SWI/SNF Complex Alterations in Tumors with Rhabdoid Features: Novel Therapeutic Approaches and Opportunities for Adoptive Cell Therapy
Abstract
1. Introduction
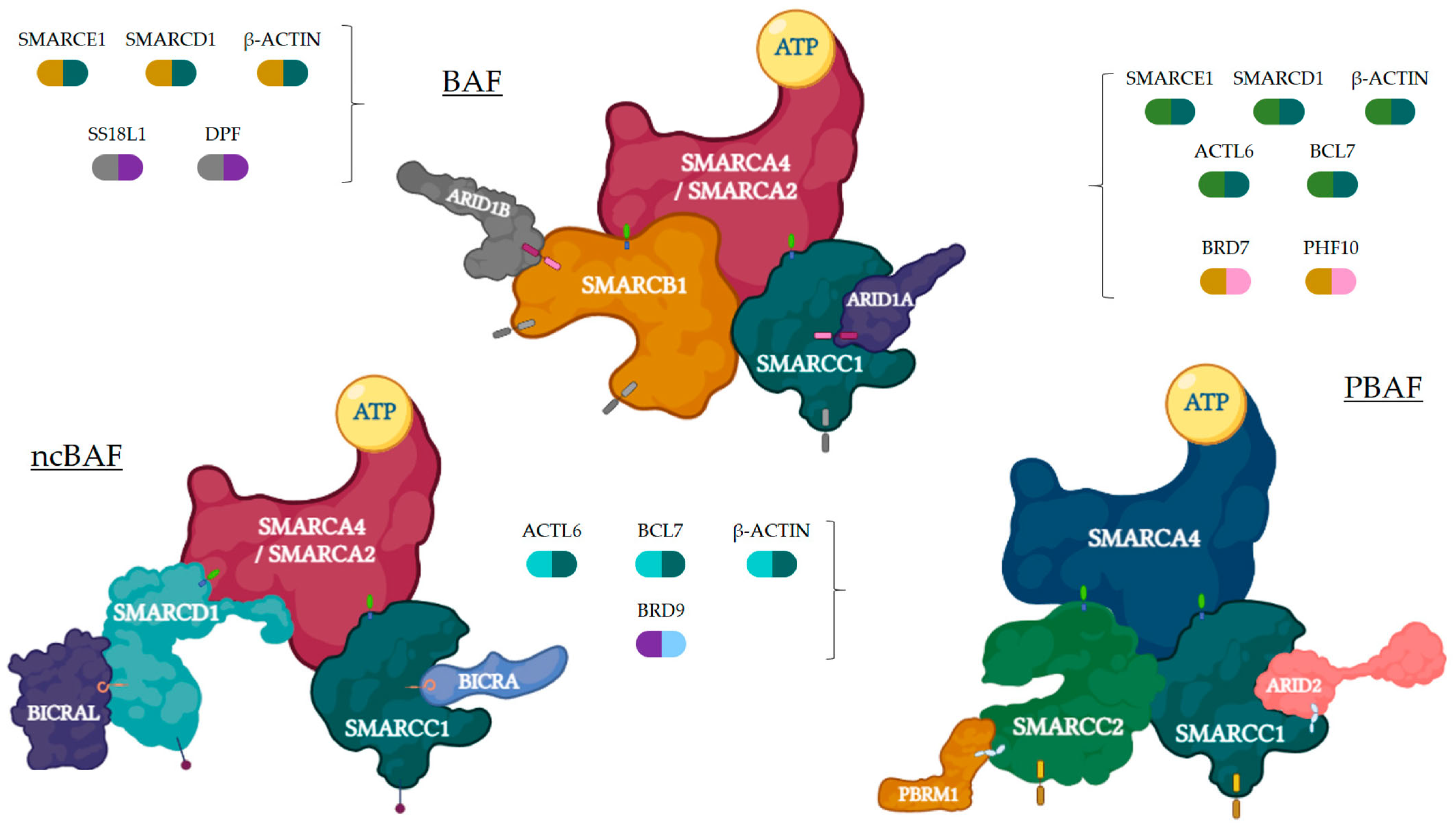
2. The SWI/SNF Complex
2.1. Description
2.2. Proteins Involved and Mechanism of Action
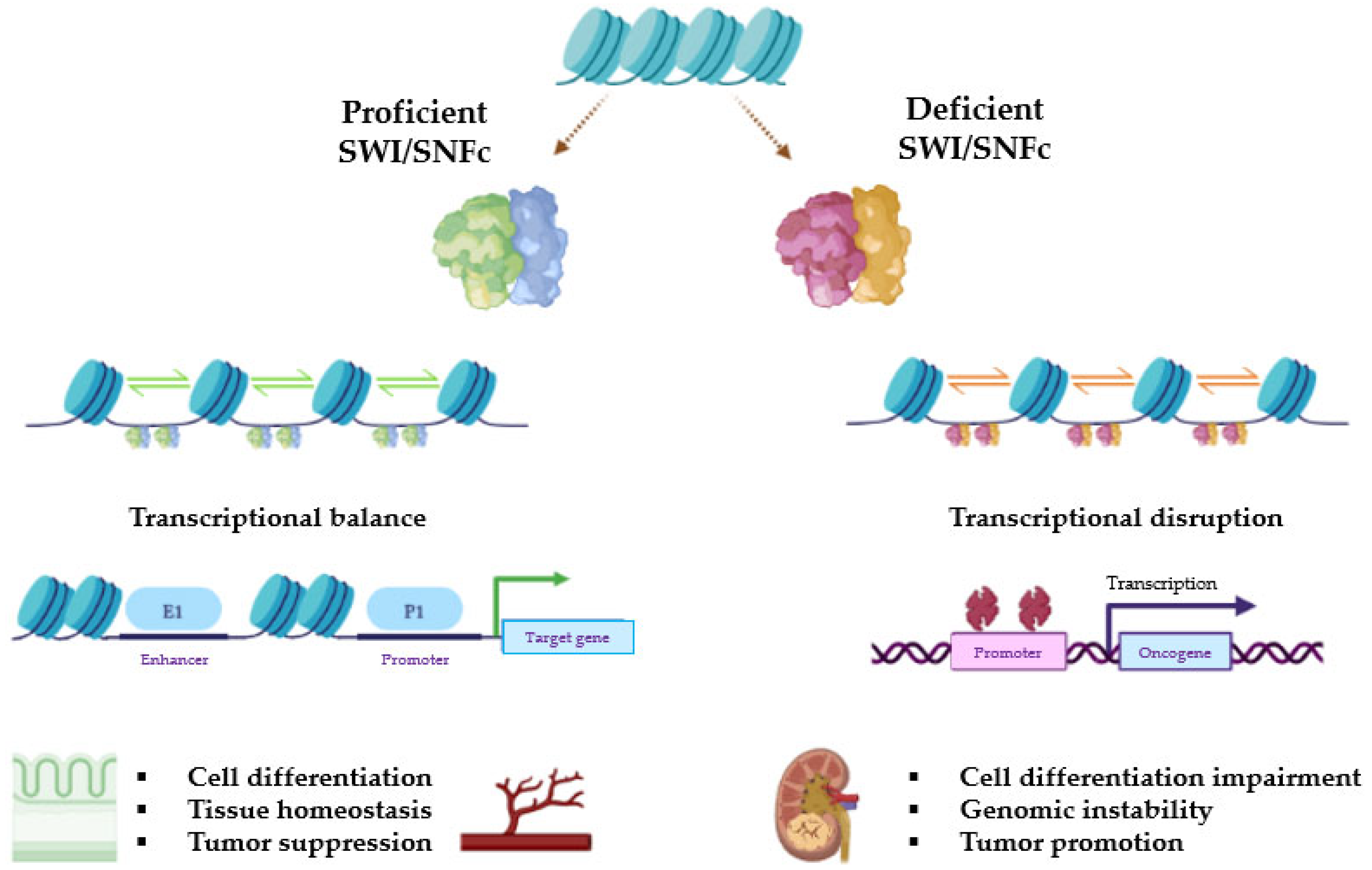
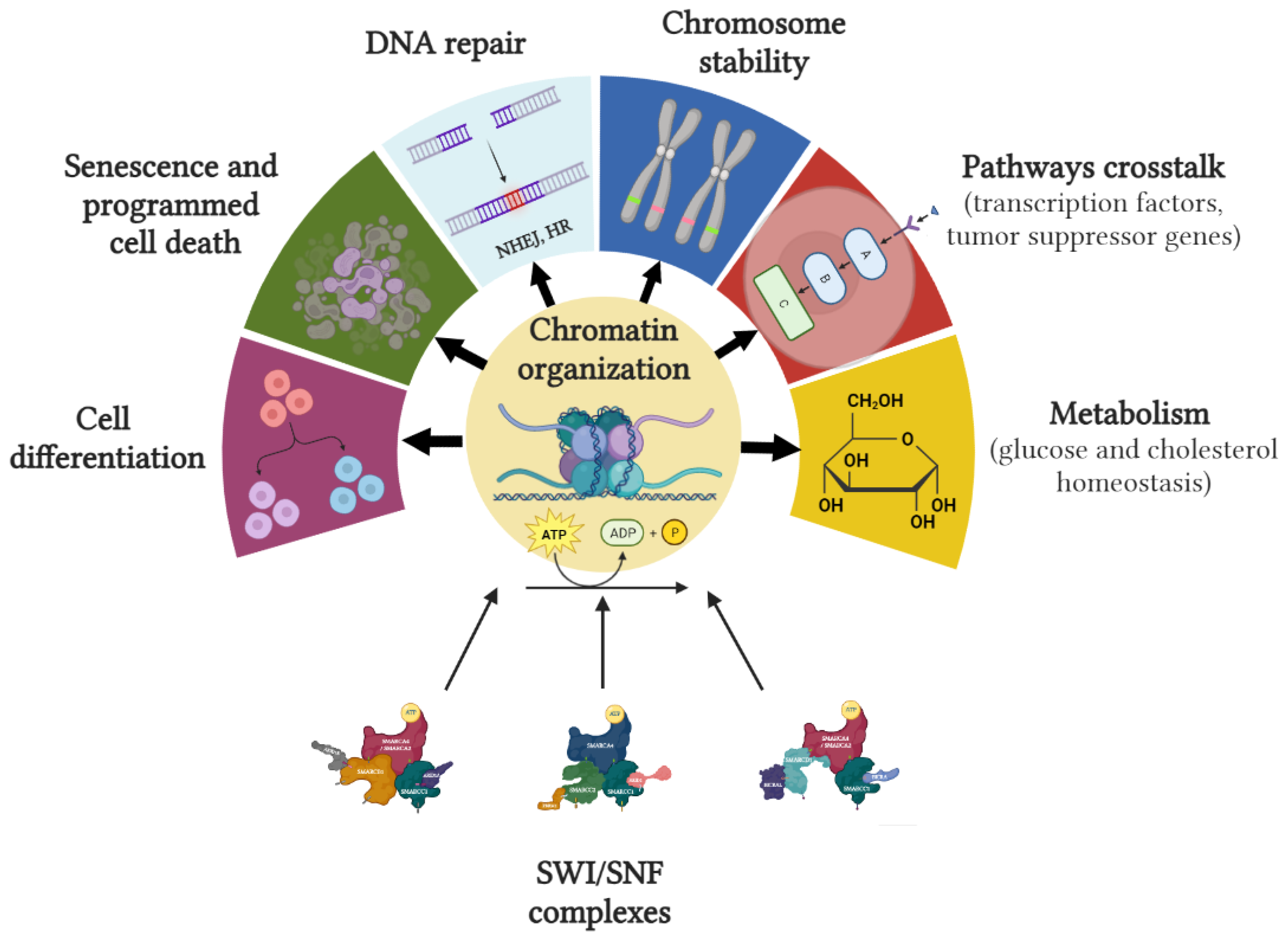
2.3. Role in Carcinogenesis
3. Rhabdoid Tumors Associated with SWI/SNF Complex Alterations
4. Therapeutic Approaches
4.1. Strategies Focusing on SWI/SNFc and Related Targets
4.1.1. Targeting SWI/SNF Subunits
4.1.2. Targeting PRC via EZH2
4.1.3. Targeting DDR Process
4.1.4. Targeting TKR
4.1.5. Targeting Kinases Involved in Cell Cycle
| Author/Year | NCT | Study Design | N | Tumor | Drug | Endpoints/Results and Grade 3–5 AEs |
|---|---|---|---|---|---|---|
| Targeting SWI/SNF complexes | ||||||
| Ongoing | 05639751 | Phase I | 86 | Advanced SMARCA4-mutant solid tumors | PRT3789 (SMARCA2 degrader) | Safety (DLT, MTD, AEs). PK, PD. Efficacy (ORR, PFS, DOR, BOR). |
| Ongoing | 04965753 | Phase I | 104 | Advanced synovial sarcoma and advanced SMARCB1-loss tumors | FHD-609 (BRD9 inhibitor) | Safety (TRAEs, AEs, DLTs). PK, PD. Efficacy (ORR, DOR, PFS, OS). |
| Ongoing | 05355753 | Phase I/II | 110 | Adolescents and adults with advanced SMARCB1-altered tumors | CFT8634 (BRD9 inhibitor) | Safety (AEs, DLTs). PK, PD. Efficacy (ORR, DOR, PFS, OS). |
| Ongoing | 03297424 | Phase I/II | 60–136 | Advanced malignancies with a known ARID1A mutation | PLX2853 (BET inhibitor) | Safety (DLT, AEs). PK, PD. Efficacy (ORR, DOR, OS, PFS). |
| Targeting PCR via EZH2 | ||||||
| Gounder et al. (2020) [63] | 02601950 | Phase II | 62 | Advanced epithelioid sarcoma with loss of INI1/SMARCB1 | Tazemetostat | Efficacy: ORR 15%; DOR not reached; median PFS 5.5 months, median OS 19 months. AEs: Anemia G3 (6%), weight loss G3 (3%). No G4-5 AEs. |
| Ongoing | 01897571 | Phase II | 420 | Advanced-stage solid tumors or B cell lymphomas | Tazemetostat | Efficacy. Safety (MTD, bioavailability). |
| Ongoing | 02601937 | Phase I | 82 | Children with MRT, ATR, RTK, and other tumors with rhabdoid features | Tazemetostat | Safety (AEs) |
| Ongoing | 03213665 | Phase II | 49 | Children R/R solid tumors, NHL or histiocytic disorders EZH2, SMARCB1, or SMARCA4-mutated | Tazemetostat | Efficacy (ORR, PFS). Safety (AEs). |
| Ongoing | 02601950 | Phase II | 250 | Adults MRT, ATRT, RTK with loss of SMARCB1 or SMARCA4 or EZH2-mutated tumors | Tazemetostat | Efficacy (ORR, DOR, PFS). Effect of tazemetostat on immune priming. |
| Ongoing | 02875548 | Phase II | 300 | Adults MRT, ATRT, RTK, synovial, or epithelioid sarcoma, mesothelioma, DLBLC | Tazemetostat | Efficacy (PFS, OS). Safety (AEs). |
| Ongoing | 05151588 | Phase II | 30 | Locally Advanced SMARCB1-deficient sinonasal carcinoma | Chemotherapy + Tazemetostat | Efficacy (BOR, PFS, OS, orbit preservation rate). Safety (AEs). |
| Ongoing | 02601937 | Phase I | 109 | Children and adolescents with R/R INI1-negative tumors or synovial sarcoma | Tazemetostat | Safety (MTD, AEs). PK. Efficacy (ORR, PFS, OS). |
| Inhibitors of DNA damage repair | ||||||
| Ongoing | 0405269 | Phase II | 40–116 | ARID1A-deficient gynecological tumors | Celasertib ± Olaparib | Efficacy (BOR) |
| Ongoing | 03682289 | Phase II | 89 | BAF250-negative solid tumors | Celasertib ± Olaparib or Durvalumab | Efficacy (ORR, DOR, PFS, OS) Safety (AEs) |
| Ongoing | 03207347 | Phase II | 57 | Adults with BAP1 and ARID1-mutant tumors | Niraparib | Efficacy (ORR, PFS, OS) |
| Ongoing | 02576444 | Phase II | 64 | Adults with cancer containing mutations in homologous DNA repair or other DDR genes, including ARID1A | Olaparib + Capivasertib | Efficacy (ORR) |
| Ongoing | 04065269 | Phase II | 40 | Adults with relapsed gynecological cancers, with or without loss of ARID1A | Olaparib + Ceralasertib (AZD6738) | Efficacy (ORR, DCR, PFS, TTP, OS) |
| Ongoing | 05523440 | Phase II | 92 | Recurrent ovarian or endometrial cancer with ARID1A mutation | Bevacizumab ± Niraparib | Efficacy (ORR, DOR, PFS). Safety (AEs) |
| Targeting tyrosine kinase receptors | ||||||
| Ongoing | 03718091 | Phase II | 223 | Adults with advanced-stage solid tumors (including an ARID1-mutant cohort) | Berzosertib (VX-970 and M6620; ATR) | Efficacy (changes in Phospo-CHK1, ɣH2AX levels and DCR |
| Ongoing | 02059265 | Phase II | 35 | Adults with recurrent or persistent gynecological cancer, with or without BAF250 loss | Dasatinib | Efficacy (ORR, PFS, OS) Safety |
| Ongoing | 04284202 | Phase II | 30 | Adults with NSCLC ARID1-mutant | Dasatinib + Toripalimab | Efficacy (PFS, OS) |
| Targeting kinases involved in the cell cycle | ||||||
| Ongoing | 02114229 | Phase II | 180 | Children and young adults with ATR and or extra-CNS MRT (with loss of SMARCB1 and/or extra-CNS MRT (with loss of SMARCB1 or SMARCA4) | Alisertib (Aurora A inhibitor) | Efficacy (ORR, PFS); PK; PD |
| Geoerger et al. (2017) [93] | 01747876 | Phase I | 32 | Children and young adults with SMARCB1-loss tumors | Ribociclib | Efficacy: ORR 0% AEs: Neutropenia G3-4 (63%), leukopenia G3-4 (38%), thrombopenia G3-4 (28%), fatigue G3-4 (3%), AST increased G3-4 (3%), anemia G3-4 (3%), decreased appetite G3-4 (3%) |
4.2. Immunotherapy Strategies
4.2.1. Immune-Checkpoint Inhibition
4.2.2. Adoptive Cell Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Kwon, H.; Imbalzano, A.N.; Khavari, P.A.; Kingston, R.E.; Green, M.R. Nucleosome Disruption and Enhancement of Activator Binding by a Human SW1/SNF Complex. Nature 1994, 370, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Shain, A.H.; Pollack, J.R. The Spectrum of SWI/SNF Mutations, Ubiquitous in Human Cancers. PLoS ONE 2013, 8, e55119. [Google Scholar] [CrossRef] [PubMed]
- Kadoch, C.; Hargreaves, D.C.; Hodges, C.; Elias, L.; Ho, L.; Ranish, J.; Crabtree, G.R. Proteomic and Bioinformatic Analysis of Mammalian SWI/SNF Complexes Identifies Extensive Roles in Human Malignancy. Nat. Genet. 2013, 45, 592–601. [Google Scholar] [CrossRef]
- Roberts, C.W.M.; Galusha, S.A.; McMenamin, M.E.; Fletcher, C.D.M.; Orkin, S.H. Haploinsufficiency of Snf5 (Integrase Interactor 1) Predisposes to Malignant Rhabdoid Tumors in Mice. Proc. Natl. Acad. Sci. USA 2000, 97, 13796–13800. [Google Scholar] [CrossRef] [PubMed]
- Klochendler-Yeivin, A.; Fiette, L.; Barra, J.; Muchardt, C.; Babinet, C.; Yaniv, M. The Murine SNF5/INI1 Chromatin Remodeling Factor Is Essential for Embryonic Development and Tumor Suppression. EMBO Rep. 2000, 1, 500–506. [Google Scholar] [CrossRef]
- Sévenet, N.; Sheridan, E.; Amram, D.; Schneider, P.; Handgretinger, R.; Delattre, O. Constitutional Mutations of the HSNF5/INI1 Gene Predispose to a Variety of Cancers. Am. J. Hum. Genet. 1999, 65, 1342–1348. [Google Scholar] [CrossRef]
- Tolstorukov, M.Y.; Sansam, C.G.; Lu, P.; Koellhoffer, E.C.; Helming, K.C.; Alver, B.H.; Tillman, E.J.; Evans, J.A.; Wilson, B.G.; Park, P.J.; et al. Swi/Snf Chromatin Remodeling/Tumor Suppressor Complex Establishes Nucleosome Occupancy at Target Promoters. Proc. Natl. Acad. Sci. USA 2013, 110, 10165–10170. [Google Scholar] [CrossRef]
- Wiegand, K.C.; Shah, S.P.; Al-Agha, O.M.; Zhao, Y.; Tse, K.; Zeng, T.; Senz, J.; McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E.; et al. ARID1A Mutations in Endometriosis-Associated Ovarian Carcinomas. N. Engl. J. Med. 2010, 363, 1532–1543. [Google Scholar] [CrossRef]
- Varela, I.; Tarpey, P.; Raine, K.; Huang, D.; Ong, C.K.; Stephens, P.; Davies, H.; Jones, D.; Lin, M.-L.; Teague, J.; et al. Exome Sequencing Identifies Frequent Mutation of the SWI/SNF Complex Gene PBRM1 in Renal Carcinoma. Nature 2011, 469, 539–542. [Google Scholar] [CrossRef]
- Brennan, B.; Stiller, C.; Bourdeaut, F. Extracranial Rhabdoid Tumours: What We Have Learned so Far and Future Directions. Lancet Oncol. 2013, 14, e329–e336. [Google Scholar] [CrossRef]
- Lu, B.; Shi, H. An In-Depth Look at Small Cell Carcinoma of the Ovary, Hypercalcemic Type (SCCOHT): Clinical Implications from Recent Molecular Findings. J. Cancer 2019, 10, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Leruste, A.; Tosello, J.; Ramos, R.N.; Tauziède-Espariat, A.; Brohard, S.; Han, Z.-Y.; Beccaria, K.; Andrianteranagna, M.; Caudana, P.; Nikolic, J.; et al. Clonally Expanded T Cells Reveal Immunogenicity of Rhabdoid Tumors. Cancer Cell 2019, 36, 597–612.e8. [Google Scholar] [CrossRef]
- Chun, H.-J.E.; Johann, P.D.; Milne, K.; Zapatka, M.; Buellesbach, A.; Ishaque, N.; Iskar, M.; Erkek, S.; Wei, L.; Tessier-Cloutier, B.; et al. Identification and Analyses of Extra-Cranial and Cranial Rhabdoid Tumor Molecular Subgroups Reveal Tumors with Cytotoxic T Cell Infiltration. Cell Rep. 2019, 29, 2338–2354.e7. [Google Scholar] [CrossRef]
- Kassabov, S.R.; Zhang, B.; Persinger, J.; Bartholomew, B. SWI/SNF Unwraps, Slides, and Rewraps the Nucleosome. Mol. Cell 2003, 11, 391–403. [Google Scholar] [CrossRef]
- Hernández-García, J.; Diego-Martin, B.; Kuo, P.H.; Jami-Alahmadi, Y.; Vashisht, A.A.; Wohlschlegel, J.; Jacobsen, S.E.; Blázquez, M.A.; Gallego-Bartolomé, J. Comprehensive Identification of SWI/SNF Complex Subunits Underpins Deep Eukaryotic Ancestry and Reveals New Plant Components. Commun. Biol. 2022, 5, 549. [Google Scholar] [CrossRef]
- Lessard, J.; Wu, J.I.; Ranish, J.A.; Wan, M.; Winslow, M.M.; Staahl, B.T.; Wu, H.; Aebersold, R.; Graef, I.A.; Crabtree, G.R. An Essential Switch in Subunit Composition of a Chromatin Remodeling Complex during Neural Development. Neuron 2007, 55, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Ronan, J.L.; Wu, J.; Staahl, B.T.; Chen, L.; Kuo, A.; Lessard, J.; Nesvizhskii, A.I.; Ranish, J.; Crabtree, G.R. An Embryonic Stem Cell Chromatin Remodeling Complex, EsBAF, Is Essential for Embryonic Stem Cell Self-Renewal and Pluripotency. Proc. Natl. Acad. Sci. USA 2009, 106, 5181–5186. [Google Scholar] [CrossRef]
- Mashtalir, N.; D’Avino, A.R.; Michel, B.C.; Luo, J.; Pan, J.; Otto, J.E.; Zullow, H.J.; McKenzie, Z.M.; Kubiak, R.L.; St. Pierre, R.; et al. Modular Organization and Assembly of SWI/SNF Family Chromatin Remodeling Complexes. Cell 2018, 175, 1272–1288.e20. [Google Scholar] [CrossRef]
- Michel, B.C.; D’Avino, A.R.; Cassel, S.H.; Mashtalir, N.; McKenzie, Z.M.; McBride, M.J.; Valencia, A.M.; Zhou, Q.; Bocker, M.; Soares, L.M.M.; et al. A Non-Canonical SWI/SNF Complex Is a Synthetic Lethal Target in Cancers Driven by BAF Complex Perturbation. Nat. Cell Biol. 2018, 20, 1410–1420. [Google Scholar] [CrossRef]
- Zhou, M.; Yuan, J.; Deng, Y.; Fan, X.; Shen, J. Emerging Role of SWI/SNF Complex Deficiency as a Target of Immune Checkpoint Blockade in Human Cancers. Oncogenesis 2021, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Pulice, J.L.; Kadoch, C. Composition and Function of Mammalian SWI/SNF Chromatin Remodeling Complexes in Human Disease. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 53–60. [Google Scholar] [CrossRef]
- Sahu, R.K.; Singh, S.; Tomar, R.S. The Mechanisms of Action of Chromatin Remodelers and Implications in Development and Disease. Biochem. Pharmacol. 2020, 180, 114200. [Google Scholar] [CrossRef]
- Romero, O.A.; Sanchez-Cespedes, M. The SWI/SNF Genetic Blockade: Effects in Cell Differentiation, Cancer and Developmental Diseases. Oncogene 2014, 33, 2681–2689. [Google Scholar] [CrossRef] [PubMed]
- Clapier, C.R.; Iwasa, J.; Cairns, B.R.; Peterson, C.L. Mechanisms of Action and Regulation of ATP-Dependent Chromatin-Remodelling Complexes. Nat. Rev. Mol. Cell Biol. 2017, 18, 407–422. [Google Scholar] [CrossRef]
- Harrod, A.; Lane, K.A.; Downs, J.A. The Role of the SWI/SNF Chromatin Remodelling Complex in the Response to DNA Double Strand Breaks. DNA Repair 2020, 93, 102919. [Google Scholar] [CrossRef]
- Mittal, P.; Roberts, C.W.M. The SWI/SNF Complex in Cancer—Biology, Biomarkers and Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 435–448. [Google Scholar] [CrossRef]
- Awad, S.; Hassan, A.H. The Swi2/Snf2 Bromodomain Is Important for the Full Binding and Remodeling Activity of the SWI/SNF Complex on H3- and H4-Acetylated Nucleosomes. Ann. N. Y. Acad. Sci. 2008, 1138, 366–375. [Google Scholar] [CrossRef]
- Khavari, P.A.; Peterson, C.L.; Tamkun, J.W.; Mendel, D.B.; Crabtree, G.R. BRG1 Contains a Conserved Domain of the SWI2/SNF2 Family Necessary for Normal Mitotic Growth and Transcription. Nature 1993, 366, 170–174. [Google Scholar] [CrossRef]
- Trouche, D.; Le Chalony, C.; Muchardt, C.; Yaniv, M.; Kouzarides, T. RB and Hbrm Cooperate to Repress the Activation Functions of E2F1. Proc. Natl. Acad. Sci. USA 1997, 94, 11268–11273. [Google Scholar] [CrossRef]
- Ruijtenberg, S.; van den Heuvel, S. G1/S Inhibitors and the SWI/SNF Complex Control Cell-Cycle Exit during Muscle Differentiation. Cell 2015, 162, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Isakoff, M.S.; Sansam, C.G.; Tamayo, P.; Subramanian, A.; Evans, J.A.; Fillmore, C.M.; Wang, X.; Biegel, J.A.; Pomeroy, S.L.; Mesirov, J.P.; et al. Inactivation of the Snf5 Tumor Suppressor Stimulates Cell Cycle Progression and Cooperates with P53 Loss in Oncogenic Transformation. Proc. Natl. Acad. Sci. USA. 2005, 102, 17745–17750. [Google Scholar] [CrossRef]
- Flores-Alcantar, A.; Gonzalez-Sandoval, A.; Escalante-Alcalde, D.; Lomelí, H. Dynamics of Expression of ARID1A and ARID1B Subunits in Mouse Embryos and in Cells during the Cell Cycle. Cell Tissue Res. 2011, 345, 137–148. [Google Scholar] [CrossRef]
- Napolitano, M.A.; Cipollaro, M.; Cascino, A.; Melone, M.A.B.; Giordano, A.; Galderisi, U. Brg1 Chromatin Remodeling Factor Is Involved in Cell Growth Arrest, Apoptosis and Senescence of Rat Mesenchymal Stem Cells. J. Cell Sci. 2007, 120, 2904–2911. [Google Scholar] [CrossRef]
- Peng, G.; Yim, E.-K.; Dai, H.; Jackson, A.P.; van der Burgt, I.; Pan, M.-R.; Hu, R.; Li, K.; Lin, S.-Y. BRIT1/MCPH1 Links Chromatin Remodelling to DNA Damage Response. Nat. Cell Biol. 2009, 11, 865–872. [Google Scholar] [CrossRef]
- Lee, H.-S.; Park, J.-H.; Kim, S.-J.; Kwon, S.-J.; Kwon, J. A Cooperative Activation Loop among SWI/SNF, γ-H2AX and H3 Acetylation for DNA Double-Strand Break Repair. EMBO J. 2010, 29, 1434–1445. [Google Scholar] [CrossRef]
- Shibata, A.; Jeggo, P.A. DNA Double-Strand Break Repair in a Cellular Context. Clin. Oncol. 2014, 26, 243–249. [Google Scholar] [CrossRef]
- Park, Y.; Chui, M.H.; Suryo Rahmanto, Y.; Yu, Z.-C.; Shamanna, R.A.; Bellani, M.A.; Gaillard, S.; Ayhan, A.; Viswanathan, A.; Seidman, M.M.; et al. Loss of ARID1A in Tumor Cells Renders Selective Vulnerability to Combined Ionizing Radiation and PARP Inhibitor Therapy. Clin. Cancer Res. 2019, 25, 5584–5594. [Google Scholar] [CrossRef]
- Vélez-Cruz, R.; Manickavinayaham, S.; Biswas, A.K.; Clary, R.W.; Premkumar, T.; Cole, F.; Johnson, D.G. RB Localizes to DNA Double-Strand Breaks and Promotes DNA End Resection and Homologous Recombination through the Recruitment of BRG1. Genes Dev. 2016, 30, 2500–2512. [Google Scholar] [CrossRef]
- de Castro, R.O.; Previato, L.; Goitea, V.; Felberg, A.; Guiraldelli, M.F.; Filiberti, A.; Pezza, R.J. The Chromatin-Remodeling Subunit Baf200 Promotes Homology-Directed DNA Repair and Regulates Distinct Chromatin-Remodeling Complexes. J. Biol. Chem. 2017, 292, 8459–8471. [Google Scholar] [CrossRef]
- Bell, A.C.; Felsenfeld, G. Methylation of a CTCF-Dependent Boundary Controls Imprinted Expression of the Igf2 Gene. Nature 2000, 405, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Euskirchen, G.M.; Auerbach, R.K.; Davidov, E.; Gianoulis, T.A.; Zhong, G.; Rozowsky, J.; Bhardwaj, N.; Gerstein, M.B.; Snyder, M. Diverse Roles and Interactions of the SWI/SNF Chromatin Remodeling Complex Revealed Using Global Approaches. PLoS Genet 2011, 7, e1002008. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, P.M.; Chambers, A.L.; Cloney, R.; Bianchi, A.; Downs, J.A. BAF180 Promotes Cohesion and Prevents Genome Instability and Aneuploidy. Cell Rep. 2014, 6, 973–981. [Google Scholar] [CrossRef]
- Oh, J.; Sohn, D.H.; Ko, M.; Chung, H.; Jeon, S.H.; Seong, R.H. BAF60a Interacts with P53 to Recruit the SWI/SNF Complex. J. Biol. Chem. 2008, 283, 11924–11934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ohta, T.; Maruyama, A.; Hosoya, T.; Nishikawa, K.; Maher, J.M.; Shibahara, S.; Itoh, K.; Yamamoto, M. BRG1 Interacts with Nrf2 to Selectively Mediate HO-1 Induction in Response to Oxidative Stress. Mol. Cell. Biol. 2006, 26, 7942–7952. [Google Scholar] [CrossRef]
- Marignani, P.A.; Kanai, F.; Carpenter, C.L. LKB1 Associates with Brg1 and Is Necessary for Brg1-Induced Growth Arrest. J. Biol. Chem. 2001, 276, 32415–32418. [Google Scholar] [CrossRef]
- Shanahan, F.; Seghezzi, W.; Parry, D.; Mahony, D.; Lees, E. Cyclin E Associates with BAF155 and BRG1, Components of the Mammalian SWI-SNF Complex, and Alters the Ability of BRG1 To Induce Growth Arrest. Mol. Cell. Biol. 1999, 19, 1460–1469. [Google Scholar] [CrossRef]
- Pfister, N.T.; Fomin, V.; Regunath, K.; Zhou, J.Y.; Zhou, W.; Silwal-Pandit, L.; Freed-Pastor, W.A.; Laptenko, O.; Neo, S.P.; Bargonetti, J.; et al. Mutant P53 Cooperates with the SWI/SNF Chromatin Remodeling Complex to Regulate VEGFR2 in Breast Cancer Cells. Genes Dev. 2015, 29, 1298–1315. [Google Scholar] [CrossRef]
- Orlando, K.A.; Nguyen, V.; Raab, J.R.; Walhart, T.; Weissman, B.E. Remodeling the Cancer Epigenome: Mutations in the SWI/SNF Complex Offer New Therapeutic Opportunities. Expert Rev. Anticancer Ther. 2019, 19, 375–391. [Google Scholar] [CrossRef]
- Biegel, J.A. Molecular Genetics of Atypical Teratoid/Rhabdoid Tumors. Neurosurg. Focus 2006, 20, E11. [Google Scholar] [CrossRef]
- Sun, X.; Wang, S.C.; Wei, Y.; Luo, X.; Jia, Y.; Li, L.; Gopal, P.; Zhu, M.; Nassour, I.; Chuang, J.-C.; et al. Arid1a Has Context-Dependent Oncogenic and Tumor Suppressor Functions in Liver Cancer. Cancer Cell 2017, 32, 574–589.e6. [Google Scholar] [CrossRef]
- Imielinski, M.; Berger, A.H.; Hammerman, P.S.; Hernandez, B.; Pugh, T.J.; Hodis, E.; Cho, J.; Suh, J.; Capelletti, M.; Sivachenko, A.; et al. Mapping the Hallmarks of Lung Adenocarcinoma with Massively Parallel Sequencing. Cell 2012, 150, 1107–1120. [Google Scholar] [CrossRef]
- Shain, A.H.; Giacomini, C.P.; Matsukuma, K.; Karikari, C.A.; Bashyam, M.D.; Hidalgo, M.; Maitra, A.; Pollack, J.R. Convergent Structural Alterations Define SWItch/Sucrose NonFermentable (SWI/SNF) Chromatin Remodeler as a Central Tumor Suppressive Complex in Pancreatic Cancer. Proc. Natl. Acad. Sci. USA 2012, 109, E252–E259. [Google Scholar] [CrossRef]
- Robinson, G.; Parker, M.; Kranenburg, T.A.; Lu, C.; Chen, X.; Ding, L.; Phoenix, T.N.; Hedlund, E.; Wei, L.; Zhu, X.; et al. Novel Mutations Target Distinct Subgroups of Medulloblastoma. Nature 2012, 488, 43–48. [Google Scholar] [CrossRef]
- Love, C.; Sun, Z.; Jima, D.; Li, G.; Zhang, J.; Miles, R.; Richards, K.L.; Dunphy, C.H.; Choi, W.W.L.; Srivastava, G.; et al. The Genetic Landscape of Mutations in Burkitt Lymphoma. Nat. Genet. 2012, 44, 1321–1325. [Google Scholar] [CrossRef]
- Wang, K.; Kan, J.; Yuen, S.T.; Shi, S.T.; Chu, K.M.; Law, S.; Chan, T.L.; Kan, Z.; Chan, A.S.Y.; Tsui, W.Y.; et al. Exome Sequencing Identifies Frequent Mutation of ARID1A in Molecular Subtypes of Gastric Cancer. Nat. Genet. 2011, 43, 1219–1223. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network. Comprehensive Molecular Characterization of Human Colon and Rectal Cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef]
- Chan-on, W.; Nairismägi, M.-L.; Ong, C.K.; Lim, W.K.; Dima, S.; Pairojkul, C.; Lim, K.H.; McPherson, J.R.; Cutcutache, I.; Heng, H.L.; et al. Exome Sequencing Identifies Distinct Mutational Patterns in Liver Fluke-Related and Non-Infection-Related Bile Duct Cancers. Nat. Genet. 2013, 45, 1474–1478. [Google Scholar] [CrossRef]
- Gokden, N.; Pfeifer, J.D.; Humphrey, P.A. Renal Cell Carcinoma with Rhabdoid Features. Am. J. Surg. Pathol. 2000, 24, 1329–1338. [Google Scholar] [CrossRef]
- Del Savio, E.; Maestro, R. Beyond SMARCB1 Loss: Recent Insights into the Pathobiology of Epithelioid Sarcoma. Cells 2022, 11, 2626. [Google Scholar] [CrossRef]
- Needs, T.; Fillman, E.P. Epithelioid Sarcoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/nbk532911/ (accessed on 23 April 2023).
- Le Loarer, F.; Zhang, L.; Fletcher, C.D.; Ribeiro, A.; Singer, S.; Italiano, A.; Neuville, A.; Houlier, A.; Chibon, F.; Coindre, J.-M.; et al. Consistent SMARCB1 Homozygous Deletions in Epithelioid Sarcoma and in a Subset of Myoepithelial Carcinomas Can Be Reliably Detected by FISH in Archival Material: SMARCB1 Homozygous Deletions in Epithelioid Sarcoma. Genes Chromosomes Cancer 2014, 53, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Gounder, M.; Schöffski, P.; Jones, R.L.; Agulnik, M.; Cote, G.M.; Villalobos, V.M.; Attia, S.; Chugh, R.; Chen, T.W.-W.; Jahan, T.; et al. Tazemetostat in Advanced Epithelioid Sarcoma with Loss of INI1/SMARCB1: An International, Open-Label, Phase 2 Basket Study. Lancet Oncol. 2020, 21, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.G.; Lyons, M.J.; Leddy, L.; Parham, D.M.; Welsh, C.T. Epithelioid Sarcoma Arising in a Long-Term Survivor of an Atypical Teratoid/Rhabdoid Tumor in a Patient with Rhabdoid Tumor Predisposition Syndrome. Pediatr. Dev. Pathol. 2021, 24, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Kohashi, K.; Yamamoto, H.; Kumagai, R.; Yamada, Y.; Hotokebuchi, Y.; Taguchi, T.; Iwamoto, Y.; Oda, Y. Differential MicroRNA Expression Profiles between Malignant Rhabdoid Tumor and Epithelioid Sarcoma: MiR193a-5p Is Suggested to Downregulate SMARCB1 MRNA Expression. Mod. Pathol. 2014, 27, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Papp, G.; Krausz, T.; Stricker, T.P.; Szendrői, M.; Sápi, Z. SMARCB1 Expression in Epithelioid Sarcoma Is Regulated by MiR-206, MiR-381, and MiR-671-5p on Both MRNA and Protein Levels: Smarcb1 Regulation by MiRNAs In Epithelioid Sarcoma. Genes Chromosomes Cancer 2014, 53, 168–176. [Google Scholar] [CrossRef]
- Sápi, Z.; Papp, G.; Szendrői, M.; Pápai, Z.; Plótár, V.; Krausz, T.; Fletcher, C.D.M. Epigenetic Regulation of SMARCB1 By MiR-206, -381 and -671-5p Is Evident in a Variety of SMARCB1 Immunonegative Soft Tissue Sarcomas, While MiR-765 Appears Specific for Epithelioid Sarcoma. A MiRNA Study of 223 Soft Tissue Sarcomas: Epigenetic Regulation of SMARCB1 by Mirnas in Soft Tissue Sarcomas. Genes Chromosomes Cancer 2016, 55, 786–802. [Google Scholar] [CrossRef]
- Msaouel, P.; Malouf, G.G.; Su, X.; Yao, H.; Tripathi, D.N.; Soeung, M.; Gao, J.; Rao, P.; Coarfa, C.; Creighton, C.J.; et al. Comprehensive Molecular Characterization Identifies Distinct Genomic and Immune Hallmarks of Renal Medullary Carcinoma. Cancer Cell 2020, 37, 720–734.e13. [Google Scholar] [CrossRef]
- Mora-Blanco, E.; Mishina, Y.; Tillman, E.; Cho, Y.J.; Thom, C.S.; Pomeroy, S.L.; Shao, W.; Roberts, C.W.M. Activation of β-catenin/TCF targets following loss of the tumor suppressor SNF5. Oncogene 2014, 33, 933–938. [Google Scholar] [CrossRef]
- Jagani, Z.; Mora-Blanco, E.L.; Sansam, C.G.; McKenna, E.S.; Wilson, B.; Chen, D.; Klekota, J.; Tamayo, P.; Nguyen, P.T.L.; Tolstorukov, M.; et al. Loss of the Tumor Suppressor Snf5 Leads to Aberrant Activation of the Hedgehog-Gli Pathway. Nat. Med. 2010, 16, 1429–1433. [Google Scholar] [CrossRef]
- Finetti, M.A.; Grabovska, Y.; Bailey, S.; Williamson, D. Translational Genomics of Malignant Rhabdoid Tumours: Current Impact and Future Possibilities. Semin. Cancer Biol. 2020, 61, 30–41. [Google Scholar] [CrossRef]
- Jackson, E.M.; Sievert, A.J.; Gai, X.; Hakonarson, H.; Judkins, A.R.; Tooke, L.; Perin, J.C.; Xie, H.; Shaikh, T.H.; Biegel, J.A. Genomic Analysis Using High-Density Single Nucleotide Polymorphism-Based Oligonucleotide Arrays and Multiplex Ligation-Dependent Probe Amplification Provides a Comprehensive Analysis of INI1/SMARCB1 in Malignant Rhabdoid Tumors. Clin. Cancer Res. 2009, 15, 1923–1930. [Google Scholar] [CrossRef]
- Schneppenheim, R.; Frühwald, M.C.; Gesk, S.; Hasselblatt, M.; Jeibmann, A.; Kordes, U.; Kreuz, M.; Leuschner, I.; Subero, J.I.M.; Obser, T.; et al. Germline Nonsense Mutation and Somatic Inactivation of SMARCA4/BRG1 in a Family with Rhabdoid Tumor Predisposition Syndrome. Am. J. Hum. Genet. 2010, 86, 279–284. [Google Scholar] [CrossRef]
- Erkek, S.; Johann, P.D.; Finetti, M.A.; Drosos, Y.; Chou, H.-C.; Zapatka, M.; Sturm, D.; Jones, D.T.W.; Korshunov, A.; Rhyzova, M.; et al. Comprehensive Analysis of Chromatin States in Atypical Teratoid/Rhabdoid Tumor Identifies Diverging Roles for SWI/SNF and Polycomb in Gene Regulation. Cancer Cell 2019, 35, 95–110.e8. [Google Scholar] [CrossRef]
- Tischkowitz, M.; Huang, S.; Banerjee, S.; Hague, J.; Hendricks, W.P.D.; Huntsman, D.G.; Lang, J.D.; Orlando, K.A.; Oza, A.M.; Pautier, P.; et al. Small-Cell Carcinoma of the Ovary, Hypercalcemic Type–Genetics, New Treatment Targets, and Current Management Guidelines. Clin. Cancer Res. 2020, 26, 3908–3917. [Google Scholar] [CrossRef]
- Matias-Guiu, X.; Prat, J.; Young, R.H.; Capen, C.C.; Rosol, T.J.; Delellis, R.A.; Scully, R.E. Human Parathyroid Hormone-Related Protein in Ovarian Small Cell Carcinoma. An Immunohistochemical Study. Cancer 1994, 73, 1878–1881. [Google Scholar] [CrossRef]
- Witkowski, L.; Carrot-Zhang, J.; Albrecht, S.; Fahiminiya, S.; Hamel, N.; Tomiak, E.; Grynspan, D.; Saloustros, E.; Nadaf, J.; Rivera, B.; et al. Germline and Somatic SMARCA4 Mutations Characterize Small Cell Carcinoma of the Ovary, Hypercalcemic Type. Nat. Genet. 2014, 46, 438–443. [Google Scholar] [CrossRef]
- Karnezis, A.N.; Wang, Y.; Ramos, P.; Hendricks, W.P.; Oliva, E.; D’Angelo, E.; Prat, J.; Nucci, M.R.; Nielsen, T.O.; Chow, C.; et al. Dual Loss of the SWI/SNF Complex ATPases SMARCA4/BRG1 and SMARCA2/BRM Is Highly Sensitive and Specific for Small Cell Carcinoma of the Ovary, Hypercalcaemic Type. J. Pathol. 2016, 238, 389–400. [Google Scholar] [CrossRef]
- Lin, D.I.; Chudnovsky, Y.; Duggan, B.; Zajchowski, D.; Greenbowe, J.; Ross, J.S.; Gay, L.M.; Ali, S.M.; Elvin, J.A. Comprehensive Genomic Profiling Reveals Inactivating SMARCA4 Mutations and Low Tumor Mutational Burden in Small Cell Carcinoma of the Ovary, Hypercalcemic-Type. Gynecol. Oncol. 2017, 147, 626–633. [Google Scholar] [CrossRef]
- Msaouel, P.; Tannir, N.M.; Walker, C.L. A Model Linking Sickle Cell Hemoglobinopathies and SMARCB1 Loss in Renal Medullary Carcinoma. Clin. Cancer Res. 2018, 24, 2044–2049. [Google Scholar] [CrossRef]
- Calderaro, J.; Masliah-Planchon, J.; Richer, W.; Maillot, L.; Maille, P.; Mansuy, L.; Bastien, C.; de la Taille, A.; Boussion, H.; Charpy, C.; et al. Balanced Translocations Disrupting SMARCB1 Are Hallmark Recurrent Genetic Alterations in Renal Medullary Carcinomas. Eur. Urol. 2016, 69, 1055–1061. [Google Scholar] [CrossRef]
- Rizzo, D.; Fréneaux, P.; Brisse, H.; Louvrier, C.; Lequin, D.; Nicolas, A.; Ranchère, D.; Verkarre, V.; Jouvet, A.; Dufour, C.; et al. SMARCB1 Deficiency in Tumors from the Peripheral Nervous System: A Link between Schwannomas and Rhabdoid Tumors? Am. J. Surg. Pathol. 2012, 36, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Eaton, K.W.; Tooke, L.S.; Wainwright, L.M.; Judkins, A.R.; Biegel, J.A. Spectrum of SMARCB1/INI1 Mutations in Familial and Sporadic Rhabdoid Tumors: SMARCB1 Mutations in Rhabdoid Tumor. Pediatr. Blood Cancer 2011, 56, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Pemov, A.; Li, H.; Presley, W.; Wallace, M.R.; Miller, D.T. Genetics of Human Malignant Peripheral Nerve Sheath Tumors. Neuro-Oncol. Adv. 2020, 2 (Suppl. S1), i50–i61. [Google Scholar] [CrossRef]
- Xu, B.; Katabi, N. Myoepithelial Carcinoma. Surg. Pathol. Clin. 2021, 14, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Baldi, G.G.; Morosi, C.; Gronchi, A.; Maestro, R. Extraskeletal Myxoid Chondrosarcoma: State of the Art and Current Research on Biology and Clinical Management. Cancers 2020, 12, 2703. [Google Scholar] [CrossRef]
- Curcio, C.; Cimera, R.; Aryeequaye, R.; Rao, M.; Fabbri, N.; Zhang, Y.; Hameed, M. Poorly Differentiated Chordoma with Whole-genome Doubling Evolving from a SMARCB1-deficient Conventional Chordoma: A Case Report. Genes Chromosomes Cancer 2021, 60, 43–48. [Google Scholar] [CrossRef]
- Leiner, J.; Le Loarer, F. The Current Landscape of Rhabdomyosarcomas: An Update. Virchows Arch. 2020, 476, 97–108. [Google Scholar] [CrossRef]
- Bharathy, N.; Cleary, M.M.; Kim, J.-A.; Nagamori, K.; Crawford, K.A.; Wang, E.; Saha, D.; Settelmeyer, T.P.; Purohit, R.; Skopelitis, D.; et al. SMARCA4 Biology in Alveolar Rhabdomyosarcoma. Oncogene 2022, 41, 1647–1656. [Google Scholar] [CrossRef]
- Helming, K.C.; Wang, X.; Roberts, C.W.M. Vulnerabilities of Mutant SWI/SNF Complexes in Cancer. Cancer Cell 2014, 26, 309–317. [Google Scholar] [CrossRef]
- Huang, P.H. Targeting SWI/SNF Mutant Cancers with Tyrosine Kinase Inhibitor Therapy. Expert Rev. Anticancer. Ther. 2017, 17, 1–3. [Google Scholar] [CrossRef]
- Xue, Y.; Meehan, B.; Macdonald, E.; Venneti, S.; Wang, X.Q.D.; Witkowski, L.; Jelinic, P.; Kong, T.; Martinez, D.; Morin, G.; et al. CDK4/6 Inhibitors Target SMARCA4-Determined Cyclin D1 Deficiency in Hypercalcemic Small Cell Carcinoma of the Ovary. Nat. Commun. 2019, 10, 558. [Google Scholar] [CrossRef]
- Geoerger, B.; Bourdeaut, F.; DuBois, S.G.; Fischer, M.; Geller, J.I.; Gottardo, N.G.; Marabelle, A.; Pearson, A.D.J.; Modak, S.; Cash, T.; et al. A Phase I Study of the CDK4/6 Inhibitor Ribociclib (LEE011) in Pediatric Patients with Malignant Rhabdoid Tumors, Neuroblastoma, and Other Solid Tumors. Clin. Cancer Res. 2017, 23, 2433–2441. [Google Scholar] [CrossRef]
- Eno, J. Immunotherapy Through the Years. J. Adv. Pract. Oncol. 2017, 8, 747–753. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- Miao, D.; Margolis, C.A.; Gao, W.; Voss, M.H.; Li, W.; Martini, D.J.; Norton, C.; Bossé, D.; Wankowicz, S.M.; Cullen, D.; et al. Genomic Correlates of Response to Immune Checkpoint Therapies in Clear Cell Renal Cell Carcinoma. Science 2018, 359, 801–806. [Google Scholar] [CrossRef]
- Pan, D.; Kobayashi, A.; Jiang, P.; Ferrari de Andrade, L.; Tay, R.E.; Luoma, A.M.; Tsoucas, D.; Qiu, X.; Lim, K.; Rao, P.; et al. A Major Chromatin Regulator Determines Resistance of Tumor Cells to T Cell–Mediated Killing. Science 2018, 359, 770–775. [Google Scholar] [CrossRef]
- Shen, J.; Ju, Z.; Zhao, W.; Wang, L.; Peng, Y.; Ge, Z.; Nagel, Z.D.; Zou, J.; Wang, C.; Kapoor, P.; et al. ARID1A Deficiency Promotes Mutability and Potentiates Therapeutic Antitumor Immunity Unleashed by Immune Checkpoint Blockade. Nat. Med. 2018, 24, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Buglioni, S.; Melucci, E.; Sperati, F.; Pallocca, M.; Terrenato, I.; De Nicola, F.; Goeman, F.; Casini, B.; Amoreo, C.A.; Gallo, E.; et al. The Clinical Significance of PD-L1 in Advanced Gastric Cancer Is Dependent on ARID1A Mutations and ATM Expression. OncoImmunology 2018, 7, e1457602. [Google Scholar] [CrossRef]
- Kim, Y.; Ahn, J.M.; Bae, W.J.; Sung, C.O.; Lee, D. Functional Loss of ARID1A Is Tightly Associated with High PD-L1 Expression in Gastric Cancer. Int. J. Cancer 2019, 145, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Jelinic, P.; Ricca, J.; Van Oudenhove, E.; Olvera, N.; Merghoub, T.; Levine, D.A.; Zamarin, D. Immune-Active Microenvironment in Small Cell Carcinoma of the Ovary, Hypercalcemic Type: Rationale for Immune Checkpoint Blockade. JNCI J. Natl. Cancer Inst. 2018, 110, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, J.; Zhou, D.; Wu, Z.; Liu, W.; Chen, Y.; Chen, G.; Zhang, J. SWI/SNF Complex Genomic Alterations as a Predictive Biomarker for Response to Immune Checkpoint Inhibitors in Multiple Cancers. Cancer Immunol. Res. 2023, 11, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.-Y.; Penel, N.; Ray-Coquard, I.L.; Schott, R.; Saada-Bouzid, E.; Bertucci, F.; Chevreau, C.M.; Bompas, E.; Coquan, E.; Cousin, S.; et al. High Clinical Benefit Rates of Pembrolizumab in Very Rare Sarcoma Histotypes: First Results of the AcSé Pembrolizumab Study. Ann. Oncol. 2019, 30, v517. [Google Scholar] [CrossRef]
- Kershaw, M.H.; Westwood, J.A.; Darcy, P.K. Gene-Engineered T Cells for Cancer Therapy. Nat. Rev. Cancer 2013, 13, 525–541. [Google Scholar] [CrossRef]
- Strickler, J.H.; Hanks, B.A.; Khasraw, M. Tumor Mutational Burden as a Predictor of Immunotherapy Response: Is More Always Better? Clin. Cancer Res. 2021, 27, 1236–1241. [Google Scholar] [CrossRef]
- Worley, B.S.; van den Broeke, L.T.; Goletz, T.J.; Pendleton, C.D.; Daschbach, E.M.; Thomas, E.K.; Marincola, F.M.; Helman, L.J.; Berzofsky, J.A. Antigenicity of Fusion Proteins from Sarcoma-Associated Chromosomal Translocations. Cancer Res. 2001, 61, 6868–6875. [Google Scholar]
- Gyurdieva, A.; Zajic, S.; Chang, Y.-F.; Houseman, E.A.; Zhong, S.; Kim, J.; Nathenson, M.; Faitg, T.; Woessner, M.; Turner, D.C.; et al. Biomarker Correlates with Response to NY-ESO-1 TCR T Cells in Patients with Synovial Sarcoma. Nat. Commun. 2022, 13, 5296. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Druta, M.; Liebner, D.A.; Schuetze, S.; Somaiah, N.; Van Tine, B.A.; Tap, W.D.; Pulham, T.; Chagin, K.; Norry, E.; et al. Pilot Study of NY-ESO-1c259 T Cells in Advanced Myxoid/Round Cell Liposarcoma. J. Clin. Oncol. 2018, 36, 3005. [Google Scholar] [CrossRef]
- Huang, X.; Park, H.; Greene, J.; Pao, J.; Mulvey, E.; Zhou, S.X.; Albert, C.M.; Moy, F.; Sachdev, D.; Yee, D.; et al. IGF1R- and ROR1-Specific CAR T Cells as a Potential Therapy for High Risk Sarcomas. PLoS ONE 2015, 10, e0133152. [Google Scholar] [CrossRef]
- Lehner, M.; Götz, G.; Proff, J.; Schaft, N.; Dörrie, J.; Full, F.; Ensser, A.; Muller, Y.A.; Cerwenka, A.; Abken, H.; et al. Redirecting T Cells to Ewing’s Sarcoma Family of Tumors by a Chimeric NKG2D Receptor Expressed by Lentiviral Transduction or MRNA Transfection. PLoS ONE 2012, 7, e31210. [Google Scholar] [CrossRef]
- Leuci, V.; Casucci, M.; Grignani, G.; Rotolo, R.; Rossotti, U.; Vigna, E.; Gammaitoni, L.; Mesiano, G.; Fiorino, E.; Donini, C.; et al. CD44v6 as Innovative Sarcoma Target for CAR-Redirected CIK Cells. OncoImmunology 2018, 7, e1423167. [Google Scholar] [CrossRef]
- Rohaan, M.W.; Borch, T.H.; van den Berg, J.H.; Met, Ö.; Kessels, R.; Geukes Foppen, M.H.; Stoltenborg Granhøj, J.; Nuijen, B.; Nijenhuis, C.; Jedema, I.; et al. Tumor-Infiltrating Lymphocyte Therapy or Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2022, 387, 2113–2125. [Google Scholar] [CrossRef]
- Huang, H.; Nie, C.; Liu, X.; Song, B.; Yue, J.; Xu, J.; He, J.; Li, K.; Feng, Y.; Wan, T.; et al. Phase I Study of Adjuvant Immunotherapy with Autologous Tumor-Infiltrating Lymphocytes in Locally Advanced Cervical Cancer. J. Clin. Investig. 2022, 132, e157726. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, S.; Helman, S.R.; Wunderlich, J.R.; Langhan, M.M.; Doran, S.L.; Kwong, M.L.M.; Somerville, R.P.T.; Klebanoff, C.A.; Kammula, U.S.; Sherry, R.M.; et al. A Phase II Study of Tumor-Infiltrating Lymphocyte Therapy for Human Papillomavirus–Associated Epithelial Cancers. Clin. Cancer Res. 2019, 25, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Creelan, B.C.; Wang, C.; Teer, J.K.; Toloza, E.M.; Yao, J.; Kim, S.; Landin, A.M.; Mullinax, J.E.; Saller, J.J.; Saltos, A.N.; et al. Tumor-Infiltrating Lymphocyte Treatment for Anti-PD-1-Resistant Metastatic Lung Cancer: A Phase 1 Trial. Nat. Med. 2021, 27, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
| Author/Year | NCT | Study Design | N | Tumor | Drug | Endpoints/Results and Grade 3–5 AEs |
|---|---|---|---|---|---|---|
| Blay et al. (2019) [103] | 03012620 | Phase II | 21 | Rare sarcomas, including rhabdoid and SMARCA4-deficient sarcomas | Pembrolizumab | ORR 15%; 1-year PFS 50% (SMARCA4-MRT) None reported G3-4 AEs |
| Ongoing | 05286801 | Phase I/II | 86 | Children (1–18 years) with R/R SMARCB1 or SMARCA4-deficient tumors | Tiragolumab and Atezolizumab | Safety (AEs); PK; Efficacy (ORR, PFS, OS, DOR) |
| Ongoing | 05407441 | Phase I/II | 49 | Children and young adults (<24 years) with INI1-neg/SMARCA4-deficient tumors | Tazemetostat + Nivolumab/Ipilimumab | Safety (AEs, MTD, RP2D) Efficacy (ORR, OS, PFS) |
| Ongoing | 04416568 | Phase II | 45 | Children, adolescents, and adults with R/R INI1-negative cancers | Nivolumab + Ipilimumab | Efficacy (ORR, PFS, OS, DCR) Safety (AEs) |
| Ongoing | 04284202 | Phase II | 30 | Adults with NSCLC ARID1-mutant | Toripalimab + Dasatinib | Efficacy (PFS, OS) |
| Ongoing | 04957615 | Phase II | 30 | Metastatic or unresectable solid tumors with ARID1A mutation | Nivolumab | Efficacy (ORR, OS, PFS) |
| Ongoing | 04953104 | Phase II | 30 | Metastatic urothelial cancer with ARID1A mutation | Nivolumab | Efficacy (ORR, OS, PFS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Castillo, J.J.; Llavata-Marti, L.; Fort-Culillas, R.; Andreu-Cobo, P.; Moreno, R.; Codony, C.; García del Muro, X.; Alemany, R.; Piulats, J.M.; Martin-Liberal, J. SWI/SNF Complex Alterations in Tumors with Rhabdoid Features: Novel Therapeutic Approaches and Opportunities for Adoptive Cell Therapy. Int. J. Mol. Sci. 2023, 24, 11143. https://doi.org/10.3390/ijms241311143
Soto-Castillo JJ, Llavata-Marti L, Fort-Culillas R, Andreu-Cobo P, Moreno R, Codony C, García del Muro X, Alemany R, Piulats JM, Martin-Liberal J. SWI/SNF Complex Alterations in Tumors with Rhabdoid Features: Novel Therapeutic Approaches and Opportunities for Adoptive Cell Therapy. International Journal of Molecular Sciences. 2023; 24(13):11143. https://doi.org/10.3390/ijms241311143
Chicago/Turabian StyleSoto-Castillo, Juan José, Lucía Llavata-Marti, Roser Fort-Culillas, Pablo Andreu-Cobo, Rafael Moreno, Carles Codony, Xavier García del Muro, Ramon Alemany, Josep M. Piulats, and Juan Martin-Liberal. 2023. "SWI/SNF Complex Alterations in Tumors with Rhabdoid Features: Novel Therapeutic Approaches and Opportunities for Adoptive Cell Therapy" International Journal of Molecular Sciences 24, no. 13: 11143. https://doi.org/10.3390/ijms241311143
APA StyleSoto-Castillo, J. J., Llavata-Marti, L., Fort-Culillas, R., Andreu-Cobo, P., Moreno, R., Codony, C., García del Muro, X., Alemany, R., Piulats, J. M., & Martin-Liberal, J. (2023). SWI/SNF Complex Alterations in Tumors with Rhabdoid Features: Novel Therapeutic Approaches and Opportunities for Adoptive Cell Therapy. International Journal of Molecular Sciences, 24(13), 11143. https://doi.org/10.3390/ijms241311143





