Dispersant and Protective Roles of Amphiphilic Poly(ethylene phosphate) Block Copolymers in Polyester/Bone Mineral Composites
Abstract
1. Introduction
2. Results and Discussion
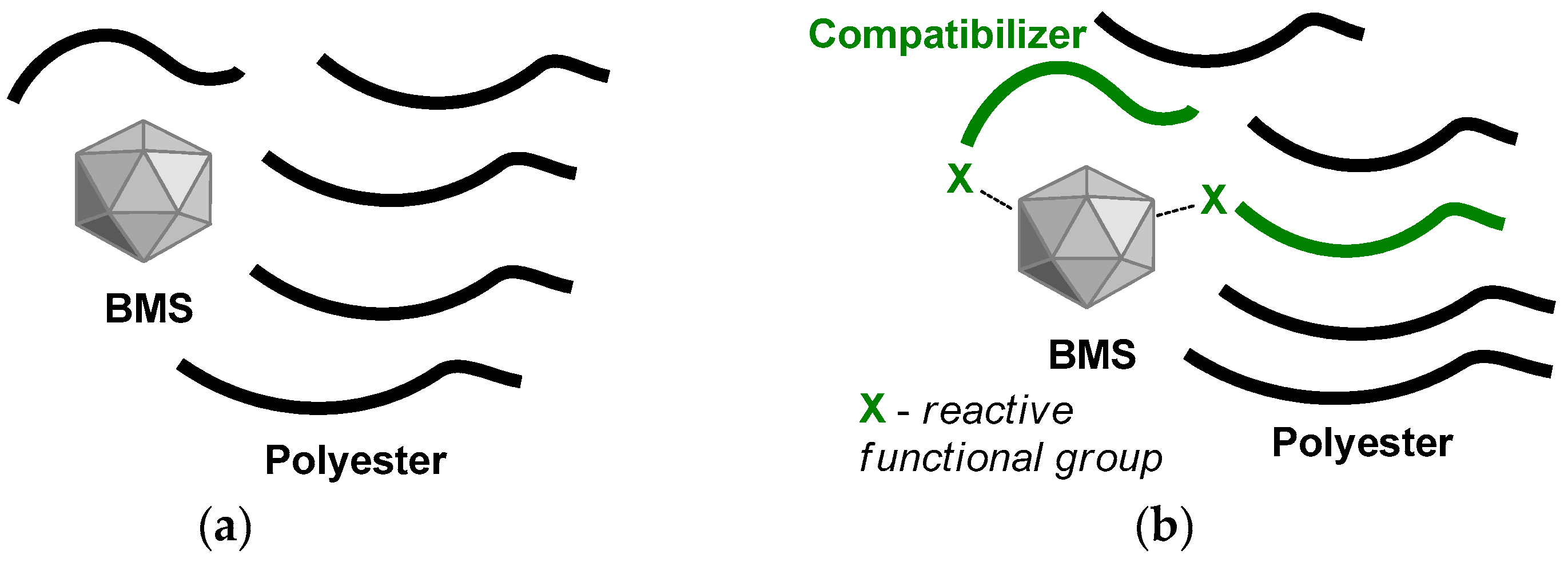
2.1. Preparation of the Composites
2.1.1. Synthesis of CAp
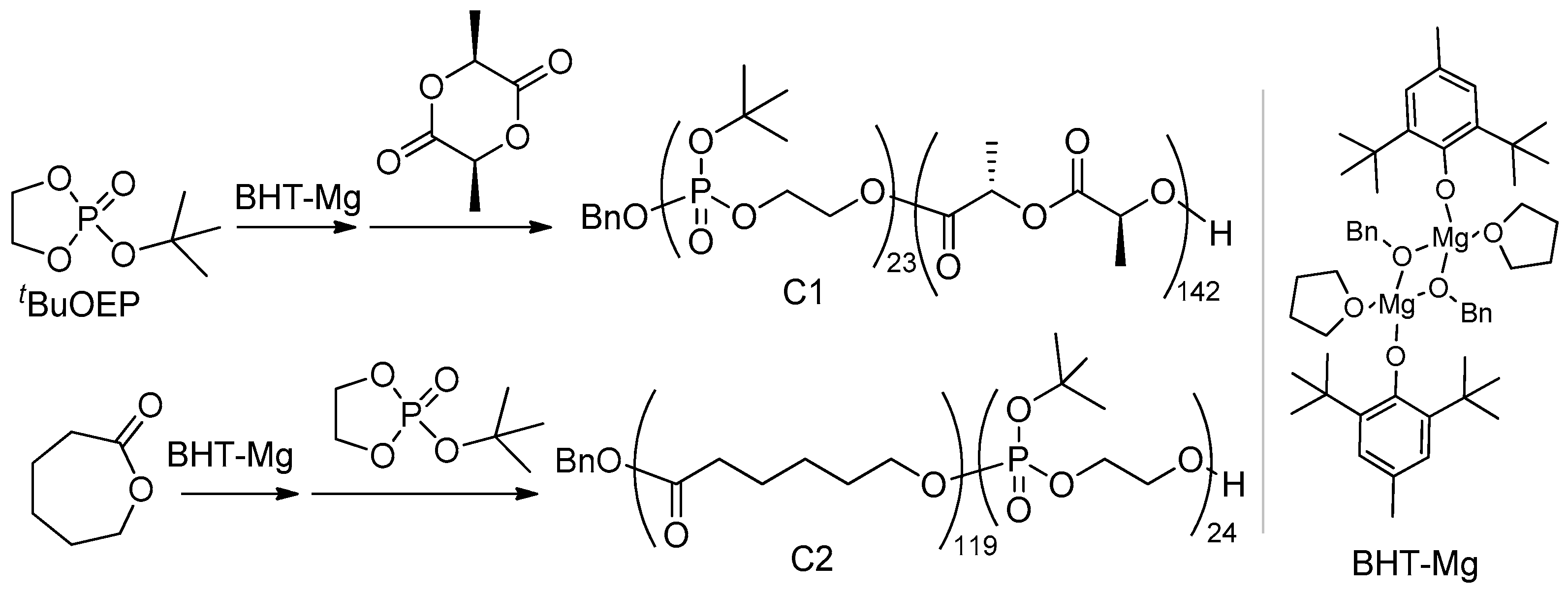
2.1.2. Synthesis of Polyester-b-poly(tBuOEP) Compatibilizer
2.1.3. Preparation and Molding of the Composites
2.2. Characteristics of the PLLA-Based Composites
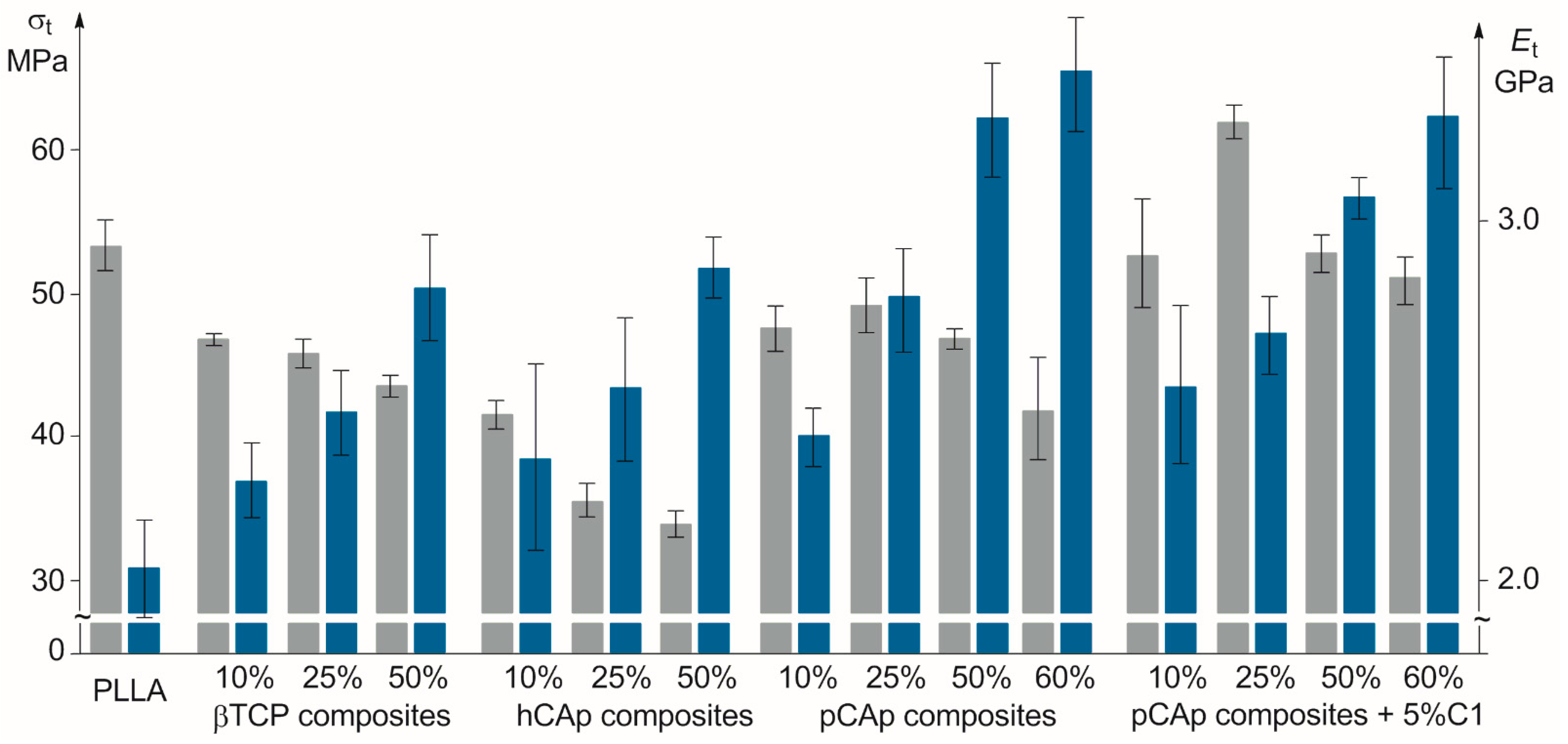
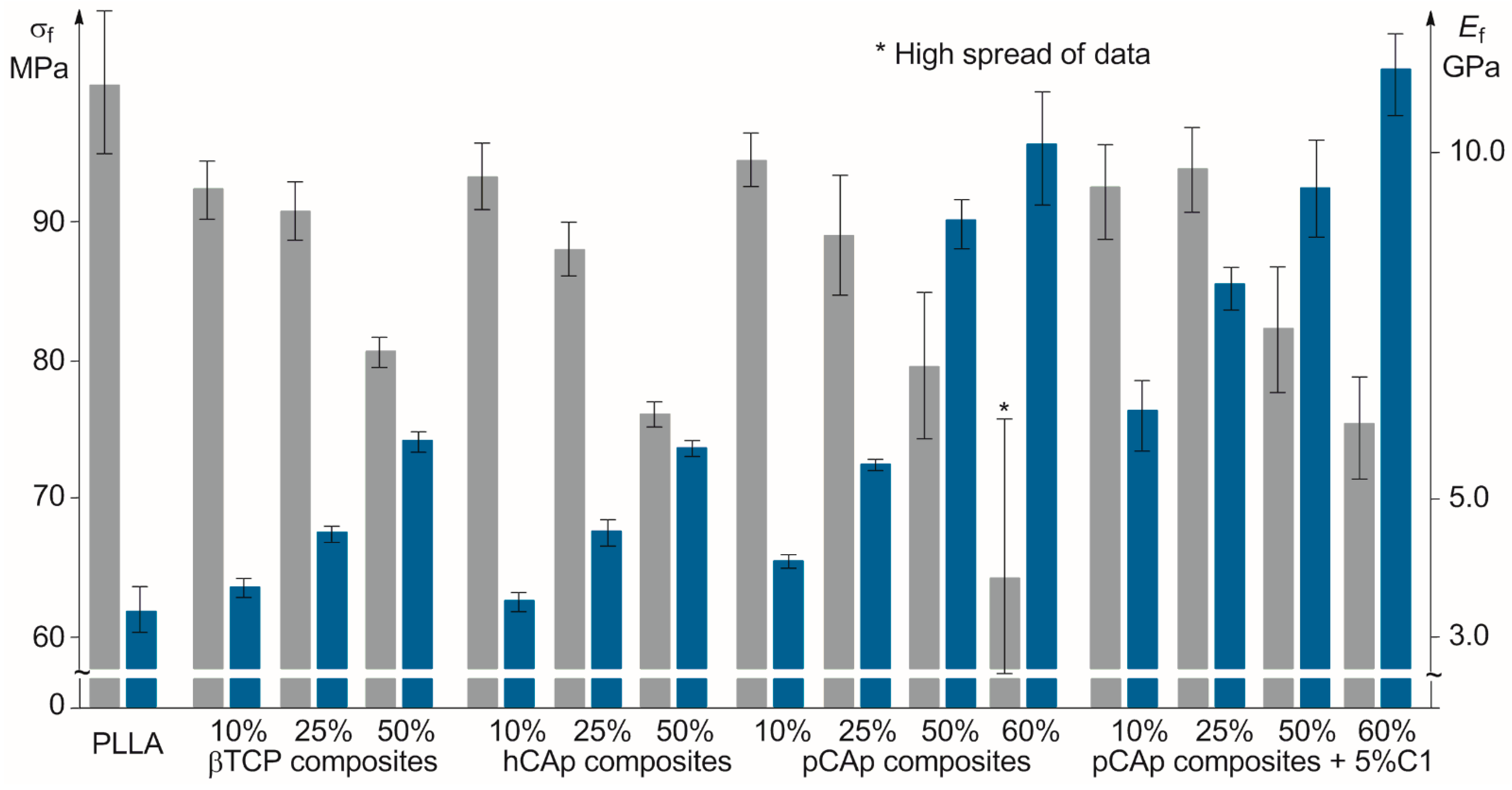
2.2.1. Mechanical Properties of PLLA/BMS Composites
2.2.2. Mechanical Properties of PLLA/C1/pCAp Composites
2.2.3. Evidence for PEPA–CAp Bonding in PLLA/C1/pCAp Composites
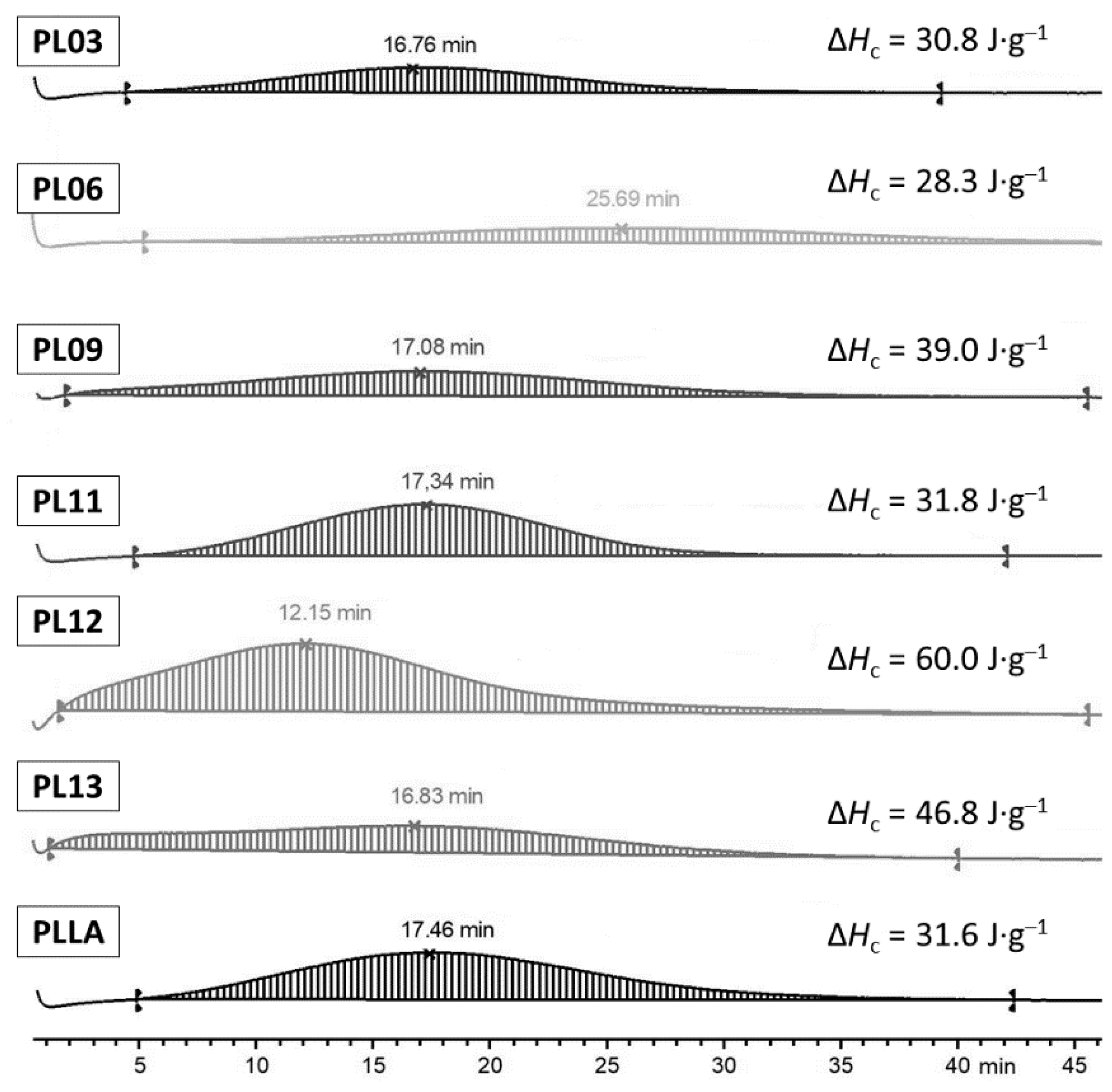
2.2.4. Thermal Properties of PLLA-Based Composites
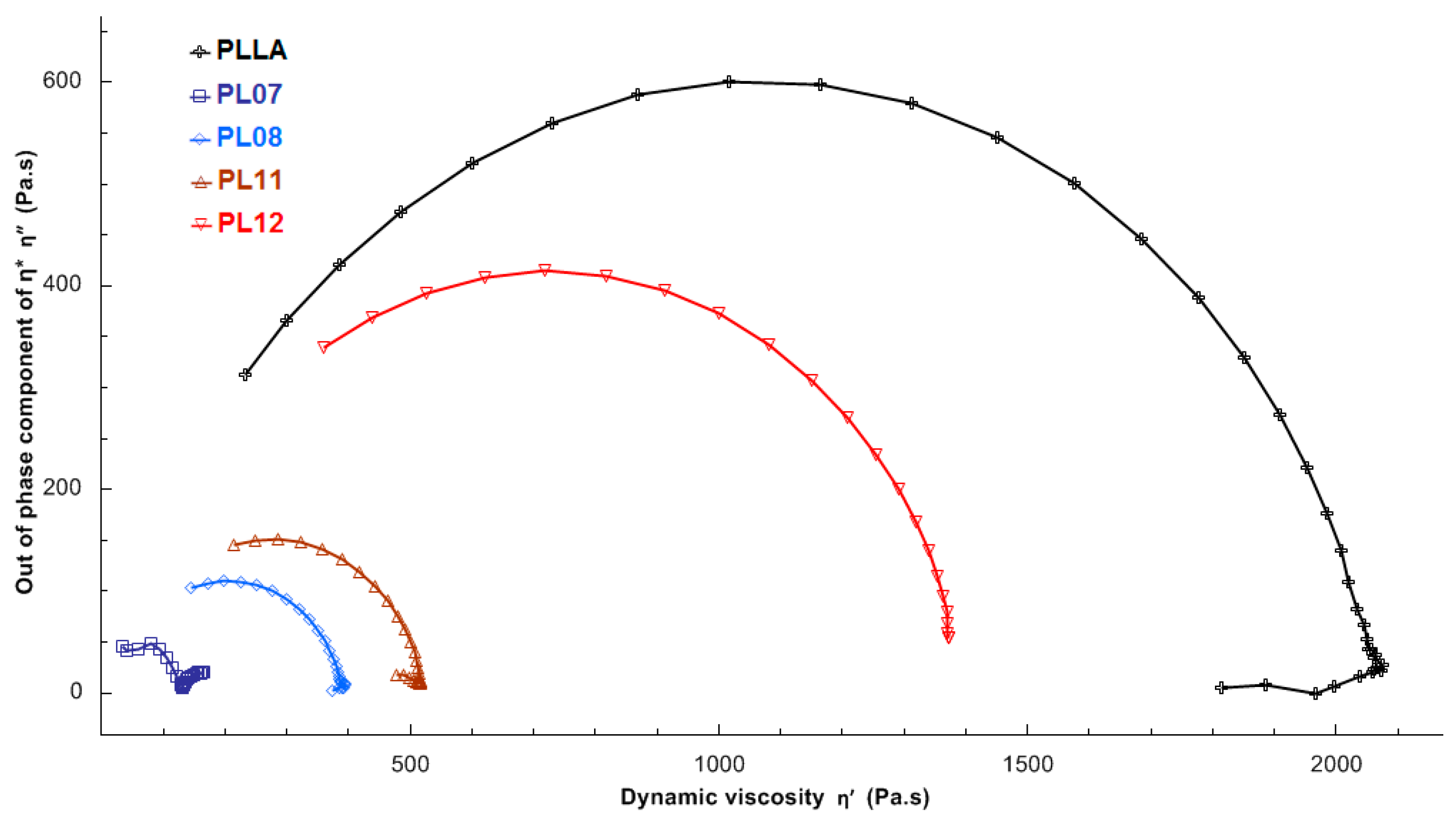
2.2.5. Rheology of PLLA-Based Composites
2.2.6. Degradation of PLLA during Molding and the Role of Compatibilizer
2.3. Characteristics of the PCL-Based Composites
3. Materials and Methods
3.1. Solvents and Reagents
3.2. Physico-Chemical Characterization
3.3. Synthesis of Copolymers
3.3.1. BnO(tBuOEP)23-b-(L-LA)142 (Copolymer C1)
3.3.2. BnO(εCL)119-b-(tBuOEP)24 (Copolymer C2)
3.4. Synthesis of CAp
3.4.1. Synthesis of hCAp
3.4.2. Synthesis of pCAp
3.5. Preparation of the Composites and Composite Samples
3.5.1. PLLA-Based Composites
3.5.2. PCL-Based Composites
3.5.3. Molding of the Composite Samples
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishikawa, K. Carbonate apatite bone replacement: Learn from the bone. J. Ceram. Soc. Jpn. 2019, 127, 595–601. [Google Scholar] [CrossRef]
- Dalí, G.C.; Lagares, D.T. Chapter 1—Nanobiomaterials in hard tissue engineering. In Applications of Nanobiomaterials; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 4, pp. 1–31. [Google Scholar] [CrossRef]
- Su, F.Y.; Pang, S.; Ling, Y.T.T.; Shyu, P.; Novitskaya, E.; Seo, K.; Lambert, S.; Zarate, K.; Graeve, O.A.; Jasiuk, I.; et al. Deproteinization of Cortical Bone: Effects of Different Treatments. Calcif. Tissue Int. 2018, 103, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Schwarcz, H.P.; Jasiuk, I. Interfacial bonding between mineral platelets in bone and its effect on mechanical properties of bone. J. Mech. Behav. Biomed. Mater. 2021, 113, 104132. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef]
- Burkhart, S.S. The evolution of clinical applications of biodegradable implants in arthroscopic surgery. Biomaterials 2000, 21, 2631–2634. [Google Scholar] [CrossRef]
- Bernardo, M.P.; da Silva, B.C.R.; Hamouda, A.E.I.; de Toledo, M.A.S.; Schalla, C.; Rütten, S.; Goetzke, R.; Mattoso, L.H.C.; Zenke, M.; Sechi, A. PLA/Hydroxyapatite scaffolds exhibit in vitro immunological inertness and promote robust osteogenic differentiation of human mesenchymal stem cells without osteogenic stimuli. Sci. Rep. 2022, 12, 2333. [Google Scholar] [CrossRef]
- Ke, D.; Bose, S. Effects of pore distribution and chemistry on physical, mechanical, and biological properties of tricalcium phosphate scaffolds by binder-jet 3D printing. Addit. Manuf. 2018, 22, 111–117. [Google Scholar] [CrossRef]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef]
- Russias, J.; Saiz, E.; Nalla, R.K.; Gryn, K.; Ritchie, R.O.; Tomsia, A.P. Fabrication and mechanical properties of PLA/HA composites: A study of in vitro degradation. Mater. Sci. Eng. C 2006, 26, 1289–1295. [Google Scholar] [CrossRef]
- Sadek, A.A.; Abd-Elkareem, M.; Abdelhamid, H.N.; Moustafa, S.; Hussein, K. Enhancement of critical-sized bone defect regeneration using UiO-66 nanomaterial in rabbit femurs. BMC Vet. Res. 2022, 18, 260. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef]
- Siddiqui, H.A.; Pickering, K.L.; Mucalo, M.R. A Review on the Use of Hydroxyapatite-Carbonaceous Structure Composites in Bone Replacement Materials for Strengthening Purposes. Materials 2018, 11, 1813. [Google Scholar] [CrossRef]
- George, S.M.; Nayak, C.; Singh, I.; Balani, K. Multifunctional Hydroxyapatite Composites for Orthopedic Applications: A Review. ACS Biomater. Sci. Eng. 2022, 8, 3162–3186. [Google Scholar] [CrossRef]
- Patil, N.A.; Kandasubramanian, B. Biological and mechanical enhancement of zirconium dioxide for medical applications. Ceram. Int. 2020, 46, 4041–4057. [Google Scholar] [CrossRef]
- Xu, M.; Girish, Y.R.; Rakesh, K.P.; Wu, P.; Manukumar, H.M.; Byrappa, S.M.; Udayabhanu; Byrappa, K. Recent advances and challenges in silicon carbide (SiC) ceramic nanoarchitectures and their applications. Mater. Today Commun. 2021, 28, 102533. [Google Scholar] [CrossRef]
- Raj, S.V.; Rajkumar, M.; Sundaram, N.M.; Kandaswamy, A. Synthesis and characterization of hydroxyapatite/alumina ceramic nanocomposites for biomedical applications. Bull. Mater. Sci. 2018, 41, 93. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Asri, R.I.M.; Alias, J.; Zulkifli, F.H.; Kadirgama, K.; Ghani, S.A.C.; Shariffuddin, J.H.M. A comprehensive review of hydroxyapatite-based coatings adhesion on metallic biomaterials. Ceram. Int. 2018, 44, 1250–1268. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Cannillo, V. Hydroxyapatite and tricalcium phosphate composites with bioactive glass as second phase: State of the art and current applications. J. Biomed. Mater. Res. 2016, 104, 1030–1056. [Google Scholar] [CrossRef]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef]
- Li, Y.; Liao, C.; Tjong, S.C. Synthetic Biodegradable Aliphatic Polyester Nanocomposites Reinforced with Nanohydroxyapatite and/or Graphene Oxide for Bone Tissue Engineering Applications. Nanomaterials 2019, 9, 590. [Google Scholar] [CrossRef] [PubMed]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos. B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Fu, Z.; Cui, J.; Zhao, B.; Shen, S.G.; Lin, K. An overview of polyester/hydroxyapatite composites for bone tissue repairing. J. Orthop. Transl. 2021, 28, 118–130. [Google Scholar] [CrossRef] [PubMed]
- On, S.-W.; Cho, S.-W.; Byun, S.-H.; Yang, B.-E. Bioabsorbable Osteofixation Materials for Maxillofacial Bone Surgery: A Review on Polymers and Magnesium-Based Materials. Biomedicines 2020, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- De Santis, R.; Guarino, V.; Ambrosio, L. 10—Composite biomaterials for bone repair. In Bone Repair Biomaterials, 2nd ed.; Pawelec, K.M., Planell, J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 273–299. [Google Scholar] [CrossRef]
- Thangavel, M.; Selvam, R.E. Review of Physical, Mechanical, and Biological Characteristics of 3D-Printed Bioceramic Scaffolds for Bone Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2022, 8, 5060–5093. [Google Scholar] [CrossRef]
- Rakmae, S.; Ruksakulpiwat, Y.; Sutapun, W.; Suppakarn, N. Effects of mixing technique and filler content on physical properties of bovine bone-based CHA/PLA composites. J. Appl. Polym. Sci. 2011, 122, 2433–2441. [Google Scholar] [CrossRef]
- Targonska, S.; Dobrzynska-Mizera, M.; Wujczyk, M.; Rewak-Soroczynska, J.; Knitter, M.; Dopierala, K.; Andrzejewski, J.; Wiglusz, R.J. New way to obtain the poly(L-lactide-co-D,L-lactide) blend filled with nanohydroxyapatite as biomaterial for 3D-printed bone-reconstruction implants. Eur. Polym. J. 2022, 165, 110997. [Google Scholar] [CrossRef]
- Backes, E.H.; Fernandes, E.M.; Diogo, G.S.; Marques, C.F.; Silva, T.H.; Costa, L.C.; Passador, F.R.; Reis, R.L.; Pessan, L.A. Engineering 3D printed bioactive composite scaffolds based on the combination of aliphatic polyester and calcium phosphates for bone tissue regeneration. Mater. Sci. Eng. C 2021, 122, 111928. [Google Scholar] [CrossRef]
- Demina, V.A.; Krasheninnikov, S.V.; Buzin, A.I.; Kamyshinsky, R.A.; Sadovskaya, N.V.; Goncharov, E.N.; Zhukova, N.A.; Khvostov, M.V.; Pavlova, A.V.; Tolstikova, T.G.; et al. Biodegradable poly(L-lactide)/calcium phosphate composites with improved properties for orthopedics: Effect of filler and polymer crystallinity. Mater. Sci. Eng. C 2020, 112, 110813. [Google Scholar] [CrossRef]
- Backes, E.H.; De Nóbile Pires, L.; Gonçalves Beatrice, C.A.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Fabrication of Biocompatible Composites of Poly(lactic acid)/Hydroxyapatite Envisioning Medical Applications. Polym. Eng. Sci. 2020, 60, 636–644. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2021, 8, 346–360. [Google Scholar] [CrossRef]
- Tayton, E.; Purcell, M.; Aarvold, A.; Smith, J.O.; Briscoe, A.; Kanczler, J.M.; Shakesheff, K.M.; Howdle, S.M.; Dunlop, D.G.; Oreffo, R.O.C. A comparison of polymer and polymer–hydroxyapatite composite tissue engineered scaffolds for use in bone regeneration. An in vitro and in vivo study. J. Biomed. Mater. Res. 2014, 102, 2613–2624. [Google Scholar] [CrossRef]
- Washington, M.A.; Balmert, S.C.; Fedorchak, M.V.; Little, S.R.; Watkins, S.C.; Meyer, T.Y. Monomer sequence in PLGA microparticles: Effects on acidic microclimates and in vivo inflammatory response. Acta Biomater. 2018, 65, 259–271. [Google Scholar] [CrossRef]
- Implants for Surgery—Calcium Phosphates—Part 3: Hydroxyapatite and β-Tricalcium Phosphate Bone Substitutes. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfstandards/detail.cfm?standard__identification_no=35613 (accessed on 5 July 2023).
- Bal, Z.; Kaito, T.; Korkusuz, F.; Yoshikawa, H. Bone regeneration with hydroxyapatite-based biomaterials. Emergent Mater. 2020, 3, 521–544. [Google Scholar] [CrossRef]
- Huang, Z.; Wan, Y.; Peng, M.; Yang, Z.; Luo, H. Incorporating nanoplate-like hydroxyapatite into polylactide for biomimetic nanocomposites via direct melt intercalation. Compos. Sci. Technol. 2020, 185, 107903. [Google Scholar] [CrossRef]
- Kuczumow, A.; Gorzelak, M.; Kosiński, J.; Lasota, A.; Blicharski, T.; Gągała, J.; Nowak, J.; Jarzębski, M.; Jabłoński, M. Hierarchy of Bioapatites. Int. J. Mol. Sci. 2022, 23, 9537. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zeng, H.; Hao, L.; Zhao, N.; Du, C.; Liao, H.; Wang, Y. Effects of hydroxyapatite microparticle morphology on bone mesenchymal stem cell behavior. J. Mater. Chem. B 2014, 2, 4703–4710. [Google Scholar] [CrossRef]
- Bohner, M.; Le Gars Santoni, B.; Döbelin, N. β-Tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Kovrlija, I.; Locs, J.; Loca, D. Octacalcium phosphate: Innovative vehicle for the local biologically active substance delivery in bone regeneration. Acta Biomater. 2021, 135, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Hayashi, K. Carbonate apatite artificial bone. Sci. Technol. Adv. Mater. 2021, 22, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Sakae, T.; Nakada, H.; Kaneda, T.; Okada, H. Confusion between Carbonate Apatite and Biological Apatite (Carbonated Hydroxyapatite) in Bone and Teeth. Minerals 2022, 12, 170. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Tavtorkin, A.N.; Legkov, S.A.; Korchagina, S.A.; Shandryuk, G.A.; Kretov, E.A.; Dmitrienko, A.O.; Ivchenko, P.V. Hydrothermal synthesis of perfectly shaped micro- and nanosized carbonated apatite. Inorg. Chem. Front. 2021, 8, 4976–4989. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, H.; Liu, Y.; Tan, J.; Chen, W.; Ying, C.; Liu, Q.; Fu, X.; Hua, S.; Wong, C.P. Homogeneously dispersed composites of hydroxyapatite nanorods and poly(lactic acid) and their mechanical properties and crystallization behavior. Compos. A Appl. Sci. Manuf. 2020, 132, 105841. [Google Scholar] [CrossRef]
- Qi, J.; Xiao, J.; Zhang, T.; Zhang, Y.; Xiong, C. Investigation of the nano-hydroxyapatite with different surface modifications on the properties of poly(lactide-co-glycolide acid)/poly(trimethylene carbonate)/nano-hydroxyapatite composites. Coll. Polym. Sci. 2021, 299, 623–635. [Google Scholar] [CrossRef]
- Qiu, X.; Chen, L.; Hu, J.; Sun, J.; Hong, Z.; Liu, A.; Chen, X.; Jing, X. Surface-modified hydroxyapatite linked by L-lactic acid oligomer in the absence of catalyst. J. Polym. Sci. A Polym. Chem. 2005, 43, 5177–5185. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Wang, Y.; Ito, Y.; Zhang, P.; Chen, X. Enhanced in Vitro Mineralization and in Vivo Osteogenesis of Composite Scaffolds through Controlled Surface Grafting of L-Lactic Acid Oligomer on Nanohydroxyapatite. Biomacromolecules 2016, 17, 818–829. [Google Scholar] [CrossRef]
- Yi, W.-J.; Qiu, Z.-S.; He, H.; Liu, B.; Wang, M.; Jiang, M.; Chao, Z.-S.; Li, L.-J.; Shen, Y.-Y.; Shen, Y. Introduction of an interface layer on hydroxyapatite whisker/poly(L-lactide) composite and its contribution for improved bioactivity and mechanical properties. Nanotechnology 2020, 31, 235703. [Google Scholar] [CrossRef]
- Diao, H.; Si, Y.; Zhu, A.; Ji, L.; Shi, H. Surface modified nano-hydroxyapatite/poly(lactide acid) composite and its osteocyte compatibility. Mater. Sci. Eng. C 2012, 32, 1796–1801. [Google Scholar] [CrossRef]
- Feng, P.; Qiu, X.; Yang, L.; Liu, Q.; Zhou, C.; Hu, Y.; Shuai, C. Polydopamine constructed interfacial molecular bridge in nano-hydroxylapatite/polycaprolactone composite scaffold. Coll. Surf. B Biointerfaces 2022, 217, 112668. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, X.; Zhang, D.; He, M.; Qin, S.; Yu, J. Preparation and properties of polylactide/hydroxyapatite/polydopamine composites. Polym. Eng. Sci. 2018, 58, 2256–2263. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, X.; Chen, X.; Zeng, X.; Luo, K. Programmable, biodegradable composite scaffolds with variable pore morphology for minimal invasive bone repair. Compos. A Appl. Sci. Manuf. 2022, 162, 107130. [Google Scholar] [CrossRef]
- Haojie, D.; Liuyun, J.; Bingli, M.; Shengpei, S. Preparation of a Highly Dispersed Nanohydroxyapatite by a New Surface-Modification Strategy Used as a Reinforcing Filler for Poly(lactic-co-glycolide). Ind. Eng. Chem. Res. 2018, 57, 17119–17128. [Google Scholar] [CrossRef]
- Carette, X.; Mincheva, R.; Gonon, M.F.; Raquez, J.-M. A simple approach for a PEG-b-PLA-compatibilized interface in PLA/HAp nanocomposite. From the design of the material to the improvement of thermal/mechanical properties and bioactivity. J. Appl. Polym. Sci. 2022, 139, e52807. [Google Scholar] [CrossRef]
- Yi, W.; Li, L.; He, H.; Hao, Z.; Liu, B.; Chao, Z.; Shen, Y. Synthesis of poly(L-lactide)/β-cyclodextrin/citrate network modified hydroxyapatite and its biomedical properties. New J. Chem. 2018, 42, 14729–14732. [Google Scholar] [CrossRef]
- Ma, Z.; Zeng, F.; Wang, J.; Yang, S.; Liu, C. Enhanced cell affinity of PHBHHx composite scaffold with polylactide-graft-hydroxyapatite as compatibilizer. Mater. Sci. Eng. C 2017, 80, 472–483. [Google Scholar] [CrossRef]
- Ko, H.-S.; Lee, S.; Lee, D.; Jho, J.Y. Mechanical Properties and Bioactivity of Poly(Lactic Acid) Composites Containing Poly(Glycolic Acid) Fiber and Hydroxyapatite Particles. Nanomaterials 2021, 11, 249. [Google Scholar] [CrossRef]
- Ko, H.-S.; Lee, S.; Jho, J.Y. Synthesis and Modification of Hydroxyapatite Nanofiber for Poly(Lactic Acid) Composites with Enhanced Mechanical Strength and Bioactivity. Nanomaterials 2021, 11, 213. [Google Scholar] [CrossRef]
- Goranova, K.L.; Overgaard, A.K.K.S.; Gitsov, I. Hydroxyapatite-poly(d,l-lactide) Nanografts. Synthesis and Characterization as Bone Cement Additives. Molecules 2021, 26, 424. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yu, L.; Feng, P.; Peng, S.; Pan, H.; Bai, X. Construction of a stereocomplex between poly(D-lactide) grafted hydroxyapatite and poly(L-lactide): Toward a bioactive composite scaffold with enhanced interfacial bonding. J. Mater. Chem. B 2022, 10, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Yang, S.; Cao, Y.; Yang, F.; Wu, J. The reactivity of surface modification of hydroxyapatite particles depends on their shape. Polym. Compos. 2020, 41, 1500–1506. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Baciu, D.; Gounari, E.; Steriotis, T.; Charalambopoulou, G.; Bikiaris, D. Biocompatible Nanobioglass Reinforced Poly(ε-Caprolactone) Composites Synthesized via In Situ Ring Opening Polymerization. Polymers 2018, 10, 381. [Google Scholar] [CrossRef]
- Moszner, N.; Salz, U.; Zimmermann, J. Chemical aspects of self-etching enamel-dentin adhesives: A systematic review. Dent. Mater. 2005, 21, 895–910. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Ivchenko, P.V. Design, Synthesis and Actual Applications of the Polymers Containing Acidic P–OH Fragments: Part 2—Sidechain Phosphorus-Containing Polyacids. Int. J. Mol. Sci. 2023, 24, 1613. [Google Scholar] [CrossRef]
- Iwasaki, Y. Bone Mineral Affinity of Polyphosphodiesters. Molecules 2020, 25, 758. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Katayama, K.; Yoshida, M.; Yamamoto, M.; Tabata, Y. Comparative physicochemical properties and cytotoxicity of polyphosphoester ionomers with bisphosphonates. J. Biomater. Sci. Polym. Ed. 2012, 24, 882–895. [Google Scholar] [CrossRef]
- Hirano, Y.; Iwasaki, Y. Bone-specific poly(ethylene sodium phosphate)-bearing biodegradable nanoparticles. Coll. Surf. B Biointerfaces 2017, 153, 104–110. [Google Scholar] [CrossRef]
- Noree, S.; Iwasaki, Y. Thermally Assisted Generation of Protein–Poly(ethylene sodium phosphate) Conjugates with High Mineral Affinity. ACS Omega 2019, 4, 3398–3404. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Yokota, A.; Otaka, A.; Inoue, N.; Yamaguchi, A.; Yoshitomi, T.; Yoshimotode, K.; Neo, M. Bone-targeting poly(ethylene sodium phosphate). Biomater. Sci. 2018, 6, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Nifant’ev, I.E.; Ivchenko, P.V. Design, Synthesis and Actual Applications of the Polymers Containing Acidic P–OH Fragments: Part 1. Polyphosphodiesters. Int. J. Mol. Sci. 2022, 23, 14857. [Google Scholar] [CrossRef] [PubMed]
- Nifant’ev, I.; Bukharova, T.; Dyakonov, A.; Goldshtein, D.; Galitsyna, E.; Kosarev, M.; Shlyakhtin, A.; Gavrilov, D.; Ivchenko, P. Osteogenic Differentiation of Human Adipose Tissue-Derived MSCs by Non-Toxic Calcium Poly(ethylene phosphate)s. Int. J. Mol. Sci. 2019, 20, 6242. [Google Scholar] [CrossRef] [PubMed]
- Otaka, A.; Kiyono, K.; Iwasaki, Y. Enhancement of osteoblast differentiation using poly(ethylene sodium phosphate). Materialia 2021, 15, 100977. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Gavrilov, D.; Tavtorkin, A.; Chinova, M.; Besprozvannykh, V.; Komarov, P.; Zaitsev, V.; Podoprigora, I.; Ivchenko, P. Antibacterial Poly(ε-CL)/Hydroxyapatite Electrospun Fibers Reinforced by Poly(ε-CL)-b-poly(ethylene phosphoric acid). Int. J. Mol. Sci. 2021, 22, 7690. [Google Scholar] [CrossRef]
- Deguchi, K.; Nomura, S.; Tsuchiya, A.; Takahashi, I.; Ishikawa, K. Effects of the carbonate content in carbonate apatite on bone replacement. J. Tissue Eng. Regener. Med. 2022, 16, 200–206. [Google Scholar] [CrossRef]
- Safarzadeh, M.; Chee, C.F.; Ramesh, S. Effect of carbonate content on the in vitro bioactivity of carbonated hydroxyapatite. Ceram. Int. 2022, 48, 18174–18179. [Google Scholar] [CrossRef]
- Pastero, L.; Bruno, M.; Aquilano, D. Habit Change of Monoclinic Hydroxyapatite Crystals Growing from Aqueous Solution in the Presence of Citrate Ions: The Role of 2D Epitaxy. Crystals 2018, 8, 308. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Komarov, P.D.; Tavtorkin, A.N.; Minyaev, M.E.; Kosarev, M.A.; Ivchenko, P.V. Synthesis in aqueous media of poly(ethylene phosphoric acids) by mild thermolysis of homopolymers and block copolymers based on tert-butyl ethylene phosphate. Eur. Polym. J. 2018, 106, 249–256. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Shlyakhtin, A.; Kosarev, M.; Karchevsky, S.; Ivchenko, P. Mechanistic Insights of BHT-Mg-Catalyzed Ethylene Phosphate’s Coordination Ring-Opening Polymerization: DFT Modeling and Experimental Data. Polymers 2018, 10, 1105. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Shlyakhtin, A.; Kosarev, M.; Gavrilov, D.; Karchevsky, S.; Ivchenko, P. DFT Visualization and Experimental Evidence of BHT-Mg-Catalyzed Copolymerization of Lactides, Lactones and Ethylene Phosphates. Polymers 2019, 11, 1641. [Google Scholar] [CrossRef]
- Shikinami, Y.; Okuno, M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly-L-lactide (PLLA): Part I. Basic Charact. Biomater. 1999, 20, 859–877. [Google Scholar] [CrossRef]
- Hong, Z.; Zhang, P.; He, C.; Qiu, X.; Liu, A.; Chen, L.; Chen, X.; Jing, X. Nano-composite of poly(l-lactide) and surface grafted hydroxyapatite: Mechanical properties and biocompatibility. Biomaterials 2005, 26, 6296–6304. [Google Scholar] [CrossRef]
- Qiu, X.; Hong, Z.; Hu, J.; Chen, L.; Chen, X.; Jing, X. Hydroxyapatite Surface Modified by l-Lactic Acid and Its Subsequent Grafting Polymerization of l-Lactide. Biomacromolecules 2005, 6, 1193–1199. [Google Scholar] [CrossRef]
- Pérez, E. Mechanical performance of in vitro degraded polylactic acid/hydroxyapatite composites. J. Mater. Sci. 2021, 56, 19915–19935. [Google Scholar] [CrossRef]
- Omar, A.A.; Mohd Hanafi, M.H.; Razak, N.H.; Ibrahim, A.; Ab Razak, N.A. A Best-Evidence Review of Bio-based Plasticizer and the Effects on the Mechanical Properties of PLA. Chem. Eng. Trans. 2021, 89, 241–246. [Google Scholar] [CrossRef]
- Li, H.; Xue, F.; Bai, J.; Bai, X.; Ye, W.; Zhang, L.; He, Q.; Ju, J.; Ding, H. In vitro degradation of self-reinforced poly(lactic acid) with beta-tricalcium phosphate composite prepared by equal channel angular pressing. Polym. Compos. 2020, 41, 4054–4063. [Google Scholar] [CrossRef]
- Torres-Huerta, A.M.; Del Angel-López, D.; Domínguez-Crespo, M.A.; Palma-Ramírez, D.; Perales-Castro, M.E.; Flores-Vela, A. Morphological and Mechanical Properties Dependence of PLA Amount in PET Matrix Processed by Single-Screw Extrusion. Polym. Plast. Technol. Eng. 2016, 55, 672–683. [Google Scholar] [CrossRef]
- Wei, J.; Sun, J.; Wang, H.; Chen, X.; Jing, X. Isothermal crystallization behavior and unique banded spherulites of hydroxyapatite/poly(L-lactide) nanocomposites. Chin. J. Polym. Sci. 2010, 28, 499–507. [Google Scholar] [CrossRef]
- Moreno de Almeida, J.F.; Nazareth da Silva, A.L.; Escócio, V.A.; Monteiro da Fonseca Thomé da Silva, A.H.; Furtado de Sousa, A.M.; Nascimento, C.R.; Bertolino, L.C. Rheological, mechanical and morphological behavior of polylactide/nano-sized calcium carbonate composites. Polym. Bull. 2016, 73, 3531–3545. [Google Scholar] [CrossRef]
- Grivet-Brancot, A.; Boffito, M.; Ciardelli, G. Use of Polyesters in Fused Deposition Modeling for Biomedical Applications. Macromol. Biosci. 2022, 22, 2200039. [Google Scholar] [CrossRef] [PubMed]
- Zavřel, F.; Novák, M.; Kroupová, J.; Beveridge, C.; Štěpánek, F.; Ruphuy, G. Development of Hot-Melt Extrusion Method to Produce Hydroxyapatite/Polycaprolactone Composite Filaments. Adv. Eng. Mater. 2022, 24, 2100820. [Google Scholar] [CrossRef]
- Liu, K.; Sun, J.; Zhu, Q.; Jin, X.; Zhang, Z.; Zhao, Z.; Chen, G.; Wang, C.; Jiang, H.; Zhang, P. Microstructures and properties of polycaprolactone/tricalcium phosphate scaffolds containing polyethylene glycol fabricated by 3D printing. Ceram. Int. 2022, 48, 24032–24043. [Google Scholar] [CrossRef]
- Nyberg, E.; Rindone, A.; Dorafshar, A.; Grayson, W.L. Comparison of 3D-Printed Poly-ε-Caprolactone Scaffolds Functionalized with Tricalcium Phosphate, Hydroxyapatite, Bio-Oss, or Decellularized Bone Matrix. Tissue Eng. A 2017, 23, 503–514. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Minyaev, M.E.; Churakov, A.V.; Karchevsky, S.G.; Birin, K.P.; Ivchenko, P.V. Mono-BHT heteroleptic magnesium complexes: Synthesis, molecular structure and catalytic behavior in the ring-opening polymerization of cyclic esters. Dalton Trans. 2017, 46, 12132–12146. [Google Scholar] [CrossRef]
- Schöttler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailänder, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Komarov, P.D.; Kosarev, M.A.; Tavtorkin, A.N.; Minyaev, M.E.; Roznyatovsky, V.A.; Ivchenko, P.V. Controlled ring-opening polymerisation of cyclic phosphates, phosphonates and phosphoramidates catalysed by hereroleptic BHT-alkoxy magnesium complexes. Polym. Chem. 2017, 8, 6806–6816. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- ISO 527-2:2012; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. ISO: Geneva, Switzerland, 2012. Available online: https://www.iso.org/ru/standard/56046.html (accessed on 3 July 2023).
- ASTM D638-14; Standard Test Method for Tensile Properties of Plastics. ASTM: West Conshohocken, PA, USA, 2022. Available online: https://www.astm.org/d0638-14.html (accessed on 3 July 2023).
- ISO 178:2010; Plastics—Determination of flexural properties. ISO: Geneva, Switzerland, 2010. Available online: https://www.iso.org/ru/standard/45091.html (accessed on 3 July 2023).
- Khan, S.A.; Prud’homme, R.K. Melt Rheology of Filled Thermoplastics. Rev. Chem. Eng. 1987, 4, 205–272. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Sadrtdinova, G.I.; Komarov, P.D.; Minyaev, M.E.; Ilyin, S.O.; Kiselev, A.V.; Samurganova, T.I.; Ivchenko, P.V. Synthesis, molecular structure and catalytic performance of heterocycle-fused cyclopentadienyl-amido CGC of Ti (IV) in ethylene (co)polymerization: The formation and precision rheometry of long-chain branched polyethylenes. Eur. Polym. J. 2022, 176, 111397. [Google Scholar] [CrossRef]
- Öhman-Mägi, C.; Holub, O.; Wu, D.; Hall, R.M.; Persson, C. Density and mechanical properties of vertebral trabecular bone—A review. JOR Spine 2021, 4, e1176. [Google Scholar] [CrossRef]
- Madupalli, H.; Pavan, B.; Tecklenburg, M.M.J. Carbonate substitution in the mineral component of bone: Discriminating the structural changes, simultaneously imposed by carbonate in A and B sites of apatite. J. Solid State Chem. 2017, 255, 27–35. [Google Scholar] [CrossRef]
- Yao, S.; Qi, M.-I.; Qi, L.; Ding, Y.; Chen, M.; Wang, Y. Investigation of EDTA concentration on the size of carbonated flowerlike hydroxyapatite microspheres. R. Soc. Open Sci. 2021, 8, 202148. [Google Scholar] [CrossRef]
- Fleet, M.E. Infrared spectra of carbonate apatites: γ2-Region bands. Biomaterials 2009, 30, 1473–1481. [Google Scholar] [CrossRef]
- Grunenwald, A.; Keyser, C.; Sautereau, A.M.; Crubézy, E.; Ludes, B.; Drouet, C. Revisiting carbonate quantification in apatite (bio)minerals: A validated FTIR methodology. J. Archaeol. Sci. 2014, 49, 134–141. [Google Scholar] [CrossRef]
- Ren, F.; Ding, Y.; Leng, Y. Infrared spectroscopic characterization of carbonated apatite: A combined experimental and computational study. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 496–505. [Google Scholar] [CrossRef]




| CAp Type | Crystal Cell Parameters, XRD | CSR, nm 1 | wt% CO32− | C/P Ratio 3 | Na/Ca Ratio 3 | ||
|---|---|---|---|---|---|---|---|
| a, Å | c, Å | FT-IR Data | TGA Data 2 | ||||
| hCAp | 9.412 | 6.944 | >1000 | 12.1 | 9.6 | 0.42 | 0.18 |
| pCAp | 9.430 | 6.915 | >1000 | 4.9 | 4.0 | 0.19 | 0.08 |
| Comp. Sample | Addition of C1, 5 wt% | BMS | BMS wt% | Tensile Test | Flexural Test | |||
|---|---|---|---|---|---|---|---|---|
| ε, % | σt, MPa | Et, GPa | σf, MPa | Ef, GPa | ||||
| PLLA | − | no filler | 0 | 2.46 ± 0.33 | 53.2 ± 1.7 | 2.18 ± 0.15 | 99.7 ± 5.6 | 3.31 ± 0.32 |
| PL01 | − | βTCP | 10 | 3.37 ± 0.24 | 47.0 ± 0.4 | 2.26 ± 0.11 | 92.3 ± 1.9 | 3.70 ± 0.13 |
| PL02 | − | βTCP | 25 | 2.60 ± 0.16 | 46.1 ± 1.1 | 2.45 ± 0.12 | 91.0 ± 1.9 | 4.47 ± 0.09 |
| PL03 | − | βTCP | 50 | 1.69 ± 0.05 | 43.6 ± 0.9 | 2.81 ± 0.15 | 80.9 ± 1.2 | 5.86 ± 0.15 |
| PL04 | − | hCAp | 10 | 3.44 ± 0.21 | 41.8 ± 1.1 | 2.33 ± 0.18 | 93.3 ± 2.1 | 3.55 ± 0.12 |
| PL05 | − | hCAp | 25 | 3.10 ± 0.81 | 36.0 ± 1.2 | 2.57 ± 0.26 | 87.9 ± 1.8 | 4.49 ± 0.15 |
| PL06 | − | hCAp | 50 | 1.33 ± 0.12 | 33.5 ± 1.0 | 2.86 ± 0.20 | 76.3 ± 0.8 | 5.76 ± 0.11 |
| PL07 | − | pCAp | 10 | 2.54 ± 0.19 | 47.8 ± 1.6 | 2.40 ± 0.09 | 94.5 ± 1.8 | 4.11 ± 0.09 |
| PL08 | − | pCAp | 25 | 1.64 ± 0.16 | 49.1 ± 2.0 | 2.78 ± 0.17 | 88.8 ± 4.1 | 5.49 ± 0.06 |
| PL09 | − | pCAp | 50 | 1.27 ± 0.06 | 47.3 ± 0.8 | 3.29 ± 0.18 | 79.3 ± 5.2 | 9.03 ± 0.38 |
| PL10 | − | pCAp | 60 | 1.08 ± 0.07 | 42.2 ± 3.6 | 3.42 ± 0.18 | 64.1 ± 13.1 1 | 10.12 ± 0.85 |
| PL11 | + | pCAp | 10 | 3.52 ± 0.91 | 52.6 ± 3.4 | 2.53 ± 0.27 | 92.6 ± 3.6 | 7.70 ± 0.52 |
| PL12 | + | pCAp | 25 | 2.54 ± 0.14 | 62.2 ± 1.4 | 2.69 ± 0.11 | 93.8 ± 3.1 | 8.09 ± 0.33 |
| PL13 | + | pCAp | 50 | 1.54 ± 0.09 | 52.9 ± 1.3 | 3.08 ± 0.06 | 82.6 ± 4.4 | 9.51 ± 0.74 |
| PL14 | + | pCAp | 60 | 1.34 ± 0.02 | 51.4 ± 1.6 | 3.35 ± 0.18 | 75.4 ± 3.7 | 11.22 ± 0.62 |
| Comp. Sample | 1st Heating | Cooling after 1st Heating | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tg, °C | ΔCp 2, J·g−1·K−1 | Tc, °C | ΔHc 2, J·g−1 | Tm, °C | ΔHm 2, J·g−1 | χc 3, % | Tg, °C | ΔCp 2, J·g−1·K−1 | |
| PLLA | 62.2 | 0.55 | 118.4 | 23.5 | 154.3 | 25.0 | 1.6 | 55.5 | 0.51 |
| PL02 | 58.6 | 0.52 | 108.7 | 24.6 | 153.0 | 27.6 | 3.2 | 55.1 | 0.55 |
| PL05 | 55.8 | 0.48 | 103.5 | 23.7 | 151.3 | 24.9 | 1.1 | 55.4 | 0.49 |
| PL07 | 56.4 | 0.35 | 109.2 | 27.7 | 155.3 | 30.9 | 3.6 | 54.5 | 0.47 |
| PL08 | 56.9 | 0.48 | 107.2 | 23.5 | 154.7 | 31.0 | 8.0 | 54.8 | 0.50 |
| PL09 | 58.6 | 0.50 | 108.1 | 23.6 | 153.1 | 25.6 | 4.3 | 53.7 | 0.49 |
| PL11 | 52.9 | 0.41 | 102.4 | 24.3 | 154.8 | 30.9 | 7.1 | 54.8 | 0.53 |
| PL12 | 56.6 | 0.33 | 107.9 | 21.8 | 155.0 | 29.2 | 7.9 | 54.3 | 0.59 |
| PL13 | 57.3 | 0.24 | 108.5 | 24.9 | 154.9 | 29.7 | 5.1 | 54.6 | 0.42 |
| Composite | Mw | Mn | ÐM | η0, Pa·s |
|---|---|---|---|---|
| PLLA | 173.62 | 90.54 | 1.92 | 1694 |
| PL07 | 111.32 | 45.69 | 1.97 | 124 |
| PL08 | 113.42 | 58.75 | 1.93 | 561 |
| PL11 | 121.23 | 61.68 | 2.44 | 212 |
| PL12 | 153.38 | 78.10 | 1.96 | 1304 |
| Comp. Sample | Addition of C1, 5 wt% | BMS | BMS wt% | Tensile Test | Flexural Test | |||
|---|---|---|---|---|---|---|---|---|
| ε, % | σt, MPa | Et, GPa | σf, MPa | Ef, GPa | ||||
| PCL | − | no filler | 0 | >100 1 | 15.0 ± 0.8 | 0.46 ± 0.03 | 23.7 ± 2.0 | 0.66 ± 0.06 |
| PC01 | − | βTCP | 50 | 16.8 ± 2.8 | 18.4 ± 1.0 | 1.53 ± 0.04 | 26.4 ± 1.2 | 1.15 ± 0.09 |
| PC02 | − | hCAp | 50 | 17.6 ± 2.6 | 15.2 ± 1.4 | 1.22 ± 0.05 | 24.4 ± 1.4 | 1.25 ± 0.03 |
| PC03 | − | pCAp | 50 | 6.2 ± 0.8 | 16.5 ± 0.4 | 1.48 ± 0.06 | 37.2 ± 1.0 | 2.55 ± 0.04 |
| PC04 | + | pCAp | 50 | 8.1 ± 0.8 | 28.3 ± 0.6 | 1.85 ± 0.15 | 41.4 ± 1.7 | 2.67 ± 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nifant’ev, I.; Tavtorkin, A.; Komarov, P.; Kretov, E.; Korchagina, S.; Chinova, M.; Gavrilov, D.; Ivchenko, P. Dispersant and Protective Roles of Amphiphilic Poly(ethylene phosphate) Block Copolymers in Polyester/Bone Mineral Composites. Int. J. Mol. Sci. 2023, 24, 11175. https://doi.org/10.3390/ijms241311175
Nifant’ev I, Tavtorkin A, Komarov P, Kretov E, Korchagina S, Chinova M, Gavrilov D, Ivchenko P. Dispersant and Protective Roles of Amphiphilic Poly(ethylene phosphate) Block Copolymers in Polyester/Bone Mineral Composites. International Journal of Molecular Sciences. 2023; 24(13):11175. https://doi.org/10.3390/ijms241311175
Chicago/Turabian StyleNifant’ev, Ilya, Alexander Tavtorkin, Pavel Komarov, Egor Kretov, Sofia Korchagina, Maria Chinova, Dmitry Gavrilov, and Pavel Ivchenko. 2023. "Dispersant and Protective Roles of Amphiphilic Poly(ethylene phosphate) Block Copolymers in Polyester/Bone Mineral Composites" International Journal of Molecular Sciences 24, no. 13: 11175. https://doi.org/10.3390/ijms241311175
APA StyleNifant’ev, I., Tavtorkin, A., Komarov, P., Kretov, E., Korchagina, S., Chinova, M., Gavrilov, D., & Ivchenko, P. (2023). Dispersant and Protective Roles of Amphiphilic Poly(ethylene phosphate) Block Copolymers in Polyester/Bone Mineral Composites. International Journal of Molecular Sciences, 24(13), 11175. https://doi.org/10.3390/ijms241311175








