Clinical and Biological Predictors of Cancer Incidence and Mortality in Patients with Stable Coronary Artery Disease
Abstract
:1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics
2.2. Follow-Up and Cancer Outcome
2.3. Association between Risk Factors for CAD and Incidence of Cancer
2.4. Association between Risk Factors for CAD and Cancer Death
2.5. Circulating Biomarkers Levels in Patients with Different Types of Cancer vs. Those without Cancer
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Study Design
4.3. Routine and Specific Biochemical Measurements
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.H.; Lo, Y.C.; Pan, W.S.; Liu, S.J.; Yeh, T.L.; Liu, L.Y. Association between coronary artery disease and incident cancer risk: A systematic review and meta-analysis of cohort studies. Peer J. 2023, 11, e14922. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Canepa, M.; Maack, C.; Ameri, P. Linking heart failure to cancer: Background evidence and research perspectives. Circulation 2018, 138, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Perez, I.E.; Taveras Alam, S.; Hernandez, G.A.; Sancassani, R. Cancer therapy-related cardiac dysfunction: An overview for the clinician. Clin. Med. Insights Cardiol. 2019, 13, 1179546819866445. [Google Scholar] [CrossRef] [Green Version]
- Rinde, L.B.; Småbrekke, B.; Hald, E.M.; Brodin, E.E.; Njølstad, I.; Mathiesen, E.B.; Løchen, M.L.; Wilsgaard, T.; Brækkan, S.K.; Vik, A.; et al. Myocardial infarction and future risk of cancer in the general population-the Tromsø Study. Eur. J. Epidemiol. 2017, 32, 193–201. [Google Scholar] [CrossRef]
- Banke, A.; Schou, M.; Videbaek, L.; Møller, J.E.; Torp-Pedersen, C.; Gustafsson, F.; Dahl, J.S.; Køber, L.; Hildebrandt, P.R.; Gislason, G.H. Incidence of cancer in patients with chronic heart failure: A long-term follow-up study. Eur. J. Heart Fail. 2016, 18, 260–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, M.; Tomoike, H.; Sumiyoshi, T.; Nagatomo, Y.; Hosoda, T.; Nagayama, M.; Ishikawa, Y.; Sawa, T.; Iimuro, S.; Yoshikawa, T.; et al. Incidence of cancers in patients with atherosclerotic cardiovascular diseases. Int. J. Cardiol. Heart Vasc. 2017, 17, 11–16. [Google Scholar] [CrossRef]
- Rajai, N.; Ahmad, A.; Toya, T.; Sara, J.D.; Herrmann, J.; Lerman, L.O.; Lerman, A. Coronary microvascular dysfunction is an independent predictor of developing cancer in patients with non-obstructive coronary artery disease. Eur. J. Prev. Cardiol. 2023, 30, 209–216. [Google Scholar] [CrossRef]
- Curigliano, G.; Cardinale, D.; Suter, T.; Plataniotis, G.; de Azambuja, E.; Sandri, M.T.; Criscitiello, C.; Goldhirsch, A.; Cipolla, C.; Roila, F. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2012, 23, vii155–vii166. [Google Scholar] [CrossRef]
- Li, N.; Huang, Z.; Zhang, Y.; Sun, H.; Wang, J.; Zhao, J. Increased cancer risk after myocardial infarction: Fact or fiction? A systemic review and meta-analysis. Cancer Manag. Res. 2019, 11, 1959–1968. [Google Scholar] [CrossRef] [Green Version]
- Das, D.; Asher, A.; Ghosh, A.K. Cancer and Coronary Artery Disease: Common Associations, Diagnosis and Management Challenges. Curr. Treat. Options Oncol. 2019, 20, 46. [Google Scholar] [CrossRef]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arfsten, H.; Cho, A.; Prausmüller, S.; Spinka, G.; Novak, J.; Goliasch, G.; Bartko, P.E.; Raderer, M.; Gisslinger, H.; Kornek, G.; et al. Inflammation-Based Scores as a Common Tool for Prognostic Assessment in Heart Failure or Cancer. Front. Cardiovasc. Med. 2021, 8, 725903. [Google Scholar] [CrossRef]
- Zhu, Y.; Bi, Y.; Liu, B.; Zhu, T. Assessment of prognostic value of preoperative neutrophil-to-lymphocyte ratio for postoperative mortality and morbidity. Front. Med. 2023, 10, 1102733. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Duan, S.; Li, Z.; Li, G.; Yu, H. Single and combined use of the platelet- lymphocyte ratio, neutrophil-lymphocyte ratio, and systemic immune-inflammation index in gastric cancer diagnosis. Front. Oncol. 2023, 13, 1143154. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Einstein, A.J.; Fazel, R.; Krumholz, H.M.; Wang, Y.; Ross, J.S.; Ting, H.H.; Shah, N.D.; Nasir, K.; Nallamothu, B.K. Cumulative exposure to ionizing radiation from diagnostic and therapeutic cardiac imaging procedures: A population-based analysis. J. Am. Coll. Cardiol. 2010, 56, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, M.J.; Afilalo, J.; Lawler, P.R.; Abrahamowicz, M.; Richard, H.; Pilote, L. Cancer risk related to low-dose ionizing radiation from cardiac imaging in patients after acute myocardial infarction. CMAJ 2011, 183, 430–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpeggiani, C.; Rossi, G.; Landi, P.; Michelassi, C.; Brambilla, M.; Cortigiani, L.; Picano, E. Long-term outcome and medical radiation exposure in patients hospitalized for cardiovascular disease. Int. J. Cardiol. 2015, 195, 30–36. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Q.; Zhong, F.; Guo, S.; Jin, Z.; Shi, W.; Chen, C.; He, J. Association between calcium channel blockers and breast cancer: A meta-analysis of observational studies. Pharmacoepidemiol. Drug Saf. 2014, 23, 711–718. [Google Scholar] [CrossRef]
- Loosen, S.H.; Schöler, D.; Luedde, M.; Eschrich, J.; Luedde, T.; Gremke, N.; Kalder, M.; Kostev, K.; Roderburg, C. Antihypertensive Therapy and Incidence of Cancer. J. Clin. Med. 2022, 11, 6624. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, Z.; Wei, S.; Liu, Z.; Pooley, K.A.; Dunning, A.M.; Svenson, U.; Roos, G.; Hosgood, H.D., 3rd; Shen, M.; et al. Shortened telomere length is associated with increased risk of cancer: A meta-analysis. PLoS ONE 2011, 6, e20466. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Yao, X.; Shen, Y. Altered mitochondrial DNA copy number contributes to human cancer risk: Evidence from an updated meta-analysis. Sci. Rep. 2016, 6, 35859. [Google Scholar] [CrossRef] [Green Version]
- Andreassi, M.G.; Botto, N. DNA damage as a new emerging risk factor in atherosclerosis. Trends Cardiovasc. Med. 2003, 13, 270–275. [Google Scholar] [CrossRef]
- Martinet, W.; Knaapen, M.W.; De Meyer, G.R.; Herman, A.G.; Kockx, M.M. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation 2002, 106, 927–932. [Google Scholar] [CrossRef] [Green Version]
- Botto, N.; Masetti, S.; Petrozzi, L.; Vassalle, C.; Manfredi, S.; Biagini, A.; Andreassi, M.G. Elevated levels of oxidative DNA damage in patients with coronary artery disease. Coron. Artery Dis. 2002, 13, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Stagliano, N.E.; Donovan, M.J.; Breitbart, R.E.; Ginsburg, G.S. Atherosclerosis and cancer: Common molecular pathways of disease development and progression. Ann. N. Y. Acad. Sci. 2001, 947, 271–292. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Lei, Y.; Chen, Y.; Cheng, M.; Wei, X.; Liu, J.; Liu, P.; Chen, R.; Yin, X.; et al. Coronary Atherosclerotic Disease and Cancer: Risk Factors and Interrelation. Front. Cardiovasc. Med. 2022, 9, 821267. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Botteri, E.; Iodice, S.; Boniol, M.; Lowenfels, A.B.; Maisonneuve, P.; Boyle, P. Tobacco smoking and cancer: A meta-analysis. Int. J. Cancer 2008, 122, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Wolin, K.Y.; Carson, K.; Colditz, G.A. Obesity and cancer. Oncologist 2010, 15, 556–565. [Google Scholar] [CrossRef]
- Pulliero, A.; Godschalk, R.; Andreassi, M.G.; Curfs, D.; Van Schooten, F.J.; Izzotti, A. Environmental carcinogens and mutational pathways in atherosclerosis. Int. J. Hyg. Environ. Health 2015, 218, 293–312. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.M.; Qiu, S.J.; Zhou, J.; et al. Systemic immuneinflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef] [Green Version]
- Fest, J.; Ruiter, R.; Mulder, M.; Groot Koerkamp, B.; Ikram, M.A.; Stricker, B.H.; van Eijck, C.H.J. The systemic immune-inflammation index is associated with an increased risk of incident cancer-A population-based cohort study. Int. J. Cancer 2020, 146, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Zhai, E.T.; Yuan, Y.J.; Wu, K.M.; Xu, J.B.; Peng, J.J.; Chen, C.Q.; He, Y.L.; Cai, S.R. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J. Gastroenterol. 2017, 23, 6261–6272. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Sideras, K.; Aziz, N.A.; Mauff, K.; Haen, R.; Roos, D.; Saida, L.; Suker, M.; van der Harst, E.; Mieog, J.S.; et al. The Systemic-immune-inflammation Index Independently Predicts Survival and Recurrence in Resectable Pancreatic Cancer and its Prognostic Value Depends on Bilirubin Levels: A Retrospective Multicenter Cohort Study. Ann. Surg. 2019, 270, 139–146. [Google Scholar] [CrossRef]
- Li, Y.; Sundquist, K.; Wang, X.; Zhang, N.; Hedelius, A.; Sundquist, J.; Memon, A.A. Association of mitochondrial DNA copy number and telomere length with prevalent and incident cancer and cancer mortality in women: A prospective Swedish population-based study. Cancers 2021, 13, 3842. [Google Scholar] [CrossRef]
- Giaccherini, M.; Gentiluomo, M.; Fornili, M.; Lucenteforte, E.; Baglietto, L.; Campa, D. Association between telomere length and mitochondrial copy number and cancer risk in humans: A meta-analysis on more than 300,000 individuals. Crit. Rev. Oncol. Hematol. 2021, 167, 103510. [Google Scholar] [CrossRef]
- Abd Radzak, S.M.; Mohd Khair, S.Z.N.; Ahmad, F.; Patar, A.; Idris, Z.; Mohamed Yusoff, A.A. Insights regarding mitochondrial DNA copy number alterations in human cancer (Review). Int. J. Mol. Med. 2022, 50, 104. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Yu, X.; Zhou, H.; Luo, Y.; Wang, W.; Wang, L. Clinical application of plasma mitochondrial DNA content in patients with lung cancer. Oncol. Lett. 2018, 16, 7074–7081. [Google Scholar] [CrossRef]
- Meng, S.; De Vivo, I.; Liang, L.; Hu, Z.; Christiani, D.C.; Giovannucci, E.; Han, J. Pre-diagnostic leukocyte mitochondrial DNA copy number and risk of lung cancer. Oncotarget 2016, 7, 27307–27312. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Forman, M.R.; Graham, B.H.; Monahan, P.O.; Giovannucci, E.L.; De Vivo, I.; Chan, A.T.; Nan, H. Association between pre-diagnostic leukocyte mitochondrial DNA copy number and survival among colorectal cancer patients. Cancer Epidemiol. 2020, 68, 101778. [Google Scholar] [CrossRef]
- Qu, F.; Chen, Y.; Wang, X.; He, X.; Ren, T.; Huang, Q.; Zhang, J.; Liu, X.; Guo, X.; Gu, J.; et al. Leukocyte mitochondrial DNA content: A novel biomarker associated with prognosis and therapeutic outcome in colorectal cancer. Carcinogenesis 2015, 36, 543–552. [Google Scholar] [CrossRef]
- Reznik, E.; Miller, M.L.; Şenbabaoğlu, Y.; Riaz, N.; Sarungbam, J.; Tickoo, S.K.; Al-Ahmadie, H.A.; Lee, W.; Seshan, V.E.; Hakimi, A.A.; et al. Mitochondrial DNA copy number variation across human cancers. Elife 2016, 5, e10769. [Google Scholar] [CrossRef] [PubMed]
- Ruta, V.M.; Man, A.M.; Alexescu, T.G.; Motoc, N.S.; Tarmure, S.; Ungur, R.A.; Todea, D.A.; Coste, S.C.; Valean, D.; Pop, M.C. Neutrophil-To-Lymphocyte Ratio and Systemic Immune-Inflammation Index-Biomarkers in Interstitial Lung Disease. Medicina 2020, 56, 381. [Google Scholar] [CrossRef]
- Nicoară, D.M.; Munteanu, A.I.; Scutca, A.C.; Mang, N.; Juganaru, I.; Brad, G.F.; Mărginean, O. Assessing the relationship between systemic immune-inflammation index and metabolic syndrome in children with obesity. Int. J. Mol. Sci. 2023, 24, 8414. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Chen, P.; Xu, W.; Wu, Y.; Che, G. Prognostic value of the pretreatment systemic immune-inflammation index (SII) in patients with non-small cell lung cancer: A meta-analysis. Ann. Transl. Med. 2019, 7, 10. [Google Scholar] [CrossRef]
- Sabatino, L.; Botto, N.; Borghini, A.; Turchi, S.; Andreassi, M.G. Development of a new multiplex quantitative real-time PCR assay for the detection of the mtDNA(4977) deletion in coronary artery disease patients: A link with telomere shortening. Environ. Mol. Mutagen. 2013, 54, 299–307. [Google Scholar] [CrossRef]
- Vecoli, C.; Borghini, A.; Pulignani, S.; Mercuri, A.; Turchi, S.; Carpeggiani, C.; Picano, E.; Andreassi, M.G. Prognostic value of mitochondrial DNA4977 deletion and mitochondrial DNA copy number in patients with stable coronary artery disease. Atherosclerosis 2018, 276, 91–97. [Google Scholar] [CrossRef] [PubMed]

| Type | Number | % |
|---|---|---|
| Prostate | 19 | 28.4 |
| Lung | 11 | 16.4 |
| Gastrointestinal | 7 | 10.4 |
| Bladder | 7 | 10.4 |
| Breast | 4 | 6.0 |
| Kidney | 4 | 6.0 |
| Brain | 3 | 4.5 |
| Leukemia | 3 | 4.5 |
| Mouth | 2 | 3.0 |
| Skin | 2 | 3.0 |
| Thyroid | 2 | 3.0 |
| Ovarian | 1 | 1.5 |
| Myeloma | 1 | 1.5 |
| Mediastinal | 1 | 1.5 |
| Cancer Event | HR | 95% CI | p-Value | ||
|---|---|---|---|---|---|
| Yes (n = 67) | No (n = 683) | ||||
| Age, years | 68 [63–72] | 65 [59–70] | 1.053 | 1.020–1.087 | 0.001 |
| Male gender, n (%) | 56 (84) | 597 (87) | 0.720 | 0.377–1.376 | 0.320 |
| Cardiovascular risk factors | |||||
| Family history, n (%) | 33 (49) | 380 (56) | 0.772 | 0.478–1.246 | 0.290 |
| Smoking habit, n (%) | 47 (70) | 414 (61) | 1.476 | 0.874–2.491 | 0.145 |
| Diabetes, n (%) | 10 (15) | 101 (15) | 1.050 | 0.536–2.057 | 0.887 |
| Hypertension, n (%) | 28 (42) | 333 (49) | 0.770 | 0.474–1.252 | 0.292 |
| Dysplipidemia, n (%) | 49 (73) | 469 (69) | 1.205 | 0.702–2.068 | 0.499 |
| Obesity, n (%) | 24 (36) | 194 (28) | 1.397 | 0.847–2.302 | 0.190 |
| Previous AMI, n (%) | 36 (52) | 354 (54) | 0.881 | 0.538–1.444 | 0.616 |
| Number of revascularization procedures | |||||
| <2, n (%) | 52 (78) | 581 (85) | Reference | ||
| ≥2, n (%) | 15 (22) | 102 (15) | 1.628 | 0.916–2.894 | 0.096 |
| Circulating biomarkers | |||||
| LTL | 1.01 [0.67–1.29] | 1.00 [0.70–1.40] | 0.952 | 0.643–1.409 | 0.806 |
| mtDNAcn | 33 [19–49] | 29 [18–45] | 1.000 | 0.994–1.005 | 0.871 |
| NLR | 2.10 [1.63–2.70] | 2.10 [1.61–2.77] | 0.927 | 0.739–1.163 | 0.512 |
| SII, 109/L | 521 [376–699] | 483 [353–642] | 1.000 | 0.999–1.001 | 0.896 |
| HR | 95% CI | p-Value | |
|---|---|---|---|
| Age | 1.071 | 1.034–1.109 | <0.001 |
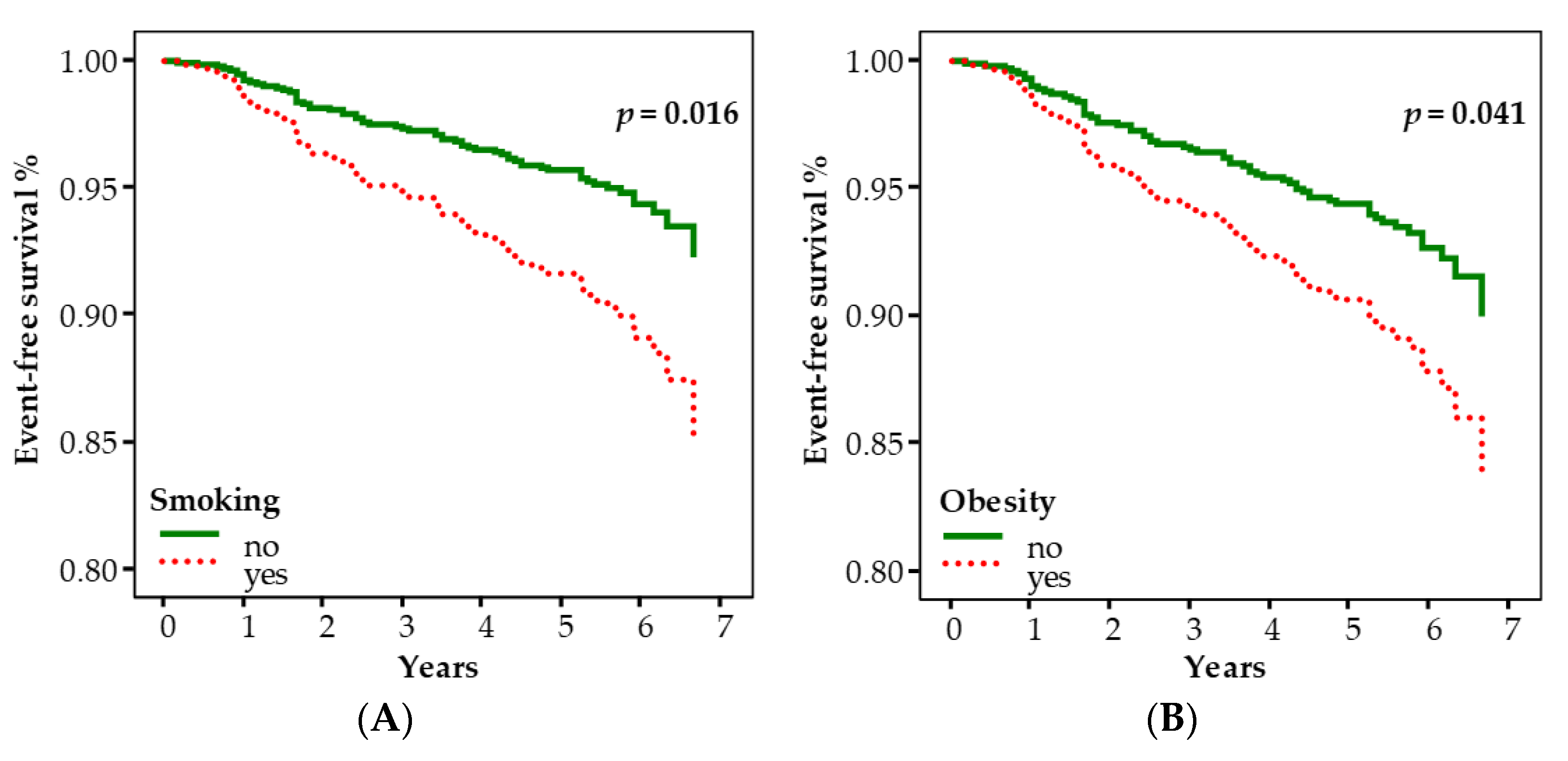
| Smoking habit | 1.994 | 1.140–3.488 | 0.016 |
| Obesity | 1.708 | 1.022–2.854 | 0.041 |
| No. of revascularization procedures ≥ 2 | 1.658 | 0.912–3.017 | 0.098 |
| LTL | 0.893 | 0.586–1.361 | 0.598 |
| mtDNAcn | 1.009 | 0.995–1.009 | 0.653 |
| NLR | 0.621 | 0.379–1.018 | 0.059 |
| SII | 1.002 | 1.001–1003 | 0.045 |
| Cancer Death | HR | 95% CI | p-Value | ||
|---|---|---|---|---|---|
| Yes (n = 19) | No (n = 731) | ||||
| Age, years | 70 [67–75] | 65 [59–70] | 1.122 | 1.049–1.200 | 0.001 |
| Male gender | 16 (84) | 637 (87) | 0.738 | 0.214–2.539 | 0.630 |
| Cardiovascular risk factors | |||||
| Family history, n (%) | 9 (47) | 404 (55) | 0.733 | 0.298–1.804 | 0.499 |
| Smoking, n (%) | 12 (63) | 449 (61) | 1.064 | 0.419–2.702 | 0.897 |
| Diabetes, n (%) | 5 (26) | 106 (15) | 2.125 | 0.765–5.902 | 0.148 |
| Hypertension, n (%) | 10 (53) | 351 (48) | 1.228 | 0.499–3.021 | 0.656 |
| Dysplipidemia, n (%) | 12 (63) | 506 (69) | 0.751 | 0.296–1.909 | 0.548 |
| Obesity, n (%) | 8 (42) | 210 (29) | 1.824 | 0.734–4.537 | 0.196 |
| Previous AMI, n (%) | 13 (68) | 377 (52) | 1.672 | 0.622–4.497 | 0.308 |
| Number of revascularization procedures | |||||
| <2, n (%) | 14 (74) | 619 (85) | Reference | ||
| ≥2, n (%) | 5 (26) | 112 (15) | 1.964 | 0.707–5.455 | 0.195 |
| Circulating biomarkers | |||||
| LTL | 1.04 [0.67–1.70] | 1.00 [0.70–1.39] | 1.402 | 0.759–2.587 | 0.280 |
| mtDNAcn | 30 [17–59] | 29 [18–45] | 0.999 | 0.987–1.011 | 0.878 |
| NLR | 2.20 [1.54–3.17] | 2.10 [1.61–2.77] | 1.020 | 0.729–1.429 | 0.907 |
| SII, 109/L | 493 [322–765] | 485 [356–649] | 1.000 | 0.998–1.001 | 0.762 |
| HR | 95% CI | p-Value | |
|---|---|---|---|
| Age | 1.132 | 1.052–1.219 | 0.001 |
| Diabetes | 1.573 | 0.524–4.725 | 0.420 |
| Obesity | 2.296 | 0.856–6.155 | 0.099 |
| No. of revascularization procedures ≥ 2 | 2.271 | 0.779–6.617 | 0.133 |
| LTL | 1.081 | 0.549–2.128 | 0.882 |
| mtDNAcn | 1.003 | 0.989–1.017 | 0.644 |
| NLR | 1.205 | 0.531–2.736 | 0.656 |
| SII | 0.999 | 0.995–1.002 | 0.534 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campolo, J.; Borghini, A.; Parolini, M.; Mercuri, A.; Turchi, S.; Andreassi, M.G. Clinical and Biological Predictors of Cancer Incidence and Mortality in Patients with Stable Coronary Artery Disease. Int. J. Mol. Sci. 2023, 24, 11091. https://doi.org/10.3390/ijms241311091
Campolo J, Borghini A, Parolini M, Mercuri A, Turchi S, Andreassi MG. Clinical and Biological Predictors of Cancer Incidence and Mortality in Patients with Stable Coronary Artery Disease. International Journal of Molecular Sciences. 2023; 24(13):11091. https://doi.org/10.3390/ijms241311091
Chicago/Turabian StyleCampolo, Jonica, Andrea Borghini, Marina Parolini, Antonella Mercuri, Stefano Turchi, and Maria Grazia Andreassi. 2023. "Clinical and Biological Predictors of Cancer Incidence and Mortality in Patients with Stable Coronary Artery Disease" International Journal of Molecular Sciences 24, no. 13: 11091. https://doi.org/10.3390/ijms241311091





