Maresin: Macrophage Mediator for Resolving Inflammation and Bridging Tissue Regeneration—A System-Based Preclinical Systematic Review
Abstract
1. Introduction
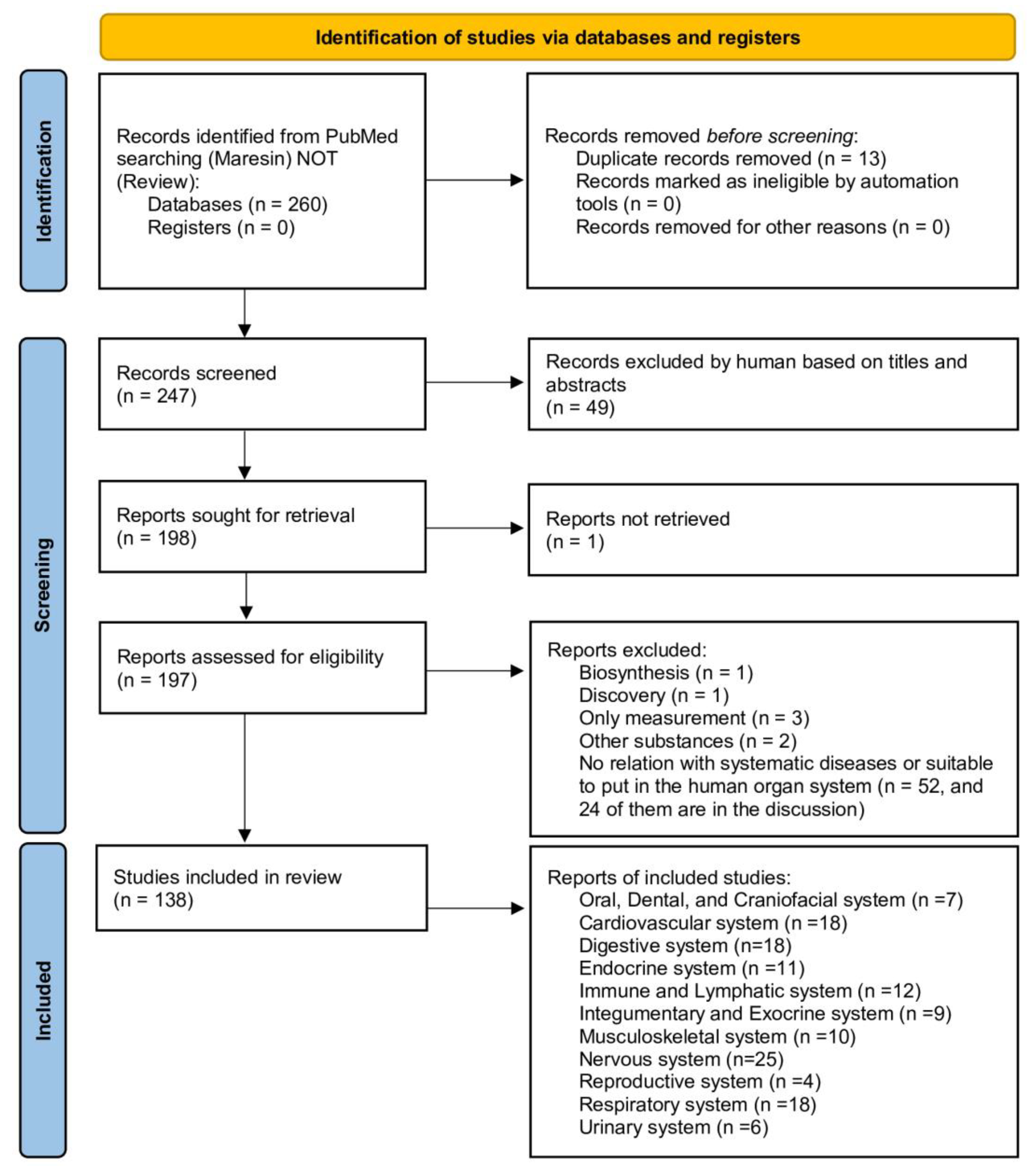
2. Materials and Methods (Including Flow Diagram)
2.1. Search Strategy
- P (Population): Human or animal with preclinical models.
- I (Intervention): Application of maresins, either locally or systematically.
- C (Comparison): Healthy or vehicle control without intervention.
- (Outcomes): Preclinical or functional outcomes.
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. Results and Discussion
3.1. Oral, Dental, and Craniofacial System
3.1.1. Periodontal Diseases
3.1.2. Human Periodontal Ligament Stem Cells (hPDLSCs)
3.1.3. Tooth Extraction and Temporomandibular Joint (TMJ) Pain
3.2. Cardiovascular System
3.2.1. Cardiomyopathy
3.2.2. Sepsis and Pulmonary Arterial Hypertension (PAH)
3.2.3. Atheroprogression, Atherosclerosis, Coronary Artery Diseases, and Vascular Inflammation
3.2.4. Blood Coagulation
3.3. Digestive System
3.3.1. Colitis
3.3.2. Hepatitis and Liver Fibrosis
3.3.3. Obesity-Related Liver Diseases
3.3.4. Non-Alcoholic Steatohepatitis and Liver Ischemia-Reperfusion Injury
3.3.5. Pancreatitis
3.4. Endocrine System
3.4.1. Obesity
3.4.2. Cold-Induced Resolution of Inflammation
3.4.3. Type 2 Diabetes Mellitus (T2DM)
3.4.4. Obesity and T2DM Treatments Related to Maresin
3.5. Immune/Lymphatic System
3.5.1. Phagocytosis and Peritonitis
3.5.2. Bacterial Infection and Sepsis
3.5.3. T Cell Differentiation and Lymphatic Obstruction
3.6. Integumentary/Exocrine System
3.6.1. Skin Inflammation, Psoriasis, Melanoma
3.6.2. Ocular Surface Inflammatory Disease
3.6.3. Adipose Tissue
3.7. Musculoskeletal System
3.7.1. Achilles Tendinopathy and Bone/Muscle Regeneration
3.7.2. Arthritis
3.8. Nervous System
3.8.1. Cognitive Disorders
3.8.2. Cerebrovascular Diseases
3.8.3. Multiple Sclerosis (MS), Spinal Cord Injury (SCI), and Amyotrophic Lateral Sclerosis (ALS)
3.8.4. Pain
3.9. Reproductive System
3.9.1. Human Milk
3.9.2. Localized Provoked Vulvodynia (LPV)
3.9.3. Polycystic Ovary Syndrome
3.9.4. Pre-Eclampsia (PE)
3.10. Respiratory System
3.10.1. Acute Respiratory Distress Syndrome (ARDS)
3.10.2. Virus and Bacterial Infection: COVID-19 and Bacterial Pneumonia
3.10.3. Chronic Rhinosinusitis and Asthma
3.10.4. Acute Lung Injury (ALI)
3.10.5. Airway Inflammation and Lung Fibrosis
3.11. Urinary System
3.11.1. Cystitis
3.11.2. Diabetic Nephropathy (DN)
3.11.3. Sepsis-Associated Acute Kidney Injury (S-AKI)
3.11.4. Renal Ischemia/Reperfusion Injury
3.12. Discussion
3.12.1. MaR1 in Human Tissue Fluid and as a Biomarker
3.12.2. Platelets’ Aggregation and Supplementation
3.12.3. Other Modulating Functions and Cancer Cell Suppressibility
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009, 206, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Dalli, J.; Karamnov, S.; Choi, A.; Park, C.K.; Xu, Z.Z.; Ji, R.R.; Zhu, M.; Petasis, N.A. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012, 26, 1755–1765. [Google Scholar] [CrossRef]
- Dalli, J.; Zhu, M.; Vlasenko, N.A.; Deng, B.; Haeggström, J.Z.; Petasis, N.A.; Serhan, C.N. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013, 27, 2573–2583. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wang, C.W.; Arnardottir, H.H.; Li, Y.; Cheng, C.Y.; Dalli, J.; Serhan, C.N. Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS ONE 2014, 9, e102362. [Google Scholar] [CrossRef]
- Freedman, C.; Tran, A.; Tourdot, B.E.; Kalyanaraman, C.; Perry, S.; Holinstat, M.; Jacobson, M.P.; Holman, T.R. Biosynthesis of the Maresin Intermediate, 13S,14S-Epoxy-DHA, by Human 15-Lipoxygenase and 12-Lipoxygenase and Its Regulation through Negative Allosteric Modulators. Biochemistry 2020, 59, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Libreros, S.; Norris, P.C.; de la Rosa, X.; Serhan, C.N. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J. Clin. Investig. 2019, 129, 5294–5311. [Google Scholar] [CrossRef]
- Dalli, J.; Sanger, J.M.; Rodriguez, A.R.; Chiang, N.; Spur, B.W.; Serhan, C.N. Identification and Actions of a Novel Third Maresin Conjugate in Tissue Regeneration: MCTR3. PLoS ONE 2016, 11, e0149319. [Google Scholar] [CrossRef]
- Chiang, N.; Riley, I.R.; Dalli, J.; Rodriguez, A.R.; Spur, B.W.; Serhan, C.N. New maresin conjugates in tissue regeneration pathway counters leukotriene D4-stimulated vascular responses. FASEB J. 2018, 32, 4043–4052. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Schjerning, A.M.; McGettigan, P.; Gislason, G. Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat. Rev. Cardiol. 2020, 17, 574–584. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Colas, R.A.; Dalli, J.; Arnardottir, H.H.; Nguyen, D.; Hasturk, H.; Chiang, N.; Van Dyke, T.E.; Serhan, C.N. Maresin 1 Biosynthesis and Proresolving Anti-infective Functions with Human-Localized Aggressive Periodontitis Leukocytes. Infect. Immun. 2015, 84, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, Y.; Liu, W. Maresin 1 regulates autophagy and inflammation in human periodontal ligament cells through glycogen synthase kinase-3beta/beta-catenin pathway under inflammatory conditions. Arch. Oral Biol. 2018, 87, 242–247. [Google Scholar] [CrossRef]
- Albuquerque-Souza, E.; Schulte, F.; Chen, T.; Hardt, M.; Hasturk, H.; Van Dyke, T.E.; Holzhausen, M.; Kantarci, A. Maresin-1 and Resolvin E1 Promote Regenerative Properties of Periodontal Ligament Stem Cells Under Inflammatory Conditions. Front. Immunol. 2020, 11, 585530. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Yu, S.H.; Fretwurst, T.; Larsson, L.; Sugai, J.V.; Oh, J.; Lehner, K.; Jin, Q.; Giannobile, W.V. Maresin 1 Promotes Wound Healing and Socket Bone Regeneration for Alveolar Ridge Preservation. J. Dent. Res. 2020, 99, 930–937. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Y.; Xiao, J.; Tian, Y.; Ma, M.; Li, X.; Li, L.; Zhang, P.; Li, M.; Wang, J.; et al. Maresin conjugates in tissue regeneration 1 prevents lipopolysaccharide-induced cardiac dysfunction through improvement of mitochondrial biogenesis and function. Biochem. Pharmacol. 2020, 177, 114005. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.Y.; Li, L.C.; Xiao, J.; Zhu, Y.M.; Tian, Y.; Sheng, Y.M.; Chen, Y.; Wang, J.G.; Jin, S.W. gammadelta T/Interleukin-17A Contributes to the Effect of Maresin Conjugates in Tissue Regeneration 1 on Lipopolysaccharide-Induced Cardiac Injury. Front. Immunol. 2021, 12, 674542. [Google Scholar] [CrossRef]
- Li, D.; Wang, M.; Ye, J.; Zhang, J.; Xu, Y.; Wang, Z.; Zhao, M.; Ye, D.; Wan, J. Maresin 1 alleviates the inflammatory response, reduces oxidative stress and protects against cardiac injury in LPS-induced mice. Life Sci. 2021, 277, 119467. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Y.; Ma, Z.; Ma, M.; Wang, D.; Xie, G.; Yin, Y.; Zhang, P.; Tao, K. Maresin 1 Mitigates Inflammatory Response and Protects Mice from Sepsis. Mediat. Inflamm. 2016, 2016, 3798465. [Google Scholar] [CrossRef]
- Gu, J.; Luo, L.; Wang, Q.; Yan, S.; Lin, J.; Li, D.; Cao, B.; Mei, H.; Ying, B.; Bin, L.; et al. Maresin 1 attenuates mitochondrial dysfunction through the ALX/cAMP/ROS pathway in the cecal ligation and puncture mouse model and sepsis patients. Lab. Investig. 2018, 98, 715–733. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Hao, Y.; Wu, C.; Fu, Y.; Su, N.; Chen, H.; Ying, B.; Wang, H.; Su, L.; et al. Maresin 1 intervention reverses experimental pulmonary arterial hypertension in mice. Br. J. Pharmacol. 2022, 179, 5132–5147. [Google Scholar] [CrossRef]
- Viola, J.R.; Lemnitzer, P.; Jansen, Y.; Csaba, G.; Winter, C.; Neideck, C.; Silvestre-Roig, C.; Dittmar, G.; Doring, Y.; Drechsler, M.; et al. Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice. Circ. Res. 2016, 119, 1030–1038. [Google Scholar] [CrossRef]
- Elder, C.T.; Filiberto, A.C.; Su, G.; Ladd, Z.; Leroy, V.; Pruitt, E.Y.; Lu, G.; Jiang, Z.; Sharma, A.K.; Upchurch, G.R., Jr. Maresin 1 activates LGR6 signaling to inhibit smooth muscle cell activation and attenuate murine abdominal aortic aneurysm formation. FASEB J. 2021, 35, e21780. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gong, Y.; Chen, T.; Li, B.; Zhang, W.; Yin, L.; Zhao, H.; Tang, Y.; Wang, X.; Huang, C. Maresin1 ameliorates ventricular remodelling and arrhythmia in mice models of myocardial infarction via NRF2/HO-1 and TLR4/NF-kB signalling. Int. Immunopharmacol. 2022, 113, 109369. [Google Scholar] [CrossRef] [PubMed]
- León, I.C.; Quesada-Vázquez, S.; Sáinz, N.; Guruceaga, E.; Escoté, X.; Moreno-Aliaga, M.J. Effects of Maresin 1 (MaR1) on Colonic Inflammation and Gut Dysbiosis in Diet-Induced Obese Mice. Microorganisms 2020, 8, 1156. [Google Scholar] [CrossRef] [PubMed]
- Marcon, R.; Bento, A.F.; Dutra, R.C.; Bicca, M.A.; Leite, D.F.; Calixto, J.B. Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. J. Immunol. 2013, 191, 4288–4298. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Zhao, E.; Wu, K.; Li, W.; Shi, L.; Wang, D.; Xie, G.; Yin, Y.; Deng, M.; et al. Maresin 1, a Proresolving Lipid Mediator, Mitigates Carbon Tetrachloride-Induced Liver Injury in Mice. Oxid. Med. Cell. Longev. 2016, 2016, 9203716. [Google Scholar] [CrossRef]
- Rodriguez, M.J.; Sabaj, M.; Tolosa, G.; Herrera Vielma, F.; Zuniga, M.J.; Gonzalez, D.R.; Zuniga-Hernandez, J. Maresin-1 Prevents Liver Fibrosis by Targeting Nrf2 and NF-kappaB, Reducing Oxidative Stress and Inflammation. Cells 2021, 10, 3406. [Google Scholar] [CrossRef]
- Laiglesia, L.M.; Lorente-Cebrian, S.; Martinez-Fernandez, L.; Sainz, N.; Prieto-Hontoria, P.L.; Burrell, M.A.; Rodriguez-Ortigosa, C.M.; Martinez, J.A.; Moreno-Aliaga, M.J. Maresin 1 mitigates liver steatosis in ob/ob and diet-induced obese mice. Int. J. Obes. 2018, 42, 572–579. [Google Scholar] [CrossRef]
- Jung, T.W.; Kim, H.C.; Abd El-Aty, A.M.; Jeong, J.H. Maresin 1 attenuates NAFLD by suppression of endoplasmic reticulum stress via AMPK-SERCA2b pathway. J. Biol. Chem. 2018, 293, 3981–3988. [Google Scholar] [CrossRef]
- Han, Y.H.; Shin, K.O.; Kim, J.Y.; Khadka, D.B.; Kim, H.J.; Lee, Y.M.; Cho, W.J.; Cha, J.Y.; Lee, B.J.; Lee, M.O. A maresin 1/RORalpha/12-lipoxygenase autoregulatory circuit prevents inflammation and progression of nonalcoholic steatohepatitis. J. Clin. Investig. 2019, 129, 1684–1698. [Google Scholar] [CrossRef] [PubMed]
- Soto, G.; Rodriguez, M.J.; Fuentealba, R.; Treuer, A.V.; Castillo, I.; Gonzalez, D.R.; Zuniga-Hernandez, J. Maresin 1, a Proresolving Lipid Mediator, Ameliorates Liver Ischemia-Reperfusion Injury and Stimulates Hepatocyte Proliferation in Sprague-Dawley Rats. Int. J. Mol. Sci. 2020, 21, 540. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tao, K.; Zhang, P.; Chen, X.; Sun, X.; Li, R. Maresin 1 protects against lipopolysaccharide/d-galactosamine-induced acute liver injury by inhibiting macrophage pyroptosis and inflammatory response. Biochem. Pharmacol. 2022, 195, 114863. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Jin, Q. Maresin-1 Inhibits Oxidative Stress and Inflammation and Promotes Apoptosis in a Mouse Model of Caerulein-Induced Acute Pancreatitis. Med. Sci. Monit. 2019, 25, 8181–8189. [Google Scholar] [CrossRef]
- Munir, F.; Jamshed, M.B.; Shahid, N.; Muhammad, S.A.; Bhandari, A.; Zhang, Q. Protective effects of maresin 1 against inflammation in experimentally induced acute pancreatitis and related lung injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G333–G341. [Google Scholar] [CrossRef]
- Martínez-Fernández, L.; González-Muniesa, P.; Laiglesia, L.M.; Sáinz, N.; Prieto-Hontoria, P.L.; Escoté, X.; Odriozola, L.; Corrales, F.J.; Arbones-Mainar, J.M.; Martínez, J.A.; et al. Maresin 1 improves insulin sensitivity and attenuates adipose tissue inflammation in ob/ob and diet-induced obese mice. FASEB J. 2017, 31, 2135–2145. [Google Scholar] [CrossRef]
- Martinez-Fernandez, L.; Gonzalez-Muniesa, P.; Sainz, N.; Escote, X.; Martinez, J.A.; Arbones-Mainar, J.M.; Moreno-Aliaga, M.J. Maresin 1 regulates insulin signaling in human adipocytes as well as in adipose tissue and muscle of lean and obese mice. J. Physiol. Biochem. 2021, 77, 167–173. [Google Scholar] [CrossRef]
- Martínez-Fernández, L.; González-Muniesa, P.; Sáinz, N.; Laiglesia, L.M.; Escoté, X.; Martínez, J.A.; Moreno-Aliaga, M.J. Maresin 1 Regulates Hepatic FGF21 in Diet-Induced Obese Mice and in Cultured Hepatocytes. Mol. Nutr. Food Res. 2019, 63, e1900358. [Google Scholar] [CrossRef]
- Sugimoto, S.; Mena, H.A.; Sansbury, B.E.; Kobayashi, S.; Tsuji, T.; Wang, C.H.; Yin, X.; Huang, T.L.; Kusuyama, J.; Kodani, S.D.; et al. Brown adipose tissue-derived MaR2 contributes to cold-induced resolution of inflammation. Nat. Metab. 2022, 4, 775–790. [Google Scholar] [CrossRef]
- Chiang, N.; de la Rosa, X.; Libreros, S.; Pan, H.; Dreyfuss, J.M.; Serhan, C.N. Cysteinyl-specialized proresolving mediators link resolution of infectious inflammation and tissue regeneration via TRAF3 activation. Proc. Natl. Acad. Sci. USA 2021, 118, e2013374118. [Google Scholar] [CrossRef]
- Colas, R.A.; Dalli, J.; Chiang, N.; Vlasakov, I.; Sanger, J.M.; Riley, I.R.; Serhan, C.N. Identification and Actions of the Maresin 1 Metabolome in Infectious Inflammation. J. Immunol. 2016, 197, 4444–4452. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Lamont, G.J.; Lamont, R.J.; Uriarte, S.M.; Wang, H.; Scott, D.A. Resolvin D1, resolvin D2 and maresin 1 activate the GSK3beta anti-inflammatory axis in TLR4-engaged human monocytes. Innate Immun. 2016, 22, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Sarabia, C.; Torres, M.; Juarez, E. Resolvin D1 (RvD1) and maresin 1 (Mar1) contribute to human macrophage control of M. tuberculosis infection while resolving inflammation. Int. Immunopharmacol. 2019, 74, 105694. [Google Scholar] [CrossRef]
- Wang, W.; Xu, R.L.; He, P.; Chen, R. MAR1 suppresses inflammatory response in LPS-induced RAW 264.7 macrophages and human primary peripheral blood mononuclear cells via the SIRT1/PGC-1alpha/PPAR-gamma pathway. J. Inflamm. 2021, 18, 8. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; Maccarrone, M.; Serhan, C.N. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 2016, 8, 353ra111. [Google Scholar] [CrossRef]
- Cezar, T.L.C.; Martinez, R.M.; Rocha, C.D.; Melo, C.P.B.; Vale, D.L.; Borghi, S.M.; Fattori, V.; Vignoli, J.A.; Camilios-Neto, D.; Baracat, M.M.; et al. Treatment with maresin 1, a docosahexaenoic acid-derived pro-resolution lipid, protects skin from inflammation and oxidative stress caused by UVB irradiation. Sci. Rep. 2019, 9, 3062. [Google Scholar] [CrossRef]
- Saito-Sasaki, N.; Sawada, Y.; Mashima, E.; Yamaguchi, T.; Ohmori, S.; Yoshioka, H.; Haruyama, S.; Okada, E.; Nakamura, M. Maresin-1 suppresses imiquimod-induced skin inflammation by regulating IL-23 receptor expression. Sci. Rep. 2018, 8, 5522. [Google Scholar] [CrossRef]
- Sáinz, N.; Fernández-Galilea, M.; Costa, A.G.V.; Prieto-Hontoria, P.L.; Barraco, G.M.; Moreno-Aliaga, M.J. n-3 polyunsaturated fatty acids regulate chemerin in cultured adipocytes: Role of GPR120 and derived lipid mediators. Food Funct. 2020, 11, 9057–9066. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Vi, L.; Zong, X.; Baht, G.S. Maresin 1 resolves aged-associated macrophage inflammation to improve bone regeneration. FASEB J. 2020, 34, 13521–13532. [Google Scholar] [CrossRef]
- Jin, S.; Chen, H.; Li, Y.; Zhong, H.; Sun, W.; Wang, J.; Zhang, T.; Ma, J.; Yan, S.; Zhang, J.; et al. Maresin 1 improves the Treg/Th17 imbalance in rheumatoid arthritis through miR-21. Ann. Rheum. Dis. 2018, 77, 1644–1652. [Google Scholar] [CrossRef]
- Yin, P.; Wang, X.; Wang, S.; Wei, Y.; Feng, J.; Zhu, M. Maresin 1 Improves Cognitive Decline and Ameliorates Inflammation in a Mouse Model of Alzheimer’s Disease. Front. Cell. Neurosci. 2019, 13, 466. [Google Scholar] [CrossRef]
- Emre, C.; Arroyo-García, L.E.; Do, K.V.; Jun, B.; Ohshima, M.; Alcalde, S.G.; Cothern, M.L.; Maioli, S.; Nilsson, P.; Hjorth, E.; et al. Intranasal delivery of pro-resolving lipid mediators rescues memory and gamma oscillation impairment in App(NL-G-F/NL-G-F) mice. Commun. Biol. 2022, 5, 245. [Google Scholar] [CrossRef]
- Yang, T.; Xu, G.; Newton, P.T.; Chagin, A.S.; Mkrtchian, S.; Carlstrom, M.; Zhang, X.M.; Harris, R.A.; Cooter, M.; Berger, M.; et al. Maresin 1 attenuates neuroinflammation in a mouse model of perioperative neurocognitive disorders. Br. J. Anaesth. 2019, 122, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sun, Z.; Wang, T.; Wang, Y.; Zhong, L.; Kong, F. Maresin 1 alleviates sevoflurane-induced neuroinflammation in neonatal rats via JAK2/STAT3/IL-6 pathways. Int. Immunopharmacol. 2022, 108, 108912. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zheng, J.; Wang, Z.; Xu, L.; Sun, D.; Song, H.; Wu, S.; Du, M.; Peng, S.; Zhang, J. Maresin 1 improves cognitive decline and ameliorates inflammation and blood-brain barrier damage in rats with chronic cerebral hypoperfusion. Brain Res. 2022, 1788, 147936. [Google Scholar] [CrossRef] [PubMed]
- Xian, W.; Wu, Y.; Xiong, W.; Li, L.; Li, T.; Pan, S.; Song, L.; Hu, L.; Pei, L.; Yao, S.; et al. The pro-resolving lipid mediator Maresin 1 protects against cerebral ischemia/reperfusion injury by attenuating the pro-inflammatory response. Biochem. Biophys. Res. Commun. 2016, 472, 175–181. [Google Scholar] [CrossRef]
- Sanchez-Fernandez, A.; Zandee, S.; Mastrogiovanni, M.; Charabati, M.; Rubbo, H.; Prat, A.; Lopez-Vales, R. Administration of Maresin-1 ameliorates the physiopathology of experimental autoimmune encephalomyelitis. J. Neuroinflamm. 2022, 19, 27. [Google Scholar] [CrossRef]
- Francos-Quijorna, I.; Santos-Nogueira, E.; Gronert, K.; Sullivan, A.B.; Kopp, M.A.; Brommer, B.; David, S.; Schwab, J.M.; Lopez-Vales, R. Maresin 1 Promotes Inflammatory Resolution, Neuroprotection, and Functional Neurological Recovery After Spinal Cord Injury. J. Neurosci. 2017, 37, 11731–11743. [Google Scholar] [CrossRef]
- Ohuchi, K.; Ono, Y.; Joho, M.; Tsuruma, K.; Ogami, S.; Yamane, S.; Funato, M.; Kaneko, H.; Nakamura, S.; Hara, H.; et al. A Docosahexaenoic Acid-Derived Pro-resolving Agent, Maresin 1, Protects Motor Neuron Cells Death. Neurochem. Res. 2018, 43, 1413–1423. [Google Scholar] [CrossRef]
- Fattori, V.; Pinho-Ribeiro, F.A.; Staurengo-Ferrari, L.; Borghi, S.M.; Rossaneis, A.C.; Casagrande, R.; Verri, W.A., Jr. The specialised pro-resolving lipid mediator maresin 1 reduces inflammatory pain with a long-lasting analgesic effect. Br. J. Pharmacol. 2019, 176, 1728–1744. [Google Scholar] [CrossRef]
- Fattori, V.; Zaninelli, T.H.; Ferraz, C.R.; Brasil-Silva, L.; Borghi, S.M.; Cunha, J.M.; Chichorro, J.G.; Casagrande, R.; Verri, W.A., Jr. Maresin 2 is an analgesic specialized pro-resolution lipid mediator in mice by inhibiting neutrophil and monocyte recruitment, nociceptor neuron TRPV1 and TRPA1 activation, and CGRP release. Neuropharmacology 2022, 216, 109189. [Google Scholar] [CrossRef]
- Gao, J.; Tang, C.; Tai, L.W.; Ouyang, Y.; Li, N.; Hu, Z.; Chen, X. Pro-resolving mediator maresin 1 ameliorates pain hypersensitivity in a rat spinal nerve ligation model of neuropathic pain. J. Pain Res. 2018, 11, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Li, Y.; Wang, J.N.; Zhao, Q.X.; Wen, S.; Wang, S.C.; Sun, T. A Novel Mechanism of Specialized Proresolving Lipid Mediators Mitigating Radicular Pain: The Negative Interaction with NLRP3 Inflammasome. Neurochem. Res. 2020, 45, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Li, Y.; Wang, J.N.; Zhao, Q.X.; Jin, J.; Wen, S.; Wang, S.C.; Sun, T. Maresin 1 Attenuates Radicular Pain Through the Inhibition of NLRP3 Inflammasome-Induced Pyroptosis via NF-κB Signaling. Front. Neurosci. 2020, 14, 831. [Google Scholar] [CrossRef]
- Wei, J.; Su, W.; Zhao, Y.; Wei, Z.; Hua, Y.; Xue, P.; Zhu, X.; Chen, Y.; Chen, G. Maresin 1 promotes nerve regeneration and alleviates neuropathic pain after nerve injury. J. Neuroinflamm. 2022, 19, 32. [Google Scholar] [CrossRef]
- Zhang, L.; Terrando, N.; Xu, Z.Z.; Bang, S.; Jordt, S.E.; Maixner, W.; Serhan, C.N.; Ji, R.R. Distinct Analgesic Actions of DHA and DHA-Derived Specialized Pro-Resolving Mediators on Post-operative Pain After Bone Fracture in Mice. Front. Pharmacol. 2018, 9, 412. [Google Scholar] [CrossRef]
- Allen, B.L.; Montague-Cardoso, K.; Simeoli, R.; Colas, R.A.; Oggero, S.; Vilar, B.; McNaughton, P.A.; Dalli, J.; Perretti, M.; Sher, E.; et al. Imbalance of proresolving lipid mediators in persistent allodynia dissociated from signs of clinical arthritis. Pain 2020, 161, 2155–2166. [Google Scholar] [CrossRef]
- Arnardottir, H.; Orr, S.K.; Dalli, J.; Serhan, C.N. Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal Immunol. 2016, 9, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Falsetta, M.L.; Wood, R.W.; Linder, M.A.; Bonham, A.D.; Honn, K.V.; Maddipati, K.R.; Phipps, R.P.; Haidaris, C.G.; Foster, D.C. Specialized Pro-resolving Mediators Reduce Pro-nociceptive Inflammatory Mediator Production in Models of Localized Provoked Vulvodynia. J. Pain 2021, 22, 1195–1209. [Google Scholar] [CrossRef]
- Levy, B.D.; Abdulnour, R.E.; Tavares, A.; Brüggemann, T.R.; Norris, P.C.; Bai, Y.; Ai, X.; Serhan, C.N. Cysteinyl maresins regulate the prophlogistic lung actions of cysteinyl leukotrienes. J. Allergy Clin. Immunol. 2020, 145, 335–344. [Google Scholar] [CrossRef]
- Ou, G.; Liu, Q.; Yu, C.; Chen, X.; Zhang, W.; Chen, Y.; Wang, T.; Luo, Y.; Jiang, G.; Zhu, M.; et al. The Protective Effects of Maresin 1 in the OVA-Induced Asthma Mouse Model. Mediat. Inflamm. 2021, 2021, 4131420. [Google Scholar] [CrossRef]
- Zhuang, R.; Yang, X.; Cai, W.; Xu, R.; Lv, L.; Sun, Y.; Guo, Y.; Ni, J.; Zhao, G.; Lu, Z. MCTR3 reduces LPS-induced acute lung injury in mice via the ALX/PINK1 signaling pathway. Int. Immunopharmacol. 2021, 90, 107142. [Google Scholar] [CrossRef]
- Han, J.; Li, H.; Bhandari, S.; Cao, F.; Wang, X.Y.; Tian, C.; Li, X.Y.; Zhang, P.H.; Liu, Y.J.; Wu, C.H.; et al. Maresin Conjugates in Tissue Regeneration 1 improves alveolar fluid clearance by up-regulating alveolar ENaC, Na, K-ATPase in lipopolysaccharide-induced acute lung injury. J. Cell. Mol. Med. 2020, 24, 4736–4747. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Bauer, C.D.; Heires, A.J.; Poole, J.A.; Wyatt, T.A.; West, W.W.; Romberger, D.J. Maresin-1 reduces airway inflammation associated with acute and repetitive exposures to organic dust. Transl. Res. 2015, 166, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, X.; Wang, X.; Yang, L.; Chen, H.; Su, N.; Wu, C.; Hao, Y.; Jin, S.; Li, H. MCTR1 Intervention Reverses Experimental Lung Fibrosis in Mice. J. Inflamm. Res. 2021, 14, 1873–1881. [Google Scholar] [CrossRef]
- Hughes, F.M., Jr.; Allkanjari, A.; Odom, M.R.; Jin, H.; Purves, J.T. Specialized pro-resolution mediators in the bladder: Receptor expression and recovery of bladder function from cystitis. Exp. Biol. Med. 2022, 247, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, B.; Wu, J.; Pu, Y.; Wan, S.; Zeng, Y.; Wang, M.; Luo, L.; Zhang, F.; Jiang, Z.; et al. Maresin 1 Alleviates Diabetic Kidney Disease via LGR6-Mediated cAMP-SOD2-ROS Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 7177889. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, J.; Wang, J.; Wang, F.; Yao, S.; Xia, H. Maresin 1 Mitigates Sepsis-Associated Acute Kidney Injury in Mice via Inhibition of the NF-kappaB/STAT3/MAPK Pathways. Front. Pharmacol. 2019, 10, 1323. [Google Scholar] [CrossRef]
- Xiao, J.; Yang, Q.; Zhang, Y.; Xu, H.; Ye, Y.; Li, L.; Yang, Y.; Jin, S. Maresin conjugates in tissue regeneration-1 suppresses ferroptosis in septic acute kidney injury. Cell Biosci. 2021, 11, 221. [Google Scholar] [CrossRef]
- Qiu, Y.; Wu, Y.; Zhao, H.; Sun, H.; Gao, S. Maresin 1 mitigates renal ischemia/reperfusion injury in mice via inhibition of the TLR4/MAPK/NF-kappaB pathways and activation of the Nrf2 pathway. Drug Des. Dev. Ther. 2019, 13, 739–745. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Van Dyke, T.E. Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S24–S29. [Google Scholar] [CrossRef]
- Genco, R.J.; Sanz, M. Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontology 2000 2020, 83, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Borgnakke, W.S. Diabetes as a potential risk for periodontitis: Association studies. Periodontology 2000 2020, 83, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Mustufvi, Z.; Twigg, J.; Kerry, J.; Chesterman, J.; Pavitt, S.; Tugnait, A.; Mankia, K. Does periodontal treatment improve rheumatoid arthritis disease activity? A systematic review. Rheumatol. Adv. Pract. 2022, 6, rkac061. [Google Scholar] [CrossRef]
- Elabdeen, H.R.; Mustafa, M.; Szklenar, M.; Ruhl, R.; Ali, R.; Bolstad, A.I. Ratio of pro-resolving and pro-inflammatory lipid mediator precursors as potential markers for aggressive periodontitis. PLoS ONE 2013, 8, e70838. [Google Scholar] [CrossRef] [PubMed]
- Rakian, A.; Rakian, R.; Shay, A.E.; Serhan, C.N.; Van Dyke, T.E. Periodontal Stem Cells Synthesize Maresin Conjugate in Tissue Regeneration 3. J. Dent. Res. 2022, 101, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Park, C.K. Maresin 1 Inhibits TRPV1 in Temporomandibular Joint-Related Trigeminal Nociceptive Neurons and TMJ Inflammation-Induced Synaptic Plasticity in the Trigeminal Nucleus. Mediat. Inflamm. 2015, 2015, 275126. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar] [CrossRef]
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef]
- Fan, J.; Watanabe, T. Atherosclerosis: Known and unknown. Pathol. Int. 2022, 72, 151–160. [Google Scholar] [CrossRef]
- Wahyuni, T.; Kobayashi, A.; Tanaka, S.; Miyake, Y.; Yamamoto, A.; Bahtiar, A.; Mori, S.; Kametani, Y.; Tomimatsu, M.; Matsumoto, K.; et al. Maresin-1 induces cardiomyocyte hypertrophy through IGF-1 paracrine pathway. Am. J. Physiol. Cell Physiol. 2021, 321, C82–C93. [Google Scholar] [CrossRef]
- Jung, T.W.; Park, H.S.; Choi, G.H.; Kim, D.; Ahn, S.H.; Kim, D.S.; Lee, T.; Jeong, J.H. Maresin 1 attenuates pro-inflammatory reactions and ER stress in HUVECs via PPARα-mediated pathway. Mol. Cell. Biochem. 2018, 448, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Welty, F.K.; Schulte, F.; Alfaddagh, A.; Elajami, T.K.; Bistrian, B.R.; Hardt, M. Regression of human coronary artery plaque is associated with a high ratio of (18-hydroxy-eicosapentaenoic acid + resolvin E1) to leukotriene B4. FASEB J. 2021, 35, e21448. [Google Scholar] [CrossRef] [PubMed]
- Keeley, E.C.; Li, H.J.; Cogle, C.R.; Handberg, E.M.; Merz, C.N.B.; Pepine, C.J. Specialized Proresolving Mediators in Symptomatic Women With Coronary Microvascular Dysfunction (from the Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD [WARRIOR] Trial). Am. J. Cardiol. 2022, 162, 1–5. [Google Scholar] [CrossRef]
- Chatterjee, A.; Sharma, A.; Chen, M.; Toy, R.; Mottola, G.; Conte, M.S. The pro-resolving lipid mediator maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells. PLoS ONE 2014, 9, e113480. [Google Scholar] [CrossRef]
- Akagi, D.; Chen, M.; Toy, R.; Chatterjee, A.; Conte, M.S. Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB J. 2015, 29, 2504–2513. [Google Scholar] [CrossRef]
- Norris, P.C.; Libreros, S.; Chiang, N.; Serhan, C.N. A cluster of immunoresolvents links coagulation to innate host defense in human blood. Sci. Signal. 2017, 10, eaan1471. [Google Scholar] [CrossRef]
- Lannan, K.L.; Spinelli, S.L.; Blumberg, N.; Phipps, R.P. Maresin 1 induces a novel pro-resolving phenotype in human platelets. J. Thromb. Haemost. 2017, 15, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.C.; Kalyanaraman, C.; Tourdot, B.E.; Conrad, W.S.; Akinkugbe, O.; Freedman, J.C.; Holinstat, M.; Jacobson, M.P.; Holman, T.R. 15-Lipoxygenase-1 biosynthesis of 7S,14S-diHDHA implicates 15-lipoxygenase-2 in biosynthesis of resolvin D5. J. Lipid Res. 2020, 61, 1087–1103. [Google Scholar] [CrossRef]
- Qiu, S.; Li, P.; Zhao, H.; Li, X. Maresin 1 alleviates dextran sulfate sodium-induced ulcerative colitis by regulating NRF2 and TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2020, 78, 106018. [Google Scholar] [CrossRef]
- Wang, H.; Shi, P.; Huang, C.; Liu, Q. Maresin 1 ameliorates iron-deficient anemia in IL-10(−/−) mice with spontaneous colitis by the inhibition of hepcidin expression though the IL-6/STAT3 pathway. Am. J. Transl. Res. 2016, 8, 2758–2766. [Google Scholar] [PubMed]
- Zhang, P.; Yin, Y.; Wang, T.; Li, W.; Li, C.; Zeng, X.; Yang, W.; Zhang, R.; Tang, Y.; Shi, L.; et al. Maresin 1 mitigates concanavalin A-induced acute liver injury in mice by inhibiting ROS-mediated activation of NF-κB signaling. Free Radic. Biol. Med. 2020, 147, 23–36. [Google Scholar] [CrossRef] [PubMed]
- López-Vicario, C.; Sebastián, D.; Casulleras, M.; Duran-Güell, M.; Flores-Costa, R.; Aguilar, F.; Lozano, J.J.; Zhang, I.W.; Titos, E.; Kang, J.X.; et al. Essential lipid autacoids rewire mitochondrial energy efficiency in metabolic dysfunction-associated fatty liver disease. Hepatology 2022, 77, 1303–1318. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Ye, T.; Fu, X.; Tan, X.; Zeng, Y.; Fan, J.; Xu, Y. Low serum Maresin-1 levels are associated with non-alcoholic fatty liver disease: A cross-sectional study. Lipids Health Dis. 2021, 20, 96. [Google Scholar] [CrossRef]
- Rius, B.; Duran-Guell, M.; Flores-Costa, R.; Lopez-Vicario, C.; Lopategi, A.; Alcaraz-Quiles, J.; Casulleras, M.; Lozano, J.J.; Titos, E.; Claria, J. The specialized proresolving lipid mediator maresin 1 protects hepatocytes from lipotoxic and hypoxia-induced endoplasmic reticulum stress. FASEB J. 2017, 31, 5384–5398. [Google Scholar] [CrossRef]
- Tang, D.; Fu, G.; Li, W.; Sun, P.; Loughran, P.A.; Deng, M.; Scott, M.J.; Billiar, T.R. Maresin 1 protects the liver against ischemia/reperfusion injury via the ALXR/Akt signaling pathway. Mol. Med. 2021, 27, 18. [Google Scholar] [CrossRef]
- Ye, J.; Peng, J.; Liu, K.; Zhang, T.; Huang, W. MCTR1 inhibits ferroptosis by promoting NRF2 expression to attenuate hepatic ischemia-reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 323, G283–G293. [Google Scholar] [CrossRef]
- Hiller-Sturmhöfel, S.; Bartke, A. The endocrine system: An overview. Alcohol Health Res. World 1998, 22, 153–164. [Google Scholar]
- Titos, E.; Rius, B.; Lopez-Vicario, C.; Alcaraz-Quiles, J.; Garcia-Alonso, V.; Lopategi, A.; Dalli, J.; Lozano, J.J.; Arroyo, V.; Delgado, S.; et al. Signaling and Immunoresolving Actions of Resolvin D1 in Inflamed Human Visceral Adipose Tissue. J. Immunol. 2016, 197, 3360–3370. [Google Scholar] [CrossRef]
- Miao, T.; Huang, B.; He, N.; Sun, L.; Du, G.; Gong, X.; Xu, Y.; Zheng, Y.; Zheng, H.; Qu, H. Decreased Plasma Maresin 1 Concentration Is Associated with Diabetic Foot Ulcer. Mediat. Inflamm. 2020, 2020, 4539035. [Google Scholar] [CrossRef]
- Shivakoti, R.; Dalli, J.; Kadam, D.; Gaikwad, S.; Barthwal, M.; Colas, R.A.; Mazzacuva, F.; Lokhande, R.; Dharmshale, S.; Bharadwaj, R.; et al. Lipid mediators of inflammation and Resolution in individuals with tuberculosis and tuberculosis-Diabetes. Prostaglandins Other Lipid Mediat. 2020, 147, 106398. [Google Scholar] [CrossRef]
- Barden, A.; Shinde, S.; Phillips, M.; Beilin, L.; Mas, E.; Hodgson, J.M.; Puddey, I.; Mori, T.A. The effects of alcohol on plasma lipid mediators of inflammation resolution in patients with Type 2 diabetes mellitus. Prostaglandins Leukot. Essent. Fat. Acids 2018, 133, 29–34. [Google Scholar] [CrossRef]
- Barden, A.; Shinde, S.; Tsai, I.J.; Croft, K.D.; Beilin, L.J.; Puddey, I.B.; Mori, T.A. Effect of weight loss on neutrophil resolvins in the metabolic syndrome. Prostaglandins Leukot. Essent. Fat. Acids 2019, 148, 25–29. [Google Scholar] [CrossRef]
- Liakh, I.; Janczy, A.; Pakiet, A.; Korczynska, J.; Proczko-Stepaniak, M.; Kaska, L.; Sledzinski, T.; Mika, A. One-anastomosis gastric bypass modulates the serum levels of pro- and anti-inflammatory oxylipins, which may contribute to the resolution of inflammation. Int. J. Obes. 2022, 46, 408–416. [Google Scholar] [CrossRef]
- Schulte, F.; Asbeutah, A.A.; Benotti, P.N.; Wood, G.C.; Still, C.; Bistrian, B.R.; Hardt, M.; Welty, F.K. The relationship between specialized pro-resolving lipid mediators, morbid obesity and weight loss after bariatric surgery. Sci. Rep. 2020, 10, 20128. [Google Scholar] [CrossRef]
- Jouvene, C.C.; Shay, A.E.; Soens, M.A.; Norris, P.C.; Haeggström, J.Z.; Serhan, C.N. Biosynthetic metabolomes of cysteinyl-containing immunoresolvents. FASEB J. 2019, 33, 13794–13807. [Google Scholar] [CrossRef]
- Caron, J.P.; Gandy, J.C.; Brown, J.L.; Sordillo, L.M. Docosahexaenoic acid-derived oxidized lipid metabolites modulate the inflammatory response of lipolysaccharide-stimulated macrophages. Prostaglandins Other Lipid Mediat. 2018, 136, 76–83. [Google Scholar] [CrossRef]
- Lastrucci, C.; Baillif, V.; Behar, A.; Al Saati, T.; Dubourdeau, M.; Maridonneau-Parini, I.; Cougoule, C. Molecular and cellular profiles of the resolution phase in a damage-associated molecular pattern (DAMP)-mediated peritonitis model and revelation of leukocyte persistence in peritoneal tissues. FASEB J. 2015, 29, 1914–1929. [Google Scholar] [CrossRef]
- Werz, O.; Gerstmeier, J.; Libreros, S.; De la Rosa, X.; Werner, M.; Norris, P.C.; Chiang, N.; Serhan, C.N. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat. Commun. 2018, 9, 59. [Google Scholar] [CrossRef]
- Korner, A.; Schlegel, M.; Theurer, J.; Frohnmeyer, H.; Adolph, M.; Heijink, M.; Giera, M.; Rosenberger, P.; Mirakaj, V. Resolution of inflammation and sepsis survival are improved by dietary Omega-3 fatty acids. Cell Death Differ. 2018, 25, 421–431. [Google Scholar] [CrossRef]
- Becker, F.; Romero, E.; Goetzmann, J.; Hasselschwert, D.L.; Dray, B.; Vanchiere, J.; Fontenot, J.; Yun, J.W.; Norris, P.C.; White, L.; et al. Endogenous Specialized Proresolving Mediator Profiles in a Novel Experimental Model of Lymphatic Obstruction and Intestinal Inflammation in African Green Monkeys. Am. J. Pathol. 2019, 189, 1953–1972. [Google Scholar] [CrossRef]
- Nichols, B.A. Conjunctiva. Microsc. Res. Tech. 1996, 33, 296–319. [Google Scholar] [CrossRef]
- Yin, X.; Yu, X.W.; Zhu, P.; Zhang, Y.M.; Zhang, X.H.; Wang, F.; Zhang, J.J.; Yan, W.; Xi, Y.; Wan, J.B.; et al. Endogenously synthesized n-3 fatty acids in fat-1 transgenic mice prevent melanoma progression by increasing E-cadherin expression and inhibiting β-catenin signaling. Mol. Med. Rep. 2016, 14, 3476–3484. [Google Scholar] [CrossRef]
- English, J.T.; Norris, P.C.; Hodges, R.R.; Dartt, D.A.; Serhan, C.N. Identification and Profiling of Specialized Pro-Resolving Mediators in Human Tears by Lipid Mediator Metabolomics. Prostaglandins Leukot. Essent. Fat. Acids 2017, 117, 17–27. [Google Scholar] [CrossRef]
- Olsen, M.V.; Lyngstadaas, A.V.; Bair, J.A.; Hodges, R.R.; Utheim, T.P.; Serhan, C.N.; Dartt, D.A. Maresin 1, a specialized proresolving mediator, stimulates intracellular [Ca2+] and secretion in conjunctival goblet cells. J. Cell. Physiol. 2021, 236, 340–353. [Google Scholar] [CrossRef]
- Olsen, M.V.; Lyngstadaas, A.V.; Bair, J.A.; Hodges, R.R.; Utheim, T.P.; Serhan, C.N.; Dartt, D.A. Signaling Pathways Used by the Specialized Pro-Resolving Mediator Maresin 2 Regulate Goblet Cell Function: Comparison with Maresin 1. Int. J. Mol. Sci. 2022, 23, 6233. [Google Scholar] [CrossRef]
- Felix-Soriano, E.; Sainz, N.; Gil-Iturbe, E.; Collantes, M.; Fernandez-Galilea, M.; Castilla-Madrigal, R.; Ly, L.; Dalli, J.; Moreno-Aliaga, M.J. Changes in brown adipose tissue lipid mediator signatures with aging, obesity, and DHA supplementation in female mice. FASEB J. 2021, 35, e21592. [Google Scholar] [CrossRef]
- Laiglesia, L.M.; Lorente-Cebrián, S.; López-Yoldi, M.; Lanas, R.; Sáinz, N.; Martínez, J.A.; Moreno-Aliaga, M.J. Maresin 1 inhibits TNF-alpha-induced lipolysis and autophagy in 3T3-L1 adipocytes. J. Cell. Physiol. 2018, 233, 2238–2246. [Google Scholar] [CrossRef]
- Dakin, S.G.; Colas, R.A.; Newton, J.; Gwilym, S.; Jones, N.; Reid, H.A.B.; Wood, S.; Appleton, L.; Wheway, K.; Watkins, B.; et al. 15-Epi-LXA(4) and MaR1 counter inflammation in stromal cells from patients with Achilles tendinopathy and rupture. FASEB J. 2019, 33, 8043–8054. [Google Scholar] [CrossRef]
- Markworth, J.F.; Brown, L.A.; Lim, E.; Castor-Macias, J.A.; Larouche, J.; Macpherson, P.C.D.; Davis, C.; Aguilar, C.A.; Maddipati, K.R.; Brooks, S.V. Metabolipidomic profiling reveals an age-related deficiency of skeletal muscle pro-resolving mediators that contributes to maladaptive tissue remodeling. Aging Cell 2021, 20, e13393. [Google Scholar] [CrossRef]
- Yao, D.; Lv, Y. A cell-free difunctional demineralized bone matrix scaffold enhances the recruitment and osteogenesis of mesenchymal stem cells by promoting inflammation resolution. Biomater. Adv. 2022, 139, 213036. [Google Scholar] [CrossRef]
- Khedgikar, V.; Charles, J.F.; Lehoczky, J.A. Mouse LGR6 regulates osteogenesis in vitro and in vivo through differential ligand use. Bone 2022, 155, 116267. [Google Scholar] [CrossRef]
- Caron, J.P.; Gandy, J.C.; Brown, J.L.; Sordillo, L.M. Omega-3 fatty acids and docosahexaenoic acid oxymetabolites modulate the inflammatory response of equine recombinant interleukin1β-stimulated equine synoviocytes. Prostaglandins Other Lipid Mediat. 2019, 142, 1–8. [Google Scholar] [CrossRef]
- Lu, J.; Feng, X.; Zhang, H.; Wei, Y.; Yang, Y.; Tian, Y.; Bai, L. Maresin-1 suppresses IL-1β-induced MMP-13 secretion by activating the PI3K/AKT pathway and inhibiting the NF-κB pathway in synovioblasts of an osteoarthritis rat model with treadmill exercise. Connect. Tissue Res. 2021, 62, 508–518. [Google Scholar] [CrossRef]
- Pistorius, K.; Ly, L.; Souza, P.R.; Gomez, E.A.; Koenis, D.S.; Rodriguez, A.R.; Foster, J.; Sosabowski, J.; Hopkinson, M.; Rajeeve, V.; et al. MCTR3 reprograms arthritic monocytes to upregulate Arginase-1 and exert pro-resolving and tissue-protective functions in experimental arthritis. EBioMedicine 2022, 79, 103974. [Google Scholar] [CrossRef]
- Do, K.V.; Hjorth, E.; Wang, Y.; Jun, B.; Kautzmann, M.I.; Ohshima, M.; Eriksdotter, M.; Schultzberg, M.; Bazan, N.G. Cerebrospinal Fluid Profile of Lipid Mediators in Alzheimer’s Disease. Cell. Mol. Neurobiol. 2023, 43, 797–811. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, X.; Hjorth, E.; Colas, R.A.; Schroeder, L.; Granholm, A.C.; Serhan, C.N.; Schultzberg, M. Pro-Resolving Lipid Mediators Improve Neuronal Survival and Increase Abeta42 Phagocytosis. Mol. Neurobiol. 2016, 53, 2733–2749. [Google Scholar] [CrossRef]
- Martinsen, A.; Tejera, N.; Vauzour, D.; Harden, G.; Dick, J.; Shinde, S.; Barden, A.; Mori, T.A.; Minihane, A.M. Altered SPMs and age-associated decrease in brain DHA in APOE4 female mice. FASEB J. 2019, 33, 10315–10326. [Google Scholar] [CrossRef]
- Wang, X.; Miao, Z.; Xu, X.; Schultzberg, M.; Zhao, Y. Reduced Levels of Plasma Lipoxin A4 Are Associated with Post-Stroke Cognitive Impairment. J. Alzheimers Dis. 2021, 79, 607–613. [Google Scholar] [CrossRef]
- Sogorb-Esteve, A.; Colas, R.A.; Dalli, J.; Rohrer, J.D. Differential Lipid Mediator Involvement in the Different Forms of Genetic Frontotemporal Dementia: Novel Insights into Neuroinflammation. J. Alzheimers Dis. 2021, 84, 283–289. [Google Scholar] [CrossRef]
- Xian, W.; Li, T.; Li, L.; Hu, L.; Cao, J. Maresin 1 attenuates the inflammatory response and mitochondrial damage in mice with cerebral ischemia/reperfusion in a SIRT1-dependent manner. Brain Res. 2019, 1711, 83–90. [Google Scholar] [CrossRef]
- Wang, Y.; Leppert, A.; Tan, S.; van der Gaag, B.; Li, N.; Schultzberg, M.; Hjorth, E. Maresin 1 attenuates pro-inflammatory activation induced by beta-amyloid and stimulates its uptake. J. Cell. Mol. Med. 2021, 25, 434–447. [Google Scholar] [CrossRef]
- Kotlega, D.; Peda, B.; Drozd, A.; Zembron-Lacny, A.; Stachowska, E.; Gramacki, J.; Szczuko, M. Prostaglandin E2, 9S-, 13S-HODE and resolvin D1 are strongly associated with the post-stroke cognitive impairment. Prostaglandins Other Lipid Mediat. 2021, 156, 106576. [Google Scholar] [CrossRef]
- Regidor, P.A.; de la Rosa, X.; Muller, A.; Mayr, M.; Gonzalez Santos, F.; Gracia Banzo, R.; Rizo, J.M. PCOS: A Chronic Disease That Fails to Produce Adequately Specialized Pro-Resolving Lipid Mediators (SPMs). Biomedicines 2022, 10, 456. [Google Scholar] [CrossRef]
- Oliveira Perucci, L.; Pereira Santos, T.A.; Campi Santos, P.; Ribeiro Teixeira, L.C.; Nessralla Alpoim, P.; Braga Gomes, K.; Pires Sousa, L.; Sant’Ana Dusse, L.M.; Talvani, A. Pre-eclampsia is associated with reduced resolvin D1 and maresin 1 to leukotriene B4 ratios in the plasma. Am. J. Reprod. Immunol. 2020, 83, e13206. [Google Scholar] [CrossRef]
- Tejera, P.; Abdulnour, R.E.; Zhu, Z.; Su, L.; Levy, B.D.; Christiani, D.C. Plasma Levels of Proresolving and Prophlogistic Lipid Mediators: Association With Severity of Respiratory Failure and Mortality in Acute Respiratory Distress Syndrome. Crit. Care Explor. 2020, 2, e0241. [Google Scholar] [CrossRef]
- Abdulnour, R.E.; Dalli, J.; Colby, J.K.; Krishnamoorthy, N.; Timmons, J.Y.; Tan, S.H.; Colas, R.A.; Petasis, N.A.; Serhan, C.N.; Levy, B.D. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc. Natl. Acad. Sci. USA 2014, 111, 16526–16531. [Google Scholar] [CrossRef]
- Regidor, P.A.; De La Rosa, X.; Santos, F.G.; Rizo, J.M.; Gracia Banzo, R.; Silva, R.S. Acute severe SARS COVID-19 patients produce pro-resolving lipids mediators and eicosanoids. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6782–6796. [Google Scholar] [CrossRef]
- Tavares, L.P.; Brüggemann, T.R.; Rezende, R.M.; Machado, M.G.; Cagnina, R.E.; Shay, A.E.; Garcia, C.C.; Nijmeh, J.; Teixeira, M.M.; Levy, B.D. Cysteinyl Maresins Reprogram Macrophages to Protect Mice from Streptococcus pneumoniae after Influenza A Virus Infection. mBio 2022, 13, e0126722. [Google Scholar] [CrossRef]
- Yu, C.X.; Shi, Z.A.; Ou, G.C.; Chen, X.J.; Liu, Q.; Zeng, D.; Nie, X.J.; Chen, J.J. Maresin-2 alleviates allergic airway inflammation in mice by inhibiting the activation of NLRP3 inflammasome, Th2 type immune response and oxidative stress. Mol. Immunol. 2022, 146, 78–86. [Google Scholar] [CrossRef]
- Gong, J.; Wu, Z.Y.; Qi, H.; Chen, L.; Li, H.B.; Li, B.; Yao, C.Y.; Wang, Y.X.; Wu, J.; Yuan, S.Y.; et al. Maresin 1 mitigates LPS-induced acute lung injury in mice. Br. J. Pharmacol. 2014, 171, 3539–3550. [Google Scholar] [CrossRef]
- Gong, J.; Liu, H.; Wu, J.; Qi, H.; Wu, Z.Y.; Shu, H.Q.; Li, H.B.; Chen, L.; Wang, Y.X.; Li, B.; et al. Maresin 1 Prevents Lipopolysaccharide-Induced Neutrophil Survival and Accelerates Resolution of Acute Lung Injury. Shock 2015, 44, 371–380. [Google Scholar] [CrossRef]
- Chen, L.; Liu, H.; Wang, Y.; Xia, H.; Gong, J.; Li, B.; Yao, S.; Shang, Y. Maresin 1 Maintains the Permeability of Lung Epithelial Cells In Vitro and In Vivo. Inflammation 2016, 39, 1981–1989. [Google Scholar] [CrossRef]
- Li, H.; Hao, Y.; Yang, L.L.; Wang, X.Y.; Li, X.Y.; Bhandari, S.; Han, J.; Liu, Y.J.; Gong, Y.Q.; Scott, A.; et al. MCTR1 alleviates lipopolysaccharide-induced acute lung injury by protecting lung endothelial glycocalyx. J. Cell. Physiol. 2020, 235, 7283–7294. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Heires, A.J.; Wyatt, T.A.; Poole, J.A.; LeVan, T.D.; Cerutis, D.R.; Romberger, D.J. Maresin-1 reduces the pro-inflammatory response of bronchial epithelial cells to organic dust. Respir. Res. 2013, 14, 51. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Chen, L.; Tan, W.; Sun, Z.; Xia, H.; Li, B.; Yu, Y.; Gong, J.; Tang, M.; et al. Maresin 1 Inhibits Epithelial-to-Mesenchymal Transition in Vitro and Attenuates Bleomycin Induced Lung Fibrosis in Vivo. Shock 2015, 44, 496–502. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, Y.; Zhao, F.; Wang, J. Maresin 1 inhibits transforming growth factor-β1-induced proliferation, migration and differentiation in human lung fibroblasts. Mol. Med. Rep. 2017, 16, 1523–1529. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Wang, L.; Jiang, L.; Qin, Z.; Zhao, Y.; Su, B. Maresin 1 Attenuates Lipopolysaccharide-Induced Acute Kidney Injury via Inhibiting NOX4/ROS/NF-kappaB Pathway. Front. Pharmacol. 2021, 12, 782660. [Google Scholar] [CrossRef]
- Dalli, J.; Serhan, C.N. Specific lipid mediator signatures of human phagocytes: Microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 2012, 120, e60–e72. [Google Scholar] [CrossRef]
- Tobón-Arroyave, S.I.; Isaza-Guzmán, D.M.; Gómez-Ortega, J.; Flórez-Alzate, A.A. Salivary levels of specialized pro-resolving lipid mediators as indicators of periodontal health/disease status. J. Clin. Periodontol. 2019, 46, 978–990. [Google Scholar] [CrossRef]
- Önal, M.A.; Fentoğlu, Ö.; Aksoy, F.; Calapoğlu, M.; Varol, E.; Orhan, H. Salivary levels of last generation specific pro-resolving lipid mediators (SPMs) (protectin and maresin) in patients with cardiovascular and periodontal disease: A case-control study. J. Periodontal Res. 2021, 56, 606–615. [Google Scholar] [CrossRef]
- Lee, C.T.; Li, R.; Zhu, L.; Tribble, G.D.; Zheng, W.J.; Ferguson, B.; Maddipati, K.R.; Angelov, N.; Van Dyke, T.E. Subgingival Microbiome and Specialized Pro-Resolving Lipid Mediator Pathway Profiles Are Correlated in Periodontal Inflammation. Front. Immunol. 2021, 12, 691216. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.Y.; Fang, X.; Teng, F.Y.; Xu, Y. Decreased Serum Maresin 1 Concentration Is Associated With Postmenopausal Osteoporosis: A Cross-Sectional Study. Front. Med. 2021, 8, 759825. [Google Scholar] [CrossRef]
- Tang, S.; Gao, C.; Long, Y.; Huang, W.; Chen, J.; Fan, F.; Jiang, C.; Xu, Y. Maresin 1 Mitigates High Glucose-Induced Mouse Glomerular Mesangial Cell Injury by Inhibiting Inflammation and Fibrosis. Mediat. Inflamm. 2017, 2017, 2438247. [Google Scholar] [CrossRef]
- Morita, Y.; Kurano, M.; Sakai, E.; Sawabe, M.; Aoki, J.; Yatomi, Y. Simultaneous analyses of urinary eicosanoids and related mediators identified tetranor-prostaglandin E metabolite as a novel biomarker of diabetic nephropathy. J. Lipid Res. 2021, 62, 100120. [Google Scholar] [CrossRef]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2021, 73, 924–939. [Google Scholar] [CrossRef]
- Singh, J.A. Treatment Guidelines in Rheumatoid Arthritis. Rheum. Dis. Clin. N. Am. 2022, 48, 679–689. [Google Scholar] [CrossRef]
- Gomez, E.A.; Colas, R.A.; Souza, P.R.; Hands, R.; Lewis, M.J.; Bessant, C.; Pitzalis, C.; Dalli, J. Blood pro-resolving mediators are linked with synovial pathology and are predictive of DMARD responsiveness in rheumatoid arthritis. Nat. Commun. 2020, 11, 5420. [Google Scholar] [CrossRef]
- Mastrogiovanni, M.; Trostchansky, A.; Naya, H.; Dominguez, R.; Marco, C.; Povedano, M.; López-Vales, R.; Rubbo, H. HPLC-MS/MS Oxylipin Analysis of Plasma from Amyotrophic Lateral Sclerosis Patients. Biomedicines 2022, 10, 674. [Google Scholar] [CrossRef]
- Wake, M.; Kobayashi, D. Associations between plasma levels of omega-3 fatty acids and subsequent allergic diseases. Clin. Nutr. ESPEN 2021, 42, 318–324. [Google Scholar] [CrossRef]
- Reddoch-Cardenas, K.M.; Sharma, U.; Salgado, C.L.; Cantu, C.; Darlington, D.N.; Pidcoke, H.F.; Bynum, J.A.; Cap, A.P. Use of Specialized Pro-Resolving Mediators to Alleviate Cold Platelet Storage Lesion. Transfusion 2020, 60 (Suppl. S3), S112–S118. [Google Scholar] [CrossRef]
- Skarke, C.; Alamuddin, N.; Lawson, J.A.; Li, X.; Ferguson, J.F.; Reilly, M.P.; FitzGerald, G.A. Bioactive products formed in humans from fish oils. J. Lipid Res. 2015, 56, 1808–1820. [Google Scholar] [CrossRef]
- Mozurkewich, E.L.; Greenwood, M.; Clinton, C.; Berman, D.; Romero, V.; Djuric, Z.; Qualls, C.; Gronert, K. Pathway Markers for Pro-resolving Lipid Mediators in Maternal and Umbilical Cord Blood: A Secondary Analysis of the Mothers, Omega-3, and Mental Health Study. Front. Pharmacol. 2016, 7, 274. [Google Scholar] [CrossRef]
- Markworth, J.F.; Kaur, G.; Miller, E.G.; Larsen, A.E.; Sinclair, A.J.; Maddipati, K.R.; Cameron-Smith, D. Divergent shifts in lipid mediator profile following supplementation with n-3 docosapentaenoic acid and eicosapentaenoic acid. FASEB J. 2016, 30, 3714–3725. [Google Scholar] [CrossRef]
- See, V.H.L.; Mas, E.; Prescott, S.L.; Beilin, L.J.; Burrows, S.; Barden, A.E.; Huang, R.C.; Mori, T.A. Effects of prenatal n-3 fatty acid supplementation on offspring resolvins at birth and 12 years of age: A double-blind, randomised controlled clinical trial. Br. J. Nutr. 2017, 118, 971–980. [Google Scholar] [CrossRef]
- Hopperton, K.E.; Trepanier, M.O.; James, N.C.E.; Chouinard-Watkins, R.; Bazinet, R.P. Fish oil feeding attenuates neuroinflammatory gene expression without concomitant changes in brain eicosanoids and docosanoids in a mouse model of Alzheimer’s disease. Brain Behav. Immun. 2018, 69, 74–90. [Google Scholar] [CrossRef]
- Souza, P.R.; Marques, R.M.; Gomez, E.A.; Colas, R.A.; De Matteis, R.; Zak, A.; Patel, M.; Collier, D.J.; Dalli, J. Enriched Marine Oil Supplements Increase Peripheral Blood Specialized Pro-Resolving Mediators Concentrations and Reprogram Host Immune Responses: A Randomized Double-Blind Placebo-Controlled Study. Circ. Res. 2020, 126, 75–90. [Google Scholar] [CrossRef]
- Al-Shaer, A.E.; Regan, J.; Buddenbaum, N.; Tharwani, S.; Drawdy, C.; Behee, M.; Sergin, S.; Fenton, J.I.; Maddipati, K.R.; Kane, S.; et al. Enriched Marine Oil Supplement Increases Specific Plasma Specialized Pro-Resolving Mediators in Adults with Obesity. J. Nutr. 2022, 152, 1783–1791. [Google Scholar] [CrossRef]
- Schlegel, M.; Körner, A.; Kaussen, T.; Knausberg, U.; Gerber, C.; Hansmann, G.; Jónasdóttir, H.S.; Giera, M.; Mirakaj, V. Inhibition of neogenin fosters resolution of inflammation and tissue regeneration. J. Clin. Investig. 2018, 128, 4711–4726. [Google Scholar] [CrossRef]
- Rao, Z.; Pace, S.; Jordan, P.M.; Bilancia, R.; Troisi, F.; Börner, F.; Andreas, N.; Kamradt, T.; Menche, D.; Rossi, A.; et al. Vacuolar (H(+))-ATPase Critically Regulates Specialized Proresolving Mediator Pathways in Human M2-like Monocyte-Derived Macrophages and Has a Crucial Role in Resolution of Inflammation. J. Immunol. 2019, 203, 1031–1043. [Google Scholar] [CrossRef]
- Chiang, N.; Shinohara, M.; Dalli, J.; Mirakaj, V.; Kibi, M.; Choi, A.M.; Serhan, C.N. Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits. J. Immunol. 2013, 190, 6378–6388. [Google Scholar] [CrossRef]
- Li, Y.; Dalli, J.; Chiang, N.; Baron, R.M.; Quintana, C.; Serhan, C.N. Plasticity of leukocytic exudates in resolving acute inflammation is regulated by MicroRNA and proresolving mediators. Immunity 2013, 39, 885–898. [Google Scholar] [CrossRef]
- Norris, P.C.; Libreros, S.; Serhan, C.N. Resolution metabolomes activated by hypoxic environment. Sci. Adv. 2019, 5, eaax4895. [Google Scholar] [CrossRef]
- Tojo, T.; Tsuruoka, M.; Kondo, T.; Yuasa, M. Evaluation of Cancer Cell Growth Suppressibility of ω-3 Fatty Acids and Their Metabolites. J. Oleo Sci. 2022, 71, 1253–1260. [Google Scholar] [CrossRef]

| 1.1 Oral, Dental, and Craniofacial System | ||||
|---|---|---|---|---|
| Preclinical Models | Administration of Maresins | (Optimal) Concentration | Efficacy | References |
| Patients with localized aggressive periodontitis (Stage IV Grade C molar-incisal pattern periodontitis) | MaR1, in vitro incubation, patient-derived neutrophils, and macrophages | 1 nM | 1. Enhanced phagocytosis (31 to 65% increase), restored bactericidal capacity (22 to 38% reduction in bacterial titers) and increased intracellular ROS generation (26 to 71% increase). | (Wang, C.W., 2015) [12] |
| Healthy human PDL cells, under the stimulation of P. gingivalis LPS | MaR1, in vitro incubation | 10 nM | 1. Increased healthy human PDL cell survival rate and autophagy, decreased its apoptosis and production (about 50% reduction) of inflammatory factors (IL-6, IL-8, TNF-α, and IL-1β). | (Du, L., 2018) [13] |
| Healthy human PDL cells, under the stimulation of IL-1β and TNF-α | MaR1 and/or RvE1, in vitro incubation | 10 nM | 1. Increased the expression of pluripotency, migration, viability, and differentiation of hPDLSCs into PDL-like cell phenotype (IL-1β + TNF-α + MaR1 + RvE1 group compared to IL-1β + TNF-α group: α-SMA increased 53%, tenomodulin increased 2.23-fold, periostin increased 76%). | (Albuquerque-Souza, E., 2020) [14] |
| Sprague Dawley rats, tooth extraction | MaR1, gelatin sponges as carriers to put into the sockets, and followed by topical use (twice a week) | 0.5 μg/μL, 0.05 μg/μL | 1. Accelerated wound closure (>33% increase), increased extraction socket bone fill (16% increase), preserved alveolar bone ridge (49% increase in both width and height) regulated M2-like macrophage surface marker CD206 (relative ratio decreased 0.26), and reduced post-operative pain scales. | (Wang, C.W., 2020) [15] |
| 1.2 Cardiovascular system | ||||
| Preclinical models | Administration of maresins | (Optimal) concentration | Efficacy | References |
| Endotoxemia (LPS), C57BL/6 mice | MCTR1, i.p. | 0.15 or 0.3 nmol/mouse (no significant difference) | 1. Improved mitochondrial biogenesis and function (MCTR1 restored the expression of Sirt-1 total and nuclear protein, ~80% increased and ~40%, respectively). | (Yang, Y., 2020) [16] |
| MCTR1, i.v. | 0.15 nmol/mouse | 1. Alleviates neutrophil infiltration (percentage of CD11b+Ly6G+ neutrophil population in the hearts reduced ~50%). | (Yang, Y., 2021) [17] | |
| C57BL/6 mice, under the stimulation of LPS | MaR1(pretreatment), i.p., followed by booster injections | 100 ng and booster injections: 100 ng every 2 days/mouse | 1. Protects the heart from injury and dysfunction (expression of cardiac injury marker lactate dehydrogenase (LDH) decreased ~20%, kinase isoenzyme (CK-MB) decreased ~53%). | (Li, D., 2021) [18] |
| Sepsis model (cecal ligation and puncture (CLP)), BALB/c mice | MaR1, i.v. | 1.1 ng | 1. Protecting mice receiving cecal ligation and puncture (CLP) from lung injury (histopathological scores reduced ~37.5%). 2. Improving liver and kidney function (level of alanine transaminase (ALT), aspartate transaminase (AST), creatinine (Cre), and blood urea nitrogen (BUN) in serum decreased ~20–50%). 3. Inhibiting production of inflammatory cytokines (IL-6, TNF-α, IL-1β decreased ~30–50%), growth of bacteria colonies, and activation of nuclear factor kappa B (NF-κb) pathway. | (Li, R., 2016) [19] |
| CLP, male C57B6/L mice | MaR1, i.p. | 10 nM | 1. Reduced the release of inflammatory cytokines in the plasma of sepsis mice (TNF-α, IL-1β, MPO, MIP-2, and IL-10 decreased ~20–30%). 2. Enhanced bacterial clearance and modulated immune cells (CFU decreased ~40%, macrophages increased ~40%, neutrophils decreased ~40%). 3. Attenuated mitochondrial dysfunction by regulating ROS associated with ALX and cAMP (ALX antagonist BOC-2 reversed the effects of MaR1). | (Gu, J., 2018) [20] |
| SuHx (Sugen 5416 injection and hypoxia exposure)-induced PAH model, male C57BL/6 mice | MaR1, i.p., followed by booster injections | 1 μg/mouse and booster injections: 100 ng every 2 days/mouse | 1. Lessening right ventricular systolic pressure (RVSP), attenuating right ventricular dysfunction (RVD), reversing abnormal changes in pulmonary vascular remodeling, and inhibiting abnormal pulmonary artery smooth muscle cells’ (PASMCs’) proliferation (enhanced apoptosis of α-SMA positive cells, decreased phosphorylation of STAT3, AKT, ERK, and FoxO1 via LGR6). | (Li, H., 2022) [21] |
| Fed with high-fat diet (HFD), Apoe−/− C57Bl/6 mice. | MaR1 and RvD2, i.p., (additional 4 weeks of HFD feeding) | MaR1: 100 ng, RvD2: 100 ng, every second day | 1. Preventing atheroprogression (decreased in necrotic core size ~30%, mac2 positive cells/plaque ~35%, vulnerability plaque index decreased ~47%; increased in total collagen ~33%, fibrous cap thickness ~20%, SMCα positive cells/plaque ~55%). 2. MaR1 and RvD2 induced a pro-resolving macrophage phenotype (increased CD206 positive macrophage ~4-fold, fold change in aortic RNA Retnla increased ~70% and Nos2 decreased ~40%, decrease in TNF-α ~40% and IL-6 ~50%, increase in TGF -β~60%). | (Viola, J.R., 2016) [22] |
| Topical elastase induced abdominal aortic aneurysm (AAA) model, C57BL/6 wild-type (WT) mice, and TGF-β2 receptor knockout (SMC-TGFβr2−/−) mice | MaR1, i.p. | 4 ng/g or 40 ng/g bodyweight | 1. Reducing abdominal aortic aneurysm (AAA) growth of smooth muscle cells mediated by LGR6 receptors (decreased ~28% in aortic diameter, expression of TGF-β2 increased ~3.1-fold, MMP2 decreased ~48%, smooth muscle alpha actin (SM-αA) increased ~34%, efferocytosis of SMC increased ~2.69-fold at 7 d/~1.53-fold at 14 d). | (Elder, C.T., 2021) [23] |
| Myocardial infarction (MI) model by left anterior descending (LAD) coronary artery ligation, male C57BL/6 mice | MaR1, i.p. | 10 ng/g every 2 days for 28 days | 1. Improving cardiac function (increased LVEF ~1.73 fold and FS ~4.8 fold, and decreased left ventricular end-diastolic volume (LVEDV) ~59%, left ventricular end-systolic volume (LVESV) ~71%, left ventricular inner dimension at end-diastolic stage (LVIDd) ~29%, and left ventricular inner dimension at end-systolic stage (LVIDs) ~37%), attenuating ventricular structural remodeling (remaining 52% of myocardial fibrosis area), decreasing ventricular electrical remodeling (decreased action potential duration (APD)90 ~37%, APD50 ~37%, electrical alternans (ALT) threshold ~25%, suppressed the decrease in effective refractory period (ERP)) and myocardial apoptosis. 2. Alleviating cardiac oxidative stress after MI by activation of NRF2/HO-1 signaling (reduced the expression of fibrotic markers and malondialdehyde (MDA) level ~17%, increased Cx43 expression ~2.5 fold and serum superoxide dismutase (SOD) level ~1.6 fold) and inhibition of TLR4/NF-kB signaling (decreased TLR4, p-p65, TNFα, and IL-6 protein levels ~30–50%, and macrophage infiltration ~36%). | (Wang, F., 2022) [24] |
| 1.3 Digestive system | ||||
| Preclinical models | Administration of maresins | (Optimal) concentration | Efficacy | References |
| Diet-induced obese (DIO) mice | MaR1, oral gavage | 50 μg/kg | 1. Relative abundance of P. xylanivorans increased. 2. IL-1β and TNF-α decreased. | (León, I.C., 2020) [25] |
| Dextran sulfate sodium (DSS) and 2,4,6-trinitrobenzene sulfonic acid-induced colitis mice | MaR1, e.v. | acute protocol: 0.1, 0.3, and 1 μg/animal chronic protocol: 0.3 μg/animal | 1. The disease activity index improved. 2. Body weight and colonic tissue damage reduced. | (Marcon, R., 2013) [26] |
| BALB/c mice | MaR1, i.p. | 0.03, 0.3, and 1 μg/animal | 1. MaR1 showed antioxidative and anti-inflammatory effects, attenuating hepatic injury, oxidative stress, and lipid peroxidation. | (Li, R., 2016) [27] |
| Diethylnitrosamine (DEN)-induced liver fibrosis rat | MaR1, i.p. | 4 ng/g | 1. The aspartate transaminase (AST) concentration decreased by about 46%. 2. The alanine transaminase (ALT) concentration decreased by about 44%. 3. The hepatic index decreased by about 12%. 1. The pro-inflammatory cytokines TNF-α and IL-1β were increased by 6- and 5.5-fold, respectively, in relation to the control group, and 4.8- and 2.4-fold, respectively, in relation to the MaR1 + DEN group. 2. The anti-inflammatory IL-10 of the MaR1 + DEN group was 3.5- and 3.4-fold, respectively, compared to the control and MaR1 group, and 5.5-fold compared to the DEN group. | (Rodríguez, M.J., 2021) [28] |
| Diet-induced obese (DIO) mice | MaR1, i.p./oral gavage | 2 μg/kg (i.p.) or 50 μg/kg (oral gavage) | 1. MaR1 decreased lipogenic enzymes and liver triglycerides content. | (Laiglesia, L.M., 2018) [29] |
| High-fat diet-induced hepatic steatosis mice | MaR1, i.p. | 35 μg/kg | 1. MaR1 ameliorated obesity-related liver steatosis by suppressing ER stress. | (Jung, T.W., 2018) [30] |
| High-fat diet-induced non-alcoholic steatohepatitis (NASH) | MaR1, i.p. | 5 μg/kg | 1. MaR1 enhanced the retinoic acid-related orphan receptor α (RORα) ability to activate the M2 polarity of liver macrophages, protecting the liver from NASH. | (Han, Y.H., 2019) [31] |
| Liver ischemia-reperfusion injury mice | MaR1, i.p. | 4 ng/g | 1. MAI (mitotic index) activity of hepatocytes was characterized by an intense cell division with 3.7- and 5.25-fold increases in the MaR1-sham and MaR1-IR groups, respectively. MaR1-IR showed an increase of 41% in cell division related to MaR1-sham livers. 2. IL-6 was increased 1.4 times in the MaR1-IR group compared to IR groups. Serum IL-6 was elevated 2.1 times in MaR1-sham with respect to the control and was 0.2 and 6 times less than the IR and MaR1-IR groups, respectively. 3. The increase in nuclear Nrf2 of the MaR1-IR group was more than 7-fold compared to the control. | (Soto, G., 2020) [32] |
| Lipopolysaccharide/d-galactosamine (LPS/D-GalN)-induced acute liver injury mice | MaR1, i.p. | 50, 100 ng | 1. MaR1 attenuated acute liver injury by ameliorating inflammation. | (Yang, W., 2022) [33] |
| Caerulein-induced pancreatitis mice | MaR1, i.p. | 0.1, 0.5, 1 μg | 1. MaR1 decreased serum levels of amylase, lipase, and inflammatory cytokines such as TNF-α, IL-1β, and IL-6. | (Lv, C., 2019) [34] |
| Cerulean-induced pancreatitis | MaR1, i.p. | 1.0 ng | 1. MaR1 alleviated inflammation of the pancreas and lungs by inhibiting the activity of NF-κB. | (Munir, F., 2019) [35] |
| 1.4 Endocrine system | ||||
| Preclinical models | Administration of maresins | (Optimal) concentration | Efficacy | References |
| High-fat diet-induced obese C57BL/6J mice | MaR1, i.p. | 2 μg/kg, 10 days | 1. Reduced subcutaneous depot weight by ~18%, serum white adipose tissue (WAT)-secreted lectin by ~21%, and fasting glucose by ~13%. | (Martínez-Fernández, L., 2017) [36] |
| Leptin-deficient ob/ob mice | 2 μg/kg, 20 days | 1. Reduced ~15% basal glucose in insulin tolerance tests (ITT). 2. Increased Glut-4 expression. 3. Reduced Dpp-4 expression. | ||
| High-fat diet-induced obese C57BL/6J mice | MaR1, oral gavage | 50 μg/kg, 10 days | 1. Reversed ~50% diet-induced increase in fasting glycemia. 2. Reduced ITT glucose levels by ~33%. 3. Partially restored muscle insulin-induced Akt phosphorylation. | (Martinez-Fernandez, L., 2021) [37] |
| Lean C57BL/6J mice | MaR1, i.p. | 50 μg/kg, 3 h | 1. Improved Akt phosphorylation in skeletal muscle and epididymal WAT. | |
| High-fat diet-induced obese C57BL/6J mice | MaR1, oral gavage | 50 µg/kg, 10 days | 1. Reversed high-fat diet-induced modulation of FGF-21 expression. | (Martinez-Fernandez, L., 2019) [38] |
| High-fat diet-induced obese C57BL/6J mice | MaR2, i.p. | 5 μg/kg, 28 days; 10 μg/kg, 26 days | 1. Downregulated plasma TNF-α levels and liver pro-inflammatory gene expression. 2. Liver weight, triglyceride levels, lipogenic gene expression, steatosis, and ALT, were not altered in 10 μg/kg (26 days) treatment. | (Sugimoto, S., 2022) [39] |
| 1.5 Immune system | ||||
| Preclinical models | Administration of maresins | (Optimal) concentration | Efficacy | References |
| Escherichia coli (E. coli)-induced peritonitis, FVB mice | A panel of MCTR3, PCTR3, RCTR3 (non-target siRNA-injected), i.p. | 50 ng each | 1. Reduced PMN numbers in the exudate by ~70% and TNF-α protein level by ~50%. | (Chiang, N., 2021) [40] |
| Regenerative model, planaria | MCTR3, suspended in water | 10 nM | 1. Planaria regeneration index increased by ~50% (CTR/ctrl). | |
| Peripheral blood mononuclear cell (PBMC)-derived human macrophages | MCTR3, suspended in PBS+/+ | 10 nM | 1. Enhanced phagocytosis of E. coli by ~50% at 60 min. | |
| PBMC-derived human macrophages | MaR1, suspended in PBS+/+ | 0.01 nM | 1. Resulted in ~90% E. coli phagocytosis. | (Colas, R.A., 2016) [41] |
| 22-OH-MaR1(1 pM) and 14-oxo-MaR1(1 pM) | 1. Resulted in ~75% and ~25% E. coli phagocytosis, respectively. | |||
| Primary human monocytes, under the stimulation of LPS and engagement of TLR4 | MaR1, suspended in RPMI | 1.0–3.0 μM | 1. Reduced ~50% release of TNF, IL-8, IL-1β, IL-12 p40. | (Gu, Z., 2016) [42] |
| 1 μM | 1. Doubled IL-10 expression. | |||
| PBMC-derived macrophages, under Mycobacterium tuberculosis infection | MaR1, suspended in RPMI | 150 nM | 1. Lowered intracellular bacterial burden by ~36% and TNF-α by more than 80%. 2. Increased bactericidal/permeability-increasing protein (BPI) expression by 66.5%. | (Ruiz, A., 2019) [43] |
| Primary human peripheral blood mononuclear cells, under stimulation of LPS | MaR1, suspended in DMEM | optimal: 100 nM | 1. Reduced ~50% of TNF-α, IL-6, IL-1β mRNA and protein levels. | (Wang, W., 2021) [44] |
| Human PBMC-purified CD8+ and CD4+ T cells | MaR1, suspended in X-VIVO 15 mediums | 10 nM | 1. Downregulated cytokines. | (Chiurchiu, V., 2016) [45] |
| Human PBMC-purified anti-CD3/CD28-stimulated T cells | 1. Reduced IL-2 production ~50%. | |||
| Human PBMC-purified naïve CD4+ cells | 1. Reduced differentiation into Th1 or Th17 cells and favored differentiation into Treg cells. | |||
| 1.6 Integumentary/exocrine system | ||||
| Preclinical models | Administration of maresins | (Optimal) concentration | Efficacy | References |
| UVB-induced skin inflammation model, hairless (HRS/J) or LysM-eGFP C57BL/6 background mice | MaR1, i.p. | 10 ng/mouse, 10 min before UVB irradiation | 1. Reduced skin edema manifested by a decrease in skin weight by ~37%. 2. Decreased neutrophil recruitment, keratinocyte apoptosis, epidermal thickness, MMP-9 activity, and collagen degradation. | (Cezar, T.L.C., 2019) [46] |
| Psoriasis model (imiquimod or IL-23 administration), C57BL/6 mice | MaR1, topical | 100 ng in 20 μg ethanol/ear | 1. Ameliorated ear swelling by ~40–50%. 2. Reduced epithelial thickness by ~33–50%. 3. Decreased dermal edema and a number of CD45+ cells and Ly-6G+ cells. | (Saito-Sasaki, N., 2018) [47] |
| Primary human adipocytes, under the stimulation of TNF-α | MaR1 | 10 nM | 1. Reversed TNF-α-induced chemerin gene expression and protein secretion back to basal level. | (Sáinz, N., 2020) [48] |
| 1.7 Musculoskeletal system | ||||
| Preclinical models | Administration of maresins | (Optimal) concentration | Efficacy | References |
| Aged (24-month-old) mice underwent tibial fracture | MaR1, i.p. | 5 µg/kg | 1. MaR1 decreased the percentage of pro-inflammatory macrophages (~52%) and serum levels of inflammatory cytokines IL-6 (~64%), IL-10 (~52%), TNFα (~60%). 2. MaR1 treatment also increased the bone volume (BV) within the fracture callus (~38%) and the relative amount of bone within the fracture callus. The ratio of bone volume and total volume increased by about 40%. 3. Bone content was higher in MaR1-treated samples. It increased by about 28%. | (Huang, R., 2020) [49] |
| Collagen-induced arthritis mice | MaR1, i.p. | 0, 20, and 100 ng | 1. Intervention of MaR1 improved the imbalanced Treg/Th17 ratio. MaR1 increased Treg cell proportion while reducing Th17 cell proportion dose dependently. | (Jin, S., 2018) [50] |
| 1.8 Nervous system | ||||
| Preclinical models | Administration of maresins | (Optimal) concentration | Efficacy | References |
| Alzheimer’s disease model (bilateral hippocampal Aβ injection), C57BL/6 mice | MaR1, i.c.v. | 0.01 µg | 1. Improved mice performance in Morris Water Maze (MWM) by reducing escape latency by ~50% and increased numbers of platform crossing and time spent in the target quadrant by ~2-fold. | (Yin, P., 2019) [51] |
| Alzheimer’s disease model, AppNL-G-F/NL-G-F mice | SPM-combined solution (RvE1, RvD1, RvD2, MaR1, and NPD1), intranasal delivery | 40 ng per LM, three times a week for 9 weeks | 1. Reduced microgliosis and recovered 57% of gamma oscillation power. | (Emre, C., 2022) [52] |
| Perioperative neurocognitive disorder model (orthopedic surgery), C57BL/6 and Ccr2RFP/+Cx3cr1GFP/+ mice | MaR1, i.p. | 100 ng | 1. Reversed surgery-reduced freezing time by ~20% in contextual fear conditioning. | (Yang, T., 2019) [53] |
| Neonatal Sprague Dawley rats, under exposure to sevoflurane | MaR1, i.p. | 10 nM, 3 days | 1. Improved MWM performances by reducing escape latency by ~75% and increasing platform crossing times, swimming distances, and staying time in the target zone, all by ~60%. | (Wu, Y., 2022) [54] |
| Chronic cerebral hypoperfusion model (2-vessel occlusion), Sprague-Dawley rats | MaR1, i.t. | 0.05 μg | 1. Decreased escape latency in MWM by ~50% maximally. 2. Alleviated blood–brain barrier (BBB) damage. | (Li, T., 2022) [55] |
| Brain ischemia/reperfusion injury model (middle cerebral artery occlusion), C57BL/6 mice | MaR1, intracerebroventricular (i.c.v.) injection | 1 ng | 1. Decreased ~40% of original infarct volume and reduced ~4% of brain water content in ischemic ipsilateral hemispheres. 2. Lowered neurological severity scores by ~50% and ~66% at 48 h and 72 h, respectively, after perfusion. | (Xian, W., 2016) [56] |
| Experimental autoimmune encephalomyelitis model, C57BL/6 mice | MaR1, i.p. | 1 μg, 21 days | 1. Lowered average EAE scores by ~40%. 2. Prevented ~72% area of myelin loss. | (Sánchez-Fernández, A., 2022) [57] |
| Spinal cord injury model, C57BL/6 mice | MaR1, i.v. | 1 μg, 7 days | 1. Raised Basso Mouse Scale scores by ~30%. 2. Reduced gait symmetry scores by ~26% and stance/width stepping variability scores by ~50%. 3. Elevated myelinated axons by ~20%. | (Francos-Quijorna, I., 2017) [58] |
| Spinal muscular atrophy model, colony bred from a pair of heterozygous SMNΔ7 mice | MaR1, i.p. | 1 mg/kg, 11–13 days | 1. Decreased righting reflex latency by ~66% and negative geotaxis test latency by ~50%. | (Ohuchi, K., 2018) [59] |
| Acute (carrageenan-induced) and chronic (complete Freund’s adjuvant (CFA)-induced) inflammatory pain model, Swiss, and LysM-eGFP mice | MaR1, i.t. | optimal: 10 ng | 1. Alleviated acute and chronic inflammatory pain in mice by reducing the difference in withdrawal thresholds between baseline (at zero-time) and after 1–5 h carrageenan stimulation under stimulation of mechanical allodynia (by max:~2 g) and thermal hyperalgesia (by max:~5 s) | (Fattori, V., 2019) [60] |
| CFA-induced overt pain model, Swiss, and LysM-eGFP mice | MaR1, i.t. | 10 ng | 1. Reduced flinches and time spent licking the paw by ~50%. | |
| LPS-induced mechanical and thermal hyperalgesia models, Swiss mice | MaR2, i.p. | optimal: 30 ng | 1. Lowered withdrawal by ~66% maximally in von Frey tests. 2. Increased latency by ~20–50% in hot plate tests. 3. Increased injured/non-injured paw weight ratio by ~25%. | (Fattori, V., 2022) [61] |
| Neuropathic pain model (spinal nerve ligation), Sprague Dawley rats | MaR1, i.t. | 100 ng/10µL | 1. Raised ipsilateral mechanical withdrawal threshold by ~50% and thermal paw withdrawal latency by ~50%. | (Gao, J., 2018) [62] |
| Radicular pain model (non-compressive lumbar disc herniation), Sprague Dawley rats | MaR1, i.t. | optimal: 100 ng | 1. Attenuated neuropathic pain 2. Reversed ~50% of mechanical stimulus-induced reduction in paw withdrawal threshold. 3. Reversed ~75% of thermal stimulus-induced paw withdrawal latency. | (Wang, Y.H., 2020) [63,64] |
| Peripheral nerve injury model (sciatic nerve crush), ICR mice | MaR1 was applied onto damaged nerves using a hemostatic gelatin sponge | 500 ng | 1. Reduced gastrocnemius atrophy by preventing ~20% loss of gastrocnemius muscle weight ratio (ipsilateral/contralateral). 2. Promoted functional recovery more effectively than nerve growth factor in rotarod, von Frey, and Hargreaves tests. | (Wei, J., 2022) [65] |
| MaR1, i.t. | optimal: 100 ng | 1. Mitigated neuropathic pain. 2. Reversed ~66% of mechanical allodynia-induced reduction in paw withdrawal threshold. 3. Reversed ~80% of thermal hyperalgesia-induced paw withdrawal latency. | ||
| MaR1, intraplantar injection | 50 ng | 1. As compared to the nerve growth factor, MaR1 did not lower the pain thresholds. | ||
| Fracture-associated post-operative pain model (tibial fracture and surgery), CD1 mice | MaR1 as peri- (500 ng, i.v.) and post-operative treatments (500 ng, i.t.) | 1. Inhibited fracture-associated post-operative pain. 2. Reversed ~55% of mechanical allodynia-induced reduction in paw withdrawal threshold. 3. Reversed ~60% of mechanical allodynia-induced increase in paw withdrawal frequency. 4. Reversed ~82% of cold allodynia-induced increase in cold score. | (Zhang, L., 2018) [66] | |
| Persistent allodynia dissociated from clinical arthritis signs (K/BxN serum-transfer model), C57BL/6 mice | MaR1, i.p. | 100 ng, repeated injections | 1. Induced amelioration of pain with later onset and longer duration. 2. Reversed ~66% of mechanical hypersensitivity-induced reduction in paw withdrawal threshold. | (Allen, B.L., 2020) [67] |
| 1.9 Reproductive system | ||||
| Preclinical models | Administration of aresins | (Optimal) concentration | Efficacy | References |
| Peritonitis mice | MaR1, i.p. | 50 ng | 1. Human milk shortened the resolution interval in mouse peritonitis, and the magnitude of PMN infiltration of MaR1 is 76% and 58%. | (Arnardottir, H., 2016) [68] |
| Vulvar pain mice | MaR1, i.p. | 1 μg/day, 4 weeks | 1. MaR1 decreased sensitivity by increasing the pain threshold and suppressed PGE2 levels. | (Falsetta, M.L., 2021) [69] |
| 1.10 Respiratory system | ||||
| Preclinical models | Administration of maresins | (Optimal) concentration | Efficacy | References |
| Human and mice precision-cut lung slices | MCTRs, i.v. | 10 ng | 1. MCTRs promote the resolution of allergic lung inflammation. 2. MCTRs blocked airway contraction in human precision-cut lung slices. | (Levy, B.D., 2020) [70] |
| BALB/c mice | MaR1, i.p. | 0.1, 1, 10 ng | 1. MaR1 suppressed the activation of the NF-κB signaling pathway, thus reducing COX-2 and ICAM-1, preventing inflammatory cell infiltration in the bronchoalveolar lavage fluid and excessive mucus production. | (Ou, G., 2021) [71] |
| Lipopolysaccharide (LPS)-induced acute lung injury mice | MCTR3 | 2 ng/g | 1. Maresin inhibited cell death, inflammatory cytokine levels, and oxidative stress through the inactivation of the ALX/PINK1-mediated mitophagy pathway, protecting against LPS-induced ALI. | (Zhuang, R., 2020) [72] |
| Lipopolysaccharide (LPS)-induced acute lung injury mice | MCTR1, i.v. | 200 ng | 1. Alveolar fluid clearance (AFC) rate increased by about 86% in the MCTR1 + LPS group compared to the LPS group. 2. MCTR1 significantly promoted AFC by upregulating epithelial sodium channel and Na+-K+-adenosine triphosphatase expression in vivo. | (Han, J., 2020) [73] |
| mice under the stimulation of inhalant dust exposure | MaR1, i.p. | 0.1, 1 ng | 1. MaR1 significantly decreased bronchoalveolar lavage neutrophil infiltration and intracellular adhesion molecule-1(ICAM-1) expression. | (Nordgren, T.M., 2015) [74] |
| Bleomycin-induced lung fibrosis mice | MCTR1, i.p. | 1 μg, 100 ng, 10 ng | 1. MCTR1 protected tissue from destroyed and enhanced survival rate best at the dose of 1 μg. | (Pan, J., 2021) [75] |
| 1.11 Urinary system | ||||
| Preclinical models | Administration of maresins | (Optimal) concentration | Efficacy | References |
| Cyclophosphamide (CP)-induced bladder inflammation mice | MaR1, i.p. | 25 μg/kg | 1. MaR1 promoted epithelial wound/barrier repair and reduced bladder inflammation and bladder weight. 2. The percentage of scratch closure of the MaR1 group is twice that of the control group. | (Hughes, F.M., 2022) [76] |
| Diabetic kidney disease (DKD) induced by high-fat diet mice | MaR1, i.p. | 4 μg/kg | 1. MaR1 alleviated DKD and glucotoxicity-induced inflammation via LGR6-mediated cAMP-SOD2 antioxidant pathway. | (Li, X., 2022) [77] |
| Sepsis mice model prepared by the CLP (cecal ligation and puncture group) method | MaR1, i.p. | 0.5, 1 ng | 1. The 7-day survival rates of the control group, the cecal ligation and puncture (CLP) group, the MaR1 low dose (LD-MaR1, 0.5 ng) group, and the MaR1 high dose (HD-MaR1, 1 ng) group were 100%, 16.67%, 58.33%, and 75%, respectively. 2. Research showed that MaR1 significantly increased the 7-day survival rate of septic mice and the anti-inflammatory factor while reducing bacterial load and pro-inflammatory cytokines. | (Sun, S., 2019) [78] |
| Sepsis-associated acute kidney injury (SA-AKI) induced by CLP mice model | MCTR1, i.v. | 200 ng | 1. MCTR1 inhibited ferroptosis and elevated the expression of Nrf2. 2. The percent survival of the CLP group is about 50% of the MCTR + CLP group. | (Xiao, J., 2021) [79] |
| Mice model underwent ischemia of the left kidney for 45 min and nephrectomy of the right kidney | MaR1, i.p. | 1.0 ng | 1. The histologic score of the IRI + MaR1 group is about 66% that of the IRI group. 2. MaR1 remarkably mitigated renal IRI-induced inflammation and oxidative stress. | (Qiu, Y., 2019) [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-C.; Yang, Y.-H.; Wang, Y.-C.; Chang, W.-M.; Wang, C.-W. Maresin: Macrophage Mediator for Resolving Inflammation and Bridging Tissue Regeneration—A System-Based Preclinical Systematic Review. Int. J. Mol. Sci. 2023, 24, 11012. https://doi.org/10.3390/ijms241311012
Liu W-C, Yang Y-H, Wang Y-C, Chang W-M, Wang C-W. Maresin: Macrophage Mediator for Resolving Inflammation and Bridging Tissue Regeneration—A System-Based Preclinical Systematic Review. International Journal of Molecular Sciences. 2023; 24(13):11012. https://doi.org/10.3390/ijms241311012
Chicago/Turabian StyleLiu, Wen-Chun, Yu-Hsin Yang, Yu-Chin Wang, Wei-Ming Chang, and Chin-Wei Wang. 2023. "Maresin: Macrophage Mediator for Resolving Inflammation and Bridging Tissue Regeneration—A System-Based Preclinical Systematic Review" International Journal of Molecular Sciences 24, no. 13: 11012. https://doi.org/10.3390/ijms241311012
APA StyleLiu, W.-C., Yang, Y.-H., Wang, Y.-C., Chang, W.-M., & Wang, C.-W. (2023). Maresin: Macrophage Mediator for Resolving Inflammation and Bridging Tissue Regeneration—A System-Based Preclinical Systematic Review. International Journal of Molecular Sciences, 24(13), 11012. https://doi.org/10.3390/ijms241311012





