The Transcriptomic Analysis of the Response of Pinus massoniana to Drought Stress and a Functional Study on the ERF1 Transcription Factor
Abstract
:1. Introduction
2. Results
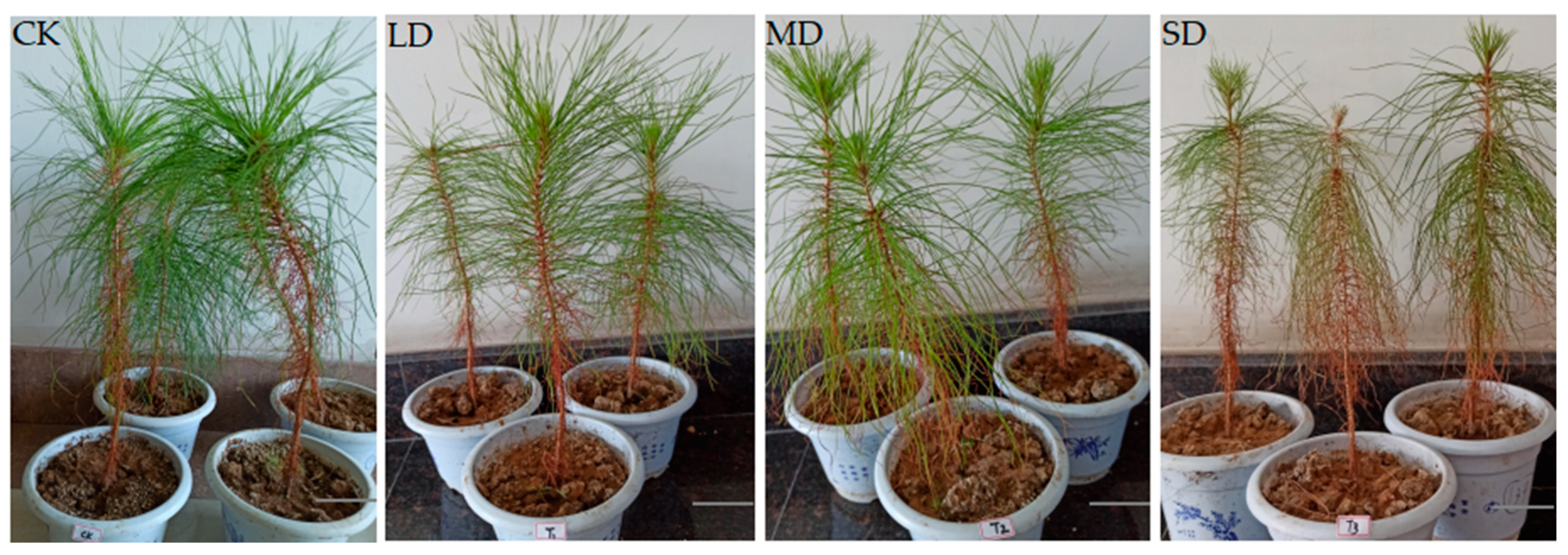
2.1. Effects of Drought Stress on Seedlings of P. massoniana
2.2. Transcriptome Sequencing and De Novo Assembly and Annotation
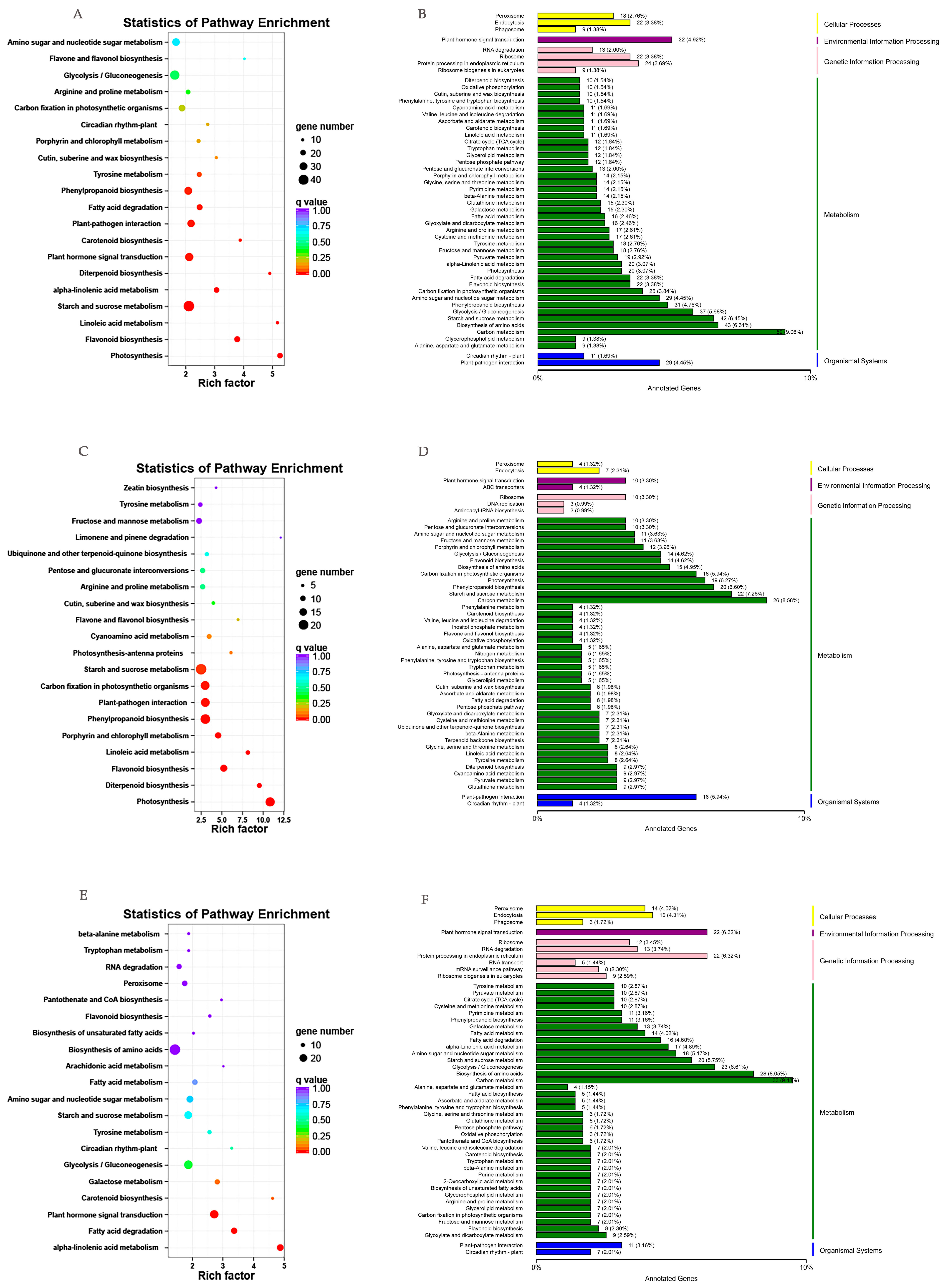
2.3. Identification of DEGs in Drought-Treated P. massoniana
2.4. RT-qPCR Validation
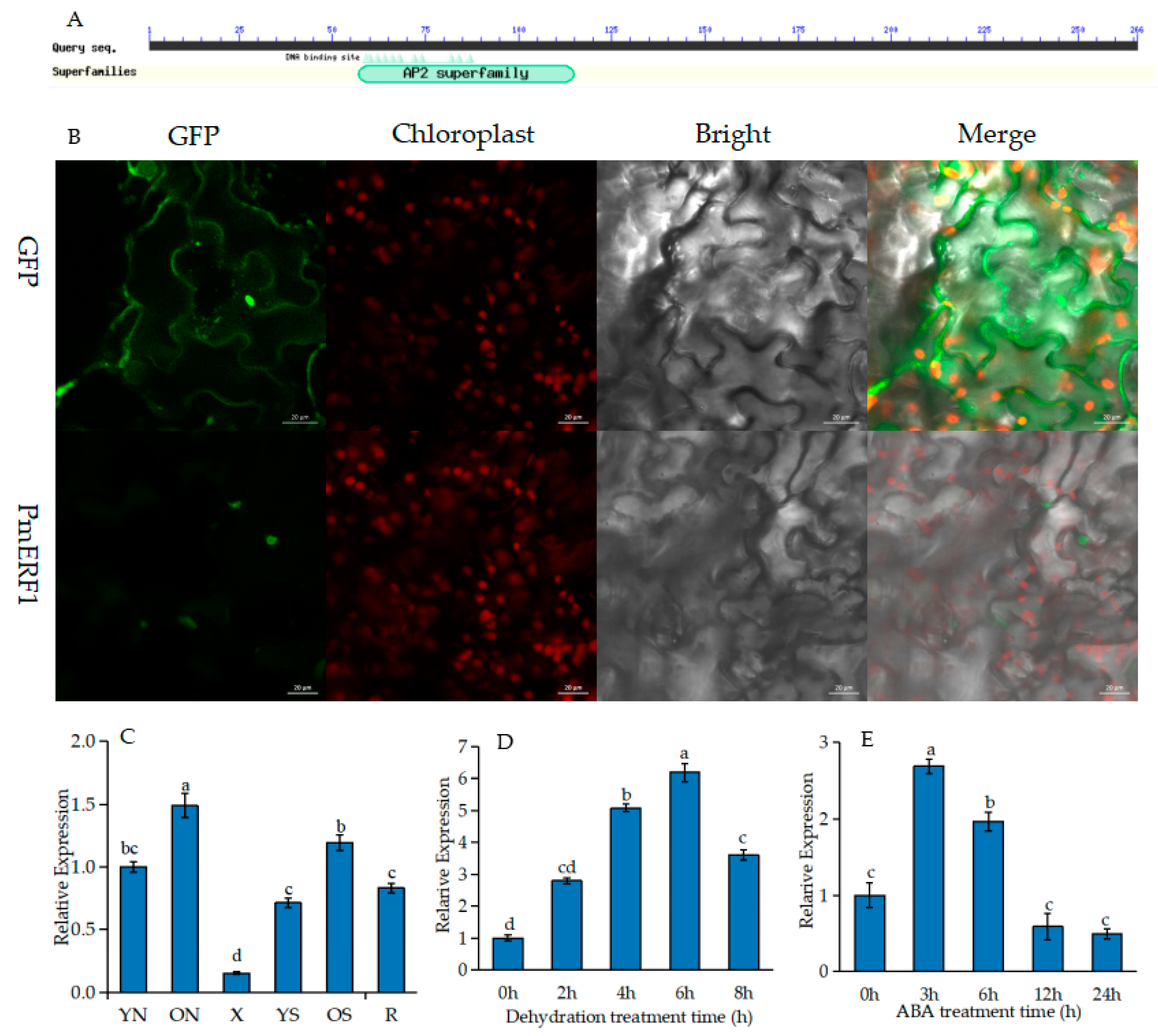
2.5. Expression Pattern Analysis of PmERF1
2.6. Drought Tolerance of OE Lines under Drought Stress
3. Discussion
4. Materials and Methods
4.1. Plant Material and Test Design
4.2. RNA Extraction, Library Construction and RNA-Seq
4.3. Analysis of Gene Expression and Differential Expression
4.4. Feature Annotation and Enrichment Analysis
4.5. RNA Extraction and qRT-PCR Analysis
4.6. Subcellular Localization
4.7. Plasmid Construction and Transformation
4.8. Molecular Verification
4.9. Drought Experiments
4.10. Determination of Photosynthetic Index, Physiological Characteristics and ABA Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.B.M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, M.P. The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Sun, M.; Huang, D.; Zhang, A.; Khan, I.; Yan, H.; Wang, X.; Zhang, X.; Zhang, J.; Huang, L. Transcriptome analysis of heat stress and drought stress in pearl millet based on Pacbio full-length transcriptome sequencing. BMC Plant. Biol. 2020, 20, 323. [Google Scholar] [CrossRef] [PubMed]
- Guerra, D.; Crosatti, C.; Khoshro, H.H.; Mastrangelo, A.M.; Mica, E.; Mazzucotelli, E. Post-transcriptional and post-translational regulations of drought and heat response in plants: A spider’s web of mechanisms. Front. Plant. Sci. 2015, 6, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, H.; Doron-Faigenboim, A.; Kelly, G.; Bourstein, R.; Attia, Z.; Zhou, J.; Moshe, Y.; Moshelion, M.; David-Schwartz, R. Transcriptome analysis of Pinus halepensis under drought stress and during recovery. Tree Physiol. 2018, 38, 423–441. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Kesawat, M.S.; Ali, A.; Lee, S.C.; Gill, S.S.; Kim, H.U. Integration of abscisic acid signaling with other signaling pathways in plant stress responses and development. Plants 2019, 8, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, Y.; Yoshida, T.; Yamaguchi-Shinozaki, K. Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Plant. Physiol. 2013, 147, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y. The Origin and Evolution of Seven Major Plant Hormone Signaling Pathways. Master’s Thesis, Shandong Agrichltural University, Tai’an, China, 2013. [Google Scholar]
- Cheng, M.C.; Liao, P.M.; Kuo, W.W.; Lin, T.P. The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant. Physiol. 2013, 162, 1566–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, S.; Mahmood, T. Functional role of DREB and ERF transcription factors: Regulating stress-responsive network in plants. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Liao, X.; Guo, X.; Wang, Q.; Wang, Y.T.; Zhao, D.; Yao, L.P.; Wang, S.; Liu, G.J.; Li, T.H. Overexpression of MsDREB 6.2 results in cytokine-deficient developmental phenotypes and enhances drought tolerance in transgenic apple plants. Plant. J. 2017, 89, 510–526. [Google Scholar] [CrossRef] [Green Version]
- Li, J.J.; Guo, X.; Zhang, M.H.; Wang, X.; Zhao, Y.; Yin, Z.G.; Zhang, Z.Y.; Wang, Y.M.; Xiong, H.Y.; Zhang, H.L.; et al. OsERF71 confers drought tolerance via modulating ABA signaling and proline biosynthesis. Plant. Sci. 2018, 270, 131–139. [Google Scholar] [CrossRef]
- Matsukura, S.; Mizoi, J.; Yoshida, T.; Todaka, D.; Ito, Y.; Maruya-ma, K.; Yamaguchi-Shinozaki, K. Comprehensive analysis of rice DREB2-type genes that encode transcription factors involved in the expression of abiotic stress-responsive genes. Mol. Genet. Genom. 2010, 283, 185–196. [Google Scholar] [CrossRef]
- Maleki, S.S.; Mohammadi, K.; Movahedi, A.; Wu, F.; Ji, K.S. Increase in cell wall thickening and biomass production by overexpres sion of PmCesA2 in Poplar. Front. Plant. Sci. 2020, 11, 110. [Google Scholar] [CrossRef] [Green Version]
- Hun, X.J.; Ou, Y.X.; Yu, F.Y. Effects of drought stress on growth and biomass of Pinus masoniana seedlings from different provenances. J. Jiangxi Agric. Univ. 2010, 32, 510–516. [Google Scholar]
- Mo, R.H.; Ding, G.J.; Luo, X.Y.; Chen, L. Response to sustained drought of seedlings of different families of Pinus massoniana. J. For. Environ. 2018, 38, 473–480. [Google Scholar]
- Wang, W.D.; Xin, H.H.; Wang, M.L.; Ma, Q.P.; Wang, L.; Kaleri, N.A.; Wang, Y.H.; Li, X.H. Transcriptomic analysis reveals the molecular mechanisms of drought-stress-induced decreases in Camellia sinensis leaf quality. Front. Plant. Sci. 2016, 7, 385. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.F.; Chen, P.Z.; Shun, X.B.; Hu, X.F.; Ji, K.S. Effects of drought on photosynthesis and related physiology of Pinus massoniana seedlings. Chin. Agric. Sci. Bull. 2021, 37, 32–38. [Google Scholar]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.G.; Wang, B.; Zhou, G.L.; Hu, H.L. Influcnces of drought stress on photosynthetic characteristics of fragile willow. J. Sichuan For. Sci. Technol. 2014, 35, 40–44. [Google Scholar]
- Moller, I.M. Plant mitochondria and oxdative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, H.; Liang, Z.S.; Han, X.L. Effects of drought stress on the morphology and growth of Ligustrum lucidum. For. Sci. 2011, 47, 48–55. [Google Scholar]
- Cui, Y.C.; Zhang, W.H.; Li, Z.P. Effects of drought and rewatering on growth and physiological characteristics of Quercus variabilis seedlings. For. Sci. 2014, 50, 66–73. [Google Scholar]
- Song, L.M.; Dai, W.R.; Ren, J.; Ma, X.L.; Ou, Y.Q.; Bi, H.F. Effect of progressive drought and subsequent water recovery on MDA accumulation and antioxidant enzyme activity in leaves of Lotus corniculatus L. J. Yunnan Agric. Univ. 2014, 29, 37–42. [Google Scholar]
- Agarwal, P.K.; Jha, B. Transcription factors in plants and ABA dependent and independent abiotic stress signaling. Biologia Plantarum 2010, 54, 201–212. [Google Scholar] [CrossRef]
- Acharya, B.R.; Assmann, S.M. Hormone interactions in stomatal function. Plant. Mol. Biol. 2009, 69, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.B.; Xu, C.F.; Liu, R.M.; Lin, Z.Z.; Cheng, L.R. Expression change of transcription factors of rice under drought stress. Chin. Agric. Sci. Bull. 2019, 35, 108–114. [Google Scholar]
- Yu, Y.W.; Yang, D.X.; Zhou, S.R.; Gu, J.T.; Wang, F.R.; Dong, J.G.; Huang, R.F. The ethylene response factor OsERF109 negatively affects ethylene biosynthesis and drought tolerance in rice. Protoplasma 2017, 254, 401–408. [Google Scholar] [CrossRef]
- Sakuma, Y.; Liu, Q.; Dubouzet, J.G.; Abe, H.; Shinozaki, K.; Yama, G.K. DNA-binding specificity of the AP2/ERF domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold- inducible gene expression. Biochem. Biophys. Res. Commun. 2002, 290, 998–1009. [Google Scholar] [CrossRef]
- Karaba, A.; Dixit, S.; Greco, R.; Aharoni, A.; Trijatmiko, K.R.; Marsch-Martinez, N.; Krishnan, A.; Nataraja, K.N.; Udayakumar, M.; Pereira, A. Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proc. Natl. Acad. Sci. USA 2007, 104, 15270–15275. [Google Scholar] [CrossRef]
- Martin-St Paul, N.; Delzon, S.; Cochard, H. Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef]
- Yoo, C.Y.; Pence, H.E.; Jin, J.B.; Miura, K.; Gosney, M.J.; Hasegaxa, P.M.; Mickelbart, M.V. The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant. Cell. 2010, 22, 4128–4141. [Google Scholar] [CrossRef] [Green Version]
- Tardieu, F. Any trait or trait-related allele can confer drought tolerance: Just design the right drought scenario. J. Exp. Bot. 2012, 63, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [Green Version]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar]
- Finn, R.D.; Tate, J.; Mistry, J.; Coggill, P.C.; Sammut, S.J.; Hotz, H.R.; Ceric, G.; Forslund, K.; Eddy, S.R.; Sonnhammer, E.L.L.; et al. The Pfam protein families’ database. Nucleic Acids Res. 2008, 36, D281–D288. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.H.; Ma, Y.Y.; Zhu, L.Z.; Chen, Y.; Ji, K.S. Selection of suitable reference genes in Pinus massoniana Lamb. under different abiotic stresses for qPCR normalization. Forests 2019, 10, 8. [Google Scholar]
- Li, Z.Q.; Wang, C.J.; Yang, A.G.; Ding, A.M.; Feng, Q.F.; Xu, J.; Jiao, H.P.; Gao, K.Y. Cloning and subcellular localization of DXS gene in tobacco. J. Anhui Agric. Sci. 2013, 41, 11957–11960. [Google Scholar]
- Li, D.D.; Song, S.Y.; Xia, X.L.; Yin, W.L. Two CBL genes from Populus euphratica confer multiple stress tolerance in transgenic triploid white poplar. Plant. Cell. Tiss. Organ. Cult. 2012, 109, 477–489. [Google Scholar] [CrossRef]




| Gene Name | Gene_id | Gene Annotations | Gene Annotations |
|---|---|---|---|
| SPI2 | c118913.graph_c0 | Phenylpropanoid biosynthesis | Peroxidase |
| NPF | c126294.graph_c2 | Amino acid transport and metabolism | Protein NRT1/PTR FAMILY |
| MYB | c123631.graph_c0 | Myb-like DNA-binding domain | Transcription |
| WRKY | c126676.graph_c0 | WRKY transcription factor 1 | Sequence-specific DNA binding transcription factor activity |
| CPS1 | c129306.graph_c0 | Copalyl diphosphate synthase | Terpene synthase, N-terminal domain |
| bZIP | c118939.graph_c1 | Basic region leucine zipper | Transcription factor HY5 |
| ERF1 | c62205.graph_c0 | Plant hormone signal transduction | Ethylene-responsive transcription factor |
| ASR | c125386.graph_c0 | ABA/WDS-induced protein | ASR protein |
| AUX/IAA | c107390.graph_c0 | Function unknown | AUX/IAA family |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, D.; Chen, P.; Zhang, C.; Yao, S.; Hao, Q.; Agassin, R.H.; Ji, K. The Transcriptomic Analysis of the Response of Pinus massoniana to Drought Stress and a Functional Study on the ERF1 Transcription Factor. Int. J. Mol. Sci. 2023, 24, 11103. https://doi.org/10.3390/ijms241311103
Zhang J, Wang D, Chen P, Zhang C, Yao S, Hao Q, Agassin RH, Ji K. The Transcriptomic Analysis of the Response of Pinus massoniana to Drought Stress and a Functional Study on the ERF1 Transcription Factor. International Journal of Molecular Sciences. 2023; 24(13):11103. https://doi.org/10.3390/ijms241311103
Chicago/Turabian StyleZhang, Jinfeng, Dengbao Wang, Peizhen Chen, Chi Zhang, Sheng Yao, Qingqing Hao, Romaric Hippolyte Agassin, and Kongshu Ji. 2023. "The Transcriptomic Analysis of the Response of Pinus massoniana to Drought Stress and a Functional Study on the ERF1 Transcription Factor" International Journal of Molecular Sciences 24, no. 13: 11103. https://doi.org/10.3390/ijms241311103
APA StyleZhang, J., Wang, D., Chen, P., Zhang, C., Yao, S., Hao, Q., Agassin, R. H., & Ji, K. (2023). The Transcriptomic Analysis of the Response of Pinus massoniana to Drought Stress and a Functional Study on the ERF1 Transcription Factor. International Journal of Molecular Sciences, 24(13), 11103. https://doi.org/10.3390/ijms241311103






