott-Raven, P.E., Eds.; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Sudhof, T.C. The Synaptic Vesicle Cycle. Annu. Rev. Neurosci. 2004, 27, 509–547. [Google Scholar] [CrossRef] [Green Version]
- Rizo, J. Molecular Mechanisms Underlying Neurotransmitter Release. Annu. Rev. Biophys. 2022, 51, 377–408. [Google Scholar] [CrossRef] [PubMed]
- Omote, H.; Moriyama, Y. Vesicular Neurotransmitter Transporters: An Approach for Studying Transporters with Purified Proteins. Physiology 2013, 28, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, J.; Li, F.; Edwards, R.H. The Mechanism and Regulation of Vesicular Glutamate Transport: Coordination with the Synaptic Vesicle Cycle. Biochim. Biophys. Acta. Biomembr. 2020, 1862, 183259. [Google Scholar] [CrossRef] [PubMed]
- Ogita, K.; Hirata, K.; Bole, D.G.; Yoshida, S.; Tamura, Y.; Leckenby, A.M.; Ueda, T. Inhibition of Vesicular Glutamate Storage and Exocytotic Release by Rose Bengal. J. Neurochem. 2001, 77, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvis, S.E.; Zamponi, G.W. Interactions between Presynaptic Ca2+ Channels, Cytoplasmic Messengers and Proteins of the Synaptic Vesicle Release Complex. Trends Pharmacol. Sci. 2001, 22, 519–525. [Google Scholar] [CrossRef]
- Catterall, W.A. Voltage-Gated Calcium Channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef]
- Vázquez, E.; Sánchez-Prieto, J. Presynaptic Modulation of Glutamate Release Targets Different Calcium Channels in Rat Cerebrocortical Nerve Terminals. Eur. J. Neurosci. 1997, 9, 2009–2018. [Google Scholar] [CrossRef]
- Nicholls, D.G. Presynaptic Modulation of Glutamate Release. Prog. Brain Res. 1998, 116, 15–22. [Google Scholar] [CrossRef]
- Millán, C.; Sánchez-Prieto, J. Differential Coupling of N- and P/Q-Type Calcium Channels to Glutamate Exocytosis in the Rat Cerebral Cortex. Neurosci. Lett. 2002, 330, 29–32. [Google Scholar] [CrossRef]
- Missler, M.; Zhang, W.; Rohlmann, A.; Kattenstroth, G.; Hammer, R.E.; Gottmann, K.; Südhof, T.C. Alpha-Neurexins Couple Ca2+ Channels to Synaptic Vesicle Exocytosis. Nature 2003, 423, 939–948. [Google Scholar] [CrossRef]
- Giansante, G.; Marte, A.; Romei, A.; Prestigio, C.; Onofri, F.; Benfenati, F.; Baldelli, P.; Valente, P. Presynaptic L-Type Ca(2+) Channels Increase Glutamate Release Probability and Excitatory Strength in the Hippocampus during Chronic Neuroinflammation. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 6825–6841. [Google Scholar] [CrossRef] [PubMed]
- Golovynska, I.; Golovynskyi, S.; Stepanov, Y.V.; Garmanchuk, L.V.; Stepanova, L.I.; Qu, J.; Ohulchanskyy, T.Y. Red and Near-Infrared Light Induces Intracellular Ca(2+) Flux via the Activation of Glutamate N-Methyl-D-Aspartate Receptors. J. Cell. Physiol. 2019, 234, 15989–16002. [Google Scholar] [CrossRef] [PubMed]
- Golovynska, I.; Golovynskyi, S.; Stepanov, Y.V.; Stepanova, L.I.; Qu, J.; Ohulchanskyy, T.Y. Red and Near-Infrared Light Evokes Ca(2+) Influx, Endoplasmic Reticulum Release and Membrane Depolarization in Neurons and Cancer Cells. J. Photochem. Photobiol. B 2021, 214, 112088. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Frattaroli, D.; Venturini, A.; Passalacqua, M.; Nobile, M.; Alloisio, S.; Tacchetti, C.; Maura, G.; Agnati, L.; Marcoli, M. Calcium-Permeable AMPA Receptors Trigger Vesicular Glutamate Release from Bergmann Gliosomes. Neuropharmacology 2015, 99, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Vergani, L.; Passalacqua, M.; Ragazzoni, M.; Venturini, A.; Cecconi, F.; Berretta, N.; Mercuri, N.; D’Amelio, M.; Maura, G.; et al. Astrocyte-Dependent Vulnerability to Excitotoxicity in Spermine Oxidase-Overexpressing Mouse. NeuroMolecular Med. 2016, 18, 50–68. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Venturini, A.; Passalacqua, M.; Guidolin, D.; Genedani, S.; Fuxe, K.; Borroto-Esquela, D.O.; Cortelli, P.; Woods, A.; Maura, G.; et al. A2A-D2 Receptor–Receptor Interaction Modulates Gliotransmitter Release from Striatal Astrocyte Processes. J. Neurochem. 2017, 140, 268–279. [Google Scholar] [CrossRef]
- Battilocchio, C.; Guetzoyan, L.; Cervetto, C.; Mannelli, L.; Frattaroli, D.; Baxendale, I.; Maura, G.; Rossi, A.; Sautsebin, L.; Biava, M.; et al. Flow Synthesis and Biological Studies of an Analgesic Adamantane Derivative That Inhibits P2X 7 -Evoked Glutamate Release. ACS Med. Chem. Lett. 2013, 4, 704–709. [Google Scholar] [CrossRef] [Green Version]
- Amaroli, A.; Arany, P.; Pasquale, C.; Benedicenti, S.; Bosco, A.; Ravera, S. Improving Consistency of Photobiomodulation Therapy: A Novel Flat-Top Beam Hand-Piece versus Standard Gaussian Probes on Mitochondrial Activity. Int. J. Mol. Sci. 2021, 22, 7788. [Google Scholar] [CrossRef]
- Bruzzone, F.; Cervetto, C.; Mazzotta, M.C.; Bianchini, P.; Ronzitti, E.; Leprince, J.; Diaspro, A.; Maura, G.; Vallarino, M.; Vaudry, H.; et al. Urotensin II Receptor and Acetylcholine Release from Mouse Cervical Spinal Cord Nerve Terminals. Neuroscience 2010, 170, 67–77. [Google Scholar] [CrossRef]
- Tang, R.; Dai, J. Biophoton Signal Transmission and Processing in the Brain. J. Photochem. Photobiol. B 2014, 139, 71–75. [Google Scholar] [CrossRef]
- Tang, R.; Dai, J. Spatiotemporal Imaging of Glutamate-Induced Biophotonic Activities and Transmission in Neural Circuits. PLoS ONE 2014, 9, e85643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Boone, K.; Tuszyński, J.; Barclay, P.; Simon, C. Possible Existence of Optical Communication Channels in the Brain. Sci. Rep. 2016, 6, 36508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarkeshian, P.; Kergan, T.; Ghobadi, R.; Nicola, W.; Simon, C. Photons Guided by Axons May Enable Backpropagation-Based Learning in the Brain. Sci. Rep. 2022, 12, 20720. [Google Scholar] [CrossRef] [PubMed]
- Moro, C.; Valverde, A.; Dole, M.; Hoh Kam, J.; Hamilton, C.; Liebert, A.; Bicknell, B.; Benabid, A.-L.; Magistretti, P.; Mitrofanis, J. The Effect of Photobiomodulation on the Brain during Wakefulness and Sleep. Front. Neurosci. 2022, 16, 942536. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervetto, C.; Amaroli, A.; Amato, S.; Gatta, E.; Diaspro, A.; Maura, G.; Signore, A.; Benedicenti, S.; Marcoli, M. Photons Induce Vesicular Exocytotic Release of Glutamate in a Power-Dependent Way. Int. J. Mol. Sci. 2023, 24, 10977. https://doi.org/10.3390/ijms241310977
Cervetto C, Amaroli A, Amato S, Gatta E, Diaspro A, Maura G, Signore A, Benedicenti S, Marcoli M. Photons Induce Vesicular Exocytotic Release of Glutamate in a Power-Dependent Way. International Journal of Molecular Sciences. 2023; 24(13):10977. https://doi.org/10.3390/ijms241310977
Chicago/Turabian StyleCervetto, Chiara, Andrea Amaroli, Sarah Amato, Elena Gatta, Alberto Diaspro, Guido Maura, Antonio Signore, Stefano Benedicenti, and Manuela Marcoli. 2023. "Photons Induce Vesicular Exocytotic Release of Glutamate in a Power-Dependent Way" International Journal of Molecular Sciences 24, no. 13: 10977. https://doi.org/10.3390/ijms241310977
APA StyleCervetto, C., Amaroli, A., Amato, S., Gatta, E., Diaspro, A., Maura, G., Signore, A., Benedicenti, S., & Marcoli, M. (2023). Photons Induce Vesicular Exocytotic Release of Glutamate in a Power-Dependent Way. International Journal of Molecular Sciences, 24(13), 10977. https://doi.org/10.3390/ijms241310977










