Therapeutic Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles to Treat PCOS
Abstract
:1. Introduction
2. Results
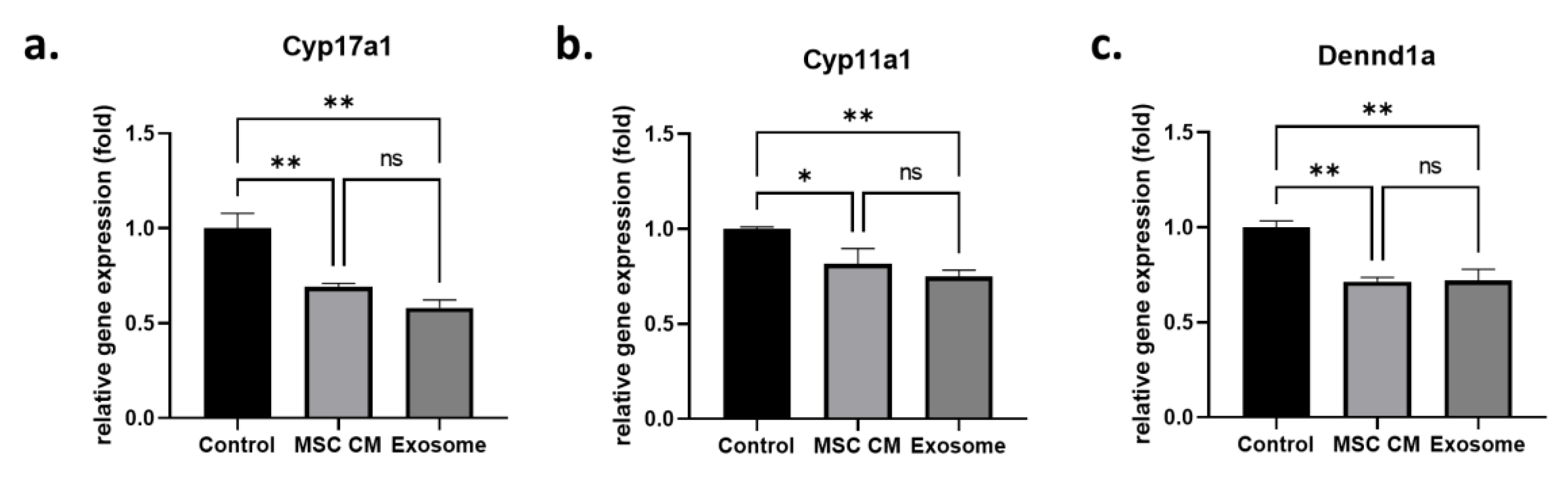
2.1. Effect of MSC-Derived Exosomes on Androgen-Producing Cells
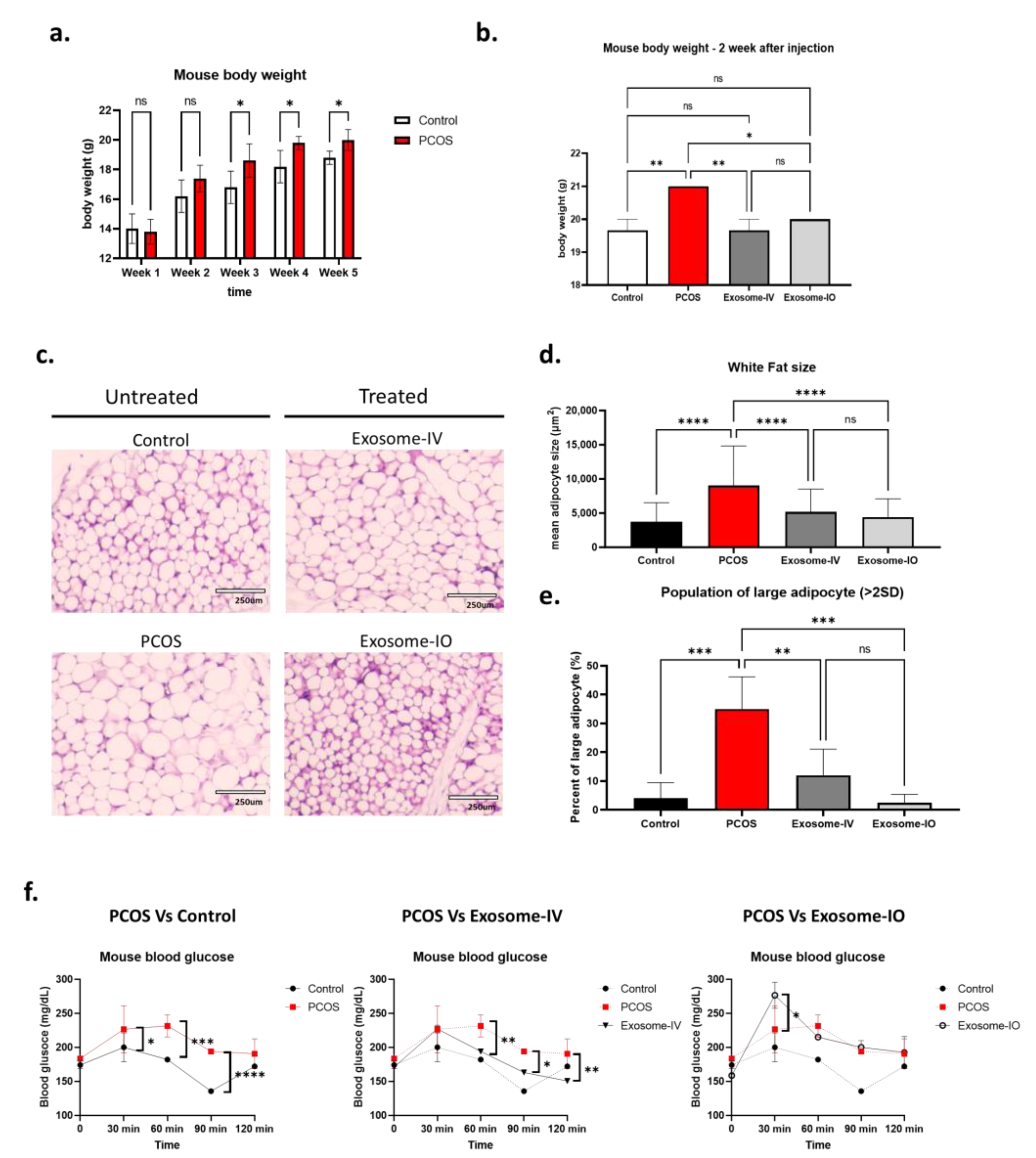
2.2. MSC-Derived Exosome Treatment Reverses Metabolism in a PCOS Mouse Model
2.3. MSC-Derived Exosomes Restore Ovarian Function in a PCOS Mouse Model
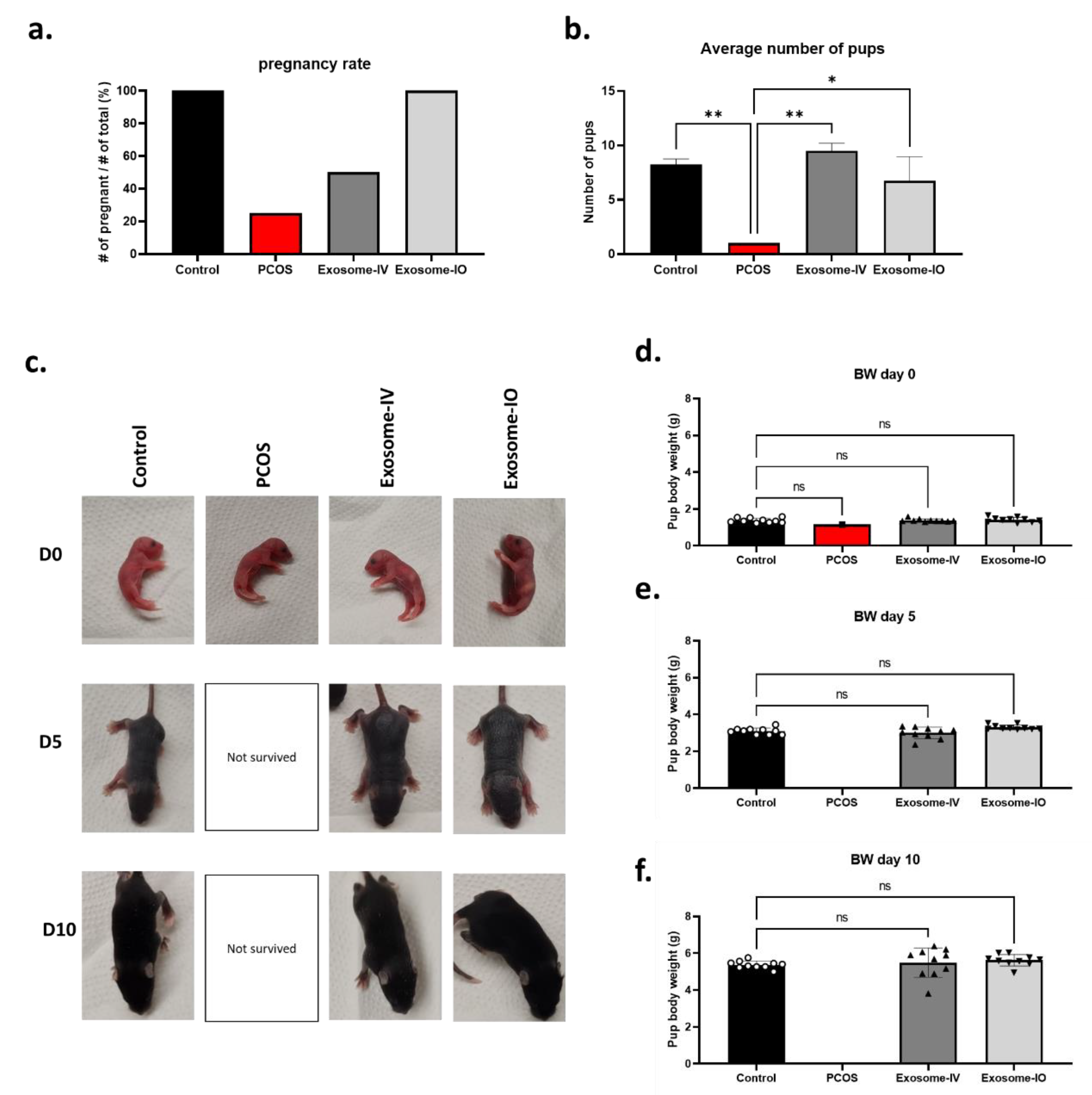
2.4. MSC-Derived Exosomes Restore Fertility in a PCOS Mouse Model
3. Discussion
3.1. MSC-Derived Exosomes Mediated Fertility Restoration in a PCOS
3.2. Metabolism Regulation by MSC-Derived Exosome Treatment
3.3. Mechanism of MSC-Derived Exosome in PCOS Treatment
3.4. Benefit of MSC-Derived Exosome Based Treatment
4. Materials and Methods
4.1. Invitro PCOS Cell Model (Human Adrenocortical Carcinoma Cell Line) Culture
4.2. Preparation of the MSC-Conditioned Media and MSC-Derived Exosomes
4.3. Treatment of H295R Cells with MSC CM
4.4. PCOS Mouse Model and Exosome Treatment
4.5. Glucose Tolerance Test (GTT)
4.6. Estrus Cycle Analysis
4.7. Serum Hormone Measurements
4.8. Breeding Experiments
4.9. Histology Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fauser, B.C.; Tarlatzis, B.C.; Rebar, R.W.; Legro, R.S.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.; et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2012, 97, 28–38.e5. [Google Scholar] [CrossRef] [PubMed]
- March, W.A.; Moore, V.M.; Willson, K.J.; Phillips, D.I.; Norman, R.J.; Davies, M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2010, 25, 544–551. [Google Scholar] [CrossRef] [Green Version]
- Helena, C.V.; Cristancho-Gordo, R.; Gonzalez-Iglesias, A.E.; Tabak, J.; Bertram, R.; Freeman, M.E. Systemic oxytocin induces a prolactin secretory rhythm via the pelvic nerve in ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R676–R681. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Colao, A.; Orio, F. Insulin-Mediated Diseases: Adrenal Mass and Polycystic Ovary Syndrome. Trends Endocrinol. Metab. 2015, 26, 512–514. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Lobo, R.A. Cancer risk and PCOS. Steroids 2013, 78, 782–785. [Google Scholar] [CrossRef]
- Lindholm, A.; Andersson, L.; Eliasson, M.; Bixo, M.; Sundstrom-Poromaa, I. Prevalence of symptoms associated with polycystic ovary syndrome. Int. J. Gynaecol. Obs. 2008, 102, 39–43. [Google Scholar] [CrossRef]
- Scicchitano, P.; Dentamaro, I.; Carbonara, R.; Bulzis, G.; Dachille, A.; Caputo, P.; Riccardi, R.; Locorotondo, M.; Mandurino, C.; Matteo Ciccone, M. Cardiovascular Risk in Women With PCOS. Int. J. Endocrinol. Metab. 2012, 10, 611–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rababa’h, A.M.; Matani, B.R.; Yehya, A. An update of polycystic ovary syndrome: Causes and therapeutics options. Heliyon 2022, 8, e11010. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International, P.N. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef] [Green Version]
- Corbould, A. Effects of androgens on insulin action in women: Is androgen excess a component of female metabolic syndrome? Diabetes Metab. Res. Rev. 2008, 24, 520–532. [Google Scholar] [CrossRef] [PubMed]
- de Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, C.W.; Zhang, L.; Sohni, A.; Doblado, M.; Wilkinson, M.F.; Chang, R.J.; Duleba, A.J. Inflammatory Stimuli Trigger Increased Androgen Production and Shifts in Gene Expression in Theca-Interstitial Cells. Endocrinology 2019, 160, 2946–2958. [Google Scholar] [CrossRef] [Green Version]
- Lang, Q.; Yidong, X.; Xueguang, Z.; Sixian, W.; Wenming, X.; Tao, Z. ETA-mediated anti-TNF-alpha therapy ameliorates the phenotype of PCOS model induced by letrozole. PLoS ONE 2019, 14, e0217495. [Google Scholar] [CrossRef] [PubMed]
- Nehir Aytan, A.; Bastu, E.; Demiral, I.; Bulut, H.; Dogan, M.; Buyru, F. Relationship between hyperandrogenism, obesity, inflammation and polycystic ovary syndrome. Gynecol. Endocrinol. 2016, 32, 709–713. [Google Scholar] [CrossRef]
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef] [Green Version]
- Sheng, H.; Wang, Y.; Jin, Y.; Zhang, Q.; Zhang, Y.; Wang, L.; Shen, B.; Yin, S.; Liu, W.; Cui, L.; et al. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008, 18, 846–857. [Google Scholar] [CrossRef]
- Tsyb, A.F.; Petrov, V.N.; Konoplyannikov, A.G.; Saypina, E.V.; Lepechina, L.A.; Kalsina, S.; Semenkova, I.V.; Agaeva, E.V. In vitro inhibitory effect of mesenchymal stem cells on zymosan-induced production of reactive oxygen species. Bull. Exp. Biol. Med. 2008, 146, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Fan, X.L.; Fang, S.B.; Lin, Y.D.; Wen, W.; Fu, Q.L. Human pluripotent stem cell-derived mesenchymal stem cells prevent chronic allergic airway inflammation via TGF-beta1-Smad2/Smad3 signaling pathway in mice. Mol. Immunol. 2019, 109, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Karam, M.; Najjar, H.; El Sabban, M.; Hamade, A.; Najjar, F. Regenerative Medicine for Polycystic Ovary Syndrome: Stem Cell-Based Therapies and Brown Adipose Tissue Activation. Stem Cell Rev. Rep. 2023, 19, 853–865. [Google Scholar] [CrossRef]
- Momin, E.N.; Mohyeldin, A.; Zaidi, H.A.; Vela, G.; Quiñones-Hinojosa, A. Mesenchymal stem cells: New approaches for the treatment of neurological diseases. Curr. Stem Cell Res. Ther. 2010, 5, 326–344. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.; Yim, H.W.; Park, H.J.; Cho, Y.; Hong, H.; Kim, N.J.; Oh, I.H. Mesenchymal Stem Cell Therapy for Ischemic Heart Disease: Systematic Review and Meta-analysis. Int. J. Stem Cells 2018, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.; Kahlenberg, S.; Hornsby, P. Therapeutic potential of mesenchymal stem cells for diabetes. J. Mol. Endocrinol. 2017, 59, R109–R120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Ma, J.; Han, J.; Zhang, W.; Ma, J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am. J. Transl. Res. 2019, 11, 6275–6289. [Google Scholar] [PubMed]
- Driscoll, J.; Patel, T. The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. J. Gastroenterol. 2019, 54, 763–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Huang, X.; Wang, H.; Liu, X.; Zhang, T.; Wang, Y.; Hu, D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res. Ther. 2015, 6, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Chapel, A.; Bertho, J.M.; Bensidhoum, M.; Fouillard, L.; Young, R.G.; Frick, J.; Demarquay, C.; Cuvelier, F.; Mathieu, E.; Trompier, F.; et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J. Gene Med. 2003, 5, 1028–1038. [Google Scholar] [CrossRef]
- Caplan, A.I. Why are MSCs therapeutic? New data: New insight. J. Pathol. 2009, 217, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef] [Green Version]
- Salgado, A.J.; Reis, R.L.; Sousa, N.J.; Gimble, J.M. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther. 2010, 5, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.Z.; Abelson, B.; Babbar, P.; Damaser, M.S. Harnessing the mesenchymal stem cell secretome for regenerative urology. Nat. Rev. Urol. 2019, 16, 363–375. [Google Scholar] [CrossRef]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef]
- Maqsood, M.; Kang, M.; Wu, X.; Chen, J.; Teng, L.; Qiu, L. Adult mesenchymal stem cells and their exosomes: Sources, characteristics, and application in regenerative medicine. Life Sci. 2020, 256, 118002. [Google Scholar] [CrossRef]
- Perocheau, D.; Touramanidou, L.; Gurung, S.; Gissen, P.; Baruteau, J. Clinical applications for exosomes: Are we there yet? Br. J. Pharmacol. 2021, 178, 2375–2392. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lin, E.Y.; Chiou, T.W.; Harn, H.J. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Ci Ji Yi Xue Za Zhi 2020, 32, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Yang, S.; Lee, J.; Park, B.; Park, K.; Jung, J.; Bae, Y.; Park, G. Combination Treatment with Human Adipose Tissue Stem Cell- derived Exosomes and Fractional CO2 Laser for Acne Scars: A 12-week Prospective, Double-blind, Randomized, Split-face Study. Soc. Publ. Acta Derm.-Venereol. 2020, 100, adv00310. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, E.; Babajani, A.; Soltani, P.; Niknejad, H. Proposed Mechanisms of Targeting COVID-19 by Delivering Mesenchymal Stem Cells and Their Exosomes to Damaged Organs. Stem Cell Rev. Rep. 2021, 17, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Rezaie, J. Potential therapeutic application of mesenchymal stem cell-derived exosomes in SARS-CoV-2 pneumonia. Stem Cell Res. 2020, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Xie, Q.; Xiong, X.; Xiao, N.; He, K.; Chen, M.; Peng, J.; Su, X.; Mei, H.; Dai, Y.; Wei, D.; et al. Mesenchymal Stem Cells Alleviate DHEA-Induced Polycystic Ovary Syndrome (PCOS) by Inhibiting Inflammation in Mice. Stem Cells Int. 2019, 2019, 9782373. [Google Scholar] [CrossRef] [Green Version]
- Kalhori, Z.; Azadbakht, M.; Soleimani Mehranjani, M.; Shariatzadeh, M.A. Improvement of the folliculogenesis by transplantation of bone marrow mesenchymal stromal cells in mice with induced polycystic ovary syndrome. Cytotherapy 2018, 20, 1445–1458. [Google Scholar] [CrossRef]
- Marti, N.; Bouchoucha, N.; Sauter, K.S.; Flück, C.E. Resveratrol inhibits androgen production of human adrenocortical H295R cells by lowering CYP17 and CYP21 expression and activities. PLoS ONE 2017, 12, e0174224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempná, P.; Hofer, G.; Mullis, P.E.; Flück, C.E. Pioglitazone inhibits androgen production in NCI-H295R cells by regulating gene expression of CYP17 and HSD3B2. Mol. Pharmacol. 2007, 71, 787–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Magoffin, D.; Munir, I.; Azziz, R. Effect of insulin and testosterone on androgen production and transcription of SULT2A1 in the NCI-H295R adrenocortical cell line. Fertil. Steril. 2009, 92, 793–797. [Google Scholar] [CrossRef] [Green Version]
- Chugh, R.M.; Park, H.S.; El Andaloussi, A.; Elsharoud, A.; Esfandyari, S.; Ulin, M.; Bakir, L.; Aboalsoud, A.; Ali, M.; Ashour, D.; et al. Mesenchymal stem cell therapy ameliorates metabolic dysfunction and restores fertility in a PCOS mouse model through interleukin-10. Stem Cell Res. Ther. 2021, 12, 388. [Google Scholar] [CrossRef]
- Chugh, R.M.; Park, H.S.; Esfandyari, S.; Elsharoud, A.; Ulin, M.; Al-Hendy, A. Mesenchymal Stem Cell-Conditioned Media Regulate Steroidogenesis and Inhibit Androgen Secretion in a PCOS Cell Model via BMP-2. Int. J. Mol. Sci. 2021, 22, 9184. [Google Scholar] [CrossRef]
- Park, H.S.; Chugh, R.M.; Pergande, M.R.; Cetin, E.; Siblini, H.; Esfandyari, S.; Cologna, S.M.; Al-Hendy, A. Non-Cytokine Protein Profile of the Mesenchymal Stem Cell Secretome That Regulates the Androgen Production Pathway. Int. J. Mol. Sci. 2022, 23, 4633. [Google Scholar] [CrossRef]
- Kauffman, A.S.; Thackray, V.G.; Ryan, G.E.; Tolson, K.P.; Glidewell-Kenney, C.A.; Semaan, S.J.; Poling, M.C.; Iwata, N.; Breen, K.M.; Duleba, A.J.; et al. A Novel Letrozole Model Recapitulates Both the Reproductive and Metabolic Phenotypes of Polycystic Ovary Syndrome in Female Mice. Biol. Reprod. 2015, 93, 69. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Q.; You, S.; Jiang, H.; Jiang, L.; He, F.; Hu, L. Efficacy of Mesenchymal Stem Cell-Derived Extracellular Vesicles in the Animal Model of Female Reproduc Diseases: A Meta-Analysis. Stem Cell Rev. Rep. 2023. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Eguchi, J.; Wada, J. Brown Adipose Tissue PPARgamma Is Required for the Insulin-Sensitizing Action of Thiazolidinediones. Acta Med. Okayama 2023, 77, 243–254. [Google Scholar] [CrossRef]
- Kasarinaite, A.; Sinton, M.; Saunders, P.T.K.; Hay, D.C. The Influence of Sex Hormones in Liver Function and Disease. Cells 2023, 12, 1604. [Google Scholar] [CrossRef] [PubMed]
- Leo, S.; Tremoli, E.; Ferroni, L.; Zavan, B. Role of Epicardial Adipose Tissue Secretome on Cardiovascular Diseases. Biomedicines 2023, 11, 1653. [Google Scholar] [CrossRef]
- Park, H.S.; Chugh, R.M.; Elsharoud, A.; Ulin, M.; Esfandyari, S.; Aboalsoud, A.; Bakir, L.; Al-Hendy, A. Safety of Intraovarian Injection of Human Mesenchymal Stem Cells in a Premature Ovarian Insufficiency Mouse Model. Cell Transpl. 2021, 30, 963689720988502. [Google Scholar] [CrossRef]
- Yakubova, A.A.; Mitusova, K.A.; Darwish, A.; Rogova, A.; Ageev, E.I.; Brodskaia, A.; Muslimov, A.R.; Zyuzin, M.V.; Timin, A.S. Calcium carbonate nanoparticles tumor delivery for combined chemo-photodynamic therapy: Comparison of local and systemic administration. J. Control Release 2023, 359, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, W.; Freeman, M.L.; Lederman, M.M.; Vasilieva, E.; Romero, R.; Margolis, L. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci. Rep. 2018, 8, 8973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.H.; Wu, K.C.; Harn, H.J.; Lin, S.Z.; Ding, D.C. Exosomes and Stem Cells in Degenerative Disease Diagnosis and Therapy. Cell Transpl. 2018, 27, 349–363. [Google Scholar] [CrossRef] [Green Version]
- Yin, K.; Wang, S.; Zhao, R.C. Exosomes from mesenchymal stem/stromal cells: A new therapeutic paradigm. Biomark. Res. 2019, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Adamiak, M.; Sahoo, S. Exosomes in Myocardial Repair: Advances and Challenges in the Development of Next-Generation Therapeutics. Mol. Ther. 2018, 26, 1635–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toh, W.S.; Lai, R.C.; Hui, J.H.P.; Lim, S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin. Cell Dev. Biol. 2017, 67, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hecker, M.; Newsted, J.L.; Murphy, M.B.; Higley, E.B.; Jones, P.D.; Wu, R.; Giesy, J.P. Human adrenocarcinoma (H295R) cells for rapid in vitro determination of effects on steroidogenesis: Hormone production. Toxicol. Appl. Pharmacol. 2006, 217, 114–124. [Google Scholar] [CrossRef]
- McAllister, J.M.; Han, A.X.; Modi, B.P.; Teves, M.E.; Mavodza, G.R.; Anderson, Z.L.; Shen, T.; Christenson, L.K.; Archer, K.J.; Strauss, J.F. miRNA Profiling Reveals miRNA-130b-3p Mediates DENND1A Variant 2 Expression and Androgen Biosynthesis. Endocrinology 2019, 160, 1964–1981. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.C.; Valenzuela, N.; Fai, S.; Bennett, S.A. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J. Vis. Exp. 2012, e4389. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-S.; Cetin, E.; Siblini, H.; Seok, J.; Alkelani, H.; Alkhrait, S.; Liakath Ali, F.; Mousaei Ghasroldasht, M.; Beckman, A.; Al-Hendy, A. Therapeutic Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles to Treat PCOS. Int. J. Mol. Sci. 2023, 24, 11151. https://doi.org/10.3390/ijms241311151
Park H-S, Cetin E, Siblini H, Seok J, Alkelani H, Alkhrait S, Liakath Ali F, Mousaei Ghasroldasht M, Beckman A, Al-Hendy A. Therapeutic Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles to Treat PCOS. International Journal of Molecular Sciences. 2023; 24(13):11151. https://doi.org/10.3390/ijms241311151
Chicago/Turabian StylePark, Hang-Soo, Esra Cetin, Hiba Siblini, Jin Seok, Hiba Alkelani, Samar Alkhrait, Farzana Liakath Ali, Mohammad Mousaei Ghasroldasht, Analea Beckman, and Ayman Al-Hendy. 2023. "Therapeutic Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles to Treat PCOS" International Journal of Molecular Sciences 24, no. 13: 11151. https://doi.org/10.3390/ijms241311151






