The Carbonic Anhydrase βCA1 Functions in PopW-Mediated Plant Defense Responses in Tomato
Abstract
:1. Introduction
2. Results
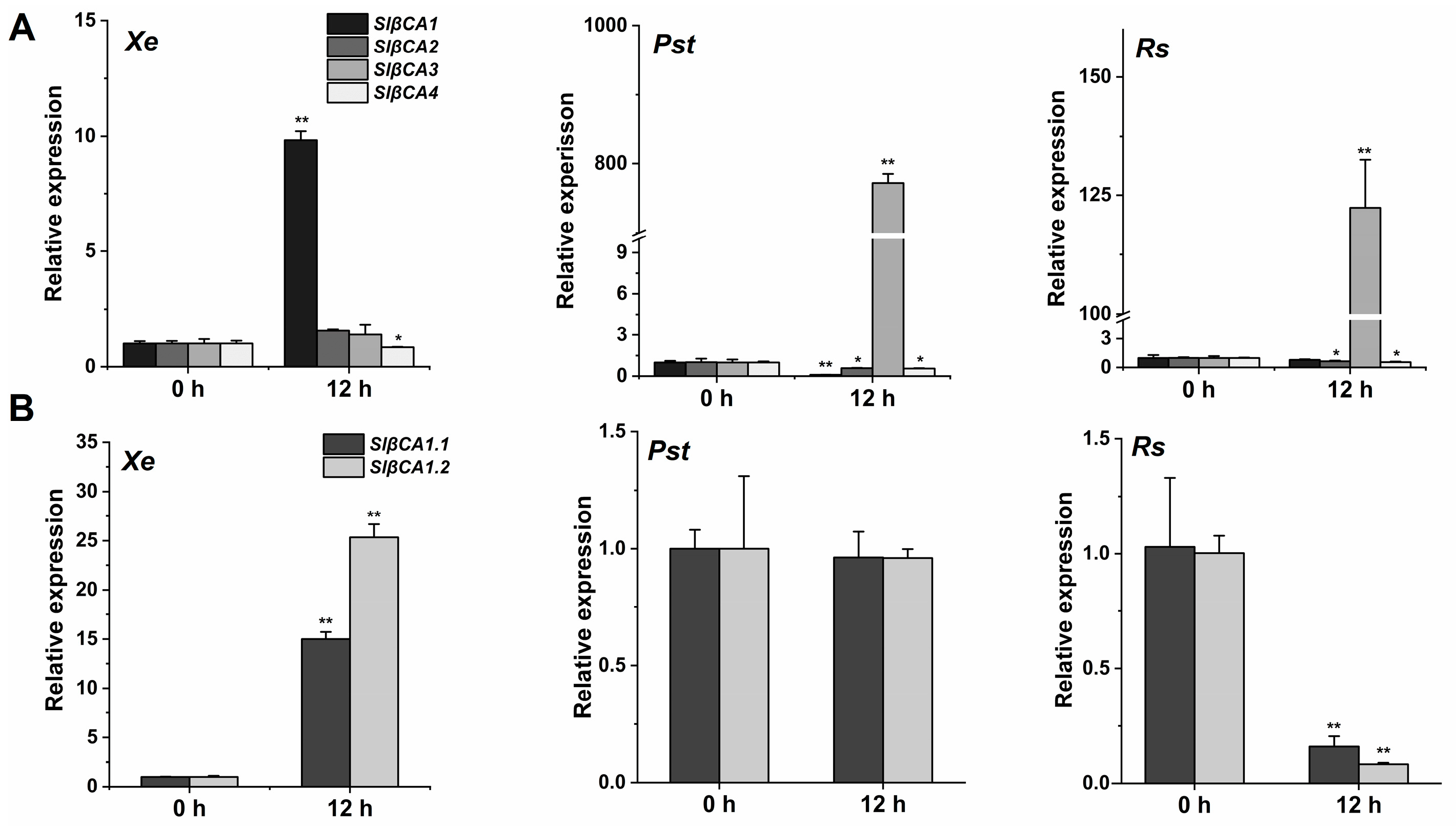
2.1. SlβCA1 Expression Was Induced by Pathogenic Bacteria
2.2. Subcellular Localization and Expression Pattern of SlβCA1
2.3. SlβCA1 Is Involved in the Positive Regulation of Immunity against Xe 85-10
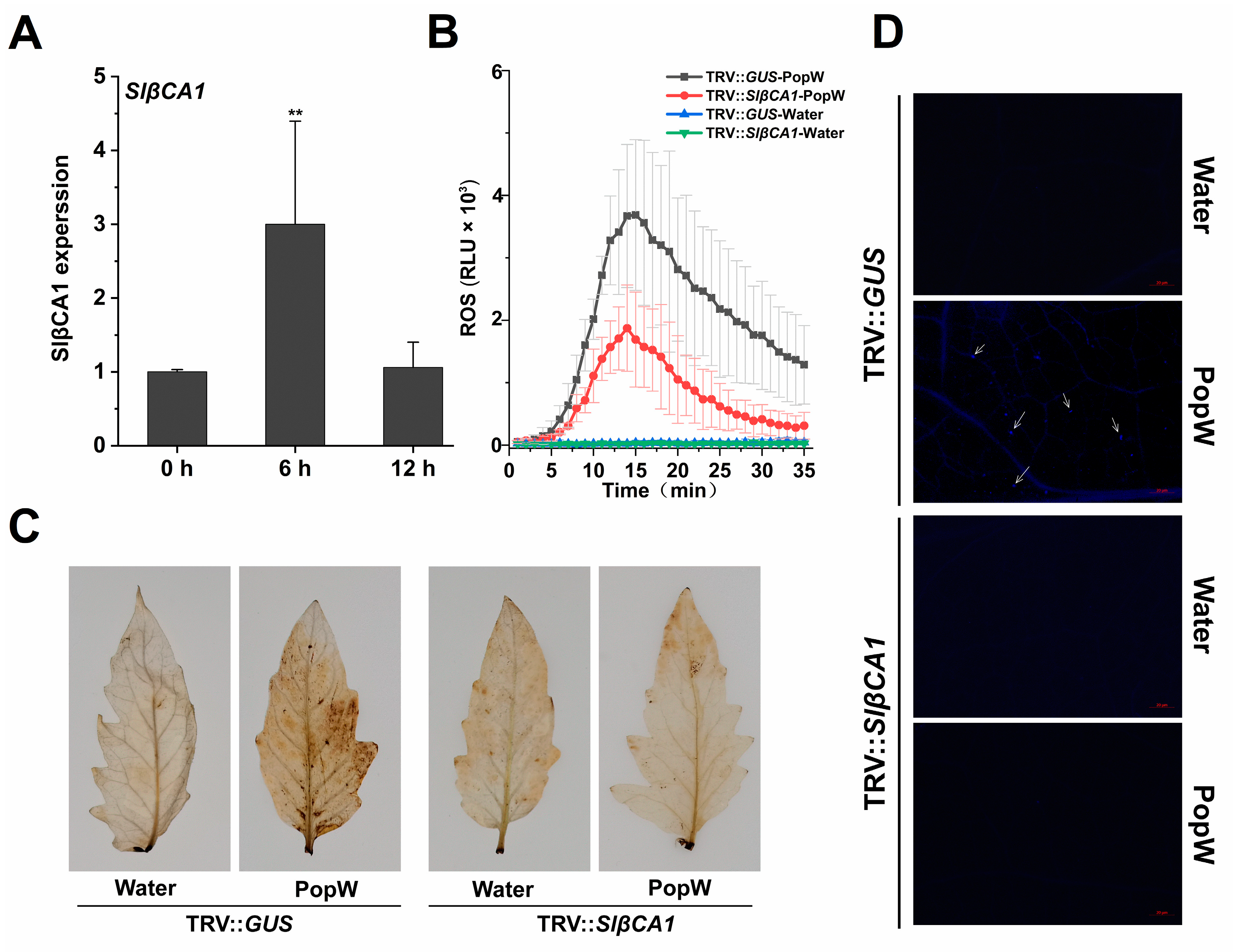
2.4. SlβCA1 Expression Was Induced by MAMP
2.5. Direct Physical Interaction of SlβCA1 with PopW
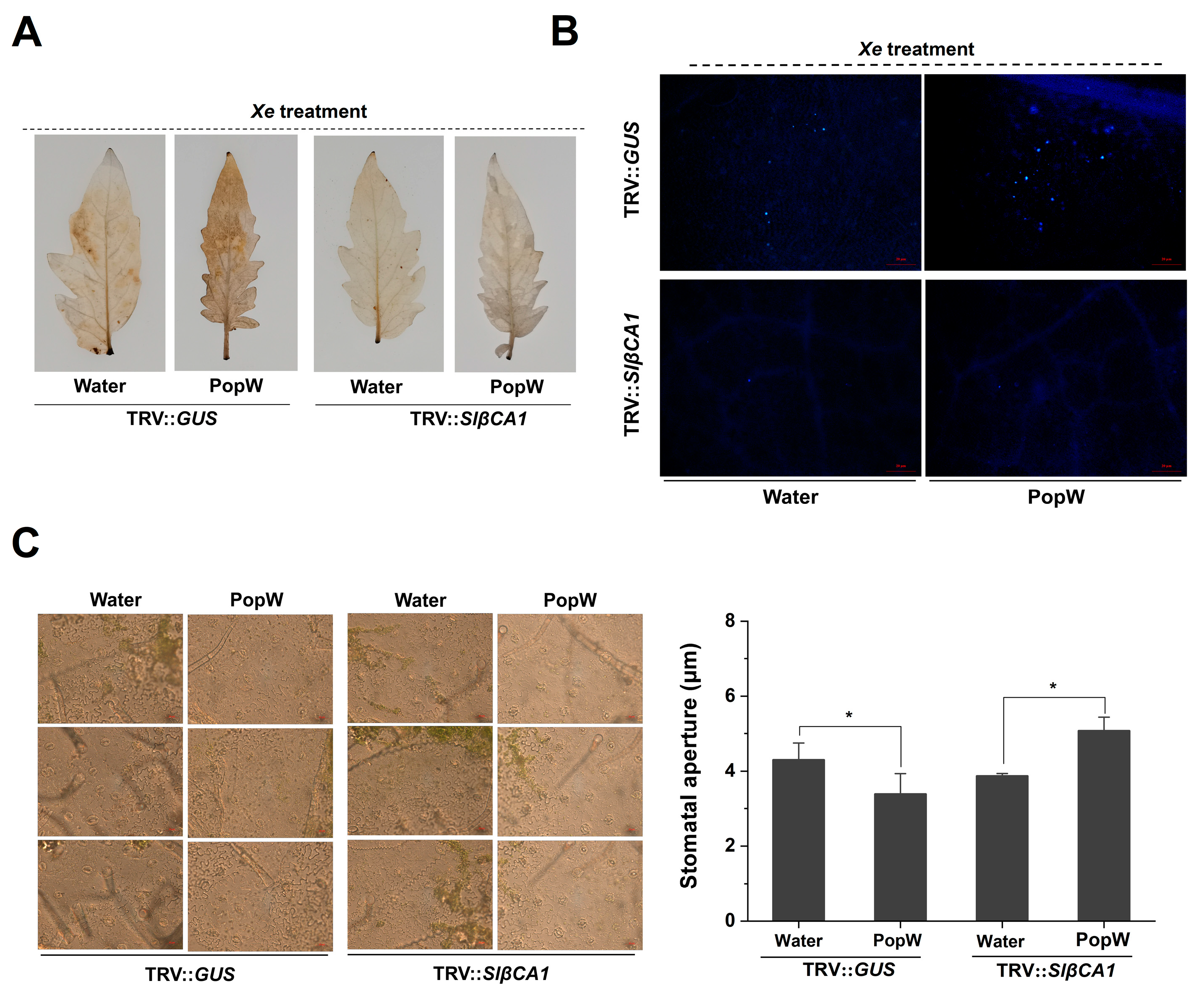
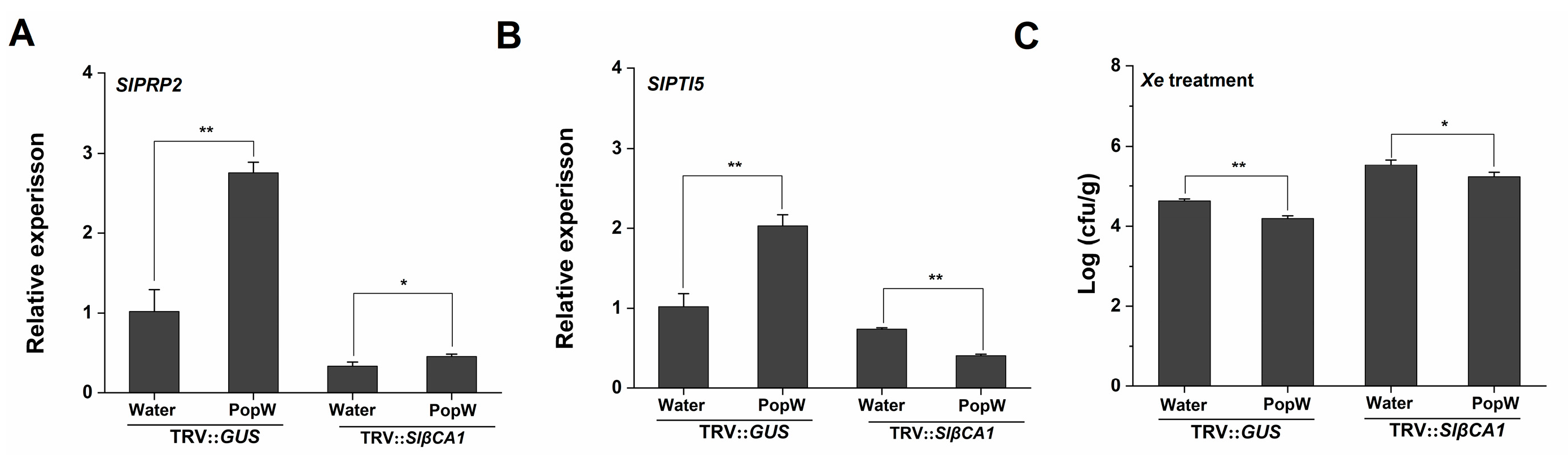
2.6. SlβCA1 Is Involved in PopW-Induced Immunity to Xe 85-10
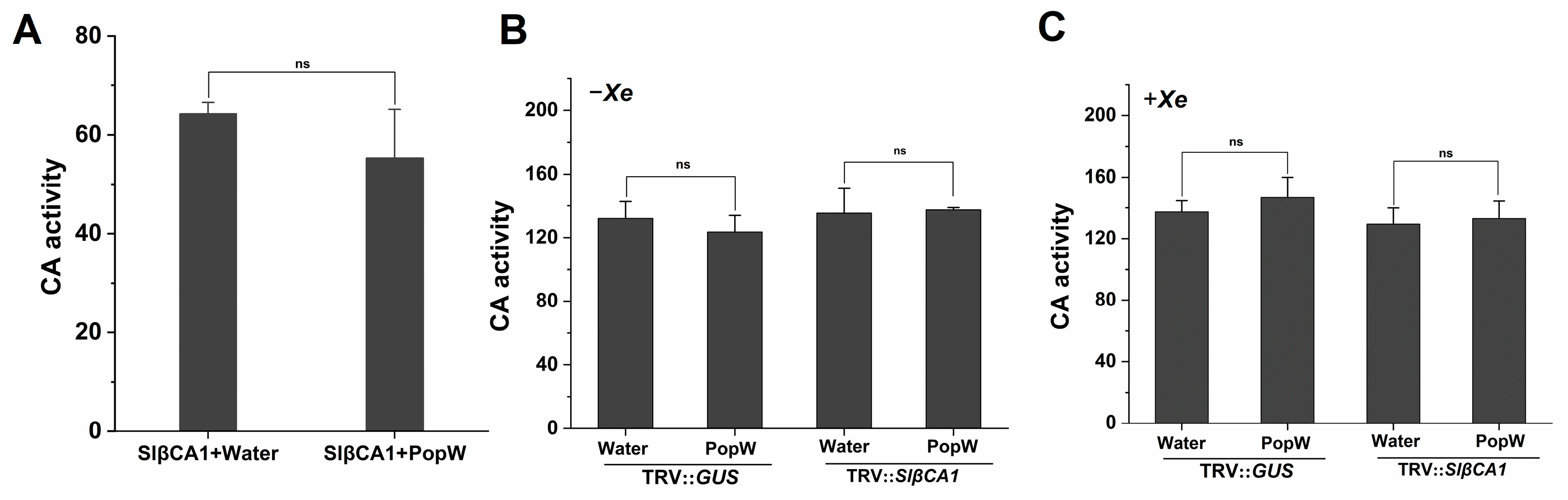
2.7. The Involvement of SlβCA1 in Immunity Is Not Related to CA Activity
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. PopW and Pathogen Acquisition and Disease Symptom Assay
4.3. Amino Acid Sequence Alignment and Tree Construction
4.4. Gene Expression Analysis by qRT-PCR
4.5. Detection of the MAMP Response
4.5.1. Measurement of ROS Production
4.5.2. Detection of Callogens
4.6. Protein Interaction Analyses
4.7. Detection of SlβCA1 Protein Localization
4.8. Determination of CA Activity
4.9. Measurement of Porosity
4.10. Statistical Analysis of the Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kourelis, J.; Marchal, C.; Posbeyikian, A.; Harant, A.; Kamoun, S. NLR immune receptor-nanobody fusions confer plant disease resistance. Science 2023, 379, 934–939. [Google Scholar] [CrossRef]
- Jones, J.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveed, Z.A.; Wei, X.; Chen, J.; Mubeen, H.; Ali, G.S. The PTI to ETI Continuum in Phytophthora-Plant Interactions. Front. Plant Sci. 2020, 11, 593905. [Google Scholar] [CrossRef] [PubMed]
- DiMario, R.J.; Quebedeaux, J.C.; Longstreth, D.J.; Dassanayake, M.; Hartman, M.M.; Moroney, J.V. The cytoplasmic carbonic anhydrases βCA2 and βCA4 are required for optimal plant growth at low CO2. Plant Physiol. 2016, 171, 280–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perales, M.; Parisi, G.; Fornasari, M.; Colaneri, A.; Villarreal, F.; González-Schain, N.; Echave, J.; Gómez-Casati, D.; Braun, H.P.; Araya, A.; et al. Gamma carbonic anhydrase like complex interact with plant mitochondrial complex I. Plant Mol. Biol. 2004, 56, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.W.; Greenstone, M. Carbonic anhydrase inhibitors for hypercapnic ventilatory failure in chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2001, 2001, CD002881. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrases-an overview. Curr. Pharm. Des. 2008, 14, 603–614. [Google Scholar] [CrossRef]
- Okabe, K.; Yang, S.Y.; Tsuzuki, M.; Miyachi, S. Carbonic anhydrase: Its content in spinach leaves and its taxonomic diversity studied with anti-spinach leaf carbonic anhydrase antibody. Plant Sci. Lett. 1984, 33, 145–153. [Google Scholar] [CrossRef]
- DiMario, R.J.; Clayton, H.; Mukherjee, A.; Ludwig, M.; Moroney, J.V. Plant carbonic anhydrases: Structures locations evolution and physiological roles. Mol. Plant 2017, 10, 30–46. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Vroegop-Vos, I.A.; Van Dijken, A.J.H.; Van der Does, D.; Zipfel, C.; Pieterse, C.M.J.; Van Wees, S.C.M. Carbonic anhydrases CA1 and CA4 function in atmospheric CO2-modulated disease. Resistance. Planta 2020, 251, 75. [Google Scholar] [CrossRef] [Green Version]
- Restrepo, S.; Myers, K.; Del-Pozo, O.; Martin, G.; Hart, A.; Buell, C.; Fry, W.; Smart, C. Gene profiling of a compatible interaction between Phytophthora infestans and Solanum tuberosum suggests a role for carbonic anhydrase. Mol. Plant Microbe Interact. 2005, 18, 913–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polesani, M.; Desario, F.; Ferrarini, A.; Zamboni, A.; Pezzotti, M.; Kortekamp, A.; Polverari, A. cDNA-AFLP analysis of plant and pathogen genes expressed in grapevine infected with Plasmopara viticola. BMC Genom. 2008, 9, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Wang, L.; Hu, D.; Riquicho, A.R.M.; Liu, T.; Hou, X.; Li, Y. Proteomic analysis of non-heading Chinese cabbage infected with Hyaloperonospora parasitica. J. Proteom. 2014, 98, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Feechan, A.; Yun, B.W.; Shafiei, R.; Hofmann, A.; Taylor, P.; Xue, P.; Yang, F.Q.; Xie, Z.S.; Pallas, J.A.; et al. S-Nitrosylation of AtSABP3 antagonizes the expression of plant immunity. J. Biol. Chem. 2009, 284, 2131–2137. [Google Scholar] [CrossRef] [Green Version]
- Slaymaker, D.H.; Navarre, D.A.; Clark, D.; del Pozo, O.; Martin, G.B.; Klessig, D.F. The tobacco salicylic acid-binding protein 3 (SABP3) is the chloroplast carbonic anhydrase which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc. Natl. Acad. Sci. USA 2002, 99, 11640–11645. [Google Scholar] [CrossRef]
- Medina-Puche, L.; Castello, M.J.; Canet, J.V.; Lamilla, J.; Colombo, M.; Tornero, P. β-carbonic anhydrases play a role in salicylic acid perception in Arabidopsis. PLoS ONE 2017, 12, e0181820. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Boisson-Dernier, A.; Israelsson-Nordström, M.; Böhmer, M.; Xue, S.; Ries, A.; Godoski, J.; Kuhn, J.M.; Schroeder, J.I. Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat. Cell Biol. 2010, 12, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Melotto, M.; Underwood, W.; He, S.Y. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 2008, 46, 101–122. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Ma, Q.; Foyer, C.H.; Lei, C.; Choi, H.W.; Zheng, C.; Li, J.X.; Zuo, Z.M.; Mei, Y.Y.; Yu, J.Q.; et al. High CO2- and pathogen-driven expression of the carbonic anhydrase βCA3 confers basal immunity in tomato. New Phytol. 2020, 229, 2827–2843. [Google Scholar] [CrossRef]
- Li, J.G.; Liu, H.X.; Cao, J.; Chen, L.F.; Gu, C.; Allen, C.; Guo, J.H. PopW of Ralstonia solanacearum a new two-domain harpin targeting the plant cell wall. Mol. Plant Pathol. 2010, 11, 371–381. [Google Scholar] [CrossRef]
- Zheng, L.; Luo, Y.M.; Xue, Q.Y.; Li, S.M.; Liu, H.X.; Guo, J.H. Control and growth promotion of PopW to cucumber downy mildew under greenhouse and field conditions. Acta Pharmacol. Sin. 2013, 43, 179–186. [Google Scholar]
- Wang, C.; Cao, J.; Wang, T.T.; Zheng, L.; Wang, C.; Liu, H.X. Induction of Protein PopW from Ralstonia solanacearum in Plants Disease Resistance. Chin. J. Ecol. 2014, 30, 186–193. [Google Scholar]
- Wang, C.; Liu, H.X.; Cao, J.; Wang, C.; Guo, J.H. Construction of transgenic tobacco expressing popW and analysis of its biological phenotype. Chin. J. Biotech. 2014, 30, 569–580. [Google Scholar]
- Wei, T.; Wang, L.; Zhou, X.; Ren, X.; Dai, X.; Liu, H. PopW activates MAMP-triggered immunity in controlling tomato bacterial spot disease. Biochem. Biophys. Res. Commun. 2015, 463, 746–750. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Zhou, X.; Wang, C.; Wang, C.; Fu, J.; Wei, T. Overexpression of a harpin-encoding gene PopW from Ralstonia solanacearum primed antioxidant defenses with enhanced drought tolerance in tobacco plants. Plant Cell Rep. 2016, 35, 1333–1344. [Google Scholar] [CrossRef]
- Adhikari, P.; Adhikari, T.B.; Louws, F.J.; Panthee, D.R. Advances and Challenges in Bacterial Spot Resistance Breeding in Tomato (Solanum lycopersicum L.). Int. J. Mol. Sci. 2020, 21, 1734. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Zhang, Y.; Liu, H.; Pan, C.Q.; Yang, M.X.; Ding, J.F.; Zhang, J.H. Isolation and characterization of Xanthomonas euvesicatoria pv euvesicatoria causing bacterial spot in Physalis pubescens in Northeast China. J. Plant Pathol. 2019, 101, 361–366. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhang, Y.X.; Gao, Z.P.; Yang, W.C. Breeding for Resistance to Tomato Bacterial Diseases in China: Challenges and Prospects. Hortic. Plant J. 2018, 4, 193–207. [Google Scholar] [CrossRef]
- Abbasi, P.A.; Weselowski, B. Efficacy of Bacillus subtilis QST 713 formulations copper hydroxide and their tank mixes on bacterial spot of tomato. Crop Prot. 2015, 74, 70–76. [Google Scholar] [CrossRef]
- Franco-Orozco, B.; Berepiki, A.; Ruiz, O.; Gamble, L.; Griffe, L.L.; Wang, S.; Birch, P.; Kanyuka, K.; Avrova, A. A new proteinaceous pathogen-associated molecular pattern (MAMP) identified in Ascomycete fungi induces cell death in Solanaceae. New Phytol. 2017, 214, 1657–1672. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.J.; Yin, Z.Y.; Li, Z.P.; Wu, Y.X.; Huang, L.L. A small cysteine-rich protein from two kingdoms of microbes is recognized as a novel pathogen-associated molecular pattern. New Phytol. 2019, 222, 995–1011. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Zhang, Z.G.; Wei, Y.D.; Collinge, D.B. Subcellular localization of H2O2 in plants H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, Y.; Mo, Z.; Liu, H.X. A Bacillus licheniformis Glycoside Hydrolase 43 Protein Is Recognized as a MAMP. Int. J. Mol. Sci. 2022, 23, 14435. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.B.; Kim, M.G.; Kang, C.H.; Park, J.H.; Lee, E.S.; Lee, S.U.; Chi, Y.H.; Paeng, S.K.; Bae, S.B.; Wi, S.D.; et al. Redox sensor QSOX1 regulates plant immunity by targeting GSNOR to modulate ROS generation. Mol. Plant 2021, 14, 1312–1327. [Google Scholar] [CrossRef]
- Seo, J.S.; Sun, H.X.; Park, B.S.; Huang, C.H.; Yeh, S.D.; Jung, C.; Chua, N.H. ELF18-induced long-noncoding RNA Associates with Mediator to Enhance Expression of Innate Immune Response Genes in Arabidopsis. Plant Cell 2017, 29, 1024–1038. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.S.; Diloknawarit, P.; Park, B.S.; Chua, N.H. ELF18-induced long noncoding RNA 1 evicts fibrillarin from mediator subunit to enhance Pathogenesis-Related Gene 1 (PR1) expression. New Phytol. 2019, 221, 2067–2079. [Google Scholar] [CrossRef]
- Sussholz, O.; Pizarro, L.; Schuster, S.; Avni, A. SlRLK-like is a malectin-like domain protein affecting localization and abundance of LeEIX2 receptor resulting in suppression of EIX-induced immune responses. Plant J. 2020, 104, 1369–1381. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Y.; Shang, Y.; Lin, H.; Wang, Y.; Cai, R.; Tang, X.; Zhou, J.M. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 2008, 146, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Michalopoulou, V.A.; Kotsaridis, K.; Mermigka, G.; Kotsifaki, D.; Kokkinidis, M.; Celie, P.H.; Jones, J.D.; Sarris, P.F. The host exocyst complex is targeted by a conserved bacterial type III effector protein that promotes virulence. Plant Cell 2020, 34, 3400–3424. [Google Scholar] [CrossRef]
- Hatch, M.D.; Burnell, J.N. Carbonic anhydrase activity in leaves and its role in the first step of C4 photosynthesis. Plant Physiol. 1990, 93, 825–828. [Google Scholar] [CrossRef] [Green Version]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant stomata function in innate immunity against bacterial invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, H.; Yamazaki, Y.; Moriyoshi, E.; Nakayasu, M.; Yamazaki, S.; Aoki, Y.; Takase, H.; Okazaki, S.; Nagano, A.J.; Kaga, A.; et al. Apoplast-localized β-Glucosidase Elevates Isoflavone Accumulation in the Soybean Rhizosphere. Plant Cell Physiol. 2023, 64, 486–500. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Yuan, Z.; Han, X.; Bao, T.; Yang, T.; Liu, Z.; Liu, H. The Carbonic Anhydrase βCA1 Functions in PopW-Mediated Plant Defense Responses in Tomato. Int. J. Mol. Sci. 2023, 24, 11021. https://doi.org/10.3390/ijms241311021
Zhao J, Yuan Z, Han X, Bao T, Yang T, Liu Z, Liu H. The Carbonic Anhydrase βCA1 Functions in PopW-Mediated Plant Defense Responses in Tomato. International Journal of Molecular Sciences. 2023; 24(13):11021. https://doi.org/10.3390/ijms241311021
Chicago/Turabian StyleZhao, Jieru, Zhixiang Yuan, Xixi Han, Tingting Bao, Tingmi Yang, Zhuang Liu, and Hongxia Liu. 2023. "The Carbonic Anhydrase βCA1 Functions in PopW-Mediated Plant Defense Responses in Tomato" International Journal of Molecular Sciences 24, no. 13: 11021. https://doi.org/10.3390/ijms241311021
APA StyleZhao, J., Yuan, Z., Han, X., Bao, T., Yang, T., Liu, Z., & Liu, H. (2023). The Carbonic Anhydrase βCA1 Functions in PopW-Mediated Plant Defense Responses in Tomato. International Journal of Molecular Sciences, 24(13), 11021. https://doi.org/10.3390/ijms241311021




