Lamin A/C Ablation Restricted to Vascular Smooth Muscle Cells, Cardiomyocytes, and Cardiac Fibroblasts Causes Cardiac and Vascular Dysfunction
Abstract
1. Introduction
2. Results
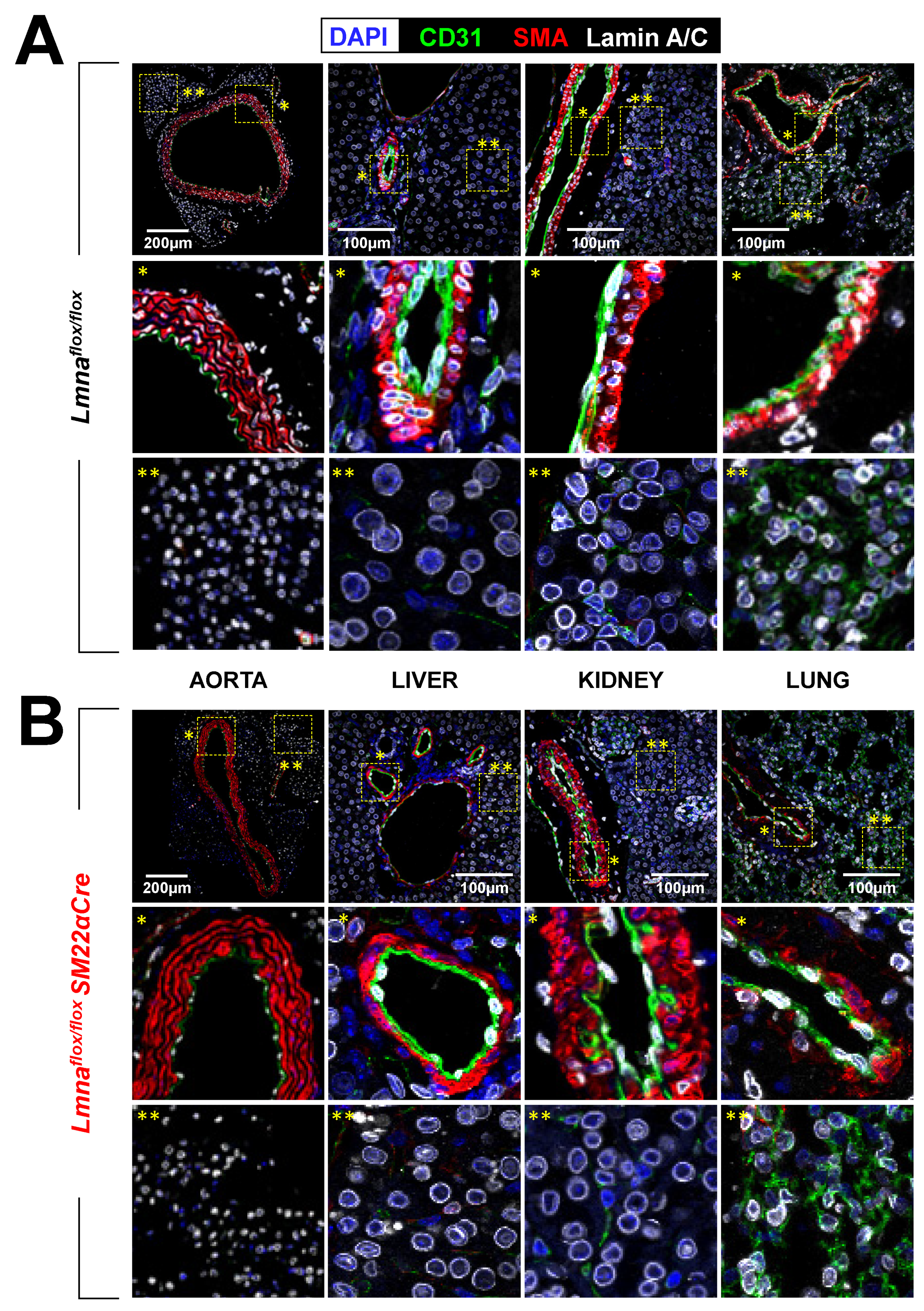
2.1. Lmnaflox/floxSM22αCre Mice with Lmna Deficiency Restricted to Vascular Smooth Muscle Cells, Cardiac Fibroblasts, and Cardiomyocytes Die Prematurely
2.2. Lmnaflox/floxSM22α-Cre Mice Develop Cardiac Fibrosis and Severe Systolic Dysfunction and Electrocardiographic Alterations
2.3. Lmnaflox/floxSM22αCre Mice Exhibit Contractile-to-Synthetic Phenotypic Switching in VSMCs and Vascular Dysfunction in the Aorta
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Longevity Studies
4.3. Hematology and Cardiac Biochemical Parameters
4.4. Histology and Immunofluorescence
4.5. Aortic RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
4.6. Western Blot
4.7. Echocardiography
4.8. Electrocardiography
4.9. Wire Myography
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerace, L.; Burke, B. Functional organization of the nuclear envelope. Annu. Rev. Cell Biol. 1988, 4, 335–374. [Google Scholar] [CrossRef]
- Stuurman, N.; Heins, S.; Aebi, U. Nuclear lamins: Their structure, assembly, and interactions. J. Struct. Biol. 1998, 122, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Broers, J.L.; Ramaekers, F.C.; Bonne, G.; Yaou, R.B.; Hutchison, C.J. Nuclear lamins: Laminopathies and their role in premature ageing. Physiol. Rev. 2006, 86, 967–1008. [Google Scholar] [CrossRef] [PubMed]
- Andrés, V.; González, J.M. Role of A-type lamins in signaling, transcription, and chromatin organization. J. Cell Biol. 2009, 187, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Dechat, T.; Pfleghaar, K.; Sengupta, K.; Shimi, T.; Shumaker, D.K.; Solimando, L.; Goldman, R.D. Nuclear lamins: Major factors in the structural organization and function of the nucleus and chromatin. Genes. Dev. 2008, 22, 832–853. [Google Scholar] [CrossRef]
- Kalukula, Y.; Stephens, A.D.; Lammerding, J.; Gabriele, S. Mechanics and functional consequences of nuclear deformations. Nat. Rev. Mol. Cell Biol. 2022, 23, 583–602. [Google Scholar] [CrossRef]
- Swift, J.; Ivanovska, I.L.; Buxboim, A.; Harada, T.; Dingal, P.D.P.; Pinter, J.; Pajerowski, J.D.; Spinler, K.R.; Shin, J.W.; Tewari, M.; et al. Nuclear Lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 2013, 341, 975–990. [Google Scholar] [CrossRef]
- Carmosino, M.; Torretta, S.; Procino, G.; Gerbino, A.; Forleo, C.; Favale, S.; Svelto, M. Role of nuclear Lamin A/C in cardiomyocyte functions. Biol. Cell 2014, 106, 346–358. [Google Scholar] [CrossRef]
- Benedicto, I.; Dorado, B.; Andrés, V. Molecular and cellular mechanisms driving cardiovascular disease in hutchinson-gilford progeria syndrome: Lessons learned from animal models. Cells 2021, 10, 1157. [Google Scholar] [CrossRef]
- Taylor, M.R.; Fain, P.R.; Sinagra, G.; Robinson, M.L.; Robertson, A.D.; Carniel, E.; Di Lenarda, A.; Bohlmeyer, T.J.; Ferguson, D.A.; Brodsky, G.L.; et al. Natural history of dilated cardiomyopathy due to lamin A/C gene mutations. J. Am. Coll. Cardiol. 2003, 41, 771–780. [Google Scholar] [CrossRef]
- Bonne, G.; Ben Yaou, R.; Beroud, C.; Boriani, G.; Brown, S.; de Visser, M.; Duboc, D.; Ellis, J.; Hausmanowa-Petrusewicz, I.; Lattanzi, G.; et al. 108th ENMC International Workshop, 3rd Workshop of the MYO-CLUSTER project: EUROMEN, 7th International Emery-Dreifuss Muscular Dystrophy (EDMD) Workshop, 13–15 September 2002, Naarden, The Netherlands. Neuromuscul. Disorder. 2003, 13, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Van Berlo, J.H.; De Voogt, W.G.; Van Der Kooi, A.J.; Van Tintelen, J.P.; Bonne, G.; Yaou, R.B.; Duboc, D.; Rossenbacker, T.; Heidbüchel, H.; De Visser, M.; et al. Meta-analysis of clinical characteristics of 299 carriers of LMNA gene mutations: Do lamin A/C mutations portend a high risk of sudden death? J. Mol. Med. 2005, 83, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Van Rijsingen, I.A.; Arbustini, E.; Elliott, P.M.; Mogensen, J.; Hermans-van Ast, J.F.; van der Kooi, A.J.; van Tintelen, J.P.; van den Berg, M.P.; Pilotto, A.; Pasotti, M.; et al. Risk factors for malignant ventricular arrhythmias in Lamin A/C mutation carriers: A European cohort study. J. Am. Coll. Cardiol. 2012, 59, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Pilotto, A.; Repetto, A.; Grasso, M.; Negri, A.; Diegoli, M.; Campana, C.; Scelsi, L.; Baldini, E.; Gavazzi, A.; et al. Autosomal dominant dilated cardiomyopathy with atrioventricular block: A lamin A/C defect-related disease. J. Am. Coll. Cardiol. 2002, 39, 981–990. [Google Scholar] [CrossRef]
- Crasto, S.; My, I.; di Pasquale, E. The Broad Spectrum of LMNA Cardiac Diseases: From Molecular Mechanisms to Clinical Phenotype. Front. Physiol. 2020, 11, 761. [Google Scholar] [CrossRef]
- Hasselberg, N.E.; Haland, T.F.; Saberniak, J.; Brekke, P.H.; Berge, K.E.; Leren, T.P.; Edvardsen, T.; Haugaa, K.H. Lamin A/C cardiomyopathy: Young onset, high penetrance, and frequent need for heart transplantation. Eur. Heart J. 2018, 39, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ren, L.; Wu, J.C. New Insights Into the Therapy for Lamin-Associated Dilated Cardiomyopathy. J. Am. Coll. Cardiol. Basic. Transl. Sci. 2022, 7, 1246–1248. [Google Scholar] [CrossRef]
- Zhang, H.; Kieckhaefer, J.E.; Cao, K. Mouse models of laminopathies. Aging Cell 2013, 12, 2–10. [Google Scholar] [CrossRef]
- Sullivan, T. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 1999, 147, 913–920. [Google Scholar] [CrossRef]
- Nikolova, V.; Leimena, C.; McMahon, A.C.; Tan, J.C.; Chandar, S.; Jogia, D.; Kesteven, S.H.; Michalicek, J.; Otway, R.; Verheyen, F.; et al. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J. Clin. Investig. 2004, 113, 357–369. [Google Scholar] [CrossRef]
- Frock, R.L.; Chen, S.C.; Da, D.F.; Frett, E.; Lau, C.; Brown, C.; Pak, D.N.; Wang, Y.; Muchir, A.; Worman, H.J.; et al. Cardiomyocyte-specific expression of lamin A improves cardiac function in Lmna−/− mice. PLoS ONE 2012, 7, 1–9. [Google Scholar] [CrossRef]
- Auguste, G.; Rouhi, L.; Matkovich, S.J.; Coarfa, C.; Robertson, M.J.; Czernuszewicz, G.; Gurha, P.; Marian, A.J. BET bromodomain inhibition attenuates cardiac phenotype in myocyte-specific lamin A/C-deficient mice. J. Clin. Investig. 2020, 130, 4740–4758. [Google Scholar] [CrossRef]
- Chai, R.J.; Werner, H.; Li, P.Y.; Lee, Y.L.; Nyein, K.T.; Solovei, I.; Luu, T.D.A.; Sharma, B.; Navasankari, R.; Maric, M.; et al. Disrupting the LINC complex by AAV mediated gene transduction prevents progression of Lamin induced cardiomyopathy. Nat. Commun. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Rouhi, L.; Auguste, G.; Zhou, Q.; Lombardi, R.; Olcum, M.; Pourebrahim, K.; Cheedipudi, S.M.; Asghar, S.; Hong, K.; Robertson, M.J.; et al. Deletion of the Lmna gene in fibroblasts causes senescence-associated dilated cardiomyopathy by activating the double-stranded DNA damage response and induction of senescence-associated secretory phenotype. J. Cardiovasc. Aging 2022, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Zheng, Y. Generation and characterization of a conditional deletion allele for Lmna in mice. Biochem. Biophys. Res. Commun. 2013, 440, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Lepore, J.J.; Cheng, L.; Min Lu, M.; Mericko, P.A.; Morrisey, E.E.; Parmacek, M.S. High-efficiency somatic mutagenesis in smooth muscle cells and cardiac myocytes in SM22α-Cre transgenic mice. Genesis 2005, 41, 179–184. [Google Scholar] [CrossRef]
- Hanna, A.; Humeres, C.; Frangogiannis, N.G. The role of Smad signaling cascades in cardiac fibrosis. Cell Signal. 2021, 77, 109826. [Google Scholar] [CrossRef] [PubMed]
- Arimura, T.; Helbling-Leclerc, A.; Massart, C.; Varnous, S.; Niel, F.; Lacene, E.; Fromes, Y.; Toussaint, M.; Mura, A.M.; Keller, D.I.; et al. Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum. Mol. Genet. 2005, 14, 155–169. [Google Scholar] [CrossRef]
- Roura, S.; Bayes-Genis, A. Vascular dysfunction in idiopathic dilated cardiomyopathy. Nat. Rev. 2009, 6, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Mathier, M.A.; Rose, G.A.; Fifer, M.A.; Miyamoto, M.I.; Dinsmore, R.E.; Castaño, H.H.; Dec, G.; Palacios, I.F.; Semigran, M.J. Coronary Endothelial Dysfunction in Patients With Acute-Onset Idiopathic Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 1998, 32, 216–224. [Google Scholar] [CrossRef]
- Sayed, N.; Liu, C.; Ameen, M.; Himmati, F.; Zhang, J.Z.; Khanamiri, S.; Moonen, J.-R.; Wnorowski, A.; Cheng, L.; Rhee, J.-W.; et al. Clinical trial in a dish using iPSCs shows lovastatin improves endothelial dysfunction and cellular cross-talk in LMNA cardiomyopathy. Sci. Transl. Med. 2020, 12, eaax9276. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, V.; Vickneson, K.; Kofidis, T.; Woo, C.C.; Lin, X.Y.; Foo, R.; Shanahan, C.M. Role of Vascular Smooth Muscle Cell Plasticity and Interactions in Vessel Wall Inflammation. Front. Immunol. 2020, 11, 599415. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, L.; Ferrer, M. Wire Myography to Study Vascular Tone and Vascular Structure of Isolated Mouse Arteries. Methods Mol. Biol. 2015, 1339, 255–276. [Google Scholar] [PubMed]







| Genotyping | Forward (5′ → 3′) | Reverse (5′ → 3′) |
|---|---|---|
| SM22αCre Transgene | GCGGTCTGGCAGTAAAAACTATC | GTGAAACAGCATTGCTGTCACTT |
| SM22αCre Internal positive control | CTAGGCCACAGAATTGAAAGATCT | GTAGGTGGAAATTCTAGCATCATCC |
| Lmnaflox/flox | AACCCAGCCTCAGAAACTGGTGGATG | GACAGCTCTCCTCTGAAGTGCTTGGA |
| Gene | Forward (5′ → 3′) | Reverse (5′ → 3′) |
|---|---|---|
| Acta2 | AAGAGGAAGACAGCACAGCC | AGCGTCAGGATCCCTCTCTT |
| Calr | CCAGAAATTGACAACCCTGAA | CCTTAAGCCTCTGCTCCTCAT |
| Cam2 | AAGTTGATGAAATGATCAGGGAAG | TGAAGTCCTAATTACTATACATGCATA |
| Cnn1 | TGGGAGTCAAGTATGCAGAG | CTGACTGGCAAACTTGTTGG |
| Hprt | CCTAAGATGAGCGCAAGTTGAA | CCACAGGACTAGAACACCTGCTAA |
| Klf4 | TTGTGACTATGCAGGCTGTG | TAGTGCCTGGTCAGTTCATC |
| Lum | TTCACTGGGCTGCAATACC | TCCCAGGATCTTACAGAAGC |
| Mmp2 | ACCTTGACCAGAACACCATC | AGCATCATCCACGGTTTCAG |
| Nox1 | CAACAGCACTCACCAATGCC | ACATCCTCACTGACTGTGCC |
| Prkg1 | ACTGCATGTGTGGTAGAAGC | GCCAGTCAGAAGCTCATACATC |
| Smtn | AGAACACCATCACCCACATC | TCTTGTCCAGGACTCCTTCG |
| Sod1 | TGGGTTCCACGTCCATCAGTA | ACCGTCCTTTCCAGCAGTCA |
| Sox9 | AGAACAAGCCACACGTCAAG | GTCTCTTCTCGCTCTCGTTC |
| Spp1 | GGTGATAGCTTGGCTTATGG | TGGGCAACAGGGATGACATC |
| Tagln | CCCAGACACCGAAGCTACTC | GACTGCACTTCTCGGCTCAT |
| Tfam | CAGGAGGCAAAGGATGATTC | CCAAGACTTCATTTCATTGTCG |
| Vcam1 | TCAAGGGTGACCAGCTCATG | TCGTTGTATTCCTGGGAGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Monte-Monge, A.; Ruiz-Polo de Lara, Í.; Gonzalo, P.; Espinós-Estévez, C.; González-Amor, M.; de la Fuente-Pérez, M.; Andrés-Manzano, M.J.; Fanjul, V.; Gimeno, J.R.; Barriales-Villa, R.; et al. Lamin A/C Ablation Restricted to Vascular Smooth Muscle Cells, Cardiomyocytes, and Cardiac Fibroblasts Causes Cardiac and Vascular Dysfunction. Int. J. Mol. Sci. 2023, 24, 11172. https://doi.org/10.3390/ijms241311172
Del Monte-Monge A, Ruiz-Polo de Lara Í, Gonzalo P, Espinós-Estévez C, González-Amor M, de la Fuente-Pérez M, Andrés-Manzano MJ, Fanjul V, Gimeno JR, Barriales-Villa R, et al. Lamin A/C Ablation Restricted to Vascular Smooth Muscle Cells, Cardiomyocytes, and Cardiac Fibroblasts Causes Cardiac and Vascular Dysfunction. International Journal of Molecular Sciences. 2023; 24(13):11172. https://doi.org/10.3390/ijms241311172
Chicago/Turabian StyleDel Monte-Monge, Alberto, Íñigo Ruiz-Polo de Lara, Pilar Gonzalo, Carla Espinós-Estévez, María González-Amor, Miguel de la Fuente-Pérez, María J. Andrés-Manzano, Víctor Fanjul, Juan R. Gimeno, Roberto Barriales-Villa, and et al. 2023. "Lamin A/C Ablation Restricted to Vascular Smooth Muscle Cells, Cardiomyocytes, and Cardiac Fibroblasts Causes Cardiac and Vascular Dysfunction" International Journal of Molecular Sciences 24, no. 13: 11172. https://doi.org/10.3390/ijms241311172
APA StyleDel Monte-Monge, A., Ruiz-Polo de Lara, Í., Gonzalo, P., Espinós-Estévez, C., González-Amor, M., de la Fuente-Pérez, M., Andrés-Manzano, M. J., Fanjul, V., Gimeno, J. R., Barriales-Villa, R., Dorado, B., & Andrés, V. (2023). Lamin A/C Ablation Restricted to Vascular Smooth Muscle Cells, Cardiomyocytes, and Cardiac Fibroblasts Causes Cardiac and Vascular Dysfunction. International Journal of Molecular Sciences, 24(13), 11172. https://doi.org/10.3390/ijms241311172





