Dihydroisotanshinone I and BMAL-SIRT1 Pathway in an In Vitro 6-OHDA-Induced Model of Parkinson’s Disease
Abstract
1. Introduction
2. Results
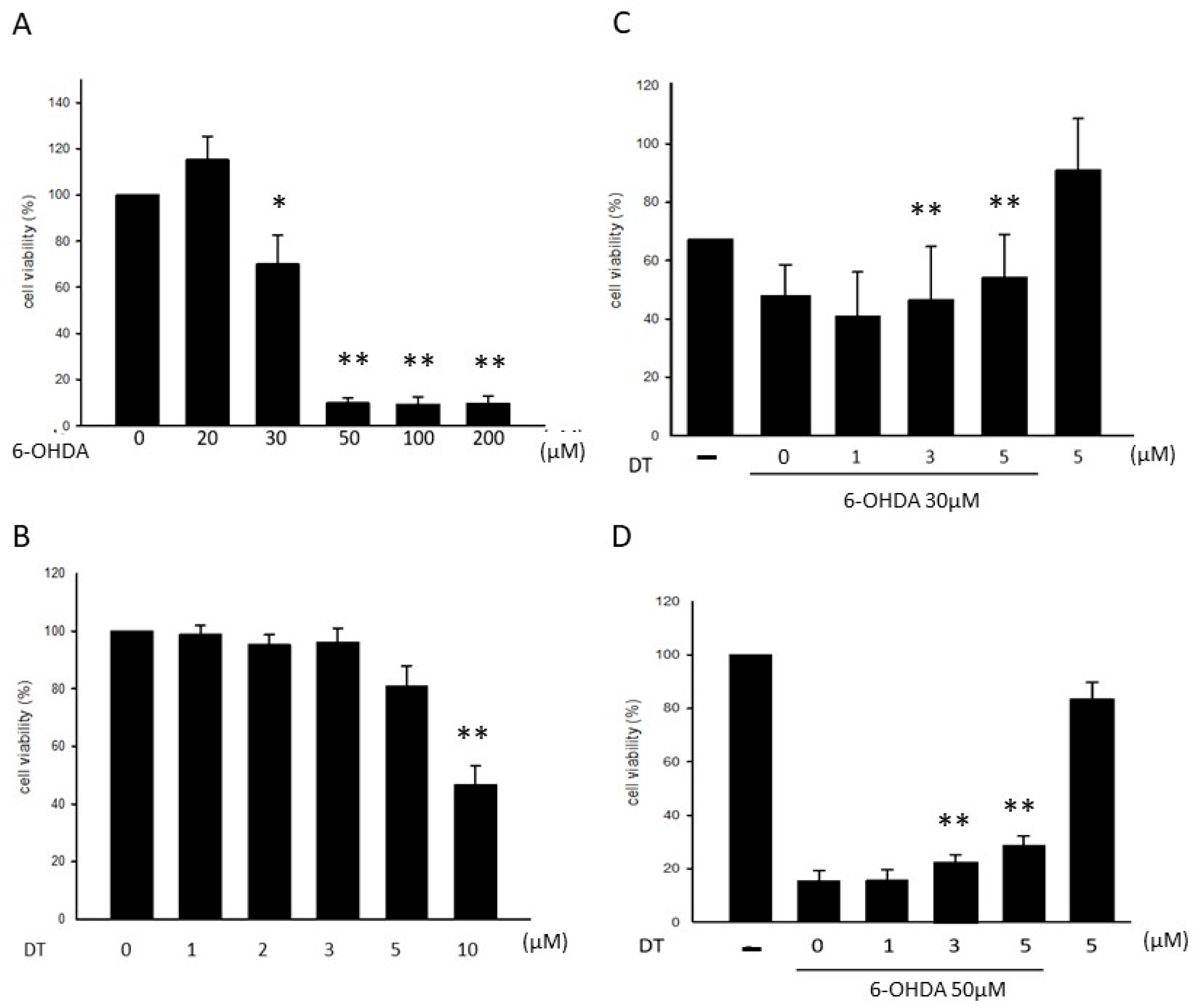
2.1. Effect of DT on the 6-OHDA-Induced Cytotoxicity
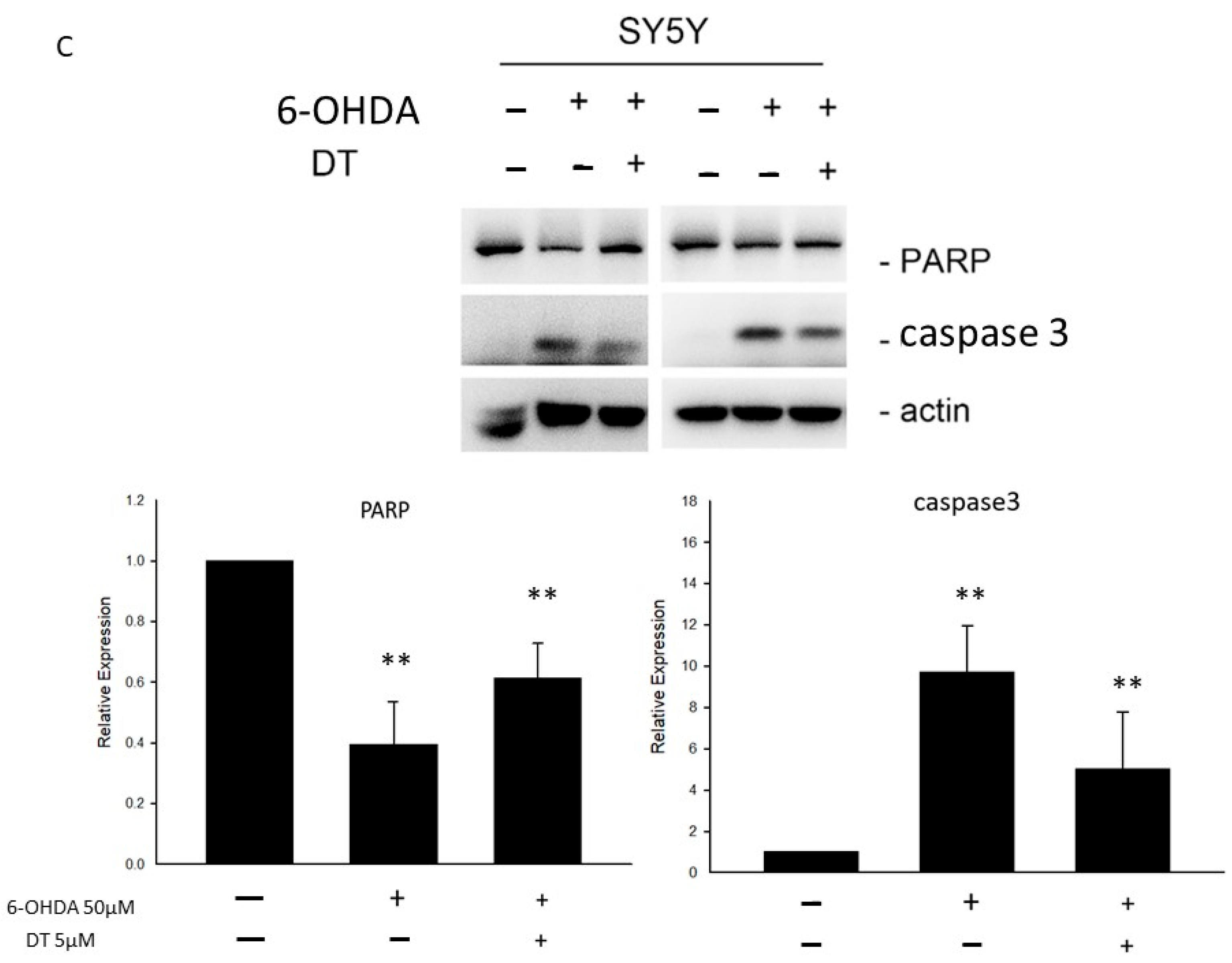
2.2. Effect of DT on Cell Apoptosis and the 6-OHDA-Induced ROS Formation
2.3. Effect of DT on the PER1, CLOCK, BMAL1, and SIRT1 Levels in the 6-OHDA-Treated SH-SY5Y Cells
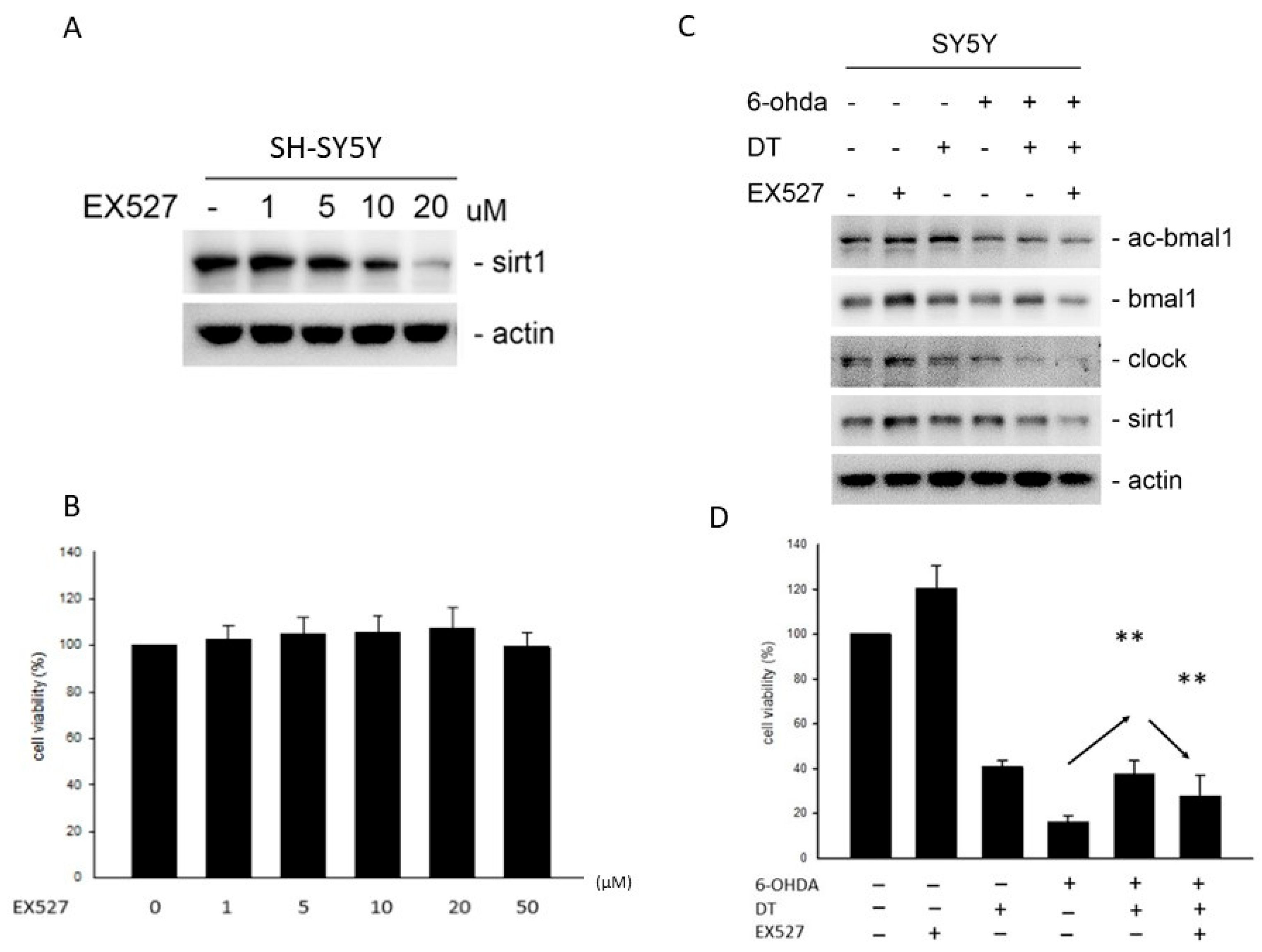
2.4. Effect of DT and the SIRT1 Inhibitor on the 6-OHDA-Induced Cell Toxicity
2.5. Effect of the DT, ROS, and SIRT1 Activators on BMAL1, SIRT1, and PARP after the 6-OHDA Treatment
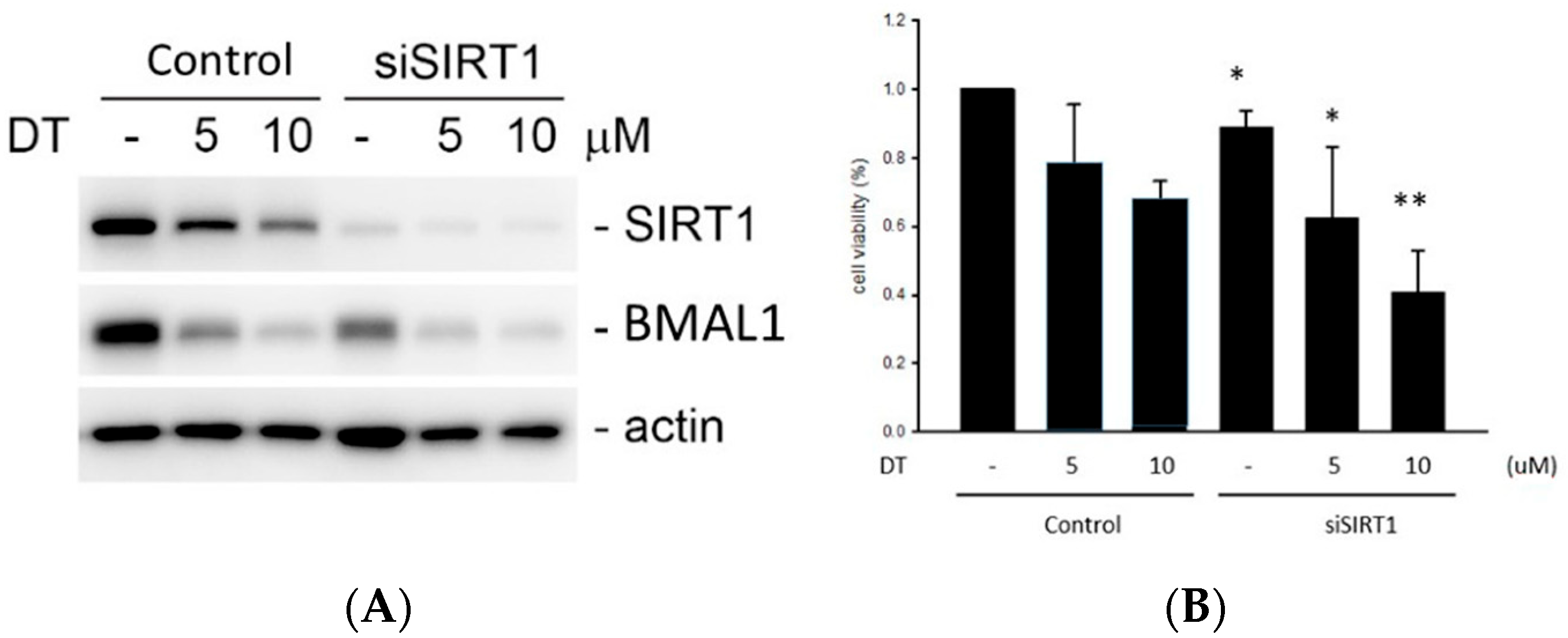
2.6. Effect of DT and SIRT1 siRNA on the BMAL1 Expression and Cell Viablity
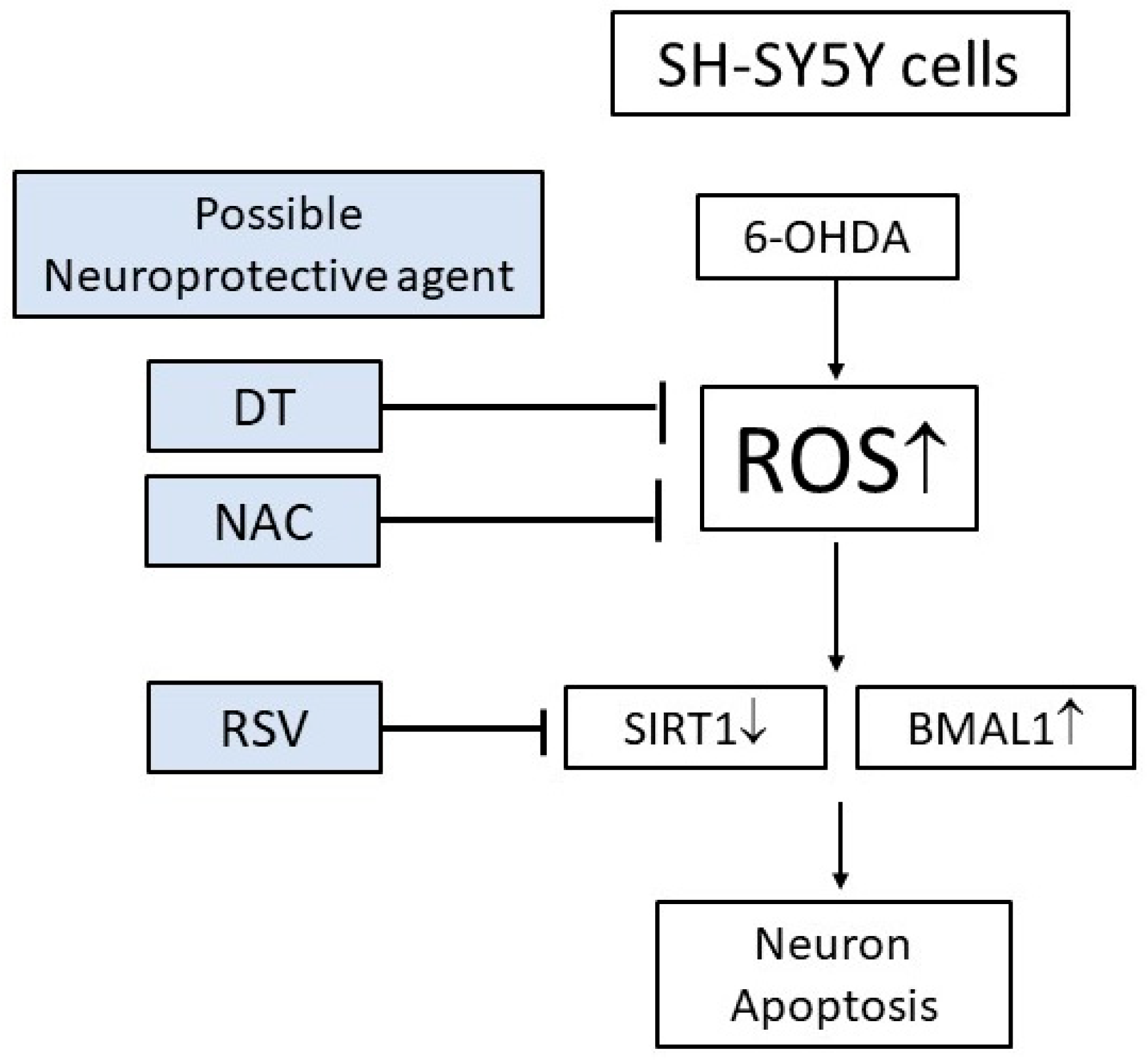
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction
4.3. Evaluation of Apoptosis and the ROS Levels Using Flow Cytometry
4.4. Western Blotting
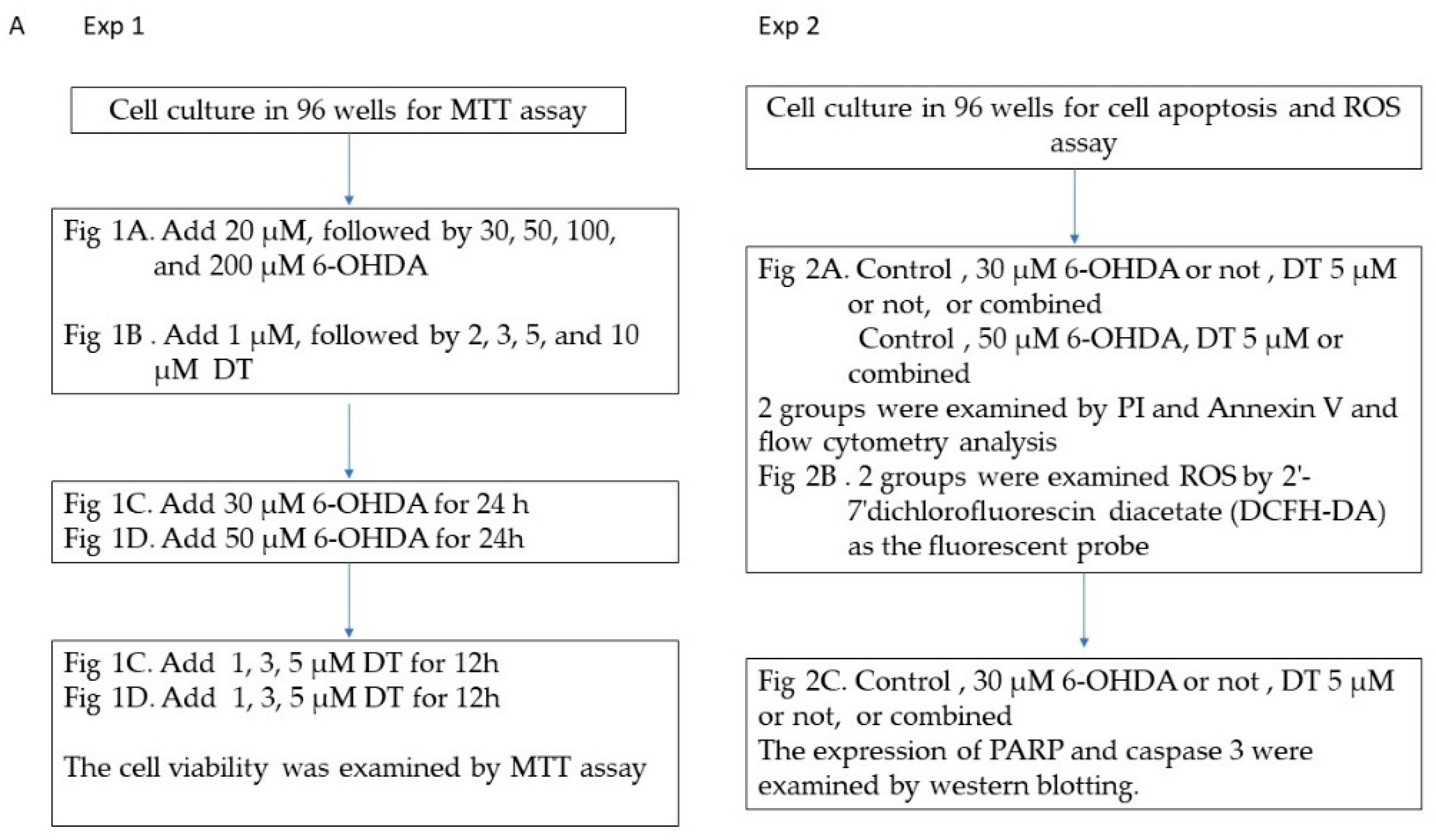
4.5. Experimental Set-Up
4.6. Statistical Analyses
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fahn, S.; Sulzer, D. Neurodegeneration and neuroprotection in Parkinson disease. NeuroRx 2004, 1, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Deleu, D.; Northway, M.G.; Hanssens, Y. An evidence-based review of Dopamine receptor agonists in the treatment of Parkinson’s disease. Neurosciences 2002, 7, 221–231. [Google Scholar] [PubMed]
- Goldenberg, M.M. Medical management of Parkinson’s disease. PT 2008, 33, 590–606. [Google Scholar]
- Factor, S.A. Current status of symptomatic medical therapy in Parkinson’s disease. Neurotherapeutics 2008, 5, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.; Coulson, E.J.; Rajnarayanan, R.; Oster, H.; Videnovic, A.; Rawashdeh, O. Sleep and circadian rhythms in Parkinson’s disease and preclinical models. Mol. Neurodegener. 2022, 17, 2. [Google Scholar] [CrossRef]
- Qian, J.; Scheer, F. Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trends Endocrinol. Metab. 2016, 27, 282–293. [Google Scholar] [CrossRef]
- Geyfman, M.; Kumar, V.; Liu, Q.; Ruiz, R.; Gordon, W.; Espitia, F.; Cam, E.; Millar, S.E.; Smyth, P.; Ihler, A.; et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc. Natl. Acad. Sci. USA 2012, 109, 11758–11763. [Google Scholar] [CrossRef]
- Breen, D.P.; Vuono, R.; Nawarathna, U.; Fisher, K.; Shneerson, J.M.; Reddy, A.B.; Barker, R.A. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014, 71, 589–595. [Google Scholar] [CrossRef]
- Lai, C.C.; Lin, P.M.; Lin, S.F.; Hsu, C.H.; Lin, H.C.; Hu, M.L.; Hsu, C.M.; Yang, M.Y. Altered expression of SIRT1 gene family in head and neck squamous cell carcinoma. Tumour. Biol. 2013, 34, 1847–1854. [Google Scholar] [CrossRef]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef]
- Sandhu, J.K.; Gardaneh, M.; Iwasiow, R.; Lanthier, P.; Gangaraju, S.; Ribecco-Lutkiewicz, M.; Tremblay, R.; Kiuchi, K.; Sikorska, M. Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6OHDA cytotoxicity. Neurobiol. Dis. 2009, 33, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Elyasi, L.; Jahanshahi, M.; Jameie, S.B.; Hamid Abadi, H.G.; Nikmahzar, E.; Khalili, M.; Jameie, M.; Jameie, M. 6-OHDA mediated neurotoxicity in SH-SY5Y cellular model of Parkinson disease suppressed by pretreatment with hesperidin through activating L-type calcium channels. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 11–17. [Google Scholar] [CrossRef]
- Ahmad, A.; Nosheen, F.; Arshad, M.U.; Saeed, F.; Afzaal, M.; Islam, F.; Imran, A.; Noreen, R.; Amer Ali, Y.; Shah, M.A. Isolation and antioxidant characterization of theaflavin for neuroprotective effect in mice model. Food Sci. Nutr. 2023, 11, 3485–3496. [Google Scholar] [CrossRef] [PubMed]
- Fiametti, L.O.; Correa, C.N.; Castro, L.M. Peptide Profile of Zebrafish Brain in a 6-OHDA-Induced Parkinson Model. Zebrafish 2021, 18, 55–65. [Google Scholar] [CrossRef]
- Wang, L.; Ma, R.; Liu, C.; Liu, H.; Zhu, R.; Guo, S.; Tang, M.; Li, Y.; Niu, J.; Fu, M.; et al. Salvia miltiorrhiza: A Potential Red Light to the Development of Cardiovascular Diseases. Curr. Pharm. Des. 2017, 23, 1077–1097. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Huang, F.; Deng, C.; Wang, Y.; Kai, G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit. Rev. Food Sci. Nutr. 2019, 59, 953–964. [Google Scholar] [CrossRef]
- Hsu, C.M.; Yang, M.Y.; Tsai, M.S.; Chang, G.H.; Yang, Y.H.; Tsai, Y.T.; Wu, C.Y.; Chang, S.F. Dihydroisotanshinone I as a Treatment Option for Head and Neck Squamous Cell Carcinomas. Int. J. Mol. Sci. 2021, 22, 8881. [Google Scholar] [CrossRef]
- Fu, L.; Han, B.; Zhou, Y.; Ren, J.; Cao, W.; Patel, G.; Kai, G.; Zhang, J. The Anticancer Properties of Tanshinones and the Pharmacological Effects of Their Active Ingredients. Front. Pharmacol. 2020, 11, 193. [Google Scholar] [CrossRef]
- Zhang, M.X.; Song, Y.; Xu, W.L.; Zhang, L.X.; Li, C.; Li, Y.L. Natural Herbal Medicine as a Treatment Strategy for Myocardial Infarction through the Regulation of Angiogenesis. Evid. Based Complement. Alternat. Med. 2022, 2022, 8831750. [Google Scholar] [CrossRef]
- Xu, X.J.; Long, J.B.; Jin, K.Y.; Chen, L.B.; Lu, X.Y.; Fan, X.H. Danshen-Chuanxiongqin Injection attenuates cerebral ischemic stroke by inhibiting neuroinflammation via the TLR2/TLR4-MyD88-NF-kappaB Pathway in tMCAO mice. Chin. J. Nat. Med. 2021, 19, 772–783. [Google Scholar]
- Bao, Z.; Zhang, H.; Jiao, H.; Chu, X.; Fu, J.; Wang, L. Dihydrotanshinone I Increase Amyloid-beta Clearance and Decrease Tau Phosphorylation via Enhancing Autophagy. Pharmacology 2020, 105, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, R.; Park, S.E.; Seong, S.H.; Paudel, P.; Fauzi, F.M.; Jung, H.A.; Choi, J.S. Monoamine Oxidase Inhibition by Major Tanshinones from Salvia miltiorrhiza and Selective Muscarinic Acetylcholine M4 Receptor Antagonism by Tanshinone I. Biomolecules 2021, 11, 1001. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Ma, J.; Zhang, Y.; Gao, Y.; Zhang, Y.; Zhang, X.; Wang, N.; Xie, Y.; Wang, J.; Zhang, J.; et al. Danshen (Salvia miltiorrhiza) injection suppresses kidney injury induced by iron overload in mice. PLoS ONE 2013, 8, e74318. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jing, H.; Yang, H.; Liu, Z.; Guo, H.; Chai, L.; Hu, L. Tanshinone I selectively suppresses pro-inflammatory genes expression in activated microglia and prevents nigrostriatal dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. J. Ethnopharmacol. 2015, 164, 247–255. [Google Scholar] [CrossRef]
- Seidl, S.E.; Potashkin, J.A. The promise of neuroprotective agents in Parkinson’s disease. Front. Neurol. 2011, 2, 68. [Google Scholar] [CrossRef]
- Visanji, N.P.; Brooks, P.L.; Hazrati, L.N.; Lang, A.E. The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol. Commun. 2013, 1, 2. [Google Scholar] [CrossRef]
- Salamon, A.; Zadori, D.; Szpisjak, L.; Klivenyi, P.; Vecsei, L. Neuroprotection in Parkinson’s disease: Facts and hopes. J. Neural Transm. 2020, 127, 821–829. [Google Scholar] [CrossRef]
- Weitzel, J.; Langer, K.; Rose, O. Effects of Generic Exchange of Levodopa Medication in Patients with Parkinson Disease. J. Patient Saf. 2022, 18, 704–710. [Google Scholar] [CrossRef]
- Maruyama, W.; Nitta, A.; Shamoto-Nagai, M.; Hirata, Y.; Akao, Y.; Yodim, M.; Furukawa, S.; Nabeshima, T.; Naoi, M. N-Propargyl-1 (R)-aminoindan, rasagiline, increases glial cell line-derived neurotrophic factor (GDNF) in neuroblastoma SH-SY5Y cells through activation of NF-kappaB transcription factor. Neurochem. Int. 2004, 44, 393–400. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. Rasagiline and selegiline modulate mitochondrial homeostasis, intervene apoptosis system and mitigate alpha-synuclein cytotoxicity in disease-modifying therapy for Parkinson’s disease. J. Neural Transm. 2020, 127, 131–147. [Google Scholar] [CrossRef]
- Farrer, M.; Hardy, J.; Hutton, M.; Maraganore, D.; Tsuboi, Y.; Wszolek, Z.K. Identifying genetic factors in Parkinson disease. JAMA 2022, 287, 715–716. [Google Scholar] [CrossRef]
- Ovidi, E.; Masci, V.L. Salvia species, interesting plants offering perspectives in Alzheimer’s disease. Curr. Tradit. Med. 2018, 4, 184–191. [Google Scholar] [CrossRef]
- Sung, P.S.; Lin, P.Y.; Liu, C.H.; Su, H.C.; Tsai, K.J. Neuroinflammation and Neurogenesis in Alzheimer’s Disease and Potential Therapeutic Approaches. Int. J. Mol. Sci. 2020, 21, 701. [Google Scholar] [CrossRef]
- Videnovic, A.; Golombek, D. Circadian Dysregulation in Parkinson’s Disease. Neurobiol. Sleep Circadian Rhythm 2017, 2, 53–58. [Google Scholar] [CrossRef]
- Leng, Y.; Musiek, E.S.; Hu, K.; Cappuccio, F.P.; Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019, 18, 307–318. [Google Scholar] [CrossRef]
- Evans, J.A.; Mendonca, P.; Soliman, K.F.A. Neuroprotective Effects and Therapeutic Potential of the Citrus Flavonoid Hesperetin in Neurodegenerative Diseases. Nutrients 2022, 14, 2228. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.S.; Li, R.; Liu, V.Y.; Fu, L.; Moore, D.D.; Ma, K.; Yechoor, V.K. Loss of BMAL1 leads to uncoupling and impaired glucose-stimulated insulin secretion in beta-cells. Islets 2011, 3, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, J.; Anwar, H.; Rasul, A.; Imran, A.; Saadullah, M.; Malik, S.A.; Shabbir, A.; Akram, R.; Sajid, F.; Zafar, S.; et al. Comparative evaluation of ethyl acetate and n-Hexane extracts of Cannabis sativa L. leaves for muscle function restoration after peripheral nerve lesion. Food Sci. Nutr. 2023, 11, 2767–2775. [Google Scholar] [CrossRef] [PubMed]
- Strathearn, K.E.; Yousef, G.G.; Grace, M.H.; Roy, S.L.; Tambe, M.A.; Ferruzzi, M.G.; Wu, Q.L.; Simon, J.E.; Lila, M.A.; Rochet, J.C. Neuro Brain Res protective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinsons disease. Brain Res. 2014, 1555, 60–77. [Google Scholar] [CrossRef]
- Zhang, P.; He, S.; Wu, S.; Li, Y.; Wang, H.; Yan, C.; Li, P. Discovering a Multi-Component Combination against Vascular Dementia from Danshen-Honghua Herbal Pair by Spectrum-Effect Relationship Analysis. Pharmaceuticals 2019, 15, 1073. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, C.; Han, B.; Wang, Z.; Meng, X.; Zhang, L.; He, J.; Fu, F. Neuroprotective effects of Danshensu on rotenone-induced Parkinson’s disease models in vitro and in vivo. BMC Complement. Med. Ther. 2020, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jackson, C.W.; Khoury, N.; Escobar, I.; Perez-Pinzon, M.A. Brain SIRT1 Mediates Metabolic Homeostasis and Neuroprotection. Front. Endocrinol. 2018, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.; Lee, C.E.; Bookout, A.L.; Lee, S.; Williams, K.W.; Anderson, J.; Elmquist, J.K.; Coppari, R. Brain SIRT1: Anatomical distribution and regulation by energy availability. J. Neurosci. 2008, 28, 9989–9996. [Google Scholar] [CrossRef] [PubMed]
- Quintas, A.; de Solis, A.J.; Diez-Guerra, F.J.; Carrascosa, J.M.; Bogonez, E. Age-associated decrease of SIRT1 expression in rat hippocampus: Prevention by late onset caloric restriction. Exp. Gerontol. 2012, 47, 198–201. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Wang, X.X.; Truong, D.; Wu, Y.C. The Critical Role of SIRT1 in Parkinson’s Disease: Mechanism and Therapeutic Considerations. Aging Dis. 2020, 11, 1608–1622. [Google Scholar] [CrossRef]
- Singh, P.; Hanson, P.S.; Morris, C.M. SIRT1 ameliorates oxidative stress induced neural cell death and is down-regulated in Parkinson’s disease. BMC Neurosci. 2017, 18, 46. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Nolan, T.; Pfaffl, M.W. Quantitative real-time RT-PCR—A perspective. J. Mol. Endocrinol. 2005, 34, 597–601. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Begum, H.; Murugesan, P.; Tangutur, A.D. Western blotting: A powerful staple in scientific and biomedical research. Biotechniques 2022, 73, 58–69. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.-C.; Sun, Y.-T.; Yang, M.-Y.; Wu, C.-Y.; Hsu, C.-M. Dihydroisotanshinone I and BMAL-SIRT1 Pathway in an In Vitro 6-OHDA-Induced Model of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 11088. https://doi.org/10.3390/ijms241311088
Su H-C, Sun Y-T, Yang M-Y, Wu C-Y, Hsu C-M. Dihydroisotanshinone I and BMAL-SIRT1 Pathway in an In Vitro 6-OHDA-Induced Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2023; 24(13):11088. https://doi.org/10.3390/ijms241311088
Chicago/Turabian StyleSu, Hui-Chen, Yuan-Ting Sun, Ming-Yu Yang, Ching-Yuan Wu, and Cheng-Ming Hsu. 2023. "Dihydroisotanshinone I and BMAL-SIRT1 Pathway in an In Vitro 6-OHDA-Induced Model of Parkinson’s Disease" International Journal of Molecular Sciences 24, no. 13: 11088. https://doi.org/10.3390/ijms241311088
APA StyleSu, H.-C., Sun, Y.-T., Yang, M.-Y., Wu, C.-Y., & Hsu, C.-M. (2023). Dihydroisotanshinone I and BMAL-SIRT1 Pathway in an In Vitro 6-OHDA-Induced Model of Parkinson’s Disease. International Journal of Molecular Sciences, 24(13), 11088. https://doi.org/10.3390/ijms241311088






