Dye Decolorization by a Miniaturized Peroxidase Fe-MimochromeVI*a
Abstract
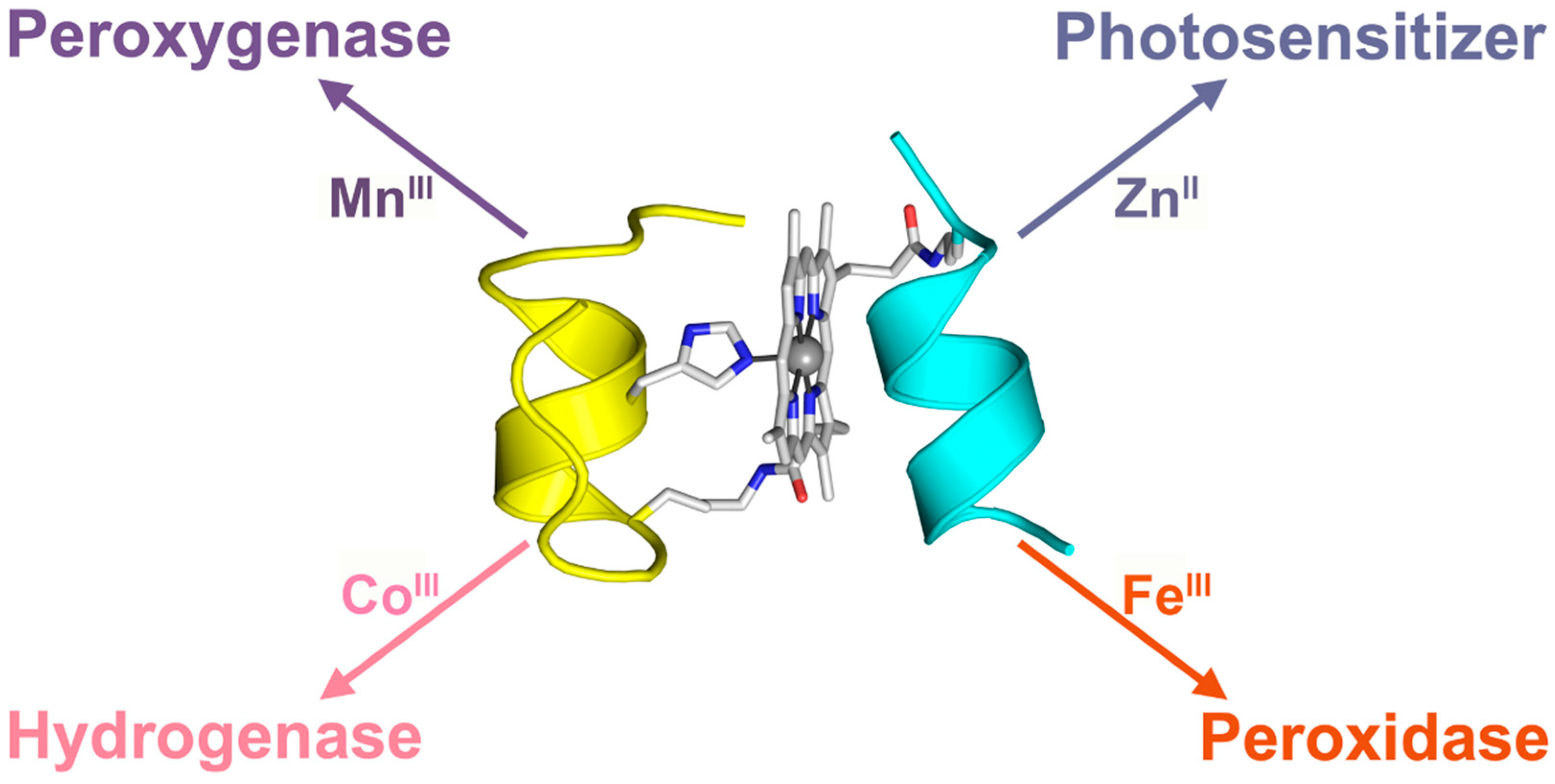
:1. Introduction
2. Results and Discussion
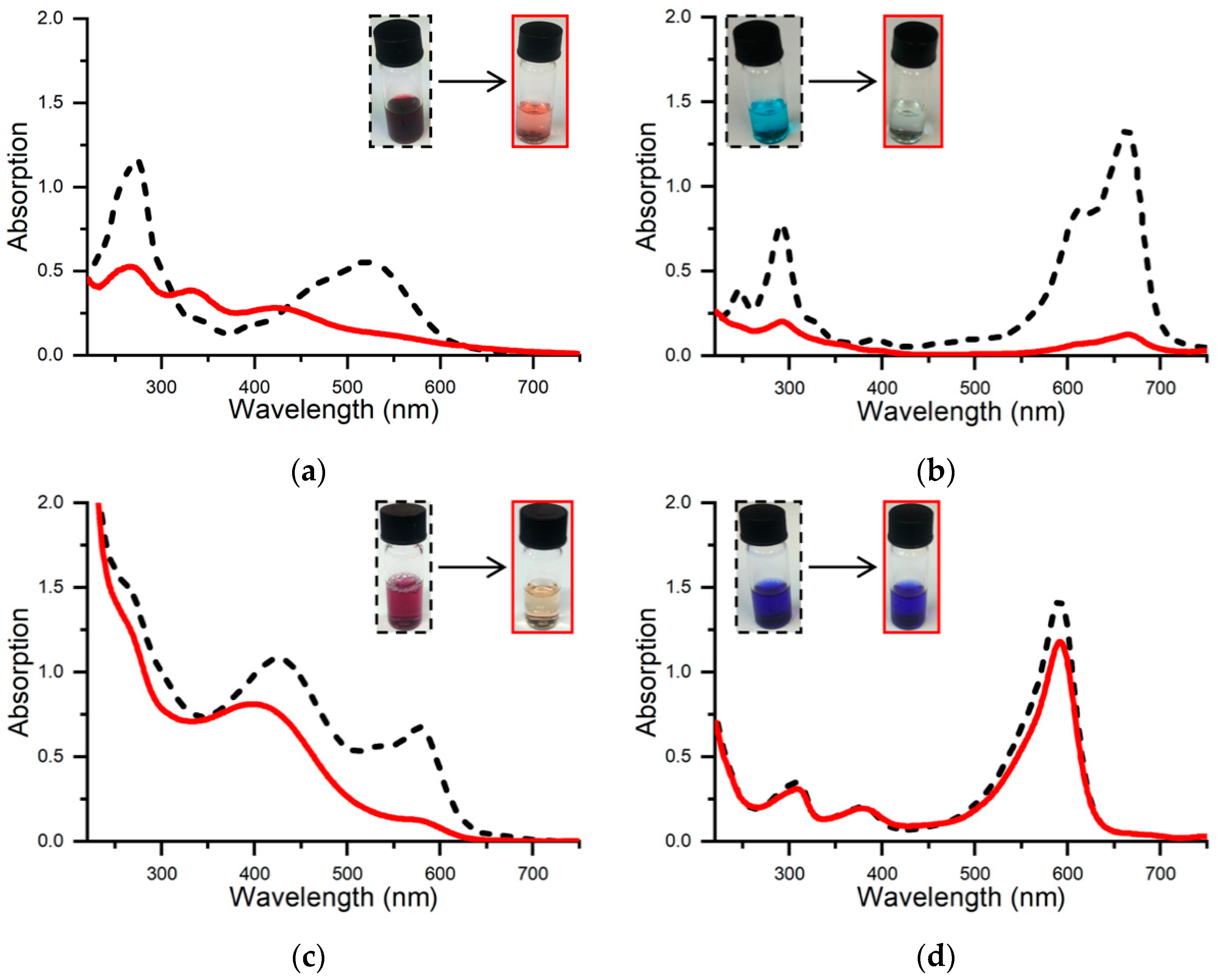
2.1. Preliminary Screening of Dye Activity
| Substrate | FeMC6*a | HRP † | SBP † | LiP † | TfuDyP † | Lac † | Inorganic Catalyst |
|---|---|---|---|---|---|---|---|
| NR | 70% 5 min | - | - | - | 0% a 1 h | 33% b 24 h | 84% c; 98% d 30 min; <5 min |
| MB | 95% 3 min | 21% e 60 min | - | 85% f 15 min | 0% a 1 h | 0% b 24 h | 90% g; 96% h 2 h; 1 h |
| XO | 82% 15 min | - | - | - | - | - | >95% i; 42% h 45 min; 1 h |
| BPB | 18% 5 min | 95% j 10 min | 85% k 60 min | 93% l 15 min | - | 14% b 24 h | 90% m 25 min |
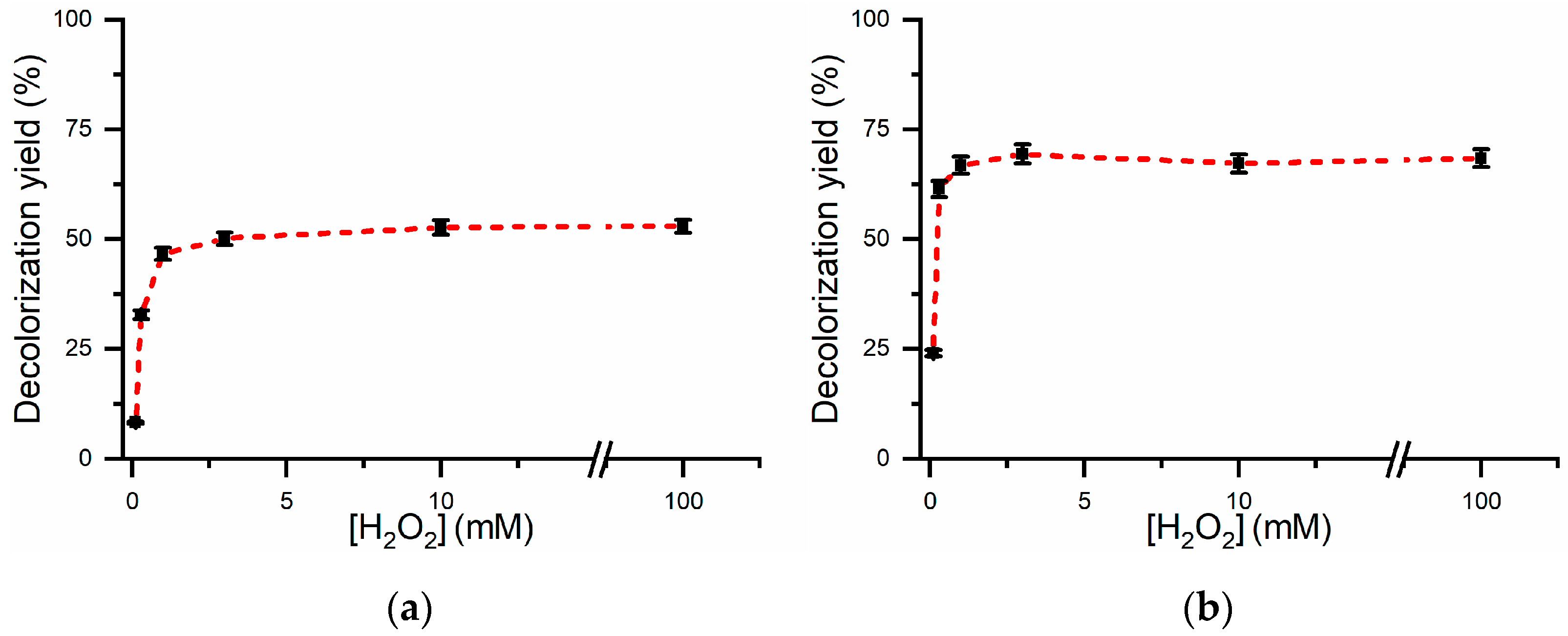
2.2. Optimization of Experimental Conditions
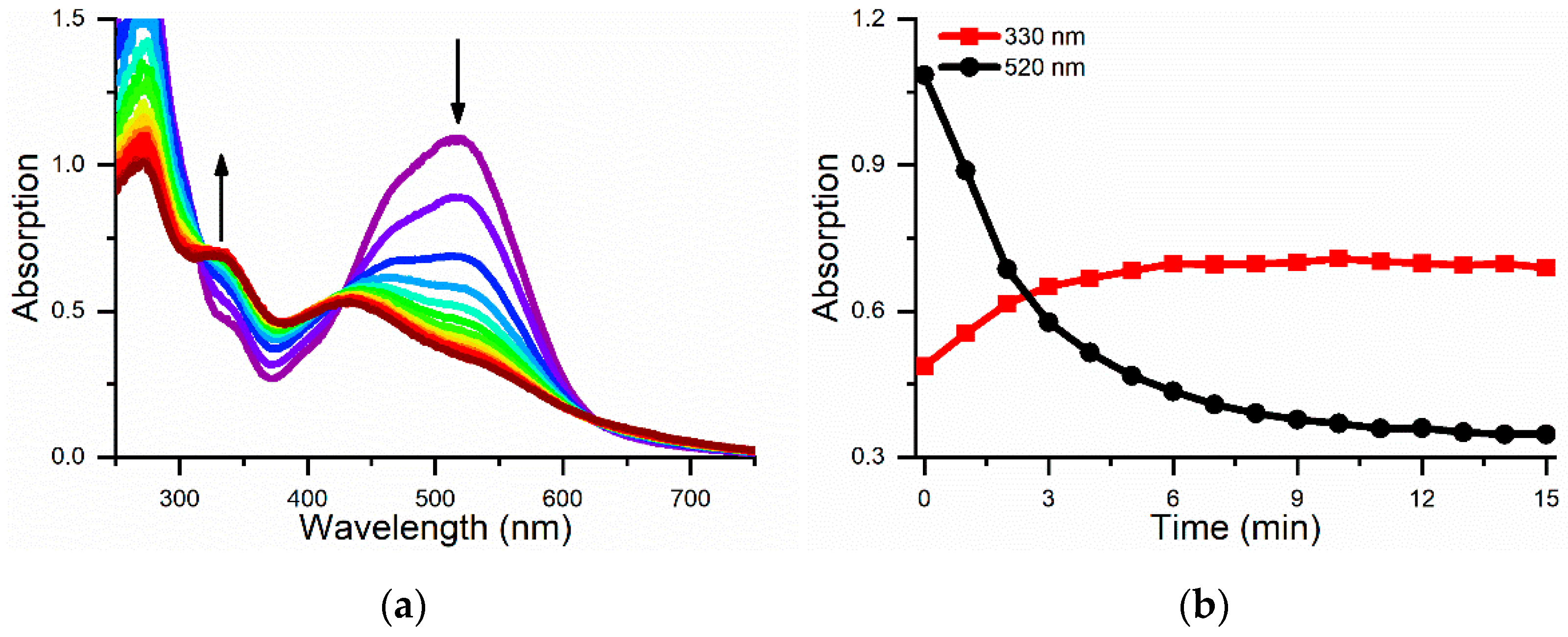
2.3. Estimation of the Catalytic Efficiency for Neutral Red Oxidation
3. Materials and Methods
3.1. Preliminary Screening of Dye Activity
3.2. Optimization of Experimental Conditions for Catalytic Activity of FeMC6*a
3.3. Catalytic Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Odabasi, M. Halogenated Volatile Organic Compounds from the Use of Chlorine-Bleach-Containing Household Products. Environ. Sci. Technol. 2008, 42, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.C.; Torres, J.A.; Castro, A.A.; da Cunha, E.F.F.; Alves de Oliveira, L.C.; Corrêa, A.D.; Ramalho, T.C. Combined Experimental and Theoretical Study on the Removal of Pollutant Compounds by Peroxidases: Affinity and Reactivity toward a Bioremediation Catalyst. J. Biomol. Struct. Dyn. 2016, 34, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N.; Hussain Shah, S.Z.; Hu, H.; Wang, W.; Zhang, X. Horseradish Peroxidase-Assisted Approach to Decolorize and Detoxify Dye Pollutants in a Packed Bed Bioreactor. J. Environ. Manag. 2016, 183, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Grönqvist, S.; Viikari, L.; Niku-Paavola, M.-L.; Orlandi, M.; Canevali, C.; Buchert, J. Oxidation of Milled Wood Lignin with Laccase, Tyrosinase and Horseradish Peroxidase. Appl. Microbiol. Biotechnol. 2005, 67, 489–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regalado, C.; García-Almendárez, B.E.; Duarte-Vázquez, M.A. Biotechnological Applications of Peroxidases. Phytochem. Rev. 2004, 3, 243–256. [Google Scholar] [CrossRef]
- Kim, S.J.; Shoda, M. Purification and Characterization of a Novel Peroxidase from Geotrichum Candidum Dec 1 Involved in Decolorization of Dyes. Appl. Environ. Microbiol. 1999, 65, 1029–1035. [Google Scholar] [CrossRef] [Green Version]
- Hatakka, A.; Lundell, T.; Hofrichter, M.; Maijala, P. Manganese Peroxidase and Its Role in the Degradation of Wood Lignin. In Applications of Enzymes to Lignocellulosics; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2003; Volume 855, pp. 230–243. ISBN 978-0-8412-3831-2. [Google Scholar]
- Wagner, M.; Nicell, J.A. Peroxidase-Catalyzed Removal of Phenols from a Petroleum Refinery Wastewater. Water Sci. Technol. 2001, 43, 253–260. [Google Scholar] [CrossRef]
- Aehle, W. (Ed.) Enzymes in Industry: Production and Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2007; ISBN 978-3-527-31689-2. [Google Scholar]
- Veitch, N.C. Horseradish Peroxidase: A Modern View of a Classic Enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef]
- Agostini, E.; Hernández-Ruiz, J.; Arnao, M.B.; Milrad, S.R.; Tigier, H.A.; Acosta, M. A Peroxidase Isoenzyme Secreted by Turnip (Brassica Napus) Hairy-Root Cultures: Inactivation by Hydrogen Peroxide and Application in Diagnostic Kits. Biotechnol. Appl. Biochem. 2002, 35, 1–7. [Google Scholar] [CrossRef]
- Young, P.R. An Improved Method for the Detection of Peroxidase-Conjugated Antibodies on Immunoblots. J. Virol. Methods 1989, 24, 227–235. [Google Scholar] [CrossRef]
- Renz, M.; Kurz, C. A Colorimetric Method for DNA Hybridization. Nucleic Acids Res. 1984, 12, 3435–3444. [Google Scholar] [CrossRef] [Green Version]
- Gross, R.A.; Kumar, A.; Kalra, B. Polymer Synthesis by In Vitro Enzyme Catalysis. Chem. Rev. 2001, 101, 2097–2124. [Google Scholar] [CrossRef]
- van Deurzen, M.P.J.; van Rantwijk, F.; Sheldon, R.A. Selective Oxidations Catalyzed by Peroxidases. Tetrahedron 1997, 53, 13183–13220. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Hu, N. Horseradish Peroxidase Immobilized in TiO2 Nanoparticle Films on Pyrolytic Graphite Electrodes: Direct Electrochemistry and Bioelectrocatalysis. Electrochimica Acta 2004, 49, 1981–1988. [Google Scholar] [CrossRef]
- Jia, J.; Wang, B.; Wu, A.; Cheng, G.; Li, Z.; Dong, S. A Method to Construct a Third-Generation Horseradish Peroxidase Biosensor: Self-Assembling Gold Nanoparticles to Three-Dimensional Sol−Gel Network. Anal. Chem. 2002, 74, 2217–2223. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Wang, H.; Yan, B.; Shen, G.; Yu, R. An Enzyme Immobilization Platform for Biosensor Designs of Direct Electrochemistry Using Flower-like ZnO Crystals and Nano-Sized Gold Particles. J. Electroanal. Chem. 2009, 627, 9–14. [Google Scholar] [CrossRef]
- Sergeyeva, T.A.; Lavrik, N.V.; Rachkov, A.E.; Kazantseva, Z.I.; Piletsky, S.A.; El’skaya, A.V. Hydrogen Peroxide—Sensitive Enzyme Sensor Based on Phthalocyanine Thin Film. Anal. Chim. Acta 1999, 391, 289–297. [Google Scholar] [CrossRef]
- Ulson de Souza, S.M.A.G.; Forgiarini, E.; Ulson de Souza, A.A. Toxicity of Textile Dyes and Their Degradation by the Enzyme Horseradish Peroxidase (HRP). J. Hazard. Mater. 2007, 147, 1073–1078. [Google Scholar] [CrossRef]
- Husain, Q. Potential Applications of the Oxidoreductive Enzymes in the Decolorization and Detoxification of Textile and Other Synthetic Dyes from Polluted Water: A Review. Crit. Rev. Biotechnol. 2006, 26, 201–221. [Google Scholar] [CrossRef]
- Bhunia, A.; Durani, S.; Wangikar, P.P. Horseradish Peroxidase Catalyzed Degradation of Industrially Important Dyes. Biotechnol. Bioeng. 2001, 72, 562–567. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, D.; Li, R.; Wang, T.; Zhu, Y. Textile Dye Biodecolorization by Manganese Peroxidase: A Review. Molecules 2021, 26, 4403. [Google Scholar] [CrossRef]
- Llena, C.; Oteo, C.; Oteo, J.; Amengual, J.; Forner, L. Clinical Efficacy of a Bleaching Enzyme-Based Toothpaste. A Double-Blind Controlled Clinical Trial. J. Dent. 2016, 44, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, I.; Riutord, P.; Tauler, P.; Tur, J.A.; Pons, A. The Whitening Effect of Enzymatic Bleaching on Tetracycline. J. Dent. 2008, 36, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, M.-C.; Poelarends, G.J. Current State and Future Perspectives of Engineered and Artificial Peroxygenases for the Oxyfunctionalization of Organic Molecules. Nat. Catal. 2020, 3, 690–702. [Google Scholar] [CrossRef]
- Barber-Zucker, S.; Mindel, V.; Garcia-Ruiz, E.; Weinstein, J.J.; Alcalde, M.; Fleishman, S.J. Stable and Functionally Diverse Versatile Peroxidases Designed Directly from Sequences. J. Am. Chem. Soc. 2022, 144, 3564–3571. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-J.; Xu, J.-K.; Wu, S.-T.; Gao, S.-Q.; Wen, G.-B.; Tan, X.; Lin, Y.-W. Design and Engineering of an Efficient Peroxidase Using Myoglobin for Dye Decolorization and Lignin Bioconversion. Int. J. Mol. Sci. 2021, 23, 413. [Google Scholar] [CrossRef]
- Liu, C.; Xu, J.; Gao, S.-Q.; He, B.; Wei, C.-W.; Wang, X.-J.; Wang, Z.; Lin, Y.-W. Green and Efficient Biosynthesis of Indigo from Indole by Engineered Myoglobins. RSC Adv. 2018, 8, 33325–33330. [Google Scholar] [CrossRef] [Green Version]
- Martin-Diaz, J.; Molina-Espeja, P.; Hofrichter, M.; Hollmann, F.; Alcalde, M. Directed Evolution of Unspecific Peroxygenase in Organic Solvents. Biotechnol. Bioeng. 2021, 118, 3002–3014. [Google Scholar] [CrossRef]
- Li, L.-L.; Yuan, H.; Liao, F.; He, B.; Gao, S.-Q.; Wen, G.-B.; Tan, X.; Lin, Y.-W. Rational Design of Artificial Dye-Decolorizing Peroxidases Using Myoglobin by Engineering Tyr/Trp in the Heme Center. Dalton Trans. 2017, 46, 11230–11238. [Google Scholar] [CrossRef]
- Liao, F.; Yuan, H.; Du, K.-J.; You, Y.; Gao, S.-Q.; Wen, G.-B.; Lin, Y.-W.; Tan, X. Distinct Roles of a Tyrosine-Associated Hydrogen-Bond Network in Fine-Tuning the Structure and Function of Heme Proteins: Two Cases Designed for Myoglobin. Mol. Biosyst. 2016, 12, 3139–3145. [Google Scholar] [CrossRef]
- Faiella, M.; Maglio, O.; Nastri, F.; Lombardi, A.; Lista, L.; Hagen, W.R.; Pavone, V. De Novo Design, Synthesis and Characterisation of MP3, A New Catalytic Four-Helix Bundle Hemeprotein. Chem.-Eur. J. 2012, 18, 15960–15971. [Google Scholar] [CrossRef]
- Lin, Y.-W. Rational Design of Heme Enzymes for Biodegradation of Pollutants toward a Green Future. Biotechnol. Appl. Biochem. 2020, 67, 484–494. [Google Scholar] [CrossRef]
- Lin, Y.-W. Rational Design of Artificial Metalloproteins and Metalloenzymes with Metal Clusters. Molecules 2019, 24, 2743. [Google Scholar] [CrossRef] [Green Version]
- Leone, L.; Chino, M.; Nastri, F.; Maglio, O.; Pavone, V.; Lombardi, A. Mimochrome, a Metalloporphyrin-Based Catalytic Swiss Knife †. Biotechnol. Appl. Biochem. 2020, 67, 495–515. [Google Scholar] [CrossRef]
- Chino, M.; Leone, L.; Zambrano, G.; Pirro, F.; D’Alonzo, D.; Firpo, V.; Aref, D.; Lista, L.; Maglio, O.; Nastri, F.; et al. Oxidation Catalysis by Iron and Manganese Porphyrins within Enzyme-like Cages. Biopolymers 2018, 109, e23107. [Google Scholar] [CrossRef]
- Maglio, O.; Nastri, F.; Lombardi, A. Structural and Functional Aspects of Metal Binding Sites in Natural and Designed Metalloproteins. In Ionic Interactions in Natural and Synthetic Macromolecules; Ciferri, A., Perico, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 361–450. ISBN 978-1-118-16585-0. [Google Scholar]
- Caserta, G.; Chino, M.; Firpo, V.; Zambrano, G.; Leone, L.; D’Alonzo, D.; Nastri, F.; Maglio, O.; Pavone, V.; Lombardi, A. Enhancement of Peroxidase Activity in Artificial Mimochrome VI Catalysts through Rational Design. ChemBioChem 2018, 19, 1823–1826. [Google Scholar] [CrossRef]
- Leone, L.; D’Alonzo, D.; Balland, V.; Zambrano, G.; Chino, M.; Nastri, F.; Maglio, O.; Pavone, V.; Lombardi, A. Mn-Mimochrome VI*a: An Artificial Metalloenzyme With Peroxygenase Activity. Front. Chem. 2018, 6, 590. [Google Scholar] [CrossRef] [Green Version]
- Zambrano, G.; Nastri, F.; Pavone, V.; Lombardi, A.; Chino, M. Use of an Artificial Miniaturized Enzyme in Hydrogen Peroxide Detection by Chemiluminescence. Sensors 2020, 20, 3793. [Google Scholar] [CrossRef]
- Renzi, E.; Piper, A.; Nastri, F.; Merkoçi, A.; Lombardi, A. An Artificial Miniaturized Peroxidase for Signal Amplification in Lateral Flow Immunoassays. Small 2023, 2207949. [Google Scholar] [CrossRef]
- Zambrano, G.; Sekretareva, A.; D’Alonzo, D.; Leone, L.; Pavone, V.; Lombardi, A.; Nastri, F. Oxidative Dehalogenation of Trichlorophenol Catalyzed by a Promiscuous Artificial Heme-Enzyme. RSC Adv. 2022, 12, 12947–12956. [Google Scholar] [CrossRef]
- D’Alonzo, D.; De Fenza, M.; Pavone, V.; Lombardi, A.; Nastri, F. Selective Oxidation of Halophenols Catalyzed by an Artificial Miniaturized Peroxidase. Int. J. Mol. Sci. 2023, 24, 8058. [Google Scholar] [CrossRef] [PubMed]
- Leone, L.; D’Alonzo, D.; Maglio, O.; Pavone, V.; Nastri, F.; Lombardi, A. Highly Selective Indole Oxidation Catalyzed by a Mn-Containing Artificial Mini-Enzyme. ACS Catal. 2021, 11, 9407–9417. [Google Scholar] [CrossRef]
- Firpo, V.; Le, J.M.; Pavone, V.; Lombardi, A.; Bren, K.L. Hydrogen Evolution from Water Catalyzed by Cobalt-Mimochrome VI*a, a Synthetic Mini-Protein. Chem. Sci. 2018, 9, 8582–8589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, J.M.; Alachouzos, G.; Chino, M.; Frontier, A.J.; Lombardi, A.; Bren, K.L. Tuning Mechanism through Buffer Dependence of Hydrogen Evolution Catalyzed by a Cobalt Mini-Enzyme. Biochemistry 2020, 59, 1289–1297. [Google Scholar] [CrossRef] [Green Version]
- Edwards, E.H.; Le, J.M.; Salamatian, A.A.; Peluso, N.L.; Leone, L.; Lombardi, A.; Bren, K.L. A Cobalt Mimochrome for Photochemical Hydrogen Evolution from Neutral Water. J. Inorg. Biochem. 2022, 230, 111753. [Google Scholar] [CrossRef]
- Chino, M.; Di Costanzo, L.F.; Leone, L.; La Gatta, S.; Famulari, A.; Chiesa, M.; Lombardi, A.; Pavone, V. Designed Rubredoxin Miniature in a Fully Artificial Electron Chain Triggered by Visible Light. Nat. Commun. 2023, 14, 2368. [Google Scholar] [CrossRef]
- Lim, J.; Mohamad, Z. Enzymes Immobilized Polymeric Supports for Wastewater Treatment Application: A Short Review. Mater. Today Proc. 2022, 65, 2946–2952. [Google Scholar] [CrossRef]
- Dave, S.; Das, J.; Shah, M.P. (Eds.) Photocatalytic Degradation of Dyes: Current Trends and Future Perspectives; Elsevier: Amsterdam, The Netherlands; Cambridge, MA, USA, 2021; ISBN 978-0-12-823876-9. [Google Scholar]
- Si, J.; Li, X.-C.; Cui, B.-K. Decolorization of Heterocycle Dye Neutral Red by White-Rot Fungus Perenniporia subacida. Desalination Water Treat. 2014, 52, 5594–5604. [Google Scholar] [CrossRef]
- Yuan, X.; Tian, G.; Zhao, Y.; Zhao, L.; Wang, H.; Ng, T.B. Degradation of Dyes Using Crude Extract and a Thermostable and PH-Stable Laccase Isolated from Pleurotus Nebrodensis. Biosci. Rep. 2016, 36, e00365. [Google Scholar] [CrossRef] [Green Version]
- Lončar, N.; Colpa, D.I.; Fraaije, M.W. Exploring the Biocatalytic Potential of a DyP-Type Peroxidase by Profiling the Substrate Acceptance of Thermobifida Fusca DyP Peroxidase. Tetrahedron 2016, 72, 7276–7281. [Google Scholar] [CrossRef]
- Alnuaimi, M.M.; Rauf, M.A.; Ashraf, S.S. Comparative Decoloration Study of Neutral Red by Different Oxidative Processes. Dyes Pigments 2007, 72, 367–371. [Google Scholar] [CrossRef]
- Pereira, A.R.; da Costa, R.S.; Yokoyama, L.; Alhadeff, E.M.; Teixeira, L.A.C. Evaluation of Textile Dye Degradation Due to the Combined Action of Enzyme Horseradish Peroxidase and Hydrogen Peroxide. Appl. Biochem. Biotechnol. 2014, 174, 2741–2747. [Google Scholar] [CrossRef]
- Ollikka, P.; Alhonmäki, K.; Leppänen, V.-M.; Glumoff, T.; Raijola, T.; Suominen, I. Decolorization of Azo, Triphenyl Methane, Heterocyclic, and Polymeric Dyes by Lignin Peroxidase Isoenzymes from Phanerochaete Chrysosporium. Appl. Environ. Microbiol. 1993, 59, 4010–4016. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.K.M.A.; Kibria, A.K.M.F.; Akter, M.; Khan, M.N.I.; Tareq, A.R.M.; Firoz, S.H. Oxidative Degradation of Methylene Blue Using Mn3O4 Nanoparticles. Water Conserv. Sci. Eng. 2017, 4, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Zucca, P.; Rescigno, A.; Pintus, M.; Rinaldi, A.C.; Sanjust, E. Degradation of Textile Dyes Using Immobilized Lignin Peroxidase-like Metalloporphines under Mild Experimental Conditions. Chem. Cent. J. 2012, 6, 161. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Diao, G. Synthesis of Porous Fe3O4 Nanospheres and Its Application for the Catalytic Degradation of Xylenol Orange. J. Phys. Chem. C 2011, 115, 18923–18934. [Google Scholar] [CrossRef]
- Kagliwal, L.D.; Singhal, R.S. Enzyme–Polysaccharide Interaction: A Method for Improved Stability of Horseradish Peroxidase. Int. J. Biol. Macromol. 2014, 69, 329–335. [Google Scholar] [CrossRef]
- Vinueza Galárraga, J.C.; dos Santos, A.F.; Bassan, J.C.; Goulart, A.J.; Monti, R. Bromophenol Blue Discoloration Using Peroxidase Immobilized on Highly Activated Corncob Powder. Rev. Cienc. Farm. Basica E Apl. 2013, 34, 321–326. [Google Scholar]
- Abdel-Khalek, A.A.; Nassar, H.F.; Abdel-Gawad, F.K.; Basem, S.M.; Awad, S. Photocatalytic Degradation of Bromophenol Blue in Wastewater Using Pure ZnO and Ag+ Doped ZnO. Quantum Matter 2016, 5, 297–304. [Google Scholar] [CrossRef]
- Ferreira-Leitão, V.S.; de Carvalho, M.E.A.; Bon, E.P.S. Lignin Peroxidase Efficiency for Methylene Blue Decolouration: Comparison to Reported Methods. Dyes Pigments 2007, 74, 230–236. [Google Scholar] [CrossRef]
- Ayala, M. Redox Potential of Peroxidases. In Biocatalysis Based on Heme Peroxidases: Peroxidases as Potential Industrial Biocatalysts; Torres, E., Ayala, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 61–77. ISBN 978-3-642-12627-7. [Google Scholar]
- Liu, Z.; Zhong, Y.; Hu, Z.; Zhang, W.; Zhang, X.; Ji, X.; Wang, X. Modification of ZIF-8 Nanocomposite by a Gd Atom Doped TiO2 for High Efficiency Photocatalytic Degradation of Neutral Red Dye: An Experimental and Theoretical Study. J. Mol. Liq. 2023, 380, 121729. [Google Scholar] [CrossRef]
- Pauliukaite, R.; Brett, C. Poly(Neutral Red): Electrosynthesis, Characterization, and Application as a Redox Mediator. Electroanalysis 2008, 20, 1275–1285. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.C.; Lin, Y.C.; Chen, S.M. Electrocatalytic Reaction of Hydrogen Peroxide and NADH Based on Poly(Neutral Red) and FAD Hybrid Film. Analyst 2011, 137, 186–194. [Google Scholar] [CrossRef]
- Pauliukaite, R.; Ghica, M.E.; Barsan, M.M.; Brett, C.M.A. Phenazines and Polyphenazines in Electrochemical Sensors and Biosensors. Anal. Lett. 2010, 43, 1588–1608. [Google Scholar] [CrossRef]
- Chen, S.-M.; Lin, K.-C. The Electrocatalytic Properties of Polymerized Neutral Red Film Modified Electrodes. J. Electroanal. Chem. 2001, 511, 101–114. [Google Scholar] [CrossRef]
- Ayala, M.; Roman, R.; Vazquez-Duhalt, R. A Catalytic Approach to Estimate the Redox Potential of Heme-Peroxidases. Biochem. Biophys. Res. Commun. 2007, 357, 804–808. [Google Scholar] [CrossRef]
- Jenkins, J.M.X.; Noble, C.E.M.; Grayson, K.J.; Mulholland, A.J.; Anderson, J.L.R. Substrate Promiscuity of a de Novo Designed Peroxidase. J. Inorg. Biochem. 2021, 217, 111370. [Google Scholar] [CrossRef]
- Bergmann, K.; O’Konski, C.T. A Spectroscopic Study of Methylene Blue Monomer, Dimer, and Complexes with Montmorillonite. J. Phys. Chem. 1963, 67, 2169–2177. [Google Scholar] [CrossRef]
- Rej, S.; Hejazi, S.M.H.; Badura, Z.; Zoppellaro, G.; Kalytchuk, S.; Kment, Š.; Fornasiero, P.; Naldoni, A. Light-Induced Defect Formation and Pt Single Atoms Synergistically Boost Photocatalytic H2 Production in 2D TiO2-Bronze Nanosheets. ACS Sustain. Chem. Eng. 2022, 10, 17286–17296. [Google Scholar] [CrossRef]




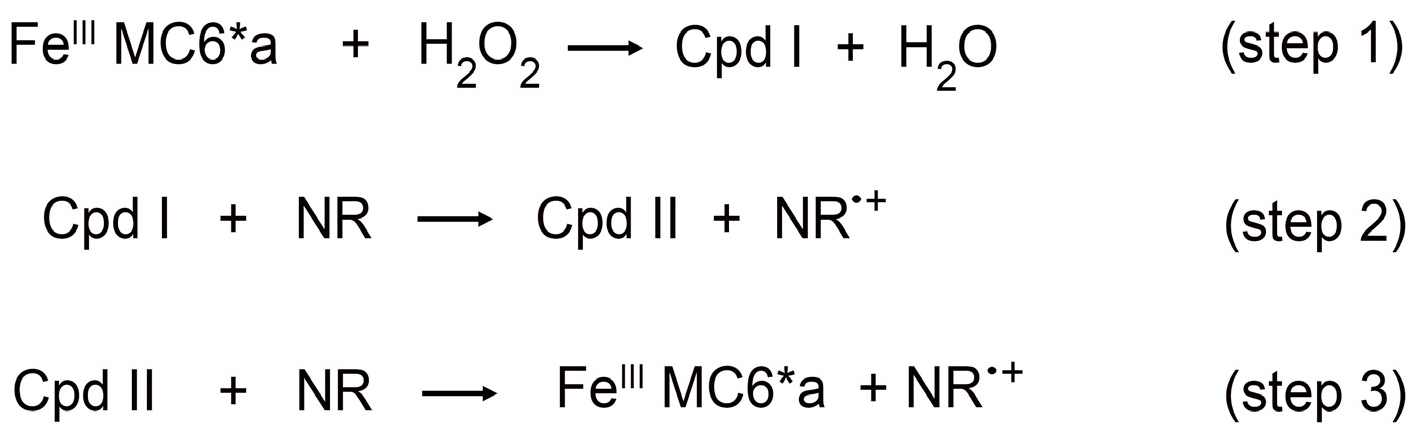
| Substrate (S) | kcat (s−1) | Km (mM) | KmH2O2 (mM) | kcat/Km (mM−1 s−1) | Reference |
|---|---|---|---|---|---|
| NR † | 28 ± 3 | 0.11 ± 0.01 | 31 ± 4 | 258 | This work |
| TCP † | 70 ± 6 | 0.10 ± 0.02 | 94 ± 8 | 700 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chino, M.; La Gatta, S.; Leone, L.; De Fenza, M.; Lombardi, A.; Pavone, V.; Maglio, O. Dye Decolorization by a Miniaturized Peroxidase Fe-MimochromeVI*a. Int. J. Mol. Sci. 2023, 24, 11070. https://doi.org/10.3390/ijms241311070
Chino M, La Gatta S, Leone L, De Fenza M, Lombardi A, Pavone V, Maglio O. Dye Decolorization by a Miniaturized Peroxidase Fe-MimochromeVI*a. International Journal of Molecular Sciences. 2023; 24(13):11070. https://doi.org/10.3390/ijms241311070
Chicago/Turabian StyleChino, Marco, Salvatore La Gatta, Linda Leone, Maria De Fenza, Angela Lombardi, Vincenzo Pavone, and Ornella Maglio. 2023. "Dye Decolorization by a Miniaturized Peroxidase Fe-MimochromeVI*a" International Journal of Molecular Sciences 24, no. 13: 11070. https://doi.org/10.3390/ijms241311070
APA StyleChino, M., La Gatta, S., Leone, L., De Fenza, M., Lombardi, A., Pavone, V., & Maglio, O. (2023). Dye Decolorization by a Miniaturized Peroxidase Fe-MimochromeVI*a. International Journal of Molecular Sciences, 24(13), 11070. https://doi.org/10.3390/ijms241311070











